Abstract
Objective
Endogenous neurosteroids that potentiate the GABAA receptor are thought to enhance the generation of sleep spindles. This study tested the hypothesis that the 5α-reductase inhibitor finasteride, an agent associated with reductions in neurosteroids, would be associated with reduced sleep spindles in men referred for polysomnography.
Methods
Spectral analysis and spindle waveform detection were performed on electroencephalographic (EEG) sleep data in the 11–16Hz sigma band, as well as several subranges, from 27 men taking finasteride and 27 matched comparison patients (ages 18 to 81 years).
Results
No significant differences between groups were observed for spectral power or sleep spindle morphology measures, including spindle density, amplitude, duration, and integrated spindle activity.
Conclusions
Contrary to our hypothesis, these findings demonstrate that finasteride is not associated with alterations in sleep spindle range activity or spindle morphology parameters.
Keywords: finasteride, sleep spindle, neurosteroid, 5α-reductase inhibitor
INTRODUCTION
Sleep spindles are waxing-waning electroencephalographic (EEG) waveforms characteristic of stage 2 non-rapid eye movement (NREM) sleep that play functionally significant roles in sleep-dependent memory consolidation and cortical development (Iber et al., 2007; Lüthi, 2013). These waveforms are generated by the reticular nucleus of the thalamus, and are potentiated by agents that are positive allosteric modulators of the GABAA receptor (De Gennaro and Ferrara, 2003). Notably, neurosteroids such as allopregnanolone and androstanediol, which are derived from progesterone and testosterone through shared stepwise enzymatic reactions with 5α-reductase and 3α-hydroxysteroid dehydrogenase, are potent endogenous allosteric modulators of GABAA receptors (Carver and Reddy, 2013). In women, increases in substrate progestins are associated with increases in sleep spindle activity, suggesting endogenous neurosteroids may affect sleep spindle generation (Baker et al., 2012; Plante and Goldstein, 2013).
However, to our knowledge there is no evidence demonstrating whether agents that inhibit the production of endogenous neurosteroids reduce sleep spindles. Finasteride is a 5α-reductase inhibitor, commonly used for benign prostatic hyperplasia and androgenic alopecia (Finn et al., 2006). This agent preferentially binds to the type 2 isoform of 5α-reductase (5αR2), which has a higher affinity for androgens compared to the type 1 isoform (5α1R) (Jin and Penning, 2001; Traish, 2012). 5αR2 is strongly expressed in the thalamic reticular nucleus in adult male rats, and is thus located anatomically in a region in which its inhibition could alter sleep spindle production (Castelli et al. 2013). Moreover, although clinically desired effects occur in peripheral tissues, finasteride crosses the blood-brain barrier and thus alters neurosteroid production in the central nervous system (CNS) (Finn et al., 2006). As evidence of its CNS effects, finasteride has been associated with mood disturbance, even at low dose (Altomare and Capella, 2002; Rahimi-Ardabili et al., 2006). These mood-related effects may be due to decreased levels of allopregnanolone (Uzunova et al., 1998), which has been demonstrated in cerebrospinal fluid and plasma levels of post-finasteride treated patients (Caruso et al., 2015). Notably, reductions in sleep spindles, particularly in males, have been associated with depression (de Maertelaer et al., 1987; Plante et al., 2013), which, although speculative, provides circumstantial connections among finasteride’s mechanism of action, neuropsychiatric effects, and its hypothesized role in reducing sleep spindle activity.
Given the functional significance of sleep spindles and their clinical utility in polysomnographic staging, this study was conducted to examine whether finasteride is associated with reductions in sleep spindles in men referred for polysomnography.
METHODS
Subjects
All patients evaluated in this study were selected using methods previously utilized in our laboratory, in accordance with a chart review protocol approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (Plante and Goldstein, 2013). To identify individuals for inclusion, all diagnostic overnight polysomnograms performed at Wisconsin Sleep™ from October 2, 2007 to July 10, 2014 were cross-referenced with finasteride medication history in the electronic medical record (EpicCare, Epic Systems Incorporated, Verona WI). Patients were excluded for a medication history of benzodiazepine and/or benzodiazepine receptor agonist use, due to known effects of these medications on sleep spindles (Puia et al., 2012; Suetsugi et al., 2001). Additional exclusion criteria based on information available in the medical record included the following: severe obstructive sleep apnea [apnea-hypopnea index (AHI)≥30/hr], total sleep time during sleep study <90 minutes, significant neurologic disorder (e.g. history of stroke), or psychotic disorder. Comparison patients not taking finasteride were age- and sex-matched using the same above exclusion criteria. Further matching was performed based on presence or absence of concomitant antidepressant medication to minimize potential confounders of depression and/or antidepressant pharmacotherapy (Dotan et al., 2008; Plante et al., 2013). Approximate duration of finasteride use was estimated using information available in the medical record. The final list of both finasteride and comparison patients was determined prior to visual inspection, processing, and analysis of the data. Based on prior studies from our laboratory (Plante and Goldstein, 2013), we estimated a sample size of 27 per group would have power of 0.80 to detect differences in spindle density between groups.
