Abstract
Background
Hepatitis C virus (HCV) is the most common cause of mixed cryoglobulinemia syndrome (MCS). The efficacy and safety of all-oral directly-acting antiviral (DAA) therapy in HCV-associated MCS (HCV-MCS) is largely unknown.
Methods
Case series of patients with HCV-MCS who were treated with sofosbuvir-based regimens and historical controls treated with pegylated interferon and ribavirin in a single healthcare network. HCV-MCS was defined by circulating cryoglobulin associated with systemic vasculitis symptoms. Renal involvement (N=7) was established by kidney biopsy (N=5) or by ≥ 2 of the following clinical findings: reduced kidney function, proteinuria, or hematuria with other causes excluded (N=2).
Results
Twelve patients received DAA therapy between December 2013 and September 2014. Median age was 61 years, 58% male, 50% had cirrhosis. Median baseline serum creatinine was 0.97 mg/dL (range 0.7 – 2.47 mg/dL.) Four patients received Rituximab concurrent with DAA therapy. Sustained virological response rate at twelve weeks (SVR12) was 83% overall. Patients with glomerulonephritis who achieved SVR12 experienced an improvement in serum creatinine and reduction in proteinuria. Cryoglobulin levels decreased in 89% of patients, with median percent decreasing from 1.5% to 0.5%, and completely disappearing in 4 of 9 cases who had cryoglobulins measured after treatment. Serious adverse events were infrequent (17%). In contrast, the historical cohort treated with pegylated interferon and ribavirin experienced only 10% SVR12 rate with 100% experiencing at least one adverse event, and 50% experiencing premature discontinuation due to adverse events.
Conclusion
SVR12 rates for sofosbuvir-based direct acting antiviral regimens in HCV-MCS were 83%, significantly higher than historical controls treated with pegylated interferon and ribavirin. Patients with glomerulonephritis experienced improvement in renal function, including those not concomitantly treated with immunosuppression.
Keywords: chronic kidney disease, nephrology, vasculitis, sustained virological response
Introduction
Hepatitis C virus (HCV) infection accounts for up to 90% of all cases of mixed cryoglobulinemia syndrome (MCS), which results from the production of polyclonal IgG and monoclonal (type II) or polyclonal (type III) IgM with rheumatoid factor activity (1–3). The clinical manifestations of HCV-associated MCS (HCV-MCS) result from small to medium vessel vasculitis and include palpable purpura, arthritis, hepatosplenomegaly, peripheral neuropathy, glomerulonephritis, and central nervous system involvement (4, 5). Serologically, HCV-MCS is characterized by a circulating cryoglobulin, hypocomplementemia and a positive rheumatoid factor. Historically, HCV-MCS has been treated with 48 weeks of pegylated interferon and ribavirin. Although few studies have systematically evaluated the efficacy of that regimen in HCV-MCS, some suggested less favorable sustained virological response (SVR) rates compared with patients without HCV-MCS (6). In a recent series of patients with HCV-MCS by Sadoun et al the addition of a first generation protease inhibitor (telaprevir or boceprevir) to pegylated interferon and ribavirin resulted in modest improvements in SVR rates up to 67%; however, in this series and others, telaprevir and boceprevir were associated with numerous hematologic, dermatologic, and renal adverse effects (7–9). These agents are now no longer being manufactured in the United States. Additionally, baseline anemia affects the large majority of patients with HCV-MCS, making treatment regimens that include ribavirin, which causes hemolysis and is cleared by the kidney, particularly challenging in this population. Nevertheless, those who do achieve SVR typically experience improvement in vasculitis symptoms, and among those with glomerulonephritis, SVR may additionally result in improvement in renal function and reduction of proteinuria (6, 10–12).
Since late 2013, several novel DAAs have been approved by the FDA, including NS5B polymerase inhibitors, second-generation NS3-4A protease inhibitors, and NS5A inhibitors (13). Interferon-free regimens that combine such novel DAAs are highly effective for the treatment of chronic HCV infection and are well tolerated with few side effects (14). There are currently no published reports documenting the efficacy of novel DAA regimens in patients with HCV-MCS. Whether patients will achieve SVR with typically prescribed treatment durations or require a longer duration of treatment is also unknown. Therefore, the aim of this study was to characterize our center’s experience using novel DAAs in patients with HCV-MCS. Since glomerulonephritis is common in HCV-MCS, we also sought to characterize the renal response to DAA therapy in the subset of patients with active glomerulonephritis.
Methods
Study design
This is a retrospective case series.
Patients
Sofosbuvir-based, interferon-free cohort
Patients with HCV-MCS who received DAA therapy between December 2013 and September 2014 were identified using the Research Patient Data Registry at Partners Healthcare System in Boston, Massachusetts. Electronic medical records were reviewed for demographic information, clinical characteristics, and laboratory and pathologic findings. Race was determined by self-report. Duration of HCV infection was determined by patient report. Cases of HCV-MCS were defined as having HCV RNA > 1000 international units (IU)/mL at baseline and circulating cryoglobulin associated with purpura, cutaneous ulcers, Raynaud’s phenomenon, arthralgias, sicca syndrome, gastrointestinal vasculitis, neurologic involvement (peripheral neuropathy and/or central nervous system), or renal involvement. All patients received one of the following regimens: sofosbuvir (400 mg daily) plus ribavirin or sofosbuvir (400 mg daily) plus simeprevir (150 mg daily). All patients received full-dose sofosbuvir. Patients treated with ribavirin received weight-based dosing in divided doses or a reduced dose adjusted for impaired renal function. Dose reductions were determined by the treating provider and were not protocolized.
Historical cohort
Patients who were treated with pegylated interferon and ribavirin prior to 2013 at the Partners Healthcare System were identified using the Research Patient Database by searching by ribavirin, interferon and diagnosis codes for cryoglobulinemia and associated symptoms. Cryoglobulin levels and clinical syndrome was confirmed by chart review, and cases of HCV-MCS were defined as above.
