Abstract
Following living-donor liver transplantation (LDLT) (and unlike deceased-donor liver transplantation (DDLT)), the liver must rapidly regenerate, and sometimes segmental graft dysfunction (SGD) is observed. Hepatic regeneration requires substantial de novo lipid synthesis, and we previously reported that expression of lipid-related genes is dysregulated in LDLT. Here, we compare serum lipid measurments in 41 LDLT recipients and 43 DDLT recipients at baseline and at serial post-transplant timepoints. In addition, we examined whether serum lipid/apolipoprotein levels correlate with degree of liver regeneration (measured using %volume increase (%VI) at 3 months) or SGD in LDLT recipients. In contrast to DDLT, lipid levels declined early after LDLT, but returned to baseline by 30 days. The odds ratio (OR) for achieving robust regeneration (>90%VI) was 2.53 (95%CI: 1.15, 5.52) for every 1 mg/dL increase in serum ApoE at 30 days. The OR of SGD for every year increase in donor age was 1.19 (95%CI: 1.02, 1.39), and 0.61 for every 1 mg/dL increase in serum HDL-C at 7 days (95%CI: 0.34, 1.11). No associations were detected between pre-operative serum lipids/apolipoproteins in LDLT donors and SGD or %VI in recipients. Conclusion: We suggest that initiation of regeneration prevents the liver from participating fully in lipid transport and metabolism. Inability to meet systemic metabolic needs may result in compromised liver function and SGD. Certain serum lipid concentrations correlate with extent of liver regeneration and function.
Keywords: apolipoprotein, cholesterol, hepatic regeneration, hepatectomy, lipid metabolism
INTRODUCTION
The liver is the primary organ responsible for the synthesis, secretion, and metabolism of lipids. Plasma lipids are largely carried in lipoproteins, macromolecular structures that include proteins called apolipoproteins, which are mostly synthesized and secreted by the liver [1]. We have shown previously that hepatic lipid genomic pathways are dysregulated in the immediate period following adult-to-adult living donor liver transplantation (LDLT).[2] In contrast to deceased donor liver transplantation (DDLT) with a whole organ, LDLT donors and recipients are both left with only a portion of liver, and functional recovery therefore depends on the liver’s unique ability to regenerate new parenchymal mass. Liver regeneration is a highly orchestrated biological process, integrating a multitude of complex interactions between inflammatory mediators, growth factors and metabolic signals.[3] In LDLT recipients, these events place an energy burden on the expanding graft, which must balance available resources between metabolic homeostasis and regeneration. LDLT grafts that fail to meet this challenge will exhibit delayed functional recovery, also known as small-for-size–syndrome (SFSS) or segmental graft dysfunction (SGD), which can contribute to graft failure, urgent re-transplantation, or patient demise.[4, 5]
Based on our previous findings, we speculated that the liver’s participation in systemic lipid transport and metabolism will be initially suppressed after LDLT, when available energy is shunted toward cell growth pathways, and that it will be subsequently restored as the graft regenerates to an appropriate size. Prior studies from other laboratories have characterized the serum levels for various lipids and apolipoproteins during periods of liver recovery in different surgical contexts, including hepatectomy, LDLT and DDLT [6–14]; however, none of these studies have directly compared LDLT and DDLT responses, and none have examined the relationship between serum lipids and graft regeneration. In this investigation, we aimed to test two related hypotheses: (1) that early serum kinetics for lipids and apolipoproteins differ between LDLT and DDLT recipients, reflecting the unique energy demands placed on the liver during early regeneration in LDLT recipients; (2) that the degree of liver regeneration in LDLT recipients and risk of SGD is associated with levels of serum lipids.
