Abstract
Endocrine disrupting chemicals (EDCs) are widespread environmental contaminants that affect many neuroendocrine functions. The brain is particularly vulnerable to EDCs during critical periods of gestational development when gonadal hormones exert organizational effects on sexually dimorphic behaviors later in life. Peripubertal development is also a time of continued neural sensitivity to organizing effects of hormones, yet little is known about EDC actions at these times. We sought to determine effects of prenatal or juvenile exposures to a class of EDCs, polychlorinated biphenyls (PCBs) at human-relevant dosages on development, physiology, and social and anxiety-related behaviors later in life, and the consequences of a second juvenile “hit” following prenatal treatment. We exposed male and female Sprague-Dawley rats to PCBs (Aroclor 1221, 1mg/kg/day, ip injection) and/or vehicle during prenatal development (embryonic days 16, 18, 20), juvenile development (postnatal days 24, 26, 28), or both. These exposures had differential effects on behaviors in sex and age-dependent ways; while prenatal exposure had more effects than juvenile, juvenile exposure often modified or unmasked the effects of the first hit. Additionally, females exhibited altered social and anxiety behavior in adolescence, while males displayed small but significant changes in sociosexual preferences in adulthood. Thus, the brain continues to be sensitive to organizing effects of EDCs through juvenile development. As humans are exposed to EDCs throughout multiple periods in their life, these findings have implications for our understanding of EDC effects on physiology and behavior.
Keywords: Endocrine-disrupting chemical, PCB, Aroclor 1221, adolescence, ultrasonic vocalization, social behavior, anxiety
Introduction
Endocrine disrupting chemicals (EDCs) are ubiquitous in our environment and alter a range of behaviors and physiological functions (Zoeller et al., 2012). One class of EDC is polychlorinated biphenyls (PCBs); while banned in the U.S. in the 1970’s, PCBs are detectable in virtually all humans (Agency for Toxic Substances and Disease Registry, 2000). PCBs, along with other environmental EDCs, have been well-studied for how early life exposures (usually prenatal or early postnatal) lead to a latent dysfunction in the adult. Nevertheless, there are other life stages when there is high vulnerability to EDCs, as well as times in life other than adulthood when outcomes should be assessed. A case in point is juvenile and adolescent development, when the brain is sensitive to organizational effects of gonadal hormones, and during which time pubertal increases in these hormones activate, and continue to organize, neural circuits that were established earlier (Ahmed et al., 2008; Cooke and Woolley, 2009; De Lorme and Sisk, 2013; Markham et al., 2007; Mohr and Sisk, 2013; Primus and Kellogg, 1990; Schulz et al., 2004; 2009; Sisk and Foster, 2004; Sisk et al., 2003). This life stage is characterized by age-specific behaviors that may be uniquely affected by prior EDC exposure; and exposure during which can lead to alterations in the adult behavioral phenotype (DellaSeta et al., 2006; Yu et al., 2011).
Initial behavioral work on developmental PCB exposures in rats showed that perinatal exposure reduced the performance of sexual behavior in adulthood (Chung and Clemens, 1999; Chung et al., 2001; Cummings et al., 2008; Steinberg et al., 2007; Wang et al., 2002). However, these behaviors are part of a much broader suite of behaviors used to communicate affective and social information to a conspecific, and which have been sparsely studied for PCB effects (Jolous-Jamshidi et al., 2010). The current study extended prior work by assessing sexually dimorphic and hormone-dependent adolescent and adult behaviors in response to PCBs. In adolescent rats, play behavior and ultrasonic vocalizations (USVs) are important features of the behavioral repertoire and communicate social status, hedonia, and social interest and memory (Auger and Olesen, 2009; Knutson et al., 1998; Meaney and Stewart, 1981; Moles et al., 2007; Scattoni et al., 2009). In adult rats, hormone-dependent sociosexual partner preference and the production of USVs can also reflect sociosexual choice and motivation distinct from overt sexual behaviors (Harding and McGinnis, 2003; Henley et al., 2011; McGinnis and Vakulenko, 2003). At both ages, anxiety-like behaviors are also hormone sensitive and sexually dimorphic (Adler et al., 1999; Bitran, 1993; Johnston and File, 1991; Marcondes et al., 2001; Mora et al., 1996; Nomikos and Spyraki, 1988) and therefore may also be targets for PCB disruption.
Humans and animals are exposed to PCBs and other EDCs throughout the lifespan, and there is evidence in humans that PCB exposure is tied to cognitive and neurodevelopmental problems when evaluated in infants and young children (Engel and Wolff, 2013). It is not possible in humans, though, to differentiate between various sensitive periods such as prenatal and adolescence on neurobehavioral outcomes. The current study directly addressed this by exposing rats to PCBs in a “two hit” model, whereby rats were exposed prenatally, in adolescence, or both, the latter enabling elucidation of the consequences of a second hit on the first. We ascertained outcomes on a range of social and anxiety-like behaviors in adolescence and adulthood. The hypothesis tested was that a second PCB hit would change the neurobehavioral developmental trajectory in a manner not predicted by either hit alone, and that due to natural sex differences in gonadal hormones, the sexes would differ in their outcomes.
Methods
PCB Treatment
The PCB mixture used in this study was Aroclor 1221 (A1221, AccuStandard, New Haven, CT, catalog number: C-221N-50MG, Lot: 23683) dissolved in vehicle (Veh, 4% dimethylsulfoxide, Sigma number D4540; Sigma, St Louis, Missouri in sesame oil). A1221 is a mix of ~45 congeners, but predominantly composed of PCBs 1, 3, 8, 4, 15, 6, and 2 in order of greatest (~%40) to least (~3%) amount (Frame et al., 1996). These congeners are lightly chlorinated (66% mono- and 26% di-chlorinated), with chlorine substitutions predominantly at ortho and para positions. For prenatal exposure, dams were injected with Veh or A1221 solution (1mg/kg, ip) on E16, E18, and E20. For juvenile exposure, pups were given an additional set of injections (1 mg/kg, ip) on P24, 26, and 28. This mixture, dosage, and injection paradigm was selected so that outcomes of the current study could be compared with findings from several other previous studies using a very similar exposure regime. This dose is not toxic to dams and does not cause fetal loss (Dickerson et al., 2011; Reilly et al., 2015; Steinberg et al., 2008; Walker et al., 2014), and is estimated to be within the range of human exposure to PCBs (Agency for Toxic Substances and Disease Registry, 2000; Centers for Disease Control, 2009; Matthews and Anderson, 1975; Patterson et al., 2009; Steinberg et al., 2008; Takagi et al., 1976).