EEG Recording and Analysis
All patients underwent routine, overnight in-laboratory polysomnography (PSG) that utilized standard electroencephalogram (EEG), electrooculogram (EOG), submental electromyogram (EMG), electrocardiogram (ECG), bilateral tibial EMG, respiratory inductance plethysmography, pulse oximetry, and a position sensor. A registered polysomnographic technologist scored sleep stages in 30 second epochs using Alice® Sleepware (Philips Respironics, Murrysville, PA) according to standard criteria (Iber et al., 2007). EEG signals were sampled at 200Hz.
Similar to prior studies (Lopez et al., 2010; Plante and Goldstein, 2013), F3-A2 was considered the a priori derivation of interest, with data from C3-A2 of secondary interest. Individual epochs with excessive artifact were removed via semi-automatic artifact rejection (performed blind to study group). Spectral analysis of NREM sleep was performed with consecutive 6-second epochs (Welch’s averaged modified periodogram with a Hamming window), resulting in a frequency resolution of 0.17Hz (Goldstein et al., 2012; Plante et al., 2012). Power spectra of artifact-free NREM epochs (stages N2 and N3) were compared for all frequency bins from 1 to 25Hz between finasteride and control groups, with the a priori analysis plan to focus the spindle detection algorithm within frequency bands in the spindle range (~11–16Hz) for which there were significant between group differences in spectral power.
Spindle Detection
Spindle detection and analyses were performed similar to prior studies in our laboratory utilizing a customized spindle detection algorithm in MATLAB (MathWorks, Natick, MA) (Ferrarelli et al., 2007, 2010; Plante et al., 2013; Plante and Goldstein, 2013). Four spindle parameters were calculated for each patient: density (number of detected spindles divided by NREM sleep time), amplitude (mean peak amplitude of all detected spindles), duration (mean duration of all detected spindles), and integrated spindle activity (ISA; integrated absolute amplitude values of each spindle divided by NREM sleep time) (Ferrarelli et al., 2010).
Statistics
Sleep architecture, spectral power, and spindle morphology parameters were compared using unpaired two-tailed t-tests. Statistical analyses were performed in MATLAB and JMP Version 11.0 (SAS Institute Inc., Cary, NC). Values in the text are reported as mean±standard deviation.
RESULTS
Subjects
Twenty-seven patients taking finasteride who met inclusion/exclusion criteria were identified and matched with 27 comparison patients. Mean daily finasteride dose was 4.15mg daily (range 0.5–5mg), with the majority (N=21) taking 5mg daily. Estimated duration of finasteride use was 23.7±24.4 months (range 1–77). Both groups demonstrated similar age (range 18–81 years), body mass indices (BMI), and sleep staging (Table 1). Five participants from each group were taking antidepressant medications at the time of PSG. The most common medical comorbidities were hyperlipidemia (6 finasteride vs. 11 controls) hypertension (11 finasteride vs. 12 controls), and obesity (BMI>30; 10 finasteride vs. 12 controls).
Table 1.
Demographic, clinical, and polysomnographic data.