PATIENTS AND METHODS
Study populations and design
This is a prospective cohort study of 41 right-lobe LDLT recipients, whose surgeries were performed at 3 transplant centers (University of Pennsylvania, Columbia University and Northwestern University) in the United States between 2006 and 2009 as part of an ancillary study to the Adult-to-Adult Living Donor Liver Transplantation (A2ALL) consortium. Institutional Review Board (IRB) approval was obtained prior to investigation (IRB805469 and IRBAAAC4418). The first portion was a matched case-control study in which 43 whole-graft DDLT recipients were selected as controls from University of Pennsylvania based on five selection criteria: donor age <55 years, recipient age <65 years, cold ischemic time (CIT) <8 hours, negative donor HCV status, and post-transplant outcome (graft intact and patient alive at 6 months). These criteria were chosen in order to control for differences in graft quality and host status. SGD was defined by either total serum bilirubin ≥10 mg/dL or INR ≥1.7 at 7 days after transplant, or graft loss within 3 months not attributed to vascular thrombosis.[15]
Assessment of graft regeneration
Graft weight was measured on the backbench and used as initial post-transplant graft volume. Final graft volume was assessed with magnetic resonance imaging (MRI) and volumetric analysis, which were performed locally at each center 3 months following LDLT. Liver regeneration was measured as the percent volume increase (%VI), i.e. how much liver tissue was generated during the post-operative period compared to the volume at the onset of liver regeneration (%VI = ([final graft volume/initial graft volume] −1) × 100). Of the 41 LDLT recipients in this study, 33 completed the necessary imaging studies required to evaluate graft regeneration in this manner. Graft volume was not assessed for DDLT recipients.
Measurement of serum lipids/apolipoproteins
Our investigation included measurement of both very low-density lipoprotein (VLDL)/low density lipoprotein (LDL)-associated lipids (including apolipoprotein-(Apo)-B, ApoC-III, Apo-E, and triglycerides), as well as those associated with the reverse cholesterol transport pathway (RCT)(including high-density lipoprotein cholesterol (HDL-C) and ApoA-I). Serum was collected immediately prior to transplant and on post-transplant days 1, 7, 14, and 30 and was stored at −80°C. Sampling success at each time-point for LDLT and DDLT is shown in Supplementary Table 1. Triglycerides, total cholesterol, HDL-C and the apolipoproteins were assayed in each available specimen using a Hitachi bioanalyzer. Calculated LDL cholesterol (cLDL-C) was determined using the Friedewald formula.[16]
Statistical methods
For comparisons of patient characteristics, continuous variables were described using means, medians, ranges and standard deviations, and hypothesis testing was performed with either the Student’s t-test (for normally distributed data) or Wilcoxon rank-sum (for data that were not normally distributed). Categorical variables were compared using Chi-squared testing. Serum lipids and apolipoproteins were evaluated with serum levels taken directly from Hitachi and absolute change from day 1 baselines. Both serum levels and changes were tested with Wilcoxon rank-sum in order to compare: (1) LDLT vs. DDLT at each individual time-point; and (2) different time-points within each group (for example: day 1 LDLT vs. day 30 LDLT). Results were represented graphically using Tukey box-and-whisker plots.
For the correlation studies, we used logistic regression. We dichotomized %VI over the median value (90%) and used it as a dependent binary variable (above-median %VI vs. below-median %VI). Different independent variables, i.e. patient characteristics and serum-derived co-variates – including donor and recipient age, BMI, cold ischemic time, MELD, and serum lipid levels – were all tested as to whether they were significantly associated with above-median %VI. Logistic regression was repeated with SGD as the categorical outcome variable. Models were tested using likelihood-ratio Chi-squared testing and McFadden’s R-squared. For all of the above testing, analyses were performed using Stata 11 and results were considered statistically significant if the type I error < 0.05.
RESULTS
Patient characteristics: LDLT vs. DDLT
Clinical parameters for the LDLT recipients and DDLT recipient controls are compared in Table 1. Donor and recipient age were used to select DDLT controls (as described above in Methods) and were therefore comparable. LDLT and DDLT were also similar with respect to donor and recipient gender and BMI. CIT and MELD, as expected, were both lower in the LDLT cohort. Hepatitis C was the most common indication for transplant in both LDLT (16/41) and DDLT (29/43). The other indications for LDLT included primary sclerosing cholangitis (n=9) and primary biliary cirrhosis (n=6), diagnoses that were not represented in the control group because no DDLT patients with these conditions met our selection criteria as listed above. LDLT outcomes were favorable overall, with 40/41 grafts intact at 90 days. 8 LDLT recipients met the criteria for SGD, 7 had subsequent recovery of graft function and the remaining recipient died of multi-organ failure on post-operative day 42. Among all LDLT recipients, total bilirubin at 7 days was significantly elevated compared to DDLT recipients (5.7 ± 4.1 vs. 2.6 ± 2.6 mg/dL; p<0.001), indicating delayed recovery of synthetic function that we have described previously.[2] We also compared characteristics for the 8 SGD recipients with the 32 normal recipients (Supplemental table 2). Significant differences included total bilirubin and INR, which were expected based on the definition of SGD, and donor age (median 28.9 in LDLT recipients without SGD vs. 47.2 in those with SGD; p<0.01).