Animal care and physiological measures
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by The University of Texas at Austin Institutional Animal Care and Use Committee. Sprague Dawley rats were purchased from Harlan Laboratories (Houston, Texas) and were housed in a humidity- and temperature- controlled room with a 12:12 reversed light cycle (lights off at noon) at 21–23C. 2–3 animals were group housed in polycarbonate cages (43 × 21 × 25cm) with aspen bedding (PJ Murphy Forest Products, Sani-Chip), a PVC tube for enrichment, and weekly cage changes. Rats were fed low phytoestrogen Harlan-Teklad 2019 Global Diet (Harlan-Teklad, Indianapolis, Indiana) ad libitum for the duration of the experiment. Upon arrival, rats were handled daily to acclimate them to their new housing conditions, and mating began at least two weeks later.
Females (3–4 month old, virgin) were mated with sexually experienced male rats (~6 months old); each male sired 2 litters, one with a Veh and one with an A1221 dam. The morning after successful mating (indicated by a sperm positive vaginal smear), termed embryonic day (E) 1, dams were singly housed. Dams were randomly assigned to either Veh (n = 6) or A1221 (n = 6), and each litter contributed no more than two animals per group. On E16, E18, and E20, during the period of sexual differentiation of the rat brain (Breedlove, 1992; Ramaley, 1979; Rhees et al., 1990; Tobet and Fox, 1989; Wagner et al., 1998), dams were weighed and injected with 0.1 mL of Veh or A1221 solution (ip, 3 hours prior to onset of dark stage). Dams were provided with nesting materials several days prior to expected parturition on E23. On the day after birth [postnatal day (P) 1], litters were culled to 6–8 pups of equal sex ratios by removing individuals with extreme anogenital index (anogenital distance/3√body weight) measures. Pups were weaned on P21 and housed with same sex littermates (2–3 per cage), and were weighed and handled for at least 5 minutes weekly. For juvenile exposure, rats were given an additional set of injections on P24, 26, and 28, when puberty is beginning and the brain is highly sensitive to organizing effects of testosterone (Döhler and Wuttke, 1975; Saksena and Lau, 1979; Schulz et al., 2009; Smyth and Wilkinson, 1994; Vetter-O’Hagen and Spear, 2011). Cagemates were given the same treatment to prevent cross-contamination.
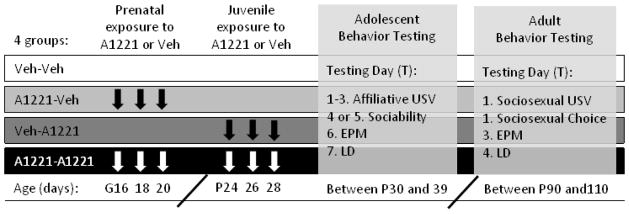
With both gestational and juvenile exposures, there are four experimental groups per sex, in a 2×2 balanced design (first hit prenatal, second hit adolescent): Veh-Veh; A1221-Veh; Veh-A1221; A1221-A1221 (Figure 1). Final Ns per group were between 9 and 12. The experimenters were blind to treatment throughout the duration of the experiment. Developmental and physiological measures were taken throughout animals’ lives. Small but significant effects of PCBs were found on body weight, age of puberty onset in females, and testes weight at time of sacrifice (described in Supplemental Materials).
Figure 1.
Experimental Design. Animals were exposed to either Vehicle (Veh) or Aroclor 1221 (A1221) prenatally and/or during juvenile development, as indicated by arrows. Behaviors were tested in adolescence (over 7 days between P30-39) and adulthood (over 4 days between P90-110). More test details are also included in Table 1. Abbreviations: G (gestational day); P (postnatal day); USV (ultrasonic vocalization); EPM (elevated plus maze); LD (light dark).
Behavioral testing
All behaviors were run in a consistent order at least one hour after lights-out under dim red light (Table 1). Adolescents were tested for social interactions with same-sex and age conspecifics and anxiety-related behaviors over 7 days, while they were between P30-39 (details in Supplemental Materials). On testing days (T)1 through T3, animals were tested for affiliative behaviors and USVs during social interactions. Adolescent rats show play behaviors and emit 50kHz USVs in anticipation of, and during, the interaction (Auger and Olesen, 2009; Knutson et al., 1998; Moles et al., 2007; Scattoni et al., 2009). These USVs are produced in multiple rewarding contexts and can be roughly divided into flat and frequency modulated (FM) calls thought to serve as communication and indicate positive affect, respectively (Burgdorf et al., 2011; Knutson et al., 2002; Wöhr and Schwarting, 2007). In addition to standard play behaviors, a previously undefined behavior was also observed and called ‘hopping’. It was frequently performed by animals immediately prior to a play bout, and consisted of an animal quickly jumping around the cage. On T4 or T5, they were tested for sociability, with half of the animals run on each day. Rats tend to show a preference for a novel over a familiar conspecific stimulus animal, and tests of this behavior can be used to indicate general social interest and memory. On T6 and T7, animals were tested for elevated plus maze (EPM) and light dark box (LD) performance, respectively. In these test, rats can chose to explore the entire novel environment, including the more exposed open arm or light side of the apparatus, or remain more hidden in the closed or dark side. Greater time spent in the exposed areas is an indicator of reduced anxiety. Females were not cycling regularly yet, and testing was not synchronized to estrous cycle.
Table 1. Behavior Testing Procedures.
