Abstract
Objective
Very few studies have examined the relationship between timing of fluoride intake and development of dental fluorosis on late-erupting permanent teeth using period-specific fluoride intake information. This study examined this relationship using longitudinal fluoride intake information from the Iowa Fluoride Study.
Methods
Participants’ fluoride exposure and intake (birth to 10 years) from water, beverages, selected food products, dietary fluoride supplements and fluoride toothpaste was collected using questionnaires sent to parents at 3- and 4- month intervals from birth to age 48 months, and every six months thereafter. Three trained and calibrated examiners used the Fluorosis Risk Index (FRI) categories to assess 16 late-erupting teeth among 465 study participants. A tooth was defined as having definitive fluorosis if any of the zones on that tooth had an FRI score of 2 or 3. Participants with questionable fluorosis were excluded from analyses. Descriptive and logistic regression analyses were performed to assess the importance of fluoride intake during different time periods.
Results
Most dental fluorosis in the study population was mild, with only 4 subjects (1%) having severe fluorosis (FRI Score 3). The overall prevalence of dental fluorosis was 27.8%. Logistic regression analyses showed that fluoride intake from each of the individual years from age 2 to 8 years plays an important role in determining the risk of dental fluorosis for most late-erupting permanent teeth. The strongest association for fluorosis on the late-erupting permanent teeth was with fluoride intake during the sixth year of life.
Conclusion
Late-erupting teeth may be susceptible to fluorosis for an extended period from about age 2 to 8 years. Although not as visually prominent as the maxillary central incisors, some of the late-erupting teeth are esthetically important and this should be taken into consideration when making recommendations about dosing of fluoride intake.
Keywords: dental fluorosis, fluoride, fluoride intake, late-erupting permanent teeth
Introduction
The effectiveness of fluoride in caries prevention has led to its widespread use in several forms, resulting in a concomitant increase in prevalence of dental fluorosis in the United States and other nations (1–4). Dental fluorosis is the result of sub-surface enamel porosity due to excessive intake of fluoride during tooth development (5). Clinically, appearance can vary from barely discernable white marks, in mild fluorosis, to confluent pitting and discoloration of affected teeth in severe forms (5).
The extent and severity of dental fluorosis are determined by the quantity and timing of fluoride intake (6–9). There is generally a dose response relationship between fluorosis and fluoride intake, meaning that, with increased fluoride intake, the prevalence and severity of fluorosis also increase. The “optimal” daily fluoride intake has been stated to be 0.05–0.07 mg F/kg-BW, but never precisely determined (1). Previous research found that consuming 0.04 to 0.06 mg/kg-BW resulted in an increased prevalence of mostly mild dental fluorosis in permanent maxillary central incisors compared with lower intakes (9). Furthermore, teeth are most susceptible to fluorosis when they are in the early maturation stage of enamel development (10). As different teeth develop at different times and individual teeth themselves develop in incremental stages, the period of maximum susceptibility to fluorosis is different for different teeth and zones. Individual regions of the teeth can have variation in prevalence and severity of dental fluorosis depending on the stage of development at the time of elevated fluoride intake. The permanent teeth (except the third molars) are considered collectively to be susceptible to development of fluorosis during the first 6–8 years of life (11–18).
Studies on the relationships between timing of fluoride intake and fluorosis have mostly focused on maxillary central incisors (7, 13–15), which are esthetically the most important teeth. These studies (7, 13–15) have found that exposure to elevated levels of fluoride in the first 2 years of life was generally the most significant risk factor for development of fluorosis in early-erupting teeth. Nonetheless, these studies also suggest that exposure to higher levels of fluoride in subsequent years, when the teeth are still developing, is associated with higher risk for developing dental fluorosis, albeit to a lesser extent. In a meta-analysis to identify “risk-periods” for development of fluorosis in maxillary permanent central incisors, Bardsen (13) concluded that duration of exposure during amelogenesis is a significant predictor of fluorosis risk and that it was difficult to single out individual periods. Evans et al. (14) reported that the period of greatest susceptibility to development of fluorosis on the maxillary central incisors was about 15–24 months for males and 21–30 months for females. Hong et al. (7) used data from the Iowa Fluoride Study and reported that each of the first four years of life were individually important for development of fluorosis, although years one and two were the most important years and were both important together. Similarly, Bardsen et al. (15) found that children exposed to higher levels of fluorides in the first and second years of life were at higher risk for developing dental fluorosis of maxillary and mandibular central incisors, and first molars, with highest risk among children exposed to high F levels during the first year of life. Pendrys et al. (12, 16) also have reported that the early-developing regions of the teeth (classified as FRI Classification I Zones, which includes incisal edges of incisors and occlusal surface of 1st molars) are susceptible to developing fluorosis based on fluoride intake during the first 6 years of life.