Table 1.
Patient characteristics for LDLT and DDLT recipients
| LDLT Recipients (n=41) | DDLT Recipient (n=43) | P* | |
|---|---|---|---|
| Recipient characteristics | |||
| Age, years; min-max (median) | 27.0–68.8 (53.4) | 19.4–65.5 (56.6) | 0.33 |
| Race, White/Black/Other | 37/3/1 | 29/8/6 | 0.06 |
| Gender, Male/Female | 25/16 | 34/9 | 0.07 |
| BMI, kg/m2; min-max (median) | 19.0–45.1 (26.1) | 20.0–42.6 (26.1) | 0.59 |
| MELD at transplant; mean (SD; min-max) | 15.8 (5.6; 6–31) | 19.8 (5.3; 8–31) | <0.01 |
| Cold ischemic time, minutes; mean (SD; min-max) | 42 (22; 15–105) | 360 (82; 181–484) | <0.001 |
| Etiology, n | |||
| Hepatitis C virus | 16 | 28 | |
| Primary sclerosing cholangitis | 9 | 1 | |
| Primary biliary cirrhosis | 7 | 0 | |
| Alcoholic cirrhosis | 2 | 4 | |
| Cryptogenic/Other | 7 | 9 | |
| Donor characteristics | |||
| Age, years; min-max (median) | 22.1–59.8 (31.4) | 8.0–55.0 (41.0) | 0.80 |
| Gender, Male/Female | 19/22 | 23/20 | 0.51 |
| BMI, kg/m2; min-max (median) | 16.4–42.4 (25.3) | 15.1–40.8 (26.7) | 0.28 |
| Liver function, POD 7; mean (SD; min-max) | |||
| Total serum bilirubin, mg/dl | 5.7 (4.1; 0.92–14.2) | 2.5 (2.6; 0.61–14.9) | <0.001 |
| International normalized ratio (INR) | 1.2 (0.2; 0.83–1.7) | 1.2 (0.3; 0.91–2.6) | 0.13 |
| Liver volumes, mean (SD; min-max) | |||
| Standard liver volume (SLV), ml | 1760 (231; 1306–2273) | - | |
| Graft weight, g | 871 (231; 550–1452) | - | |
| Graft weight/SLV, % | 50.6 (12.9; 29.6–81.7) | - | |
| Graft weight/recipient weight, % | 1.10 (0.30; 0.54–1.86) | - | |
| Final liver volume, ml | 1639 (462; 1087–3764) | - | |
| Regeneration, mean (SD; min-max) | - | ||
| Volume Increase, % | 96.9 (56.5; 20.1–296.2) | - | |
| Absolute growth, g | 772 (453; 265–2814) | - | |
| Perioperative complication, n | |||
| Death, within 90 days | 1 | 0 | |
| Graft loss, within 90 days | 0 | 0 | |
Hypothesis testing was performed with either Wilcoxon ranksum or Student's ttest for continuous variables, Chi-squared for categorical variables.
Abbreviations: living-donor liver transplantation (LDLT); deceased-donor liver transplantation (DDLT); body-mass index (BMI); standard deviation (SD).