In both cohorts, renal involvement was defined either by the histologic finding of cryoglobulinemic glomerulonephritis with a membranoproliferative glomerulonephritis pattern of injury on kidney biopsy, or by the presence of at least two of the following clinical signs: proteinuria, hematuria, and reduced estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 without alternative cause for chronic kidney disease. The use of immunosuppression was determined by chart review. Baseline eGFR was calculated based on the serum creatinine measurement prior to treatment initiation using the Chronic Kidney Disease Epidemiology Collaboration formula (15). Proteinuria was quantified by the ratio of urine protein to urine creatinine on a spot urine sample, when available. Otherwise, semi-quantitative measurement by urinalysis was recorded. The presence of urinary heme was determined by dipstick analysis. The serologic findings that are associated with HCV-MCS, including the cryoglobulin percentage (cryocrit), serum complement levels, anti-nuclear antibody, and rheumatoid factor were recorded and, where available the values obtained most proximal to initiation of DAA therapy are presented. DAA therapy was prescribed at the discretion of the treating physician; treatment regimens depended on the genotype, presence of cirrhosis, and prior treatment experience. Administration of immunosuppressive therapy prior to, during, and after antiviral therapy was also recorded. All serum creatinine values obtained during outpatient visits within our healthcare system were collected for the twelve months prior to therapy initiation, during therapy and up to twelve months after therapy. Patients were considered to have cirrhosis based on liver biopsy findings (Metavir stage 4 or Ishak stage 5 or 6) in the absence of liver biopsy, any two of the following criteria: platelet count <140,000 per µl, presence of esophageal varices on esophagogastroduodenoscopy, evidence of cirrhosis and/or portal hypertension and/or ascites by imaging studies, or FibroSure® or transient elastography (Fibroscan®) result compatible with Metavir stage 4 fibrosis (16, 17). Cure of HCV infection was defined as SVR12, or an undetectable HCV RNA 12 weeks after the completion of therapy (18). Follow-up values are those obtained 12 weeks after treatment discontinuation (SVR12 visit).
Adverse events were determined by chart review; all clinical notes and laboratory results from the time of treatment initiation until four weeks after treatment completion were reviewed to determine adverse effects. Clinical outcomes, including resolution of HCV-MCS symptoms and laboratory findings were determined by chart review for up to 24 weeks of follow-up.
Statistical Analysis
Baseline characteristics of all patients with HCV-MCS treated with novel DAA therapy were described using count and percent or median and range. Baseline and follow-up values were compared using paired samples t-test. Ninety-five percent confidence intervals were calculated using the exact formula. Signs and symptoms experienced during DAA therapy use were determined from chart review and the number of patients experiencing each potential adverse effect is tabulated in Table 2. All analyses were performed with Stata version 13.1 (StataCorp, College Station, Texas, USA). For all comparisons, a two sided P value of < 0.05 was considered to indicate statistical significance. The institutional review board of Partners Healthcare System approved this retrospective study; the need for informed consent was waived.
Table 2.
Adverse events experienced during treatment
| Adverse event | Number of cases |
|---|---|
| Renal | |
| Hyperkalemia > 5.5 mEq/dL† | 1 |
| Rise in creatinine ≥ 0.3mg/dL | 1 |
| Hematologic | |
| Decrease in hemoglobin ≥ 1.0g/dL | 2 |
| Anemia requiring ESA | 0 |
| Fatigue | 1 |
| Dizziness/Lightheadedness | 1 |
| Insomnia/Agitation/Anxiety†† | 3 |
| Infection††† | 2 |
| Photosensitivity | 2 |
| Rash | 1 |
| Vitiligo | 1 |
| Palpitations | 1 |
| Xerosis | 1 |
| Xerophthalmia | 1 |
| Nausea | 1 |
| Headache | 1 |
| Hypoglycemia | 1 |
| Hypokalemia | 1 |
Legend: Eight of twelve patients experienced at least one adverse event. The frequency count of each adverse event is listed above.
Patient required emergency room evaluation and treatment with intravenous hydration and intravenous calcium despite absence of electrocardiogram changes. Hyperkalemia was then managed with low potassium diet, oral bumetanide daily and twice weekly sodium polysterene.
One patient experienced severe worsening of anxiety requiring cessation of sofosbuvir and ribavirin at week 10 of 12.
Infections included one urinary tract infection and pneumonia. Both were treated with antibiotics and resolved without complications.
Abbreviations: ESA = erythropoietin stimulating agent
Results
Twelve patients with HCV-MCS who were treated with sofosbuvir-based, interferon-free antiviral therapy were identified. Patient characteristics for this cohort are shown in Table 1. The median duration of HCV infection prior to initiation of DAA therapy was 30,years, range 10–53 years. Fifty percent were treatment naïve; the remainder had failed at least one prior antiviral treatment attempt. Fifty percent had evidence of cirrhosis. All patients had type II cryoglobulinemia, as defined by Brouet et al., with a monoclonal IgM kappa.(19) The main clinical features associated with HCV-MCS in these patients included kidney involvement (58%), purpura (50%), arthralgias (50%), neuropathy (33%), Raynauds phenomenon (17%), and sicca (8%) (Table 1). The distribution of genotypes is also shown in Table 1. Median pre-treatment viral load was 3,590,000 IU/mL (Range 544,000 – 16,100,000 IU/mL). Baseline laboratory tests and serologies are also shown in Table 1.
Table 1.