Studies have shown that prevalence and severity of fluorosis are typically higher in late-erupting teeth because of increased exposure to fluoride with increasing age (19, 20). However, very few studies have examined the relationship between timing of fluoride intake and fluorosis of the late-erupting teeth. Studying this relationship can improve our understanding of the biologic mechanisms of dental fluorosis and help in optimizing fluoride intake and reducing risk of dental fluorosis. Teeth affected by severe fluorosis can be at greater risk for dental caries (21, 22), and esthetics sometimes can be a concern in less severe forms of fluorosis. The mesial portions of canines and premolars often are visible for people with a broad smile, making them esthetically important. Previous research on the impact of dental fluorosis on esthetic perceptions has found that some people are more likely to perceive teeth as unattractive when the teeth were affected by conditions that lead to discoloration of teeth, including fluorosis (23). Teeth were sometimes perceived to be unattractive when they did not have a uniform color due to opacities or mild fluorosis limited to some, but not all, portions of the teeth resulting in a contrast in color (24, 25). On the other hand, teeth were considered to be more attractive when the teeth were affected by mild fluorosis (TF score 1) (25). A review article (24) on the relationship between dental fluorosis and esthetic perceptions, including Oral Health-Related Quality of Life (OHRQoL), concluded that recent studies with methodological improvements to assess quality of life (QoL) showed mild fluorosis was not a concern for most individuals and sometimes it was even associated with improved QoL.
Among the few studies that examined the relationship between timing of fluoride intake and fluorosis on late-erupting permanent teeth, Larsen et al. (17) studied 110 Greenland children, with some receiving fluoride supplements and some not. They reported the following age ranges to be the periods of increased fluorosis risk for the late-erupting permanent teeth: maxillary canines 3.5y to 6.5y, mandibular canines 3.5y to 5.5y, maxillary first and second premolars – 3.5y to 8.5y and mandibular first premolars 3.5y to 6.5y. For both maxillary and mandibular second molars, the age range was 5.5 to 8.5 years. Ishii and Suckling (18) studied Japanese children who were accidentally exposed to drinking water with high fluoride levels (7.8 ppm) and reported that 2.5y – 3.5y was a period of high risk for upper first premolars. Pendrys et al. (12) examined risk factors for fluorosis among children living in a fluoridated Connecticut community using the Fluorosis Risk Index (FRI)(26). They found that, for FRI classification II zones (that include cervical regions of incisors, as well as many of the zones on late-erupting teeth), the first four years of life were the most important period for development of fluorosis. For FRI Classification II, they found improper use of dietary fluoride supplements to be the most important risk factor (with ORs of 19.28 and 9.86 for supplement use from 1–4 and 1–6 years of age, respectively). They did not find a significant relationship between infant formula use and FRI-II dental fluorosis. They reported that frequent tooth brushing with fluoride toothpaste from 1–8 years was associated with higher odds (OR = 2.63) of developing fluorosis. In an analysis of risk factors among a non-fluoridated Connecticut population, Pendrys et al. (16) reported that children who received fluoride supplements from 2–8 years of age had greater risk of fluorosis of FRI-II zones (OR = 7.97) compared to those who did not receive supplements. Those who began brushing with fluoridated toothpaste in the first 2 years of life and brushed more than once a day were more likely to develop dental fluorosis (OR = 4.23) on FRI-II zones than those who started brushing after 2 years of age.
These studies have several limitations, however, including that there were no period-specific fluoride intake estimates. Also, many only assessed fluoride intake measures from a single source- fluoride supplements in the Larsen et al. (17) study and short-term exposure to high levels of fluoride in drinking water in the Ishii et al. study (18). Also, while the Pendrys et al. (12, 16) studies examined the effects of fluoride intake from many sources, they were based on retrospective intake estimates from many years earlier. Moreover, these studies are from the 1980s and fluorides are even more widely available now. Therefore, it is worthwhile to continue to assess the relationships and refine the estimates about the importance of timing of dental fluorosis risk associated with more contemporary exposure patterns. The purpose of this paper is to report findings concerning the relationships between fluorosis on late-erupting permanent teeth and period-specific fluoride intake from birth to 10 years collected as part of the longitudinal Iowa Fluoride Study.