Lipid/apolipoprotein kinetics in LDLT and DDLT recipients
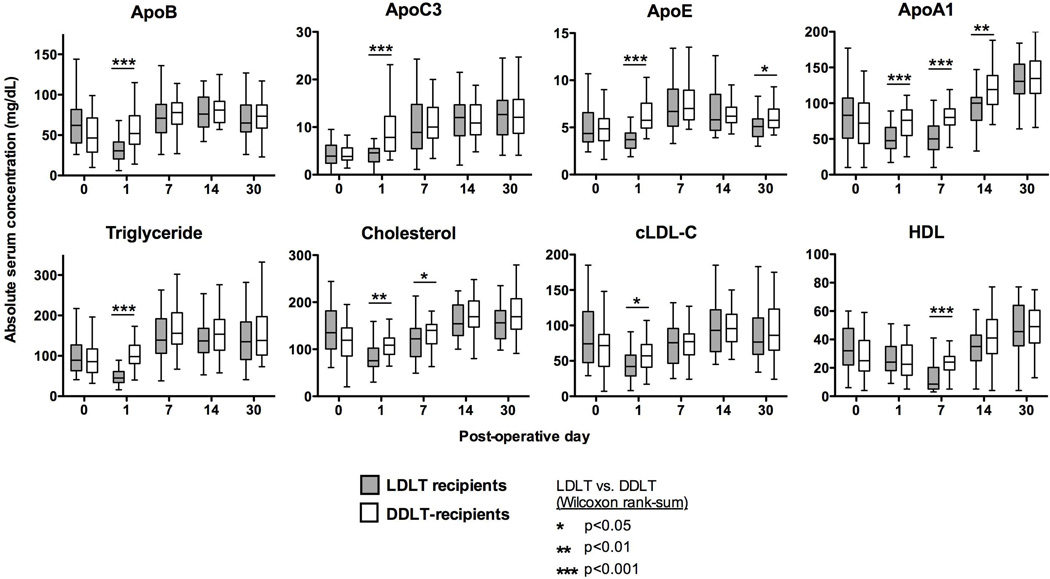
Lipid profiles for DDLT and LDLT were statistically similar pre-operatively, and again similar after 30 days post-transplant (Supplemental Table 3). Figure 1 shows the serum values measured at each individual time-point, and demonstrates that differences between the two groups were most pronounced on post-transplant day 1. At that time, LDLT recipients had significant reductions in triglycerides, total cholesterol, cLDL-C, apoB, apoE, and apoA-I compared to pre-transplant baselines (P<0.001 for all of the above; these P values not noted in Figure 1), whereas in DDLT recipients these remained similar to their pre-transplant serum concentrations, and apoC-III and apoE levels had actually increased (P<0.001 and P<0.01 respectively; these P values not noted in Figure 1). Accordingly, LDLT values for the majority of the measured lipids were lower than those for DDLT recipients on post-operative day 1 (P values are noted in Figure 1).
Figure 1. Absolute serum lipid levels for DDLT and LDLT recipients.
Serum lipid levels are shown as Tukey plots for each of the 5 measured timepoints in DDLT and LDLT recipients. Significant differences between the groups are noted with an asterisk; however, for visual simplification we have not included notation for significant differences within each group (example: LDLT day 0 vs. LDLT day 1). Abbreviations: cLDL = calculated low density lipoprotein.
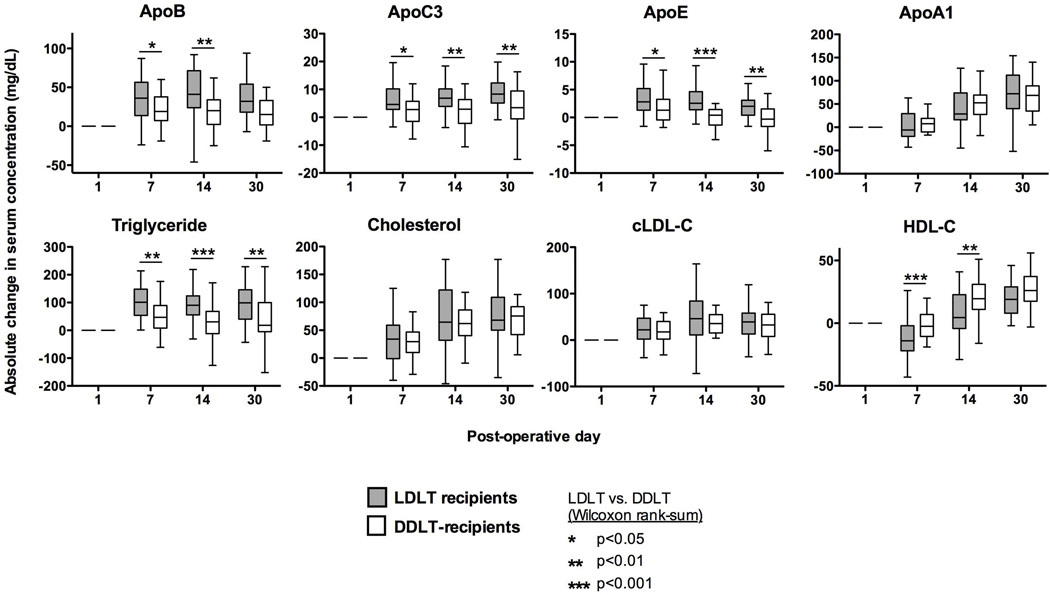
Figure 2 shows the absolute changes in serum concentration for the various components already mentioned. LDLT recipients had greater absolute changes in ApoB, ApoC-III and ApoE and triglycerides compared to DDLT. Notably, absolute change in total cholesterol was similar for LDLT and DDLT, These results implied that LDLT recipients had a change in their VLDL/LDL composition in comparison to DDLT recipients, specifically an enrichment of triglyceride. In both groups, VLDL/LDL components seemed to have more rapid kinetics than RCT components.