Adolescent (Adol.) animals were between 30 and 39 days old, and adult animals were between 90 and 110 days old.
| Test Name (&interpretation) | Test Design | Age at day of test (T) | Stages of Test | Duration of Test | Outcomes Measured |
|---|---|---|---|---|---|
| Affiliative Behavior (motivation for playful social interactions) |
|
Adol. (T1-3) | Isolation | 4 hours | T1, 2, and 3: Number of frequency modulated (FM) and Flat Ultrasonic vocalizations (USVs). T3: Number, duration, and latency to nape contact, hop, and wrestle |
| Pre-Stimulus | 5 min (T1, 2) | ||||
| Stimulus | 10 min (T1-3) | ||||
| Post-Stimulus | 5 min (T1,2) | ||||
| Sociability (attraction to and memory of same-sex social interactions) |

|
Adol. (T4 or 5) | Habituate to chamber | 5 min | Time and latency to reach area proximate to stimulus chamber |
| 1: Familiar vs Empty | 10 min | ||||
| 2: Familiar vs Novel | 10 min | ||||
| Sociosexual USVs (motivation for sexual interactions) |

|
Adult (T1) | Pre-Stimulus | 5 min | Number of FM and Flat USVs |
| Stimulus | 10 min | ||||
| Post-Stimulus | 5 min | ||||
| Sociosexual Choice (preferences for opposite-sex interactions) |

|
Adult (T1) | Habituate to chamber | 5 min | Time in and latency to reach area proximate to stimulus chamber |
| Hormone/No-Hormone | 10 min | ||||
| Elevated Plus Maze (EPM) (anxiety-like behavior) |

|
Adol. (T6) & Adult (T3) | Habituate to room | 30 min | Time and number of entries into open arms |
| Test | 5 | ||||
| Light Dark Box (LD) (anxiety-like behavior) |

|
Adol. (T7) & Adult (T4) | Habituate to room | 30 min | Time and number of entries into light side |
| Test | 5 |
As adults, these same rats were tested for sociosexual interactions with receptive opposite-sex conspecifics and anxiety-related behaviors over 4 days between P90-110 (details in Supplemental Materials). Behavior testing began on the evening of proestrus in females, and receptivity was confirmed with a stimulus male animal. On T1 sociosexual USVs and partner preference were tested, in that order. As with adolescent tests, flat and FM calls in the 50 kHz range may serve to communicate with the conspecific and indicate hedonia, respectively (Burgdorf et al 2011). Preference for spending time near a hormone- over a non-hormone treated potential mate is also indicative of sociosexual motivation. Two and three days after sociosexual testing, on T3 and 4, animals were tested for EPM and LD performance, respectively (during diestrus in females). Sexes and litters were balanced across apparatus, testing time, and stimulus animal.
Analysis and Statistics
Final Ns were as follows: for males, Veh-Veh (12); A1221-Veh (10); Veh-A1221 (10); A1221-A1221 (9) and for females, Veh-Veh (10); A1221-Veh (9); Veh-A1221 (12); A1221-A1221 (10). No effects of birth litter were observed in any of the significant effects, so individual rats were used as the unit of analysis for statistical purposes, and there were no cohort effects. Any outliers were identified via Grubbs test (maximum of one per group) and were removed. Within each test, 0–2 animals were identified as outliers across all groups; in the sociosexual test, 4 animals were excluded from analysis because they did not leave the center chamber. Individual behavioral and physiological measures were analyzed using a 2 × 2 analysis of variance (ANOVA) within each sex to determine any main effects of prenatal or juvenile treatment and interactions, with appropriate follow-up t-tests to identify the source of detected interactions. If measures failed to meet normality or homogeneity assumptions (as indicated by Shapiro Wilks and Levene’s tests), a non-parametric Kruskal-Wallis test was used and is indicated in the results as a ‘KW’. In this case, an interaction was identified by testing for effects of one treatment while holding the other constant and vice versa.
Effect sizes of main effects and interactions from ANOVAs are indicated with Eta squared (η2 = treatment variance/corrected total variance); when multiplied by 100, η2 indicates the percent of variance in the dependent variable that is attributable to each effect. Effect sizes of follow-up t-tests were determined by Cohen’s d (d = the difference between two means divided by a standard deviation for the data). Analysis was completed using SPSS, GraphPad Prism and http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-SMD1.php, with significant differences demarcated in figures and tables as *, p < 0.05 and **, p < 0.01.
Results
Juvenile Behavior
PCB exposures primarily affected female, but not male, adolescent animals, and were dominated by interactions between prenatal and juvenile exposure (Table 2).
Table 2.
Summary of Adolescent Results
| Males
|
Females
|
|||||
|---|---|---|---|---|---|---|
| Effect of Prenatal A1221 | Effect of Juvenile A1221 | Effect of Prenatal (in intx) | Effect of Prenatal A1221 | Effect of Juvenile A1221 | Effect of Prenatal (in intx) | |
| Affiliative Behavior | ||||||
|
| ||||||
| T1, Pre Stimulus, FM USV | - | - | - | - | - | - |
| T1, Pre Stimulus, Flat USV | - | - | - | - | - | - |
| T1, Stimulus, FM USV | - | - | - | - | - | - |
| T1, Stimulus, Flat USV | - | - | - | - | - | - |
| T1, Post/Pre Stimulus, FM USV | - | - | - | - | - |
 ** −1.29 ** −1.29 |
| T1, Post/Pre Stimulus, Flat USV | - | - | - | - | - | - |
| T2, Pre Stimulus, FM USV | - | - | - | - | - | - |
| T2, Pre Stimulus, Flat USV | - | - | - |
 * 0.12 * 0.12 |
- | - |
| T2, Stimulus, FM USV | - | - | - | - | - | - |
| T2, Stimulus, Flat USV | - | - | - | - | - | - |
| T2, Post Stimulus, FM USV | - | - | - | - | - | - |
| T2, Post Stimulus, Flat USV | - | - | - | - | - | - |