Baseline demographics and clinical characteristics of patients with HCV-MCS treated with sofosbuvir-based direct-acting antiviral therapy (n=12)
| Parameter | Median (Range) or count (%) |
|---|---|
| Age, years | 61 (37–73) |
| Male (%) | 7 (58%) |
| Race (%) | |
| Caucasian | 6 (50%) |
| African American | 2 (17%) |
| Hispanic | 4 (33%) |
| Cirrhotic | 6 (50%) |
| Hepatocellular carcinoma (prior) | 1 (8%) |
| Diabetic (%) | 0% |
| Hypertensive (%) | 10 (83%) |
| Number of anti-hypertensive medications | 2 (0–5) |
| BMI (kg/m2) | 28.3 (22.7–34.1) |
| Duration of HCV infection (years) | 30 (10–53) |
| Genotype | |
| 1a | 5 (42%) |
| 1b | 2 (17%) |
| 1 untypeable | 1 (8%) |
| 2b | 2 (17%) |
| 3 | 1 (8%) |
| 4 | 1 (8%) |
| Prior treatment experience | |
| Previously treated | 6 (50%) |
| Treatment Naïve | 6 (50%) |
| Treatment regimen prescribed | |
| SOF/SIM x 12 weeks | 8 (67%) |
| SOF/RBV x 12 weeks | 2 (17%) |
| SOF/RBV x 24 weeks | 2 (17%) |
| Duration of known cryoglobulinemia (years) | 5 (0.5–21) |
| Baseline clinical presentation Φ | |
| Glomerulonephritis | 7 (58%) |
| Purpura | 6 (50%) |
| Arthralgia | 6 (50%) |
| Peripheral neuropathy | 4 (33%) |
| Raynaud’s phenomenon | 2 (17%) |
| Sicca | 1 (8%) |
| Renal arteritis/infarct | 1 (8%) |
| Baseline laboratory studies | Median (Range) |
| Serum Creatinine (mg/dl) | 0.97 (0.70–2.47) |
| eGFR (ml/min/1.73m2) | 65 (26 – 108) |
| eGFR < 30 | 1 (8%) |
| eGFR 30–45 | 1 (8%) |
| eGFR 45–60 | 3 (25%) |
| eGFR > 60 | 7 (58%) |
| Hemoglobin (g/dL) | 12.8 (9.5–15.1) |
| ALT (U/L) | 41 (18–207) |
| AST (U/L) | 53 (23–222) |
| Baseline Serologic findings* | |
| Cryoglobulin percentage | 1.5% (0.5–4%) |
| Baseline C3 Value (mg/dL) | 79 (51–111) |
| Baseline C4 Value (md/dL) | 11 (2–29) |
| Positive rheumatoid factor | 9/10 (90%) |
Legend: HIV = Human Immunodeficiency Virus, HBV = Hepatitis B Virus, HCV = Hepatitis C Virus, BMI = body mass index, IVDU = intravenous drug use, SOF = sofosbuvir, SIM = simeprevir, RBV = ribavirin, PEGIFN = pegylated interferon, eGFR = estimated glomerular filtration rate, ALT = alanine aminotransferase, AST = aspartate transferase
Pre-treatment complement levels were available for 11 of 12 cases, rheumatoid factor levels were available for 10 of 12 cases.
Note that percentages are inclusive and add up to greater than 100%
Normal range for ALT (10–55 U/L), AST (10–40 U/L), C3 ( 81–157 mg/dL), C4 (12–39mg/dL).
Treatment efficacy and safety
Eight of 12 patients (67%) received sofosbuvir paired with simeprevir, four (33%) received sofosbuvir and ribavirin. Three received full weight-based dosing of ribavirin, one required dose reduction due to renal impairment. Most cases were treated with twelve weeks of therapy (Table 1). Data on the Q80K polymorphism were not available for any of the patients treated with sofosbuvir and simeprevir.
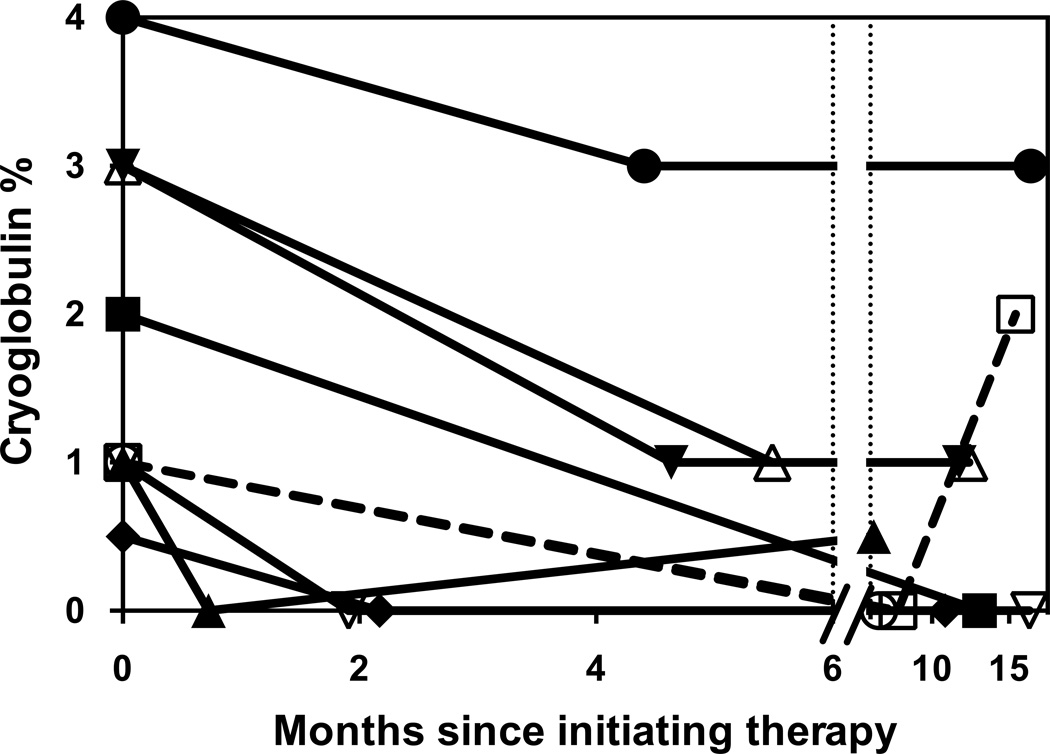
All patients had undetectable HCV RNA by week 4 and remained undetectable while on treatment. SVR12 was achieved in 10/12 (83%, 95% confidence interval 55–95%). There were two relapses after treatment discontinuation; the first had genotype 1 non-subtypable infection and had been treated with sofosbuvir and simeprevir for 12 weeks; the second had genotype 4 infection and had been treated with sofosbuvir and ribavirin for 12 weeks. Alanine aminotransferase levels decreased from a median of 42 U/L (range 18–207) pre-treatment to 20 U/L (range 10–41) on average after treatment (range 12–24 weeks post-treatment), and albumin increased from 3.8g/dL (range 3.0–4.9g/dL) to 4.0g/dL (range 3.7–4.9g/dL) after treatment. Hemoglobin increased from 12.8g/dL (9.5–15.1) to 13.2g/dL (10.1–15.8). Nine patients had cryoglobulin levels re-checked during or after treatment; median cryglobulin levels decreased from 1.5% (range 0.5–4%) to 0.5% (range 0–2%); cryoglobulins levels disappeared in 4 of 9 cases who had cryoglobulins measured after treatment (Table 3). The timeline and durability of decline in cryoglobulin levels are shown in Figure 1. All patients experienced a decrease in cryoglobulin levels except one patient who experienced a relapse of viremia (Figure 1).