Figure 2. Relative lipid levels for DDLT and LDLT recipients.
Serum lipid levels are shown relative to post-transplant day 1 using Tukey plots for each of the 5 measured timepoints in DDLT and LDLT recipients. Significant differences between the groups are noted with an asterisk; however, for visual simplification we have not included notation for significant differences within each group (example: LDLT day 0 vs. LDLT day 1). Abbreviations: cLDL = calculated low density lipoprotein.
Modeling correlations between lipids/apolipoproteins and graft outcomes
Four variables were found to be significantly associated with superior graft regeneration, i.e. whether %VI would exceed the median value. These were: 30 day serum levels for ApoE, ApoC-III, and triglycerides, as well as donor BMI (Table 2). When these were each combined into a multi-termed equation, adjusting for donor and recipient age, only ApoE retained independent association (OR 2.53 per 1 mg/dl increase, 95%CI 1.15, 5.5). According to this equation, relative increases in 30-day ApoE of 1mg/ml between individuals corresponded to 2.5 times greater odds of achieving a %VI that exceeded the median. This model had good fitness, with a likelihood ratio of 12.7 (P<0.01) and a pseudo-R-squared of 0.34.
Table 2.
Predictors of higher liver regeneration at 3 months, logistic regression
| Odds ratio | 95% CI | Likelihood ratio |
P | Pseudo-R^2 | ||
|---|---|---|---|---|---|---|
| ApoE, mg/dL* | 2.20 | 1.07 | 4.50 | 7.59 | 0.01 | 0.20 |
| Donor BMI (kg/m^2) | 0.81 | 0.67 | 0.98 | 6.14 | 0.01 | 0.13 |
| ApoC-III, mg/dL* | 1.21 | 1.00 | 1.46 | 5.03 | 0.03 | 0.13 |
| Triglycerides, mg/dL* | 1.02 | 1.00 | 1.03 | 3.93 | 0.05 | 0.11 |
serum expression at 30 days
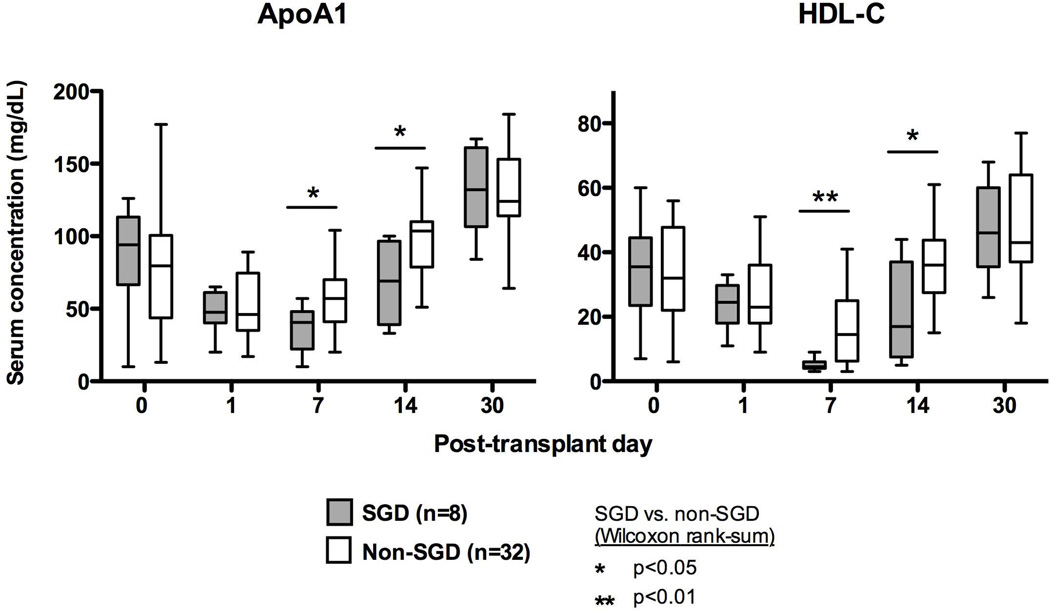
Results from a similar logistic regression analysis for SGD are shown in Table 3, and they suggested that serum levels of HDL-C and its major protein ApoA-I are inversely associated with odds of having SGD. As demonstrated in Figure 3, HDL-C and ApoA-I were lower in SGD patients on post-LDLT days 7 and 14, whereas ApoB lipoproteins were unaffected (not shown). Donor age was also significantly associated with SGD.