| Finasteride (N = 27) | Controls (N = 27) | p* | |
|---|---|---|---|
|
|
|||
| Age (years) | 60.8 (15.6) | 60.7 (15.5) | 0.98 |
| BMI (kg/m2) | 30.1 (7.0) | 31.0 (4.5) | 0.58 |
| TST (min) | 317.4 (81.4) | 338.5 (65.8) | 0.30 |
| WASO (min) | 124.5 (49.4) | 111.3 (49.6) | 0.33 |
| SE (%) | 68.0 (13.5) | 72.5 (12.3) | 0.21 |
| SOL (min) | 20.9 (19.1) | 17.7 (22.1) | 0.57 |
| N1 (%) | 13.9 (9.2) | 15.0 (10.6) | 0.68 |
| N2 (%) | 64.6 (9.0) | 64.9 (9.2) | 0.88 |
| N3 (%) | 4.8 (7.0) | 5.6 (6.6) | 0.65 |
| REM (%) | 16.8 (6.8) | 14.5 (6.6) | 0.20 |
| REML (min) | 144.6 (74.7) | 162.5 (92.7) | 0.44 |
| AHI (#/hr) | 5.9 (5.8) | 5.1 (3.8) | 0.57 |
| PLMAI (#/hr) | 4.1 (4.3) | 3.6 (2.5) | 0.57 |
BMI, body mass index; TST, total sleep time; WASO, wake after sleep onset; AI, arousal index; SE, sleep efficiency (TST/time in bed); SOL, sleep onset latency; N1/2/3, NREM stage 1/2/3 (% of TST); REM, stage REM (% of TST); REML, REM latency (time from sleep onset to first REM sleep epoch).
Values are displayed as mean (standard deviation).
p-value derived using 2-tailed, independent samples t-tests.
EEG analyses
Following artifact rejection, greater than 90% of epochs were retained in each group, and the percent of retained epochs did not differ between groups (finasteride vs. comparison: 91.7±2.6% vs. 91.4±2.9%, p=0.77). Spectral analysis demonstrated no significantly different frequency bins from 1–25Hz between groups. Exploratory analysis of detected spindles (11–16Hz) demonstrated no significant differences between groups for spindle density (1.81±0.97 vs. 1.86±0.65 counts/min, p=0.81), amplitude (19.02±6.27 vs. 19.79±6.64 μV, p=0.66), duration (0.70±0.19 vs. 0.71±0.18 seconds, p=0.75) or ISA (3.62±2.09 vs. 3.55±2.64, p=0.92) (Figure 1). Further subgroup analysis of participants taking finasteride 5mg versus controls further revealed no differences between groups in any measure of spindle density or morphology. Results were unchanged when utilizing data from C3-A2, as well as when slower (e.g. ~11 to 13–14Hz) and faster (13–14 to 16Hz) spindle subranges were examined on an exploratory basis.
Figure 1.
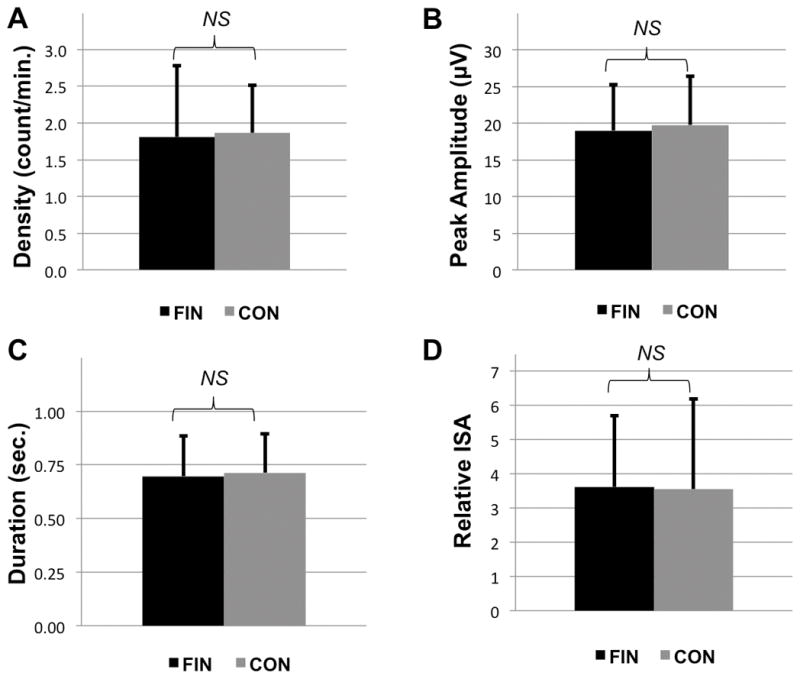
Sleep spindle parameters for patients taking finasteride (FIN) and matched control patients (CON) derived from F3-A2 via 11–16Hz automated spindle detection. (A) Density, (B) Mean Peak Amplitude, (C) Duration, and (D) Integrated Spindle Activity (ISA). All data plotted ± SD; NS = not significant.
Given that prior research has demonstrated age-related declines in sleep spindle characteristics (Nicolas et al. 2001; Peters et al. 2014), correlations with age were also explored. Consistent with the prior literature, each of the four spindle parameters across all participants was significantly negatively correlated with age (r = −0.46 to −0.66, p<0.001). Correlations were similar for finasteride and control participants when evaluated separately. Duration of finasteride use, however, was not related to spindle characteristics in this sample (r = −0.04 to −0.25, all p>0.2).