Table 3.
Clinical symptoms, serology, and immunosuppression required before and after direct-acting antiviral therapy
| Pt. | Duration Cryo (years) |
Pretreatment | Regimen | SVR | Post-treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Serology | On treatment Immuno- suppression |
Persistent Symptoms |
Serology | Immuno- suppression |
||||
| 1 | 5 | NHBCL, Neuropathy, Reynauds, Arthralgia, GN |
Cryo - 4% C3 - 57 C4 - 2 RF - Positive |
Rituxumab | SOF/SIM | YES | Neuropathy GN |
Cryo - 3 C3 - 148 C4 - 6 RF - Positive |
None |
| 2 | 21 | Neuropathy, Sicca, Reynaud’s, Purpura, GN |
Cryo - 2% C3 - 72 C4 - 16 RF - Positive |
None | SOF/SIM | YES | Reynaud’s, GN |
Cryo - Negative C3 - ND C4 - ND RF - ND |
None |
| 3 | 1 | Purpura, Skin Ulcers, Arthralgias, GN |
Cryo - 1% C3 - 99 C4 - 6 RF - Positive |
None | SOF/RIBA | YES | Arthralgia | Cryo - Trace C3 - 90 C4 - 18 RF - Positive |
None |
| 4 | 10 | GN | Cryo - 3% C3 - 82 C4 - 13 RF - Positive |
None | SOF/SIM | YES | GN | Cryo - 1% C3 - 115 C4 - 16 RF - ND |
None |
| 5 | 5 | Neuropathy, Purpura, GN |
Cryo - 0.5% C3 - 111 C4 - 29 RF ND |
None | SOF/SIM | YES | None | Cryo - Negative C3 - ND C4 - ND RF - ND |
None |
| 6 | 3 | GN | Cryo - 2% C3 - ND C4 - ND RF - Positive |
None | SOF/SIM | YES | None | Cryo - ND C3 - ND C4 - ND RF - ND |
None |
| 7 | 7 | Arthralgia, GN | Cryo - 1% C3 - 51 C4 - 11 RF - Negative |
Ustekinumab (psoriasis) |
SOF/SIM | NO | Arthralgia, GN | Cryo - Negative C3 - 85 C4 - 15 RF - Negative |
Ustekinumab (psoriasis) |
| 8 | 5 | Purpura, Arthralgia | Cryo - 2% C3 - 79 C4 - 2 RF - Positive |
None | SOF/SIM | YES | None | Cryo - ND C3 - ND C4 - ND RF - ND |
None |
| 9 | 0.5 | Neuropathy, Purpura |
Cryo - 0.5% C3 - 66 C4 - 5 RF - Positive |
Rituxumab | SOF/RIBA | YES | Neuropathy | Cryo - ND C3 - ND C4 - ND RF - ND |
Rituximab |
| 10 | 6 | Purpura, Arthralgia, Renal Artery vasculitis |
Cryo - 1% C3 - 89 C4 - 12 RF - Positive |
Rituxumab | SOF/RIBA | NO | Purpura Arthralgia, Renal Artery vasculitis |
Cryo - 2% C3 - 103 C4 - 6 RF - Negative |
Rituximab and Prednisone |
| 11 | 1 | Arthritis | Cryo - 3% C3 - 89 C4 - 27 RF - Positive |
Rituxumab | SOF/RIBA | YES | Arthritis | Cryo - 1% C3 - 110 C4 - 30 RF - Positive |
Rituximab |
| 12 | 1 | Arthritis | Cryo - 1% C3 - 65 C4 - 9 RF - Positive |
None | SOF/SIM | YES | None | Cryo - Negative C3 - 124 C4 - 21 RF - ND |
None |
Legend: Normal range for serum C3 (81–157 mg/dL), C4 (12–39mg/dL). Abbreviations: NHBCL = non-Hodgkin’s B Cell Lymphoma, GN = glomerulonephritis, RF = rheumatoid factor, SOF = Sofosbuvir, SIM = Simeprevir, SVR = sustained virological response
Figure 1. Change in cryoglobulin levels over time in patients treated with sofosbuvir-based direct-acting antiviral therapy.
Patients who achieved SVR12 are shown with solid lines. The two patients who relapsed are shown with dashed lines. The first data-point at time 0 shows the baseline pre-treatment cryoglobulin percent measurement for each of the 9 patients with serial cryoglobulin percent measured. The second data-point shows the nadir and the time-to-nadir in days since initiating therapy. The final data-point for each patient shows the cryoglobulins percentage at last follow-up visit. Three patients did not have repeat cryoglobulins measured after initiating treatment and are excluded from this figure.
Mild adverse events were common during treatment; eight (67%) reported experiencing at least one adverse event during therapy (Table 2). Only two (17%) experienced serious adverse events; one patient taking sofosbuvir and ribavirin experienced hyperkalemia requiring a brief emergency department evaluation and another on sofosbuvir and ribavirin experienced severe worsening of anxiety and insomnia that led to treatment discontinuation at week 10 of 12. Both patients who experienced serious adverse events achieved SVR12. No patients required erythropoiesis stimulating agents or blood transfusion (Table 2).
