TO THE EDITOR
Vitiligo and alopecia areata (AA) share a similar pathogenesis, as they are both IFN-γ-driven and dependent on CD8+ T cells1,2. Here we report a case of rapid, but not durable, repigmentation and hair regrowth in a 35-year-old male with concurrent vitiligo and AA during treatment with oral ruxolitinib. His AA started at the age of 16 as patchy hair loss on his arms, trunk, and scalp. Several years later, he reported macular depigmentation on his face, trunk, and extremities, as well as notable overlap with alopecic lesions. He initially participated in a randomized, placebo-controlled trial at the University of Massachusetts to test the efficacy of oral simvastatin in the treatment of vitiligo. During six months of follow up while taking placebo he demonstrated no evidence of spontaneous repigmentation. Eighteen months later, he enrolled in a phase-2 open-label clinical trial at Columbia University to evaluate the efficacy of ruxolitinib (Jakafi®, Incyte, Wilmington, DE) in moderate to severe AA.
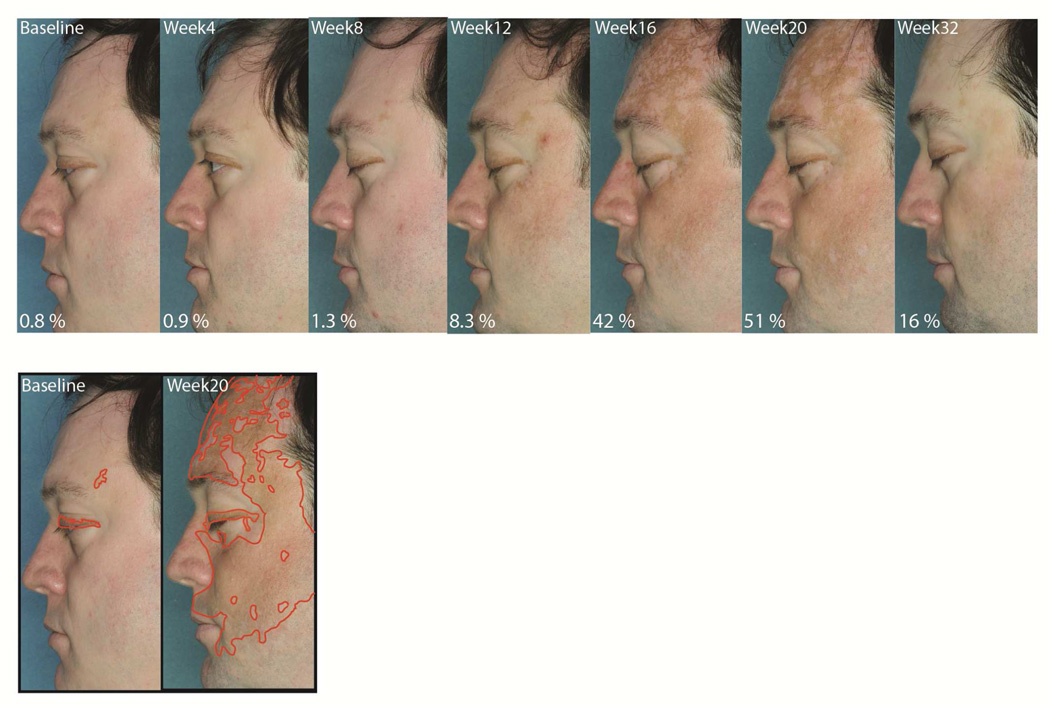
His baseline skin examination at that time revealed widespread, near-complete depigmentation of his face, as well as lesions on his trunk and extremities. He also had patches of non-scarring alopecia on his scalp and extremities. He began treatment with ruxolitinib 20mg orally twice daily for a total of twenty weeks. Four weeks after initiating treatment, he experienced some hair regrowth on his frontoparietal scalp, and after twelve weeks he had significant improvement (85% scalp hair compared to 63% at baseline). At that time he also began to note the appearance of pigmented macules, and at week 20 he exhibited a large amount of repigmentation on his face and other areas (51% facial pigmentation compared to 0.8% at baseline). Twelve weeks after discontinuing ruxolitinib, while his hair regrowth was maintained, much of the regained pigment had regressed (Figure 1).
Figure 1. Vitiligo repigmentation during treatment with ruxolitinib.
Screening skin examination reveals near-complete depigmentation of the patient’s face at baseline. The first evidence of skin repigmentation appeared after 12 weeks of therapy, which continued until week 20, when ruxolitinib was discontinued. Follow up visit 12 weeks after stopping the treatment shows recurrent depigmentation in the majority of previously repigmented areas. Pigmented areas of the face were outlined using the free-hand selection tool followed by calculation of the % selected area using ImageJ software.