DISCUSSION
Contrary to our hypothesis, our findings demonstrate that the use of finasteride at typical doses is not associated with alterations in sleep spindles in adult men referred for polysomnography. Although these results are negative, they are of significant clinical interest. First, as demonstrated in Table 1 and consistent with prior literature (Cunningham and Hirshkowitz 1995), this study suggests that the use of finasteride is unlikely to significantly affect sleep staging through alterations in sleep spindles, and thus should not have meaningful effects on polysomnographic scoring or interpretation. Second, our findings suggest that the neurocognitive side effects associated with finasteride are unlikely to be related to alterations in sleep spindles, as our findings do not indicate this agent reduces these functionally significant waveforms during NREM sleep.
Strengths of this investigation include the use of an adequately powered sample, exclusion of exogenous agents that might affect sleep spindles, and careful matching of comparison patients to minimize confounds such as age. It is possible that our a priori power estimations underestimated the sample size necessary to detect a difference between groups, since they were based on a study examining the effects of exogenous progestins on sleep spindles, and androstanediol is a less potent modulator of the GABAA receptor compared to allopregnanolone (Reddy and Jian, 2010). However, the very similar mean values for all spindle parameters between groups with variances that are proportionally much greater than the differences in means, suggests even a very large study would still fail to reject the null hypothesis of no difference between groups. Additionally, our results cannot be extended to other 5α-reductase inhibitors such as dutasteride, which non-selectively inhibits 5αR1 and 5αR2, both of which are expressed in the thalamic reticular nucleus (Traish, 2012; Agís-Balboa et al., 2006; Castelli et al., 2013). Finally, because data were derived from a clinical population, failure to find differences between groups as a result of some other uncontrolled factor, including medication non-adherence, cannot be definitively excluded. However, the strong null findings of this study suggest future investigation using a more rigorous study design is unlikely to be fruitful.
Several considerations may reconcile these findings with existing literature that has demonstrated significant alterations of sleep spindles via other neuromodulators and pharmacologic agents (De Gennaro and Ferrara, 2003; Baker et al., 2012; Plante and Goldstein, 2013). First, as mentioned above, while GABAergic neurosteroids have shared enzymatic reactions that may be affected by finasteride, both level of potency of a given neurosteroid and specific GABAA receptor subunit affinity for neurosteriods may vary (Reddy and Jian, 2010). Similarly, the extent to which synthetic neurosteroids versus enzymatic inhibitors of neurosteroid pathways impact GABAergic activity, and correspondingly the extent of direct and indirect effects on sleep spindle characteristics, remains unclear (Lambert et al., 2001). Given the reported sex differences in sleep spindles across a number of studies (Genzel et al., 2012; Plante et al., 2013; Bódizs et al., 2014), as well as the previously described reduced potentiation of the GABAA receptor by androstanediol relative to allopregnanolone, the magnitude of EEG effects induced by finasteride may vary between men and women. It is also possible that larger doses of finasteride are required to observe significant alterations of sleep spindles. However, it appears less likely that effects vary from short-term to prolonged exposure given the lack of significant correlations with duration of finasteride use observed here. Finally, the extent to which EEG in general reflects neurosteroid modulation afforded by these different agents is not fully elucidated (Rupprecht, 2003). In addition to general scalp EEG limitations, most prominently the restricted spatial resolution, the clinical polysomnography protocol utilized in the present study may not fully capture sleep characteristics in a naturalistic home environment or be generalizable to other clinical and non-clinical populations (Bae et al., 2009). Future research would benefit from addressing these considerations to further the interpretation of these findings.
Acknowledgments
We thank Drs. Meredith Rumble and Ruth Benca, who provided oversight and maintenance of the IRB protocol under which this study was conducted. Dr. Plante is supported by NIMH (K23MH099234), the Brain and Behavior Research Foundation, and the American Sleep Medicine Foundation.
FUNDING
Dr. Plante is supported by NIMH (K23MH099234; Bethesda, MD, USA), the Brain and Behavior Research Foundation (New York, NY, USA), and the American Sleep Medicine Foundation (Darien, IL, USA). None of the funding sources had any further role in the study design, data collection, analysis and interpretation of the data, and the decision to submit the paper for publication.