| T3, Stimulus, FM USV | - | - | - | - | - | - |
| T3, Stimulus, Flat USV | - | - | - | - | - | - |
| T3, Number of Hops | - | - | - | - | - | - |
| T3, Number of Nape Contact | - | - | - | - | - | - |
| T3, Number of Wrestle | - | - | - | - | - |
 ** 1.70 ** 1.70 |
| T3, Latency to Hop | - | - |
 * 0.36 * 0.36 |
- |
 * 0.15 * 0.15 |
- |
| T3, Latency to Nape Contact | - | - | - | - | - | - |
| T3, Latency to Wrestle | - | - | - | - | - | - |
|
| ||||||
| Sociability | ||||||
|
| ||||||
| Stage 1, Time in Social Zone | - | - | - | - | - | - |
| Stage 1, Latency to Social Zone | - | - | - | - |
 * 0.13 * 0.13 |
- |
| Stage 2, Time in Novel Zone | - | - | - | - | - | - |
| Stage 2, Latency to Novel Zone | - | - | - | - | - | - |
| Stage 2, Time in Familiar Zone | - | - | - | - | - | - |
| Stage 2, Latency to Familiar Zone | - | - | - | - | - | - |
|
| ||||||
| Anxiety-like Behaivor | ||||||
|
| ||||||
| Elevated Plus, Time in Open | - | - | - | - | - |
 ** 1.23 ** 1.23 |
| Elevated Plus, Entries into Open | - | - | - | - | - |
 ** 1.48 ** 1.48 |
| Light Dark Box, Time in Light | - | - | - | - | - | - |
| Light Dark Box, Entries into Light | - | - | - |
 ** 0.18 ** 0.18 |
- | - |
The direction (
 increase or
increase or
 decrease in measure), significance (*p < 0.05, **p < 0.01), and size (η2 for main effects or d for pair-wise interaction follow ups) of effects are shown. Interactions are due to an effect of prenatal exposure detected only in animals also exposed as juveniles; for simplicity, only this effect is shown in the interaction (intx) column. Abbreviations: T (testing day); FM (frequency modulated); USV (ultrasonic vocalization); A1221 (aroclor 1221)
decrease in measure), significance (*p < 0.05, **p < 0.01), and size (η2 for main effects or d for pair-wise interaction follow ups) of effects are shown. Interactions are due to an effect of prenatal exposure detected only in animals also exposed as juveniles; for simplicity, only this effect is shown in the interaction (intx) column. Abbreviations: T (testing day); FM (frequency modulated); USV (ultrasonic vocalization); A1221 (aroclor 1221)
Affiliative USVs
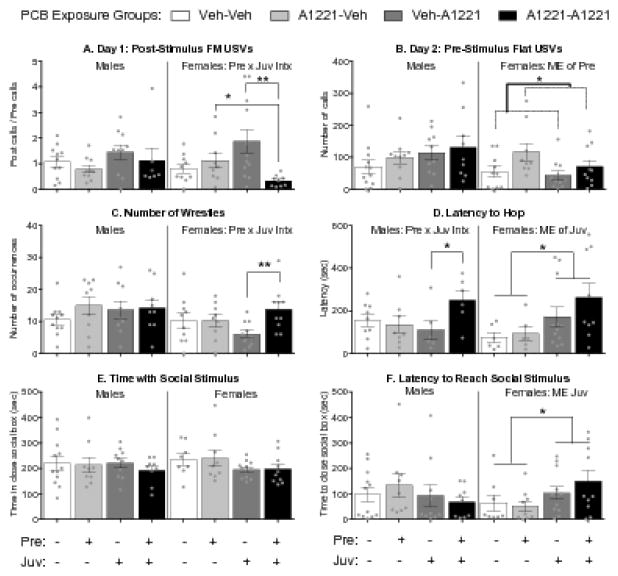
PCB treatments did not affect the numbers of flat or frequency modulated (FM) USVs produced while interacting with stimulus animals on any day of testing (Supplemental Figure 2). However, they did affect calling in experimental female, but not male, animals in a subset of tests (Fig 2). On Day 1 of testing (T1), prenatal and juvenile exposure interacted to affect the number of FM calls following the first conspecific interaction (KW, p < 0.05, η2 = 0.17, Fig 2a), such that females exposed to two hits called less than those exposed to a single prenatal (t(16) = 2.62, p < 0.05, d = −1.24) or single juvenile (t(19) = 2.93, p < 0.01, d = −1.29) hit. On Day 2, a main effect of prenatal exposure was found on flat calls in the Pre-stimulus session (KW, p < 0.05, η2 = 0.12, Fig 2b), with more calls in the prenatal A1221 than the Veh females.
Figure 2.
Effects of PCBs on adolescent affiliative and sociability behavior with a same-sex and age conspecific. Frequency modulated (FM) or flat ultrasonic vocalization (USV) production (A, B); play behaviors (C, D); and time spent with, and latency to reach, the stimulus animal in Stage 1 of the sociability test (E, F) are shown. Bars are mean ± SEM, with dots indicating individual data points. Within-sex main effects (ME) of, or interactions between, prenatal (Pre) or juvenile (Juv) exposure are described in each subtitle, with specific group differences indicated by *, p < 0.05 or **, p < 0.01.
Affiliative behavior
PCBs tended to reduce play behavior, significantly so in females (Fig 2). The number of wrestles was affected in females, but not males (Fig 2c). An interaction between prenatal and juvenile exposure was found (F(1,36) = 4.14, p < 0.05, η2 = 0.10) such that females exposed to A1221 during both prenatal and juvenile development showed more wrestles than those only exposed as juveniles (t(19) = 3.28, p < 0.01, d = 1.70). While the number of hops was not affected by PCBs (data not shown), the latency to perform the behavior was modified, in both males and females (Fig 2d). In males, an interaction between prenatal and juvenile exposure was found (F(1,25) = 4.36, p < 0.05, η2 = 0.14), such that males exposed during both prenatal and juvenile development showed a longer latency than those only exposed as juveniles (t(10) = 2.26, p < 0.05, d = 0.36). In females, a main effect of juvenile exposure was found (KW, p < 0.05, η2 = 0.15,), such that latency to hop was longer in juvenile A1221 compared to Veh females, regardless of their prenatal exposure. No effects were found on nape contacts (data not shown). Positive correlations between USV production and the performance of play behaviors are discussed in the Supplemental materials.
Sociability
In juveniles, no effects of PCBs were seen on time spent in the area immediately proximate to the stimulus animal in the first stage of the test where they were given a choice between sides with or without a stimulus animal in it (Fig 2e). However, females (but not males) exposed to juvenile PCBs showed a longer latency to reach that area near the stimulus animal (KW, p < 0.05, η2 = 0.13, Fig 2f). On the second stage of the test, animals were given a choice between the familiar and a novel stimulus animal. In this stage, prenatal and juvenile exposures did not affect time spent in, or latency to reach, the area immediately proximate to either stimulus animals or both stimulus animals combined (data not shown).