HCV-MCS Glomerulonephritis
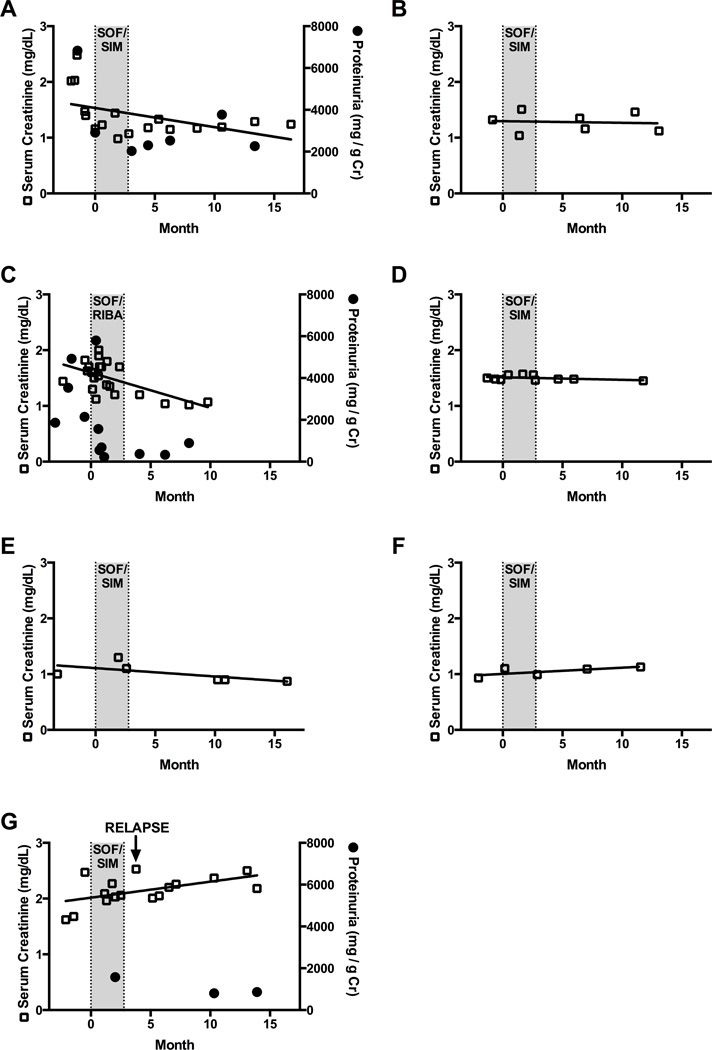
Seven patients had evidence of renal involvement. Five had a kidney biopsy demonstrating membranoproliferative pattern of injury with cryoglobulin deposits noted on electron microscopy, and two were diagnosed clinically (Table 4). The length of time from onset of kidney disease to DAA therapy ranged from less than six months to 17 years. Four patients with glomerulonephritis were treatment experienced. Three had been intolerant of interferon-based therapies and one relapsed after completion of telaprevir, pegylated interferon, and ribavirin combination therapy. Immunosuppressive regimens that included combinations of rituximab, corticosteroids, and plasmapheresis were used in six of the eight patients at some point prior to initiation of DAA therapy; only one patient was receiving ongoing immunosuppression concurrent with antiviral therapy (Table 3). Individual eGFR changes in patients with active glomerulonephritis are shown in Figure 2 (a–g). Three patients had proteinuria quantified before and after therapy; there was a reduction in proteinuria in all cases that had available quantitative measures before and after therapy (Figure 2a, 2c, 2g).
Table 4.
Demographics, clinical characteristics, and follow-up of patients with kidney involvement
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Age / gender | 59 M | 73 F | 52 M | 58 M | 59 F | 60 M | 69 M |
| Race | Hispanic | Hispanic | White | Hispanic | White | White | White |
| HCV Viral Load (million IU/ml) |
1.24 | 4.82 | 7.19 | 1.87 | 3.48 | 1.35 | 0.33 |
| Genotype | 1a | 1b | 2b | 1a | 1b | 1a | 1 |
| Duration of HCV infection (years) | 30 | 53 | 30 | 28 | 45 | 35 | 25 |
| Diabetes | No | No | No | No | No | No | No |
| Cirrhosis | No | Yes | No | Yes | Yes | No | Yes |
| Hypertension | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| # of medications pre-treatment | 2 | 1 | 4 | 4 | 4 | 3 | 5 |
| Prior Antiviral Therapy | None | Intolerant of IFN/RBV |
None | Relapse on TLV/IFN/RBV |
Intolerant of IFN/RBV |
Intolerant of IFN |
None |
| Serologic findings | |||||||
| ANA titre | 1:40, speckled | 1:5120, speckled | Negative | Negative | 1:40, speckled | ND | 1:160, speckled |
| C3 C4 | Low | Low | Normal | Low | Normal | ND | Low |
| Cryoglobulin type | Type II | Type II | Type II | Type II | Type II | Type II | Type II |
| Cryoglobulin percent | 4% | 2% | 1% | 3% | 0.5% | 2% | 1% |
| Rheumatoid Factor | Positive | Positive | Positive | Positive | ND | Positive | Negative |
| Clinical Symptoms | NHBCL†, Neuropathy, Raynaud’s, Arthralgia, GN |
Neuropathy Purpura, Sicca Raynaud’s, GN |
Purpura, Skin ulcers, GN Arthralgias |
GN | Purpura, Neuropathy, GN |
GN | Arthralgia, GN |
| Onset of kidney disease | 3 years | 17 years | 1 year | 10 years | 2.5 years | 3 years | 7 years |
| Kidney Biopsy Findings | ND | MPGN | ND | MPGN | MPGN | MPGN | MPGN |
| Immunosuppression prior to HCV treatment |
CS 2011–14 CYC, 2012–14 RTX 2011-present |
RTX 2004–06 | None | RTX 2010–11 | CS 2012–13 RTX 2012–13 |
Pheresis 2011 RTX 2011–12 |
RTX 2009–2010 Ustekinumab 2012-present (psoriasis) |
| Immunosuppression concurrent with HCV treatment |
RTX | None | None | None | None | None | Ustekinumab (psoriasis) |
| Immunosuppressin after treatment | None | None | None | None | None | None | Ustekinumab (psoriasis) |
| Active GN†† | Yes | Yes | Yes | Yes | Remission | Remission | Yes |
| Antivirals Prescribed | SOF/SIM | SOF/SIM | SOF/RBV | SOF/SIM | SOF/SIM | SOF/SIM | SOF/SIM |
| Treatment Duration (weeks) | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| ESA use required during treatment | No | No | No | No | No | No | No |
| SVR | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Baseline Laboratory Tests Serum Creatinine (mg/dl) |
1.40 | 1.32 | 1.7 | 1.48 | 1.00 | 0.93 | 2.47 |
| Estimated GFR (ml/min) | 55 | 40 | 45 | 52 | 62 | 89 | 26 |
| Serum Albumin (g/dL) | 3.0 | 3.7 | 3.3 | 3.0 | 4.4 | 4.4 | 3.0 |
| ALT/AST (U/L) | 32/23 | 47/96 | 53/51 | 41/70 | 98/112 | 30/33 | 41/70 |
| Urine Protein (mg/gCr) ††† | 6827 | ND | 2141 | 3+ (UA) | Negative (UA) | 1+ (UA) | 1574 |
| Urine Heme | 3+ | ND | 2+ | 3+ | 1+ | Negative | Negative |
| Post-treatment Laboratory Tests | |||||||