Methods
This study used data from the Iowa Fluoride Study (IFS), a longitudinal study of a birth cohort, approved by the University of Iowa’s Institutional Review Board. Detailed study methods of the IFS and study sample characteristics were described in previous publications (27–29). Briefly, a total of 1,882 mothers with newborns provided informed consent and completed baseline questionnaires between 1992 and 1995. Subsequently, the mothers were sent questionnaires on a regular basis, which included detailed series of items concerning children’s fluoride exposures and ingestion during the preceding weeks from various sources, including water, beverages, selected food products, dietary fluoride supplements and fluoride toothpaste (27–29). This information was collected using questionnaires sent to parents at mostly 3- and 4-month intervals from birth to age 48 months, with questionnaires every six months thereafter. Fluoride intake in milligrams per kilogram bodyweight (mg F/kg BW) per day was estimated based on parents’ responses to the fluoride questions, assay of participants’ water fluoride levels, and parent-reported body weights of the children. Area-Under-the-Curve (AUC) fluoride intake estimates for various cumulative time periods were calculated by the trapezoidal method. Participants were required to have three valid fluoride intake estimates during the first year, two during the second year, and at least one per year thereafter.
Dental Examinations
Three trained and calibrated examiners used the Fluorosis Risk Index (FRI) categories (26) to assess four zones of the buccal/facial surfaces (incisal edge/occlusal table, occlusal third, middle third, and cervical third) of the 16 late-erupting teeth, which included four canines, eight premolars and four second molars. Zones were categorized as no fluorosis (FRI Score=0), questionable fluorosis if <50% of the zone had white striations (FRI Score=1), definitive fluorosis when >50% of zone had white striations (FRI Score=2), or severe fluorosis when a zone displayed pitting or staining (FRI Score=3) (26). Cervical zones that were not yet visible, due to incomplete eruption, were assigned a score of zero (no fluorosis). The four FRI scores on each tooth were used to determine if the tooth had definitive fluorosis (any zone with FRI=2 or 3). Teeth were then aggregated by tooth type (canine, 1st premolar, 2nd premolar, 2nd molar), where any individual with 2 or more teeth (per tooth type) showing definitive fluorosis was defined as having fluorosis for that tooth type. Subjects with only a single tooth exhibiting definitive fluorosis were excluded from analyses. A similar aggregation of FRI-II zones was used (those which develop during the third through sixth years of life and include the cervical thirds of incisors, middle thirds of canines, and incisal, middle and occlusal cusp areas of premolars and second molars), and individuals with 2 or more teeth exhibiting definitive fluorosis on those specific zones were defined as having FRI-II fluorosis. Again, subjects with only 1 tooth showing definitive fluorosis were excluded from analyses. Also, we computed the sum of all FRI scores on all zones of 1) canines, 2) 1st premolars, 3) 2nd premolars, 4) 2nd molars 5) all 16 late-erupting teeth and 6) FRI-II zones of the later-erupting teeth. These sum scores were used to compute correlations with fluoride intakes and are presented in Figure 1.
Fig. 1.
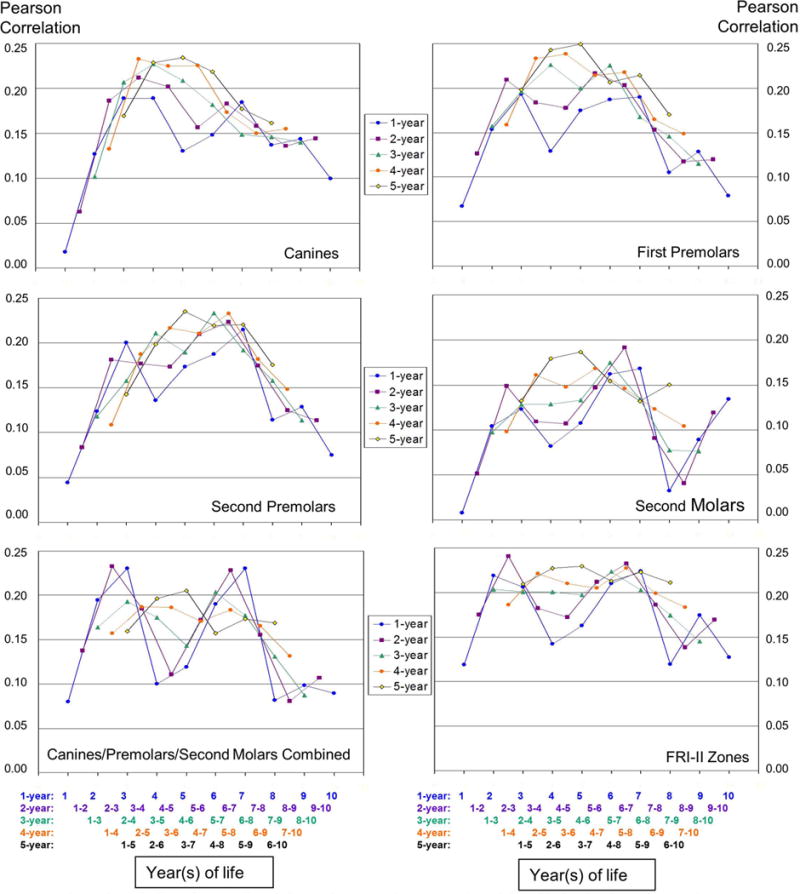