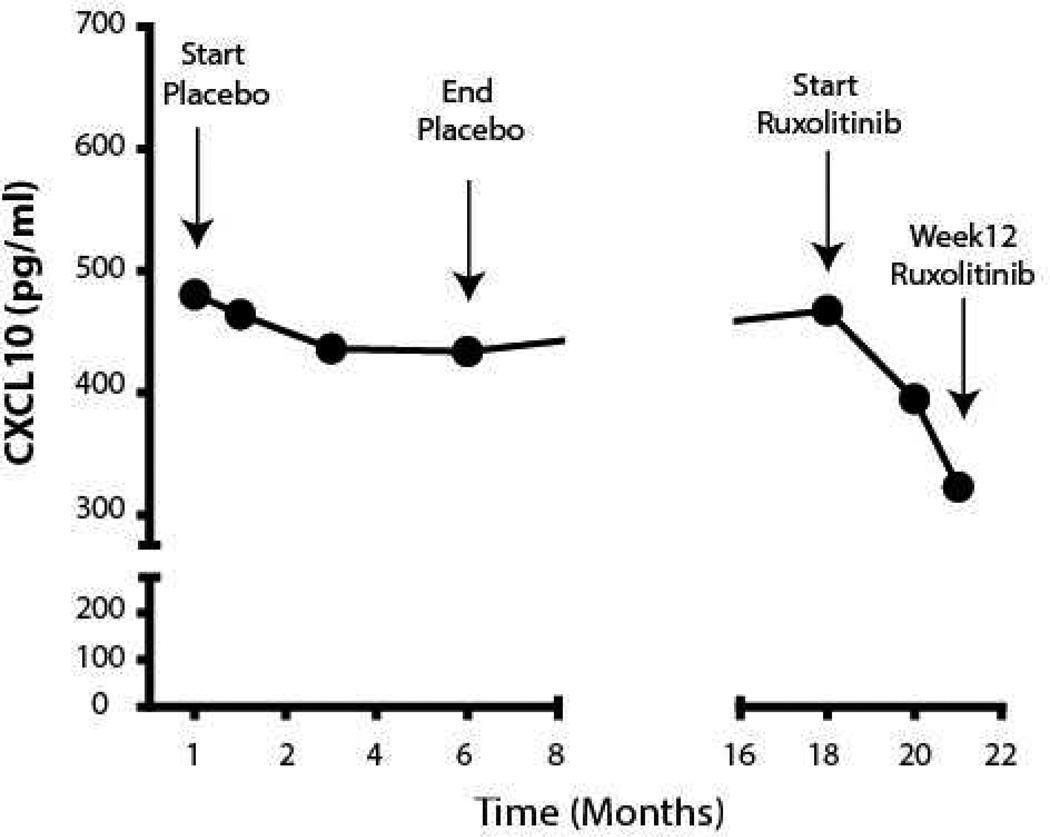
Ruxolitinib is a potent small-molecule Janus kinase (JAK) inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of intermediate- or high-risk myelofibrosis and polycythemia vera. It interferes with IFN-γ signaling by preferential inhibition of JAK1 and JAK23,4. We previously demonstrated that ruxolitinib eliminated the IFN signature and effectively reversed hair loss in three patients and a mouse model of AA2. We also reported that CXCL10, an IFN-γ induced chemokine, is critical for autoreactive T cell recruitment to the skin during the progression and maintenance of vitiligo, and hypothesized that targeting the IFN-γ-CXCL10 cytokine axis might be an effective treatment by reducing the production of CXCL101. Interestingly, measuring the patient’s serum CXCL10 level by enzyme-linked immunosorbent assay (ELISA) revealed that it was initially elevated and stable for over 1 year, but was reduced after treatment with ruxolitinib (Figure 2).
Figure 2. Decrease in serum CXCL10 after initiating treatment with ruxolitinib.
Serum samples were analyzed by enzyme-linked immunosorbent assay (ELISA). CXCL10 level was elevated and remained stable while the patient was taking placebo in the first trial, but decreased after initiating treatment with ruxolitinib.
There are currently no FDA-approved treatments for vitiligo, and standard off-label treatments are limited in efficacy. Recently, significant repigmentation was reported in a patient with vitiligo after treatment with tofacitinib, an oral JAK 1/3 inhibitor5. Additional studies will be needed to determine whether ruxolitinib, or other JAK inhibitors, are safe and effective long-term treatments for vitiligo.
Acknowledgements
We thank Julissa Borbon for assistance with study coordination. We thank Canfield Scientific for assistance with clinical photography. This project was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the NIH, under award number AR061437, and research grants from the Vitiligo Research Foundation, Kawaja Family Vitiligo Research Initiative Award, and Dermatology Foundation Stiefel Scholar Award (to JEH); and by the Physician-Scientist Career Development Award from the Dermatology Foundation, the Louis V. Gerstner, Jr Scholars Program, and the Irving Scholars Program from the Irving Institute for Clinical and Translational Research/CUMC CTSA (to AJ). This work was supported in part by US Public Health Service National Institutes of Health NIAMS grants R21AR061881 (to AMC and RC), U01AR067173 (to AMC) and P30AR044535 (the Columbia University Skin Disease Research Center), as well as the Locks of Love Foundation and the Alopecia Areata Initiative.
Abbreviations
- AA
Alopecia areata
- JAK
Janus kinase
- FDA
Food and Drug Administration
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The single center, proof-of-concept clinical trial entitled “An Open-Label Pilot Study to Evaluate the Efficacy of ruxolitinib in Moderate to Severe Alopecia Areata,” is registered at ClinicalTrials.gov identifier NCT01950780. The protocol for this intervention trial was reviewed and approved by the Institutional Review Board at Columbia University and conducted under the Declaration of Helsinki principles.
Conflict of Interest Disclosure: The authors have no conflict of interest to declare
References
- 1.Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014 Feb 12;6(223):223ra23. doi: 10.1126/scitranslmed.3007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014 Sep;20(9):1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015 Jan 29;372(5):426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintás-Cardama A, Kantarjian H, Cortes J, et al. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011 Feb;10(2):127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 5.Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015 Jun 24; doi: 10.1001/jamadermatol.2015.1520. [DOI] [PubMed] [Google Scholar]