| Serum Creatinine (mg/dl) | 1.15 | 1.35 | 1.04 | 1.48 | 0.9 | 1.09 | 2.01 |
| Estimated GFR (ml/min) | 69 | 39 | 82 | 52 | 70 | 73 | 33 |
| Serum Albumin (g/dL) | 3.6 | 4.0 | 3.9 | 3.9 | 4 | 4.9 | 3.5 |
| ALT/AST (U/L) | 14/23 | 17/36 | 22/41 | 21/30 | 16/21 | 22/23 | 28/44 |
| Urine Protein (mg/gCr) | 2533 | ND | 400 | 3+ | Negative (UA) | ND | 800 |
| Cryoglobulin percent | 3% | 2% | trace | 1% | 0% | ND | 0% |
Abbreviations: Hisp = Hispanic, W = White, HCV = hepatitis C Virus, IU = international units, NHBCL = non-Hodgkin’s B Cell Lymphoma, RTX = Rituximab, GN = glomerulonephritis, ND = not done, CS = corticosteroids, CYC = cyclophosphamide, Sof = sofosbuvir, RBV = ribavirin, IFN = interferon, Sim = simeprevir, TLV = telaprevir, ALT = alanine transaminase, AST = Aspartate transaminase, ESA = erythropoetin stimulating agens, MPGN = membranoproliferative glomerulonephritis, UA = urinalysis, Pheresis = plasmapheresis.
Normal range – ALT (10–55 U/L), (AST 10–40 U/L), Albumin (3.3–5.0 g/dl)
The diagnosis of non-Hodgkin’s lymphoma was based on the World Health Organization (WHO) criteria. (Harris NL, Jaffe ES, Diebold J, Flandrin G, World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: J Clin Oncol 1999;17:3835–3849.)
Active glomerulonephritis was defined as either the presence of proteinuria and hematuria or a reduction in eGFR > 25% in the last 3 months.
Urine protein measured by spot protein to creatinine ratio and expressed in mg per gram urinary creatinine (mg/gCr). When unavailable, urinalysis (UA) measures are provided.
Figure 2. Serum creatinine and proteinuria trends in 7 cases of HCV-MCS with renal involvement.
Serum creatinine (hollow squares) and proteinuria (dark circles, panels A,C,G) values obtained 3 months prior to direct-acting antiviral therapy initiation, through treatment period (gray) and through last follow-up visit. Patients 1–6 (panels A-F) achieved SVR12. Patient 7 (Panel G) relapsed four weeks after completing therapy. Abbreviations: SOF=sofosbuvir, SIM = simeprevir, RBV = ribavirin. SVR12 = sustained virological response 12 weeks after completion of therapy.
Analysis of the historical cohort
Prior to 2013 at our institution, ten patients with HCV-MCS received pegylated interferon and ribavirin. Their baseline characteristics are shown in Table 5. It is notable that in this cohort, only 1 of 10 achieved SVR; this 53 year old Caucasian non-cirrhotic male with genotype 3 infection had purpura, arthritis and neuropathy at baseline and achieved full clinical remission without the need for immunosuppression at any time before or after antiviral therapy. All patients in the historical cohort treated with pegylated interferon and ribavirin experienced side effects with 50% needing to discontinue therapy prematurely due to the severity of side effects (Supplemental Table). None of the 9 patients who did not achieve SVR had any improvement in HCV-MCS symptoms and none were able to be removed from immunosuppression. Cryoglobulin levels in the historical cohort treated with pegylated interferon and ribavirin did not decline except in the one patient who achieved SVR, pre-treatment median 2.5% (range 0.5–16%) and post-treatment median 4% (range 0–9%).
Discussion
To our knowledge, this is the first published series of patients with HCV-MCS who were treated with novel all-oral direct-acting antiviral therapies, including sofosbuvir and simeprevir. We observed an on-treatment viral suppression rate of 100% and an SVR12 rate of 83%, which, despite wide confidence intervals (55–95%), is comparable to the SVR12 rate reported with similar regimens in other non-cryoglobulinemic real-world cohorts.(20, 21) These findings suggest that in the age of novel direct-acting antiviral therapy, HCV may not be more difficult to cure in the setting of cryoglobulinemic vasculitis. Previous studies suggest that kidney involvement is frequently associated with unfavorable clinical response in HCV-MCS (22), but remarkably, in this series 6 of 7 (86%) of patients with kidney involvement achieved SVR12. Furthermore, patients with active glomerulonephritis who were successfully treated with direct-acting antiviral therapy experienced an improvement in eGFR and reduction in proteinuria, particularly in those whose onset of proteinuria was recent.