Footnotes
CONFLICT OF INTEREST
Dr. Plante has received royalties from Cambridge University Press.
References
- Agís-Balboa RC, Pinna G, Zhubi A, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A. 2006;103(39):14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia. J Dermatol. 2002;29(10):665–669. doi: 10.1111/j.1346-8138.2002.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Bae CJ, Lee JK, Foldvary-Schaefer N. The use of sleep studies in neurologic practice. Semin Neurol. 2009;29(4):305–319. doi: 10.1055/s-0029-1237123. [DOI] [PubMed] [Google Scholar]
- Baker FC, Sassoon SA, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–545. doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bódizs R, Gombos F, Ujma PP, Kovács I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Front Hum Neurosci. 2014;8:952. doi: 10.3389/fnhum.2014.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, Abbiati F, Giatti S, et al. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J Steroid Biochem Mol Biol. 2015;146:74–79. doi: 10.1016/j.jsbmb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 2013;230(2):151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Casti A, Casu A, et al. Regional distribution of 5α-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology. 2013;38(2):281–293. doi: 10.1016/j.psyneuen.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GR, Hirshkowitz M. Inhibition of steroid 5 alpha-reductase with finasteride: sleep-related erections, potency, and libido in healthy men. J Clin Endocrinol Metab. 1995;80(6):1934–1940. doi: 10.1210/jcem.80.6.7775644. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- De Maertelaer V, Hoffman G, Lemaire M, et al. Sleep spindle activity changes in patients with affective disorders. Sleep. 1987;10(5):443–451. doi: 10.1093/sleep/10.5.443. [DOI] [PubMed] [Google Scholar]
- Dotan Y, Suraiya S, Pillar G. Sleep spindles in post traumatic stress disorder: significant importance of selective serotonin reuptake inhibitors. Harefuah. 2008;147(10):763–767. 839–840. [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Kiefer T, Renner L, et al. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology. 2012;37(7):987–998. doi: 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Goldstein MR, Plante DT, Hulse BK, et al. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr Scand. 2012;125(6):468–477. doi: 10.1111/j.1600-0447.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1. Westchester, IL: 2007. [Google Scholar]
- Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15(1):79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Harney SC, Belelli D, Peters JA. Neurosteroid modulation of recombinant and synaptic GABAA receptors. Int Rev Neurobiol. 2001;46:177–205. doi: 10.1016/s0074-7742(01)46063-6. [DOI] [PubMed] [Google Scholar]
- Lopez J, Hoffmann R, Armitage R. Reduced sleep spindle activity in early-onset and elevated risk for depression. J Am Acad Child Adolesc Psychiatry. 2010;49(9):934–943. doi: 10.1016/j.jaac.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A. Sleep Spindles: Where They Come From, What They Do. Neuroscientist. 2013;20(3):243–256. doi: 10.1177/1073858413500854. [DOI] [PubMed] [Google Scholar]
- Nicolas A, Petit D, Rompré S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol. 2001;112(3):521–527. doi: 10.1016/s1388-2457(00)00556-3. [DOI] [PubMed] [Google Scholar]
- Peters KR, Ray LB, Fogel S, et al. Age differences in the variability and distribution of sleep spindle and rapid eye movement densities. PLoS One. 2014;9(3):e91047. doi: 10.1371/journal.pone.0091047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, et al. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density EEG investigation. BMC Psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Goldstein MR. Medroxyprogesterone acetate is associated with increased sleep spindles during non-rapid eye movement sleep in women referred for polysomnography. Psychoneuroendocrinology. 2013;38:3160–3166. doi: 10.1016/j.psyneuen.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Goldstein MR, Landsness EC, et al. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146(1):120–125. doi: 10.1016/j.jad.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Gullo F, Dossi E, et al. Novel modulatory effects of neurosteroids and benzodiazepines on excitatory and inhibitory neurons excitability: a multi-electrode array recording study. Front Neural Circuits. 2012;6:94. doi: 10.3389/fncir.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, et al. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006;6:7. doi: 10.1186/1472-6904-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334(3):1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Suetsugi M, Mizuki Y, Ushijima I, et al. The effects of diazepam on sleep spindles: a qualitative and quantitative analysis. Neuropsychobiology. 2001;43(1):49–53. doi: 10.1159/000054865. [DOI] [PubMed] [Google Scholar]
- Traish AM. 5α-reductases in human physiology: an unfolding story. Endocr Pract. 2012;18(6):965–975. doi: 10.4158/EP12108.RA. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95(6):3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]