Anxiety-like Behavior
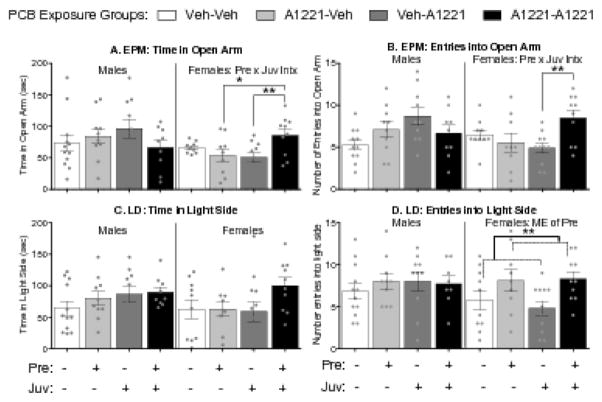
Prenatal and juvenile PCB exposure interacted to affect anxiety behavior in females, but not males (Fig 3). In the elevated plus maze, prenatal x juvenile exposure interactions were found for time in the closed arm (F(1,36) = 4.71, p < 0.05, η2 = 0.10), such that females exposed to PCBs during both prenatal and juvenile development showed less anxiety than those exposed only as juveniles (t(15) = 2.58, p < 0.05, d = −0.81). The expected inverse was also found with time in the open arm. A prenatal x juvenile exposure interaction was found for this measure, (F(1,35) = 8.00, p < 0.01, η2 = 0.17, Fig 3a), however this dataset failed Levene’s test of homogeneity, so a Kruskal-Wallis analysis was used to determine where effects of one exposure depended on the level of the other. In that measure, females exposed to PCBs during both prenatal and juvenile development showed less anxiety than those exposed only as juveniles (KW, p < 0.01, d = 1.24). In addition, females exposed to PCBs during both prenatal and juvenile development showed less anxiety than those exposed only prenatally (KW, p = 0.05, d = 1.01). A similar effect was found in open arm entries (F(1,36) = 8.22, p < 0.01, η2 = 0.17, Fig 3b), such that the double-hit females entered the open arm more than females only exposed as juveniles (t(19) = 3.39, p < 0.01, d = 1.48). In the light/dark box, no effects of PCBs were observed on the time spent in the light side of the box (Fig 3c). However, females exposed to PCBs during prenatal development showed more entries into the light side than females not exposed at that time, independent of any subsequent juvenile exposure (F(1,36) = 7.86, p < 0.01, η2 = 0.18, Fig 3d).
Figure 3.
Effects of PCBs on adolescent anxiety-like behavior. Results from elevated plus maze (EPM, A and B) and light dark box (LD, C and D) are shown (mean ± SEM), with dots indicating individual data points. Within-sex main effects (ME) of, or interactions between, prenatal (Pre) or juvenile (Juv) exposure are described in each subtitle, with specific group differences indicated by *, p < 0.05 or **, p < 0.01.
Adult Behavior
PCB effects on adult behavior were observed in males, and were dominated by main effects of the single prenatal and juvenile hits that affected behavior in opposite directions (an increase or decrease in performance, Table 3).
Table 3.
Summary of Adult Results
| Males
|
Females
|
|||||
|---|---|---|---|---|---|---|
| Effect of Prenatal A1221 | Effect of Juvenile A1221 | Prenatal x Juvenile Intx | Effect of Prenatal A1221 | Effect of Juvenile A1221 | Prenatal x Juvenile Intx | |
| Sociosexual USVs | ||||||
|
| ||||||
| Pre Stimulus, FM USV | - | - | - | - | - | - |
| Pre Stimulus, Flat USV |
 * 0.13 * 0.13 |
- | - | - | - | - |
| Stimulus, FM USV | - | - | - | - | - | - |
| Stimulus, Flat USV | - | - | - | - | - | - |
| Post Stimulus, FM USV | - | - | - | - | - | - |
| Post Stimulus, Flat USV | - | - | - | - | - | - |
|
| ||||||
| Sociosexual Choice | ||||||
|
| ||||||
| Time in Hormone Stim Zone | - | - | - | - | - | - |
| Time in No Hormone Stim Zone |
 ** 0.28 ** 0.28 |
 * 0.10 * 0.10 |
- | - | - | - |
| Time in Either Stim Zones | - |
 ** 0.19 ** 0.19 |
- | - | - | - |
| Percent Time with Hormone Stim |
 ** 0.22 ** 0.22 |
- | - | - | - | - |
|
| ||||||
| Anxiety-like Behavior | ||||||
|
| ||||||
| Elevated Plus, Time in Open | - | - | - | - | - | - |
| Elevated Plus, Entries into Open | - | - | - | - | - | - |
| Light Dark Box, Time in Light | - | - | - | - | - | - |
| Light Dark Box, Entries into Light | - | - | - | - | - | - |
The direction (
 increase or
increase or
 decrease in measure), significance (*p < 0.05, **p < 0.01), and size (η2 for main effects) of effects are shown. Abbreviations: FM (frequency modulated); USV (ultrasonic vocalization); A1221 (aroclor 1221); Intx (interaction)
decrease in measure), significance (*p < 0.05, **p < 0.01), and size (η2 for main effects) of effects are shown. Abbreviations: FM (frequency modulated); USV (ultrasonic vocalization); A1221 (aroclor 1221); Intx (interaction)
Sociosexual USVs
Prenatal exposure increased the number of flat calls in the session preceding the opposite sex interaction in males, independent of juvenile exposure (F(1,37) = 5.82, p < 0.05, η2 = 0.13, Supplemental Figure 3). Other aspects of USV behavior with an opposite sex conspecific were unaffected by treatments.