Correlation of person-level fluorosis scores (sum scores) and fluoride intake (mg F/kg BW) considered as 1-, 2-, 3-, 4-, and 5-year cumulative periods. Values on Y-axis are correlation coefficients. Values on x- axis are years of life. For Example: When 5-year cumulative periods were considered, the highest correlations for all tooth types considered separately and together, as well for FRI II zones, were found with fluoride intake from third to seventh year period (so that means from age 2 to 7 years, or about 24 to 84 months).
Examinations were conducted using a portable dental chair, mouth mirror and exam light. The teeth were dried lightly using gauze. Fluorosis was differentiated from non-fluorosis opacities and white spot lesions using Russell’s criteria (30) and based on location, color, and texture of the lesions (31), respectively. A total of 550 participants were examined for fluorosis on all permanent teeth at about age 13.
Data Analysis
Descriptive statistics were used to examine the fluorosis outcomes, demographic characteristics, fluoride intakes and weights of study participants. Differences in questionnaire response rates led to differences in sample sizes across the age groups in the analyses. Pearson’s correlation analyses were performed to assess the correlations among pairs of fluoride intakes during the first 10 years. Correlation analyses also were performed to examine the relationships between estimated fluoride intake (mg F/kg BW) and FRI scores (sum scores for all zones) on the late-erupting teeth considered separately by tooth type and together, as well for all FRI II zones considered together. Logistic regression analysis was used to assess the relationships between fluoride intake during various cumulative periods of 1, 2, 3, 4 and 5 years duration and prevalence of dental fluorosis. Regression analyses compared individuals with FRI scores of 2 (definitive) or 3 (severe) on at least two teeth to individuals with scores of 0 or 1 on all teeth. Participants having only 1 tooth with definitive fluorosis were omitted from the regression analyses. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). A statistical significance level (alpha) of 0.05 was used throughout.
Results
A total of 465 children (male=235, female=230) with period-specific fluoride intake information and examinations for dental fluorosis on late-erupting permanent teeth were included in this analysis. The mean age at the time of dental exams was 13.5 years (median=13.3, range 12.4–16.0). Approximately, 70% of participants’ mothers and 55% of fathers had a 2-year college degree or higher level of education. The participants were mostly from middle to high income families, with 65% having annual family income of $60,000 or more in 2007. Participants were predominantly non-Hispanic whites (95%). Other participants were white Hispanic (2.6%), black (1.5%), and Asian, Native American or mixed race (1.0% combined). Most dental fluorosis in the study population was very mild or mild, with only 4 participants (0.9%) having severe fluorosis (FRI Score 3). The overall prevalence of dental fluorosis was 27.8% (participants with 2 or more teeth showing definitive fluorosis) and 31.8% (when participants with only one affected tooth were excluded).
Table 1 summarizes the mean number of responses per subject per year, estimated daily area-under-the-curve (AUC) fluoride intakes and mean body weights for the study participants. The mean number of responses per year declined from 4.01 in the first year of life to 1.66 in the tenth year of life. The mean total daily fluoride intakes during the first two years of life were 0.40 and 0.48 mgF, respectively, and increased to 0.65 to 0.71 mg per day during each of the other eight years. However, when fluoride intake in mg F/kg-BW was considered, the highest fluoride intake was in the first year of life (mean 0.055 mg F/kg BW) and it decreased as the children grew older to a mean of 0.023 mg F/kg BW in the 9–10 year time point.
Table 1.