Table 3.
Predictors of SGD, logistic regression
| Odds ratio |
95% CI | Likelihood ratio |
P | Pseudo-R^2 | ||
|---|---|---|---|---|---|---|
| HDL, mg/dL* | 0.68 | 0.45 | 1.02 | 13.46 | <0.001 | 0.33 |
| ApoA-I, mg/dL** | 0.94 | 0.90 | 0.99 | 9.71 | <0.01 | 0.32 |
| HDL-C, mg/dL** | 0.88 | 0.80 | 0.97 | 9.60 | <0.01 | 0.32 |
| Donor age, years | 1.12 | 1.03 | 1.22 | 9.20 | <0.01 | 0.21 |
| ApoA-I, mg/dL* | 0.93 | 0.89 | 0.99 | 9.14 | <0.01 | 0.23 |
| ApoC-III, mg/dL* | 0.83 | 0.68 | 1.00 | 5.32 | 0.02 | 0.13 |
serum expression at 7 days
serum expression at 14 days
Figure 3. Differences in serum lipid levels for recipients with SGD.
Day 7 and day 14 levels of apoA-I and HDL were significantly lower in patients with SGD. Data are displayed with Tukey plots and comparisons were performed with Wilcoxon ranksum.
Logistic regression results were then used to construct a multinomial equation that was adjusted for recipient age. The final equation included HDL concentration at 7 days (OR 0.61, 95%CI 0.34, 1.11) and donor age (OR 1.19, 95%CI 1.02, 1.40), with a likelihood ratio of 21.9 (P<0.001) and a McFadden’s R-squared of 0.54. According to this model, the odds of having SGD increase almost 20% with each 1 year increase in donor age, and decrease almost 40% with every 1 mg/dL increase in serum HDL-C at 7 days.
Of note, pre-operative serum lipid/apolipoprotein levels from LDLT donors (Supplemental Table 4) were included in the above analyses but did not significantly associate with either %VI or SGD.
DISCUSSION
Our prior work demonstrated down-regulation of lipid-related genomic pathways at the onset of liver regeneration.[2] This observation led to our hypothesis that the energy demands placed on the regenerating graft by the activation of cell proliferation pathways would shunt resources away from basal metabolic functions, and postulated that serum lipid/apolipoprotein expression could be used to further study the process of graft regeneration in LDLT subjects. The current investigation compared peripheral expression peri-operatively in LDLT and DDLT recipients and sought to correlate these measures with graft growth and function. Significant findings included: (1) that LDLT recipients had lower levels of peripheral lipid expression in comparison to DDLT at post-transplant day 1, though they eventually recovered to the same serum concentrations by 30 days; (2) that the odds of achieving above-median regeneration at 3 months increases with serum ApoE expression at 30 days; and (3) that the odds of having SGD increases with donor age and decreases with the serum HDL-C concentration measured 1 week post-transplant.
The observational comparisons between LDLT and DDLT subjects are important because they highlight expression kinetics unique to a regenerating graft. We anticipated the initial drop in serum lipid levels for LDLT recipients based on our genomic analyses. Yet, despite this early nadir, LDLT and DDLT recipient lipid profiles were mostly similar by day 7, and nearly indistinguishable by day 30 (Supplementary Table 3). This suggested that despite smaller graft volumes, LDLT recipients had increases in serum lipid expression after day 1 that served to “catch up” and fully compensate for initially lower levels. Additionally, the changes noted for VLDL/LDL components (Figure 2), suggested a relative enrichment of triglycerides for LDLT subjects, a compositional change not present in DDLT. Taken together, these findings are reminiscent of the classic studies done by Delahunty and Rubinstein in a rat hepatectomy model, which demonstrated that the regenerating liver “rapidly acquires the ability to mobilize triglycerides at a rate equal to that of a much larger normal liver.”[17] Further studies are needed to help clarify whether the shifts in peripheral VLDL/LDL composition and expression level represent cause or effect of liver regeneration.