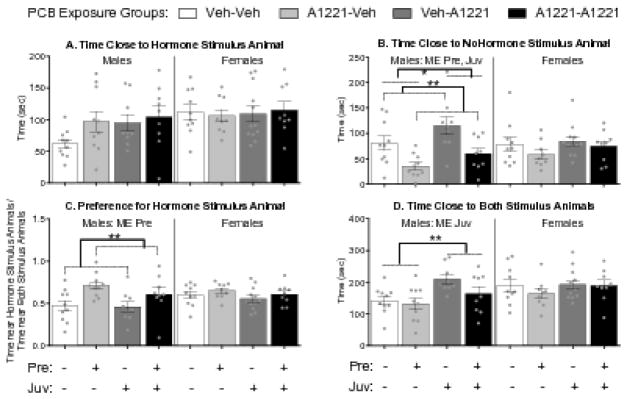
Sociosexual Choice
PCBs altered the preference for an opposite sex stimulus animal in males but not females. Males exposed to PCBs prenatally spent less time near a no-hormone stimulus animal than animals not exposed prenatally, independent of juvenile exposure (F(1,34) = 15.55, p < 0.01, η2 = 0.28, Fig 4b). As no significant effects were seen on time spent near a hormone stimulus animal (Fig 4a), this effect drove the preference for a hormone-treated stimulus animal in prenatally exposed males (F(1,34) = 10.17, p < 0.01, η2 = 0.17 = 0.22, Fig 4c). A separate main effect of juvenile exposure was found on time spent near the stimulus animals. Juvenile exposed males spent more time with the no-hormone female than did unexposed males (F(1,34) = 5.37, p < 0.05, η2 = 0.10, Fig 4b). This effect, together with the nonsignificant increase in time spent with the hormone-treated stimulus females, resulted in the observation that males with juvenile exposure spent more time with both stimulus animals than males not exposed at that time (F(1,34) = 8.65, p < 0.01, η2 = 0.19, Fig 4d).
Figure 4.
Effects of PCBs on sociosexual choice. Time near (A, B, D) and preference for (C) stimulus animals are shown (mean ± SEM), with dots indicating individual data points. Within-sex main effects (ME) of prenatal (Pre) or juvenile (Juv) exposure are described in each subtitle, with specific group differences indicated by *, p < 0.05 or **, p < 0.01.
Anxiety-like Behavior
No effects of PCBs were found on adult anxiety behavior, in either elevated plus maze or light dark box tests (Supplemental Figure 4).
Discussion
The current study utilized a two-hit model of exposure to PCBs to differentiate between different critical stages of exposure, ages at analysis, and sexes of subject on behavioral outcomes. The most consistent effects of PCBs were disruptions of female adolescent social and anxiety-like behavior. These results add to the literature on EDC effects on behavior in several novel and important ways. First, animals exposed during prenatal or juvenile development had unique sets of behavioral phenotypes. Second, the age at which testing was conducted and the sex of the animal also played important roles in the measured outcomes: effects of developmental PCB exposures on behaviors were predominantly manifested in females when tested in adolescence, while adult behaviors were affected in males. Third, the two-hit model revealed that the effects of prenatal exposure could be modified by juvenile exposure, suggesting that early exposure changes the developmental trajectory of the brain in ways that may not be discernible unless animals experience an additional exposure later in life. Fourth, only a subset of behaviors was affected, underscoring the importance of thorough behavioral testing to assess neurobehavioral outcomes. Humans are exposed to PCBs and other EDCs at multiple stages of development, and these results suggest that it is important to consider these factors in assessing exposures and potential neurobiological effects.
Effects of A1221 on Adolescent Social Behavior
Females exposed to prenatal PCBs produced more USVs in the session preceding introduction of the playmate on Day 2 than animals not exposed at that time. As the short-term isolation and placement into the recording chamber could serve as cues for the impending play session, this finding could indicate an increased anticipation of play after prenatal exposure. While no other main effects of prenatal exposure were found in additional measures of play or social interest or memory, juvenile exposure revealed additional prenatal effects. Specifically, prenatal exposure to PCBs was associated with more wrestling during play and fewer USVs post-play, but only in females that were also exposed as juveniles. The ethological meaning of post-play USV production is unclear but, considering the other prosocial effects of prenatal exposure, perhaps it indicates reduced arousal after a period of heightened engagement. Juvenile exposure also revealed an effect of prenatal exposure on the latency to hop during play interactions in adolescent males; however, the behavioral significance of this effect is limited, as no other measures of prosocial behavior were affected in male adolescents.
Prenatal PCB exposure in humans, as indicated by levels in umbilical cord blood, has been correlated with sex-dependent shifts in the performance of masculine and feminine play behavior in children (Vreugdenhil et al., 2002; Winneke et al., 2014). To our knowledge, no other studies have assessed the effects of prenatal PCBs on the performance of play behavior in rodents. In a related measure, prenatal exposure to other PCBs (PCB 47 and 77 mix, 2–4mg/kg, from mating until weaning) altered olfactory investigations of conspecifics in juvenile males (Jolous-Jamshidi et al., 2010). Although the PCB literature is limited, comparisons to results found using another EDC, bisphenol A (BPA) are illuminating. While PCBs have multiple mechanisms of action, both A1221 and BPA can be estrogenic in some contexts. As in our study, BPA (~150 ug/kg oral to dam E0-P1) altered specific aspects of social interactions in juvenile male and female mice (Wolstenholme et al., 2011). However, a longer exposure period (E0-P21) of high and low doses in mice and rats shows inconsistent results (Dessì-Fulgheri et al., 2002; Ferguson et al., 2014; Porrini et al., 2005). Therefore, it appears that effects of prenatal exposure to these EDCs on adolescent social behavior are moderate and depend on the exposure period, the sex of the animal tested, and the specific behavior that was tested.
In contrast to prenatal exposure, juvenile exposure tended to reduce social motivation in adolescent females. Specifically, it increased the latency to hop in play interactions and reach the social stimulus in sociability tests in adolescent female animals. Thus, the timing of the PCB exposure has major implications for the direction of the effect on social behavior. Sex and age-dependent effects of postnatal EDC exposure on social behavior are also found in the literature. For example, juvenile female voles exposed to BPA from P8-P14 spent more time investigating a novel same-sex stimulus animal, while exposed juvenile males spent less time performing the same behavior than controls (Sullivan et al., 2014). However, juvenile BPA exposure (P23-30) had no effect on play and social interaction in juvenile male or female rats (Yu et al., 2011). Again, the choice of EDC and species differences may explain the opposite direction of the effects. We are unaware of any studies on links between PCBs in juveniles on social behavior in adolescent humans.