Mean numbers of responses, daily fluoride intake and body weight estimates by year
| Age in years | Number of Participants1 | Mean Number of Responses2 (SD) | Daily fluoride intake in mg (SD) | Daily fluoride intake in mg/kg bw (SD) | Mean body weight in kg |
|---|---|---|---|---|---|
| 0–1 | 422 | 4.01 (0.47) | 0.40 (0.29) | 0.055 (0.042) | 7.9 (1.7) |
| 1–2 | 388 | 2.80 (0.58) | 0.48 (0.23) | 0.046 (0.024) | 10.8 (2.1) |
| 2–3 | 429 | 2.52 (0.86) | 0.65 (0.32) | 0.052 (0.027) | 13.0 (2.6) |
| 3–4 | 412 | 2.42 (0.98) | 0.71 (0.34) | 0.049 (0.025) | 15.2 (3.2) |
| 4–5 | 406 | 1.78 (0.71) | 0.67 (0.34) | 0.042 (0.024) | 17.3 (5.1) |
| 5–6 | 419 | 1.76 (0.60) | 0.67 (0.34) | 0.038 (0.021) | 19.5 (5.5) |
| 6–7 | 409 | 1.83 (0.62) | 0.67 (0.34) | 0.033 (0.018) | 22.3 (6.6) |
| 7–8 | 387 | 1.73 (0.65) | 0.67 (0.34) | 0.028 (0.015) | 25.5 (8.2) |
| 8–9 | 388 | 1.63 (0.60) | 0.71 (0.39) | 0.027 (0.015) | 28.9 (10.0) |
| 9–10 | 397 | 1.66 (0.53) | 0.67 (0.35) | 0.023 (0.012) | 31.9 (10.8) |
The number of participants who returned completed fluoride intake questionnaires for different reporting time periods
Mean number of number of questionnaire responses per subject per year
Table 2 presents the Pearson correlation coefficients between pairs of annual fluoride intakes (mg F/kg BW) for years 1 to 10 (from birth to age of 10). In general, the magnitudes of correlation were much higher for years that were closer together and lower for years that were farther apart. All of the correlations that were examined were moderate, yet statistically significant. The magnitudes of correlations show that the fluoride intakes were largely stable through the study period.
Table 2.
Pearson correlation coefficients (Number of observations) for annual fluoride intakes (mg F/kg bw) from birth to age 10 years
| Year 0–1 | Year 1–2 | Year 2–3 | Year 3–4 | Year 4–5 | Year 5–6 | Year 6–7 | Year 7–8 | Year 8–9 | Year 9–10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year 0–1 | 1 (416) |
0.62*
(356) |
0.51*
(386) |
0.41*
(367) |
0.57*
(361) |
0.57 *
(373) |
0.51*
(366) |
0.55*
(348) |
0.50*
(345) |
0.51*
(360) |
| Year 1–2 | 1 (380) |
0.60*
(360) |
0.53*
(345) |
0.52 *
(340) |
0.52*
(347) |
0.51*
(337) |
0.49*
(320) |
0.53 *
(322) |
0.43 *
(325) |
|
| Year 2–3 | 1 (425) |
0.73*
(387) |
0.62*
(377) |
0.58*
(386) |
0.56*
(380) |
0.50*
(363) |
0.49*
(366) |
0.46*
(365) |
||
| Year 3–4 | 1 (410) |
0.68*
(377) |
0.63*
(380) |
0.55*
(371) |
0.58*
(358) |
0.53 *
(349) |
0.49*
(359) |
|||
| Year 4–5 | 1 (403) |
0.74*
(374) |
0.70*
(362) |
0.65*
(352) |
0.62*
(351) |
0.55*
(356) |
||||
| Year 5–6 | 1 (415) |
0.72*
(381) |
0.72*
(365) |
0.70*
(363) |
0.60*
(367) |
|||||
| Year 6–7 | 1 (406) |
0.74*
(362) |
0.73*
(353) |
0.65*
(360) |
||||||
| Year 7–8 | 1 (385) |
0.79*
(347) |
0.71*
(346) |
|||||||
| Year 8–9 | 1 (388) |
0.72 *
(347) |
||||||||
| Year 9–10 | 1 (397) |
Indicates p < 0.0001
Figure 1 illustrates the correlations between person-level fluorosis scores (sum scores) and fluoride intake (mg F/kg BW) considered as 1-, 2-, 3-, 4-, and 5-year cumulative periods for the four late-erupting tooth types, considered individually and together, as well as combined FRI-II zones. In general, correlations were higher and the results smoothed when fluoride intake over longer periods of time were considered. When correlations between 1-year fluoride intakes and fluorosis scores were considered, the highest correlations were with intake during the third year of life for canines and first premolars, and seventh year of life for second premolars and second molars. For all late-erupting teeth considered together, intakes during the third and seventh years of life had the highest correlations. For FRI-II zones, the highest correlations were with fluoride intakes during the second and seventh years of life.