The goals of treatment for HCV-MCS are achieving SVR, achieving symptomatic remission of MCS, and minimizing the use of immunosuppression. The link between antiviral therapy and improvement in kidney function was first demonstrated by Johnson et al in the first series describing the association between MPGN and HCV.(10) The four patients treated with interferon in this initial series achieved dramatic reduction in proteinuria while on treatment. Recent series have shown that treatment with ribavirin and interferon is associated with significant improvement in eGFR and proteinuria in patients who achieve SVR but not in those who relapse.(23) Our historical cohort confirms that SVR12 was difficult to achieve in patients with HCV-MCS treated with pegylated interferon and ribavirin, and no cases of HCV-MCS symptomatically improved in the absence of SVR12. Because pooled analysis has shown that the majority of cases of HCV-MCS treated with ribavirin and pegylated interferon relapse,(24) immunosuppression is often added up front to control symptoms of HCV-MCS. A large, prospective series showed improved outcomes in those in which rituximab was added to antiviral therapy (ribavirin and pegylated interferon) compared to those treated with antiviral therapy alone, particularly in those with kidney involvement.(25)
Prior to this series, there has only been one published letter describing three cases of HCV genotype 1 infection complicated by cryoglobulinemia that were treated with sofosbuvir.(26) They reported that clearance of cryoglobulins lagged behind viral clearance by 4–6 weeks in 2 of 3 patients; SVR rate was not reported.(26) In our study, we found that viral cure can be achieved in a very high proportion of patients receiving sofosbuvir-based direct-acting antiviral regimens, cryoglobulin levels decreased, reaching a nadir at a median of 4.6 months (range 22 days – 13 months) (Figure 1). Several patients were able to decrease immunosuppression; in fact, the only patient who needed an increase in immunosuppression experienced virologic relapse (Patient 10, Table 4). Serious adverse events were much less common than seen with pegylated interferon and ribavirin combined with the first generation protease inhibitors telaprevir and boceprevir. In series of patients with HCV-MCS treated with first generation protease inhibitors, pegylated interferon and ribavirin, 47% experienced a serious adverse event and 93% required erythropoietin stimulating agents to manage anemia.(7)
This study has several limitations. First, the number of patients with HCV-MCS glomerulonephritis is small; however, it parallels the size of other studies that have had important influence in the field of HCV-MCS.(27–29) Despite the small size of this initial cohort, it was relatively diverse in its ethnic and racial composition: 54% were white non-Hispanic, 31% Hispanic, and 15% African American; however, none of the patients with renal involvement were African American. Thus, a larger cohort is needed to determine whether these initial results are generalizable to particular at risk cohorts.
Although there were few serious adverse events, the number of cases is too small to provide definitive assessment of the safety of combination direct-acting antiviral therapy in HCV-MCS. Furthermore, although all serious adverse events occurred in patients on regimens that contained ribavirin, the small numbers preclude assessment of the relative safety of different treatment regimens in patients with HCV-MCS. All but one patient in this cohort had a baseline eGFR > 30mL/min/1.73m2. There are currently no dosing guidelines for sofosbuvir in patients with eGFR < 30mL/min/1.73m2; thus, these findings may not apply to patients with more advanced renal failure. The patient with eGFR < 30 mL/min/1.73m2 received full dose sofosbuvir and did not experience any adverse events. Since the clinical data were collected retrospectively, there are several patients for whom it was impossible to determine the quantitative changes in proteinuria since it was not always routinely quantified. However, eGFR data are available before and after treatment in all cases and demonstrate the potential for improvement in renal function. Because this is a retrospective cohort, a major limitation is incomplete data; not all patients had complement levels and cryoglobulin levels repeated after treatment (Table 3). Since kidney biopsy was not performed in all cases of glomerulonephritis, it is possible that misclassification occurred; however, biopsy is not uniformly performed for classic cases of HCV-MCS associated glomerulonephritis, so we used the clinical definition most commonly used in other studies (30).
Previous studies have suggested that the presence of circulating cryoglobulin is an independent risk factor for nonresponse to antiviral therapy with interferon-based regimens (6). It has been hypothesized that high levels of inflammatory chemokines or the use of immunosuppression in patients with HCV-MCS have predisposed them to nonresponse to interferon-based therapies that relied on host immune response (31, 32). However, the ability of novel direct-acting antivirals to directly and profoundly halt viral replication and production may have overcome the performance gap previously seen in HCV-MCS. Viral clearance occurs rapidly on novel direct-acting antiviral regimens, much more so than previously reported for interferon-based regimens; the vast majority of patients have undetectable viral load by week 4 on treatment (33). Our cohort displayed similar viral kinetics, with all patients’ HCV RNA becoming undetectable within 4 weeks, no on-therapy viral breakthrough, and an SVR rate of 83%. We believe that future study in patients with HCV-MCS associated glomerulonephritis should prospectively characterize changes in eGFR and proteinuria that occur with therapy and determine the timeline of these changes.
In summary, in light of their efficacy and safety, direct-acting antiviral therapies should be strongly considered in the initial management of HCV-MCS. Future studies will need to clarify the role of immunosuppressive agents in light of the availability of rapidly-acting antiviral agents.
Supplementary Material
Acknowledgments
Funding: NIH K24 DK078772 (RTC), NIH U19 AI082630 (RTC, AYK), ASN Foundation for Kidney Research Fellows Award (MES)
List of abbreviations
- HCV
Hepatitis C Virus
- MCV
Mixed Cryoglobulinemia Syndrome
- HCV-MCV
Hepatitis C Virus Associated Mixed Cryoglobulinemia Syndrome
- SVR
Sustained virological response
- SVR12
Sustained viralogical response rate at twelve weeks
- DAA
Direct acting antiviral
- eGFR
Estimated glomerular filtration rate
- RNA
Ribonucleic Acid
- IU
International units
- IQR
Interquartile range
Table abbreviations
- HIV
Human Immunodeficiency Virus
- HBV
Hepatitis B Virus
- SOF
Sofosbuvir
- SIM
Simeprevir
- RBV
Ribavirin
- PEG-IFN
Pegylated interferon
- ALT
Alanine aminotransferase
- AST
Aspartate transferase
- ESA
Erythropoietin stimulating agent
- CS
Corticosteroids
- RTX
Rituximab
- CYC
Cytoxan
- Pheresis
Plasmapheresis
Footnotes
Conflict of interest statement:
MES declares receiving research support from Gilead Sciences and service on a scientific advisory board for Abbvie.
AKB declares employment at UpToDate
AYK declares participation in scientific advisory boards for Abbvie and Bristol-Myers Squibb and receiving research support from Abbvie and Gilead Sciences
RTC declares research grants from Gilead Sciences, Bristol Myers Squibb, Abbvie, Merck, Janssen, and Mass Biologics.
RT has served on an advisory board for Merck.
JW, MVL, JLV, ALL, DS, MT, WWW, NH have no conflicts of interest to declare.
Contributor Information
Meghan E. Sise, Email: msise@partners.org.