Effects of A1221 on Adult Sociosexual Behavior
Unlike adolescent behaviors, which were primarily affected in female rats, changes in adult behaviors were observed only in males. Sociosexual interest and motivation, as assessed in tests of USV production in response to a receptive opposite sex stimulus animal, was not affected by 1mg/kg prenatal A1221. This is in agreement to findings with a similar PCB exposure, where only a 0.5mg/kg (but not 1mg/kg) dose affected flat USVs produced after a sociosexual interaction (Davaeva et al., 2013). In preference tests, prenatal exposure reduced the amount of time spent by adult males near the no-hormone-treated stimulus animal. While this was associated with an increased preference for the hormone-treated animal, the meaning of this effect is difficult to interpret because even vehicle-treated animals did not show the expected preference for hormone-treated stimulus animals over non-hormone animals. Perhaps this is because of experimental factors such as time of day tested, or that rats were sexually naïve. In contrast to the current study of preference behavior, studies on the performance of sexual behavior have found that A1221 and mixes of indicator congeners tend to increase the latency to perform sex behavior (Chung and Clemens, 1999; Colciago et al., 2009; Faass et al., 2013; Steinberg et al., 2007). Therefore, it appears that prenatal PCBs affect specific aspects of sexual behavior.
Juvenile exposure tended to affect general partner preference. Juvenile PCB-exposed males spent more time with the no-hormone and (non-significantly) hormone treated stimulus animals, resulting in an overall increase in time spent with both social stimuli. To our knowledge, no other studies have assessed effects of juvenile PCB exposure on sociosexual behavior in adulthood. While lactational PCB exposure decreased typical partner preference (Cummings et al., 2008), adult exposure had no effect on sex behavior in female rats (Chung et al., 2001), suggesting the importance of developmental stage in efficacy of exposure. An increase in adult same-sex sociability was found in response to peripubertal BPA exposure (P23-30); however the effect was present in female, but not male mice (Yu et al., 2011). Together, these studies suggest that the juvenile brain remains sensitive to EDCs, but effects are manifested only for certain measures of sociosexual behavior and motivation.
Effects of A1221 on Anxiety Behavior
When tested in adolescence, females but not males showed consistent effects of prenatal PCBs on anxiety. As a main effect in the LD box and an interaction in the EPM test, adolescent females exposed to PCBs prenatally showed a reduction in anxiety-like behavior. This reduction in anxiety in prenatally exposed adolescent females could underlie the increased social motivation observed in the same group. This is in contrast to anxiogenic effects reported after exposure of adolescent male and female mice to a mixture of non-dioxin like PCBs containing PCB 153 (10 or 100 ng/kg oral to dam P1-21)(Elnar et al., 2012). In humans, Inuit preschoolers with relatively higher levels of PCB 153 in umbilical cord blood had greater levels of anxiety and reduced positive affect. However, A1221 does not contain any PCB 153, so these studies suggest congener-specific effects of PCBs on adolescent anxiety.
Unlike in adolescence, no effects of exposure were found in tests of anxiety-like behaviors in adult animals in the current study. Age specific effects of PCBs are consistent with the human literature as well; heavily chlorinated PCBs increase depressive symptoms in older adults (Fitzgerald et al., 2008) but not young adults (Strøm et al., 2014). Therefore, some effects of PCBs on affective behavior may be missed if testing only occurs at a single age. Alternatively, it is possible that the previous adolescent testing in the same environment prevented us from observing effects in adulthood. Indeed, a low dose lactational exposure to a non-dioxin like mix of PCBs resulted in an increase in anxiety behavior in adult male and female mice (Elnar et al., 2012). More experiments are needed to determine if these discrepancies are the result of species, dose, or mixture. No main effects of juvenile exposure were seen in tests of anxiety in adolescent or adult animals.
A unique phenotype emerges from exposure to PCBs during juvenile development
Juvenile PCB exposure had effects that were different from those induced by prenatal exposure, so we must consider the possibility that the juvenile brain is uniquely sensitive to some effects of A1221. PCBs are known to act on several different receptors and enzymes, and these systems undergo developmental change. Therefore, it is not surprising that effects of prenatal exposure are unique when compared to juvenile exposure, and are modulated by a second hit. Interactions between prenatal and juvenile exposure were observed in female adolescent affiliative and anxiety-like behaviors and, in all cases, were the result of differences between animals exposed to a single juvenile hit compared to those exposed to the double hit. As such, juvenile exposure unmasked an effect of prenatal exposure. Therefore, we can conclude that prenatal PCB exposure changes the developmental trajectory of the brain in some ways that are undetectable except when animals experience an additional exposure later in life.
Conclusion
This is the first study to demonstrate that the behavioral phenotype is uniquely affected by a single hit of PCBs given either prenatally or during juvenile development. Moreover, in the two-hit PCB model, the second juvenile hit sometimes unmasked effects of a single prenatal hit to reveal an emergent phenotype. The most consistent effects of PCBs were an increase in prosocial and reduction in anxiety-like behavior in adolescent females. However, we should not conclude that PCBs affect behavior in a positive way, but instead, that exposure caused the behaviors to be performed in an atypical manner. The performance of these behaviors must be considered from the perspective of a conspecific rat, a process that we cannot do well as humans. Therefore, although effects were moderate and specific to certain behaviors, they highlight the need for continued testing across a range of behaviors, ages, and in both sexes.
Supplementary Material
Highlights.
Prenatal PCB exposure alters social and anxiety behaviors, especially in females.
Juvenile PCB exposure affects male sociosexual preference behaviors.
Prenatal and juvenile PCB exposures have both individual and interacting effects.
Adolescence may be a second critical period for PCB exposure effects.