With respect to our correlation studies, we believe these findings are significant because they represent potential serum-derived markers for LD graft regeneration and function. We do acknowledge however, that the timepoints for the significant independent variables are relatively late – ApoE correlates with regeneration at 30 days, at which point most of the regeneration has already occurred, and HDL-C correlates (inversely) with SGD at 7 days, at which point the graft dysfunction is usually clinically apparent. More useful markers could include serum-derived measurements on post-transplant day 1, or donor serum markers that could be screened pre-operatively. Unfortunately neither emerged from our analysis. We also noted that the correlated variables differed for SGD and %VI, the former involved RCT components and the latter VLDL/LDL, and both at different timepoints. This seemed somewhat paradoxical because of presumed overlap between graft growth and graft function. However, function and regeneration were not related in other analyses we performed. Total bilirubin and albumin were not significantly related to %VI, nor was SGD itself. It should also be noted that there were no significant differences in regeneration for the SGD subjects when compared to those without SGD (Supplementary Table 2).
Several prior reports have already examined lipid expression following DDLT, LDLT or hepatectomy. The most comprehensive review of DDLT lipid kinetics was performed in a study by Malmendier, et al, which found that RCT components (HDL, ApoA-I and ApoA2) exhibited decreases from baseline during days 1–5 followed by a subsequent reconstitution, whereas VLDL/LDL-associated factors (TG, apoB and apoC) all increased compared to preoperative levels starting on post-operative day 1.[9] Another study, by Armstrong, et al confirmed that serum levels of ApoA-I fall after transplant and correlated with subsequent liver function using MEGX.[10] Two studies to date measured serum lipid expression in LDLT. The first, Tanaka, et al, studied cholesterol, LCAT, and ApoA-I at in 20 pediatric LDLT recipients and found that esterification of cholesterol is an index of hepatic functional recovery post-LDLT.[13] The other study, by Ishida, et al, evaluated cholesterol expression in 40 patients and found that cholesterol expression initially declined, but improved within 20 days to 100mg/dL.[14] Katsuramaki, et al reported associations between apoA-I levels and liver synthetic function in a group of 100 patients who underwent liver hepatectomy for various indications. The group found decreases in ApoA-I post-operatively that were restored by day 14, and correlations between ApoA-I and prealbumin at days 7 and 14.[6, 7] Our data in the present study are in general agreement with all of these prior findings, with the additional contribution of a more comprehensive apolipoprotein panel and a direct comparision of LDLT and DDLT cohorts.
This study has several notable limitations. For practical concerns, we were only able to measure serum values at a fixed number of timepoints, and we were only able to obtain a single imaging study per patient for post-transplant volumetrics. It is therefore possible that alterations in serum concentrations could have occurred at points not measured, or that correlations were missed due to unavailable %VI at earlier timepoints. There are also several influences over lipid expression that could confound our conclusions, including most importantly patient nutritional status (timing of re-initiation of enteral feeding, use of parenteral nutrition) and immunosuppression. Nutritional status was not captured in our dataset and was therefore not available for study. Immunosuppression with corticosteroids and tacrolimus has been associated with hyperlipidemia.[18]
In summary, our data indicate that LDLT recipients have lower concentrations of lipid/lipoprotein than DDLT immediately after transplantation. LDLT grafts seemed to make up for the early drop by post-transplant day 7 and serum lipids were normalized by day 30. We believe this occurs as graft size increases and the liver is better able to balance between the competing energy demands for regeneration and metabolism. Grafts that do not achieve this balance in time are at risk for SGD. Serum apoE at 30 days is a marker for liver regeneration and recovery of full metabolic function and SGD is very unlikely to be present if HDL is normal at 7 days. Our findings suggest that measuring serum lipids could help predict the potential for robust regeneration and SGD in the post-transplant setting.
Supplementary Material
Acknowledgments
Grant support
Supported by funds from NIDDK 5-U01-DK-062494-12 (AS, KMO).