Allyson K. Bloom, Email: akbloom@mgh.harvard.edu.
Jessica Wisocky, Email: Jessica_Wisocky@dfci.harvard.edu.
Ming V. Lin, Email: mvlin@mgh.harvard.edu.
Jenna L. Gustafson, Email: jlgustafson@mgh.harvard.edu.
Andrew L. Lundquist, Email: alundquist@mgh.harvard.edu.
David Steele, Email: dsteele@mgh.harvard.edu.
Michael Thiim, Email: mthiim@mgh.harvard.edu.
Winfred W. Williams, Email: wwwilliams@mgh.harvard.edu.
Nikroo Hashemi, Email: nhashemi@partners.org.
Arthur Y. Kim, Email: akim1@mgh.harvard.edu.
Ravi Thadhani, Email: rthadhani@mgh.harvard.edu.
Raymond T. Chung, Email: Chung.Raymond@mgh.harvard.edu.
References
- 1.Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631–657. doi: 10.1016/s0272-6386(03)00828-x. [DOI] [PubMed] [Google Scholar]
- 2.Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, et al. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 3.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 4.Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379:348–360. doi: 10.1016/S0140-6736(11)60242-0. [DOI] [PubMed] [Google Scholar]
- 5.Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. doi: 10.1186/1750-1172-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gragnani L, Fognani E, Piluso A, Boldrini B, Urraro T, Fabbrizzi A, Stasi C, et al. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: A prospective, controlled, open-label, cohort study. Hepatology. 2014 doi: 10.1002/hep.27623. [DOI] [PubMed] [Google Scholar]
- 7.Saadoun D, Resche Rigon M, Pol S, Thibault V, Blanc F, Pialoux G, Karras A, et al. PegIFNalpha/ribavirin/protease inhibitor combination in severe hepatitis C virus-associated mixed cryoglobulinemia vasculitis. J Hepatol. 2015;62:24–30. doi: 10.1016/j.jhep.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Gordon SC, Muir AJ, Lim JK, Pearlman B, Argo CK, Ramani A, Maliakkal B, et al. Safety Profile of Boceprevir and Telaprevir in Chronic Hepatitis C: Real-World Experience From HCV-TARGET. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauss S, Hueppe D, Alshuth U. Renal impairment is frequent in chronic hepatitis C patients under triple therapy with telaprevir or boceprevir. Hepatology. 2014;59:46–48. doi: 10.1002/hep.26602. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 11.Rossi P, Bertani T, Baio P, Caldara R, Luliri P, Tengattini F, Bellavita P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis: long-term remission after antiviral therapy. Kidney Int. 2003;63:2236–2241. doi: 10.1046/j.1523-1755.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 12.Alric L, Plaisier E, Thebault S, Peron JM, Rostaing L, Pourrat J, Ronco P, et al. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis. 2004;43:617–623. doi: 10.1053/j.ajkd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration Label. [Accessed March 9, 2015]; http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204671s000lbl.pdf.
- 14.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 17.Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887–1896. doi: 10.1373/clinchem.2006.070961. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida EM, Sulkowski MS, Gane EJ, Herring RW, Jr, Ratziu V, Ding X, Wang J, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41–45. doi: 10.1002/hep.27366. [DOI] [PubMed] [Google Scholar]
- 19.Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 20.al DDe “Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network” AASLD. 2014 Abstract 46. [Google Scholar]
- 21.Jenson Dea. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: Real-world experience in a diverse, longitudinal observational cohort. AASLD. 2014 Abstract 45. [Google Scholar]
- 22.Fabrizi F. Hepatitis C virus, cryoglobulinemia, and kidney: novel evidence. Scientifica (Cairo) 2012;2012:128382. doi: 10.6064/2012/128382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadoun D, Resche-Rigon M, Thibault V, Piette JC, Cacoub P. Antiviral therapy for hepatitis C virus--associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54:3696–3706. doi: 10.1002/art.22168. [DOI] [PubMed] [Google Scholar]
- 24.Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623–637. doi: 10.1053/j.ajkd.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Saadoun D, Resche Rigon M, Sene D, Terrier B, Karras A, Perard L, Schoindre Y, et al. Rituximab plus Peg-interferon-alpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood. 2010;116:326–334. doi: 10.1182/blood-2009-10-248518. quiz 504–325. [DOI] [PubMed] [Google Scholar]
- 26.Stine JG, Cornella S, Shah NL. Treatment of chronic hepatitis C complicated by mixed cryoglobulinemia with new protease inhibitor, sofosbuvir. Ann Rheum Dis. 2014;73:e64. doi: 10.1136/annrheumdis-2014-206180. [DOI] [PubMed] [Google Scholar]
- 27.Roccatello D, Baldovino S, Rossi D, Giachino O, Mansouri M, Naretto C, Di Simone D, et al. Rituximab as a therapeutic tool in severe mixed cryoglobulinemia. Clin Rev Allergy Immunol. 2008;34:111–117. doi: 10.1007/s12016-007-8019-0. [DOI] [PubMed] [Google Scholar]
- 28.Quartuccio L, Soardo G, Romano G, Zaja F, Scott CA, De Marchi G, Fabris M, et al. Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxford) 2006;45:842–846. doi: 10.1093/rheumatology/kel004. [DOI] [PubMed] [Google Scholar]
- 29.Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:835–842. doi: 10.1002/art.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadoun D, Resche Rigon M, Thibault V, Longuet M, Pol S, Blanc F, Pialoux G, et al. Peg-IFNalpha/ribavirin/protease inhibitor combination in hepatitis C virus associated mixed cryoglobulinemia vasculitis: results at week 24. Ann Rheum Dis. 2014;73:831–837. doi: 10.1136/annrheumdis-2012-202770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelli A, Fallahi P, Ferrari SM, Corrado A, Sebastiani M, Giuggioli D, Miccoli M, et al. Parallel increase of circulating CXCL11 and CXCL10 in mixed cryoglobulinemia, while the proinflammatory cytokine IL-6 is associated with high serum Th2 chemokine CCL2. Clin Rheumatol. 2013;32:1147–1154. doi: 10.1007/s10067-013-2246-y. [DOI] [PubMed] [Google Scholar]
- 32.Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, Charles ED, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175–1182. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804–811. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.