When fluoride intakes during 2-year cumulative periods were considered, the highest correlations were between intakes in the third and fourth years for canines and fifth and sixth years for first premolars. For both second premolars and second molars, the highest correlations were with intakes in the sixth and seventh years. When all the late-erupting teeth were considered together, intakes during second and third, as well as sixth and seventh, years had higher correlations than other periods. For FRI-II zones, the correlation was highest for the second and third years.
When 3-year cumulative periods of fluoride intake were considered, the highest correlations were for fluoride intakes from the third to fifth years for canines and first premolars. First premolar fluorosis was also highly correlated with the fifth to seventh years of fluoride intake. For second premolars, second molars, all tooth types together and all FRI-II zones, the correlations were highest for the fifth to seventh year cumulative period.
When 4-year cumulative periods were considered, the highest correlations were found for fluoride intake during years 2 to 5 for canines, 3 to 6 for first premolars, 5 to 8 for second premolars and 4 to 7 for second molars, respectively. When all late-erupting teeth were considered together, higher correlations were found for both the second to fifth and third to sixth years, which had similar correlation values. For FRI II zones, the correlation was highest for the fifth to eighth year period. When 5-year cumulative periods were considered, the highest correlations for all tooth types considered separately and together, as well for FRI II zones, were found with fluoride intake from third to seventh year period.
Tables 3 summarizes separate logistic regression analyses examining the relationships between the prevalence of dental fluorosis on each of the four late-erupting tooth types and fluoride intake defined as the daily area-under-the-curve (AUC) fluoride intake for each of the 1- year periods between birth and 10 years of age, while Table 4 presents results for each 2-, 3-, 4- and 5-year period. Results include the prevalence of dental fluorosis, odds ratios, p-values and C-statistic values. Sample sizes vary for different analysis due to some fluoride intake data being unavailable. Fluorosis was defined as the participant having definitive fluorosis (FRI score of 2 or 3) on at least one FRI zone of at least two teeth for the given analysis. Participants with definitive fluorosis on only one tooth were not included in the analysis, nor were participants with missing scores on any non-cervical zones and having no definitive fluorosis elsewhere. Individuals with only scores of 0 or 1 on all teeth were used as the comparison group. Using these criteria, approximately, 17.5%, 21.0%, 22.2%, and 15.7% of the participants had definitive fluorosis (on canines, first and second premolars, and second molars, respectively. The odds ratios represent a change in prevalence of dental fluorosis with an incremental increase of 0.01 mg/kg-body-weight of daily fluoride intake.
Table 3.
Logistic regression statistics for fluorosis prevalence (2 or more teeth vs. none) - with yearly mgF/kg fluoride intake