Acknowledgments
We gratefully acknowledge Bethany Hart, Isaac Miller-Crews, Spurthi Tarugu, Michael Reilly, Viktoria Topper, Alexandra Garcia, and Weiling Yin for their help with data analysis and tissue collection. This work was funded by grants from NIEHS: RO1-ES020662 (ACG), T32-ES07247 (MRB), F32-ES023291 (MRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polychlorinated Biphenyls. 2000. [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Olesen KM. Brain sex differences and the organization of juvenile social play behavior. J Neuroendocrinol. 2009;21:519–525. doi: 10.1111/j.1365-2826.2009.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D. Treatment with an Anabolic-Androgenic Steroid Affects Anxiety-Related Behavior and Alters the Sensitivity of Cortical GABAA Receptors in the Rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Sexual dimorphism in the vertebrate nervous system. Journal of Neuroscience. 1992;12:4133–4142. doi: 10.1523/JNEUROSCI.12-11-04133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. 2011;35:1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Fourth National Report on Human Exposure to Environmental Chemicals. 2009. [Google Scholar]
- Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol. 1999;62:664–670. doi: 10.1007/s001289900925. [DOI] [PubMed] [Google Scholar]
- Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev Neurobiol. 2009;69:141–152. doi: 10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiol Behav. 2008;95:471–475. doi: 10.1016/j.physbeh.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Davaeva VY, Reilly MP, Chen JK, Otto KJ, Thompson LM, Crews DP, Gore AC. Gestational exposure of rats to endocrine disrupting chemicals alters sociosexual behaviors in adulthood. Society for Neuroscience Abstract 2013 [Google Scholar]
- De Lorme KC, Sisk CL. Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian hamsters. Physiol Behav. 2013;112–113:1–7. doi: 10.1016/j.physbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaSeta D, Minder I, Belloni V, Aloisi AM, Dessì-Fulgheri F, Farabollini F. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm Behav. 2006;50:301–307. doi: 10.1016/j.yhbeh.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Dessì-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Elnar AA, Diesel B, Desor F, Feidt C, Bouayed J, Kiemer AK, Soulimani R. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (Sigma 6 NDL-PCBs) in mice. Toxicology. 2012;299:44–54. doi: 10.1016/j.tox.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wolff MS. Causal inference considerations for endocrine disruptor research in children’s health. Annu Rev Public Health. 2013;34:139–158. doi: 10.1146/annurev-publhealth-031811-124556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faass O, Ceccatelli R, Schlumpf M, Lichtensteiger W. Developmental effects of perinatal exposure to PBDE and PCB on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen Comp Endocrinol. 2013;188:232–241. doi: 10.1016/j.ygcen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Law CD, Kissling GE. Developmental treatment with ethinyl estradiol, but not bisphenol A, causes alterations in sexually dimorphic behaviors in male and female Sprague Dawley rats. Toxicol Sci. 2014;140:374–392. doi: 10.1093/toxsci/kfu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, Jansing RL, Hwang SA, Hicks HE. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame GM, Wagner RE, Carnahan JC, Brown JF, Jr, May RJ, Smullen LA, Bedard DL. Comprehensive, quantitative, congener-specific analyses of eight aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- Harding SM, McGinnis MY. Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats. Horm Behav. 2003;43:327–335. doi: 10.1016/s0018-506x(02)00049-1. [DOI] [PubMed] [Google Scholar]
- Henley CL, Nunez AA, Clemens LG. Hormones of choice: the neuroendocrinology of partner preference in animals. Front Neuroendocrinol. 2011;32:146–154. doi: 10.1016/j.yfrne.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Jolous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol Lett. 2010;199:136–143. doi: 10.1016/j.toxlet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Anderson MW. Effect of chlorination on the distribution and excretion of polychlorinated biphenyls. Drug Metabolism and Disposition. 1975;3:371–380. [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol Behav. 2003;80:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Mohr MA, Sisk CL. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proc Natl Acad Sci USA. 2013;110:4792–4797. doi: 10.1073/pnas.1219443110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27:691–696. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- Porrini S, Belloni V, DellaSeta D, Farabollini F, Giannelli G, Dessì-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65:261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Ramaley JA. Development of Gonadotropin regulation in the prepubertal mammal. Biol Reprod. 1979;20:1–31. doi: 10.1093/biolreprod/20.1.1. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav. 2015;73:47–55. doi: 10.1016/j.yhbeh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Saksena SK, Lau IF. Variations in serum androgens, estrogens, progestins, gonadotropins and prolactin levels in male rats from prepubertal to advanced age. Exp Aging Res. 1979;5:179–194. doi: 10.1080/03610737908257197. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: a finishing school for male social behavior. Ann N Y Acad Sci. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Smyth C, Wilkinson M. A Critical Period for Glutamate Receptor-MediatedInduction of Precocious Puberty in Female Rats. J Neuroendocrinol. 1994;6:275–284. doi: 10.1111/j.1365-2826.1994.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, Halldorsson TI. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ Int. 2014;68:41–48. doi: 10.1016/j.envint.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, Patisaul HB. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster) Endocrinology. 2014;155:3867–3881. doi: 10.1210/en.2014-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Otake T, Kataoka M, Murata Y, Aburada S. Studies of the transfer and distribution of [14C]polychlorinated biphenyls from maternal to fetal and suckling rats. Toxicol Appl Pharmacol. 1976;38:549–558. doi: 10.1016/0041-008x(76)90186-1. [DOI] [PubMed] [Google Scholar]
- Tian YH, Hwan Kim S, Lee SY, Jang CG. Lactational and postnatal exposure to polychlorinated biphenyls induces sex-specific anxiolytic behavior and cognitive deficit in mice offspring. Synapse. 2011;65:1032–1041. doi: 10.1002/syn.20934. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Fox TO. Sex- and hormone-dependent antigen immunoreactivity in developing rat hypothalamus. Proc Natl Acad Sci USA. 1989;86:382–386. doi: 10.1073/pnas.86.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The Effects of Gonadectomy on Age-and Sex-Typical Patterns of Ethanol Consumption in Sprague–Dawley Rats. Alcohol Clin Exp Res. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil HJI, Lanting CI, Mulder PGH, Boersma ER, Weisglas-Kuperus N. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr. 2002;140:48–56. doi: 10.1067/mpd.2002.119625. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol. 2014;28:99–115. doi: 10.1210/me.2013-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Fang J, Nunez AA, Clemens LG. Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol Behav. 2002;75:689–696. doi: 10.1016/s0031-9384(02)00673-x. [DOI] [PubMed] [Google Scholar]
- Winneke G, Ranft U, Wittsiepe J, Kasper-Sonnenberg M, Fürst P, Krämer U, Seitner G, Wilhelm M. Behavioral sexual dimorphism in school-age children and early developmental exposure to dioxins and PCBs: a follow-up study of the Duisburg Cohort. Environ Health Perspect. 2014;122:292–298. doi: 10.1289/ehp.1306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SRJ, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE. 2011;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Schwarting RKW. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS ONE. 2007;2:e1365. doi: 10.1371/journal.pone.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Tai F, Song Z, Wu R, Zhang X, He F. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ Toxicol Pharmacol. 2011;31:88–99. doi: 10.1016/j.etap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Saal, Vom FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.