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation
- CIT
cold ischemic time
- cLDL
calculated low-density lipoprotein
- DDLT
deceased-donor liver transplantation
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- IRB
institutional review board
- LDLT
living donor liver transplantation
- LDL
low-density lipoprotein
- RCT
reverse cholesterol transport pathway
- SFSS
small-for-size syndrome
- SGD
segmental graft dysfunction
- VLDL
very-low density lipoprotein
- %VI
percent volume increase
References
- 1.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96(12):1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 2.de Jonge J, Kurian S, Shaked A, Reddy KR, Hancock W, Salomon DR, et al. Unique early gene expression patterns in human adult-to-adult living donor liver grafts compared to deceased donor grafts. Am J Transplant. 2009;9(4):758–772. doi: 10.1111/j.1600-6143.2009.02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2) Suppl 1:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 4.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5(11):2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 5.Olthoff KM. Small size and disease severity in living donation: a difficult match. Liver Transpl. 2009;15(5):457–459. doi: 10.1002/lt.21737. [DOI] [PubMed] [Google Scholar]
- 6.Katsuramaki T, Hirata K, Kimura Y, Nagayama M, Meguro M, Kimura H, et al. Changes in serum levels of apolipoprotein A-1 as an indicator of protein metabolism after hepatectomy. Wound Repair Regen. 2002;10(1):77–82. doi: 10.1046/j.1524-475x.2002.10602.x. [DOI] [PubMed] [Google Scholar]
- 7.Katsuramaki T, Mizuguchi T, Nagayama M, Kimura Y, Furuhata T, Yamaguchi K, et al. Analysis of the changes pattern of serum apolipoprotein A-1 after hepatectomy. Hepatogastroenterology. 2006;53(72):924–927. [PubMed] [Google Scholar]
- 8.Kawamoto M, Mizuguchi T, Nagayama M, Nobuoka T, Kawasaki H, Sato T, et al. Serum lipid and lipoprotein alterations represent recovery of liver function after hepatectomy. Liver Int. 2006;26(2):203–210. doi: 10.1111/j.1478-3231.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 9.Malmendier CL, Lontie JF, Mathe D, Adam R, Bismuth H. Lipid and apolipoprotein changes after orthotopic liver transplantation for end-stage liver diseases. Clin Chim Acta. 1992;209(3):169–177. doi: 10.1016/0009-8981(92)90165-m. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong VW, Schutz E, Kaltefleiter M, Luy M, Helmhold M, Wieland E, et al. Relationship of apolipoproteins AI, B and lipoprotein Lp(a) to hepatic function of liver recipients during the early post-transplant period. Eur J Clin Invest. 1995;25(7):485–493. doi: 10.1111/j.1365-2362.1995.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 11.Palombo JD, Lopes SM, Zeisel SH, Jenkins RL, Albers JJ, Blackburn GL, et al. Effectiveness of orthotopic liver transplantation on the restoration of cholesterol metabolism in patients with end-stage liver disease. Gastroenterology. 1987;93(6):1170–1177. doi: 10.1016/0016-5085(87)90241-1. [DOI] [PubMed] [Google Scholar]
- 12.Cooper ME, Akdeniz A, Hardy KJ. Effects of liver transplantation and resection on lipid parameters: a longitudinal study. Aust N Z J Surg. 1996;66(11):743–746. doi: 10.1111/j.1445-2197.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka A, Sano K, Tanaka K, Honda K, Uemoto S, Takada Y, et al. Short-term changes in lipid and protein metabolism in liver transplants from living-related donors. Am J Surg. 1993;166(1):32–38. doi: 10.1016/s0002-9610(05)80578-1. [DOI] [PubMed] [Google Scholar]
- 14.Ishida H, Furusawa M, Ishizuka T, Tojimbara T, Nakajima I, Fuchinoue S, et al. Short-term changes in cholesterol metabolism in 40 patients with liver transplants from living related donors. Transpl Int. 2002;15(2–3):142–144. doi: 10.1007/s00147-002-0387-z. [DOI] [PubMed] [Google Scholar]
- 15.Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16(8):943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 17.Delahunty TJ, Rubinstein D. Accumulation and release of triglycerides by rat liver following partial hepatectomy. J Lipid Res. 1970;11(6):536–543. [PubMed] [Google Scholar]
- 18.Li HY, Li B, Wei YG, Yan LN, Wen TF, Zhao JC, et al. Higher tacrolimus blood concentration is related to hyperlipidemia in living donor liver transplantation recipients. Dig Dis Sci. 2012;57(1):204–209. doi: 10.1007/s10620-011-1817-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.