| Fluoride Intake Age | Canines1 | 1st Premolars1 | 2nd Premolars1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Prevalence (%) | O.R.2 | P | c | N | Prev. (%) | O.R.2 | P | c | N | Prev. (%) | O.R.2 | P | c | |
| Overall Prevalance | 337 | 17.5 | 372 | 21.0 | 343 | 22.2 | |||||||||
| 0–1 | 297 | 18.9 | 1.03 | 0.40 | 0.546 | 328 | 22.6 | 1.04 | 0.22 | 0.554 | 305 | 23.6 | 1.00 | 0.95 | 0.491 |
| 1–2 | 280 | 18.6 | 1.10 | 0.12 | 0.565 | 302 | 21.5 | 1.08 | 0.14 | 0.552 | 280 | 22.9 | 1.04 | 0.53 | 0.507 |
| 2–3 | 308 | 16.9 | 1.14 | 0.02 | 0.629 | 340 | 20.3 | 1.12 | 0.03 | 0.612 | 314 | 21.7 | 1.17 | 0.002 | 0.647 |
| 3–4 | 297 | 17.8 | 1.17 | 0.008 | 0.653 | 327 | 20.8 | 1.13 | 0.03 | 0.609 | 306 | 21.6 | 1.16 | 0.006 | 0.645 |
| 4–5 | 286 | 16.8 | 1.18 | 0.004 | 0.671 | 320 | 20.9 | 1.16 | 0.007 | 0.622 | 295 | 22.0 | 1.15 | 0.02 | 0.616 |
| 5–6 | 294 | 17.3 | 1.26 | 0.0003 | 0.696 | 331 | 21.5 | 1.20 | 0.003 | 0.643 | 309 | 23.0 | 1.22 | 0.002 | 0.652 |
| 6–7 | 291 | 17.9 | 1.22 | 0.007 | 0.638 | 324 | 21.9 | 1.20 | 0.008 | 0.611 | 301 | 23.3 | 1.23 | 0.004 | 0.626 |
| 7–8 | 274 | 17.9 | 1.24 | 0.02 | 0.614 | 303 | 21.1 | 1.13 | 0.16 | 0.573 | 287 | 22.3 | 1.17 | 0.07 | 0.577 |
| 8–9 | 282 | 17.7 | 1.20 | 0.06 | 0.587 | 311 | 21.9 | 1.16 | 0.08 | 0.566 | 289 | 23.5 | 1.12 | 0.19 | 0.548 |
| 9–10 | 284 | 18.7 | 1.24 | 0.08 | 0.597 | 314 | 21.7 | 1.17 | 0.14 | 0.577 | 299 | 23.7 | 1.13 | 0.24 | 0.564 |
| Fluoride Intake Age | 2nd Molars1 | Canines/Premolars/2ndMolars1,3 | FRI-II Teeth4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Prev. (%) | O.R.2 | P | c | N | Prevalence (%) | O.R.2 | P | c | N | Prevalence (%) | O.R.2 | P | c | |
| Overall Prevalance | 313 | 15.7 | 269 | 43.9 | 352 | 31.8 | |||||||||
| 0–1 | 280 | 16.1 | 1.04 | 0.29 | 0.563 | 238 | 46.2 | 1.03 | 0.28 | 0.548 | 310 | 33.9 | 1.05 | 0.08 | 0.563 |
| 1–2 | 254 | 17.7 | 1.14 | 0.03 | 0.593 | 220 | 45.0 | 1.08 | 0.17 | 0.552 | 285 | 33.0 | 1.10 | 0.07 | 0.560 |
| 2–3 | 287 | 16.0 | 1.12 | 0.04 | 0.628 | 247 | 42.5 | 1.18 | 0.003 | 0.636 | 322 | 31.1 | 1.16 | 0.002 | 0.629 |
| 3–4 | 278 | 15.8 | 1.18 | 0.006 | 0.688 | 238 | 43.3 | 1.16 | 0.02 | 0.620 | 309 | 31.4 | 1.16 | 0.004 | 0.628 |
| 4–5 | 272 | 15.8 | 1.20 | 0.002 | 0.692 | 229 | 44.1 | 1.15 | 0.02 | 0.619 | 304 | 31.9 | 1.11 | 0.03 | 0.597 |
| 5–6 | 282 | 15.6 | 1.21 | 0.004 | 0.682 | 241 | 44.4 | 1.21 | 0.003 | 0.653 | 313 | 32.9 | 1.20 | 0.002 | 0.633 |
| 6–7 | 274 | 16.1 | 1.34 | 0.0003 | 0.678 | 235 | 45.5 | 1.22 | 0.008 | 0.609 | 309 | 33.7 | 1.19 | 0.007 | 0.597 |
| 7–8 | 261 | 15.3 | 1.36 | 0.003 | 0.655 | 224 | 44.6 | 1.24 | 0.02 | 0.597 | 289 | 33.2 | 1.16 | 0.06 | 0.568 |
| 8–9 | 263 | 17.5 | 1.33 | 0.004 | 0.643 | 228 | 45.2 | 1.14 | 0.14 | 0.561 | 296 | 33.4 | 1.12 | 0.15 | 0.549 |
| 9–10 | 266 | 17.3 | 1.55 | 0.0007 | 0.680 | 230 | 46.5 | 1.21 | 0.11 | 0.578 | 297 | 34.3 | 1.07 | 0.46 | 0.547 |
Requires FRI scoring on occlusal, incisal and middle thirds of all teeth of that tooth type to be considered no fluorosis.
Odds ratio reflects change per 0.01 mg/kg increase in fluoride intake.
Must have all 48 zones scored (o,i,m) out of 48 to be considered no fluorosis.
Up to 6 missing zones permitted to be considered no fluorosis. If 1st premolars have been extracted, up to 16 missing zones permitted.
Table 4.
Logistic regression statistics for fluorosis prevalence (2 or more teeth vs. none) - with cumulative mgF/kg fluoride intake over 2 or more years.
| Fluoride Intake Age | Canines1 | 1st Premolars1 | 2nd Premolars1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Prev. (%) | O.R.2 | P | c | N | Prev. (%) | O.R.2 | P | c | N | Prev. (%) | O.R.2 | P | c | |
| 2–4 | 282 | 16.7 | 1.18 | 0.02 | 0.639 | 310 | 20.3 | 1.14 | 0.02 | 0.617 | 291 | 21.3 | 1.18 | 0.004 | 0.655 |
| 3–5 | 268 | 17.5 | 1.24 | 0.002 | 0.691 | 298 | 20.8 | 1.17 | 0.02 | 0.626 | 277 | 21.3 | 1.21 | 0.003 | 0.658 |
| 4–6 | 260 | 16.5 | 1.27 | 0.0007 | 0.711 | 295 | 21.4 | 1.24 | 0.001 | 0.653 | 273 | 22.3 | 1.24 | 0.002 | 0.659 |
| 5–7 | 269 | 17.8 | 1.29 | 0.0008 | 0.692 | 302 | 22.5 | 1.22 | 0.004 | 0.635 | 283 | 24.0 | 1.27 | 0.002 | 0.657 |
| 6–8 | 256 | 18.0 | 1.24 | 0.03 | 0.614 | 284 | 22.2 | 1.19 | 0.04 | 0.591 | 269 | 23.4 | 1.23 | 0.02 | 0.606 |
| 7–9 | 250 | 18.0 | 1.23 | 0.05 | 0.601 | 276 | 21.4 | 1.12 | 0.23 | 0.560 | 262 | 22.9 | 1.14 | 0.18 | 0.558 |
| 8–10 | 250 | 18.8 | 1.24 | 0.07 | 0.590 | 278 | 22.3 | 1.19 | 0.10 | 0.576 | 263 | 24.3 | 1.13 | 0.24 | 0.546 |
| 2–5 | 257 | 17.1 | 1.24 | 0.003 | 0.679 | 285 | 20.7 | 1.20 | 0.008 | 0.639 | 265 | 21.5 | 1.24 | 0.002 | 0.673 |
| 3–6 | 247 | 17.0 | 1.32 | 0.0004 | 0.729 | 278 | 20.9 | 1.23 | 0.004 | 0.653 | 260 | 21.5 | 1.27 | 0.002 | 0.678 |
| 4–7 | 239 | 16.7 | 1.29 | 0.002 | 0.703 | 271 | 22.1 | 1.25 | 0.003 | 0.644 | 252 | 23.0 | 1.27 | 0.003 | 0.655 |
| 5–8 | 244 | 17.6 | 1.29 | 0.005 | 0.664 | 272 | 22.4 | 1.21 | 0.02 | 0.615 | 259 | 23.9 | 1.25 | 0.02 | 0.629 |
| 6–9 | 234 | 18.4 | 1.20 | 0.09 | 0.596 | 260 | 22.7 | 1.16 | 0.12 | 0.574 | 246 | 24.0 | 1.16 | 0.13 | 0.573 |
| 7–10 | 228 | 19.3 | 1.27 | 0.047 | 0.600 | 252 | 22.2 | 1.15 | 0.22 | 0.562 | 241 | 23.7 | 1.13 | 0.26 | 0.544 |
| 2–6 | 237 | 16.5 | 1.30 | 0.002 | 0.716 | 267 | 20.6 | 1.25 | 0.003 | 0.666 | 250 | 21.6 | 1.29 | 0.002 | 0.691 |
| 3–7 | 228 | 17.1 | 1.33 | 0.001 | 0.712 | 256 | 21.5 | 1.23 | 0.01 | 0.642 | 240 | 22.1 | 1.29 | 0.003 | 0.669 |
| 4–8 | 223 | 17.0 | 1.34 | 0.002 | 0.691 | 250 | 22.4 | 1.29 | 0.004 | 0.635 | 236 | 22.9 | 1.32 | 0.004 | 0.645 |
| 5–9 | 224 | 17.9 | 1.27 | 0.02 | 0.648 | 250 | 22.8 | 1.19 | 0.05 | 0.597 | 238 | 24.4 | 1.20 | 0.06 | 0.596 |
| 6–10 | 215 | 19.5 | 1.23 | 0.09 | 0.597 | 239 | 23.4 | 1.17 | 0.13 | 0.576 | 228 | 24.6 | 1.15 | 0.22 | 0.559 |
| 2–7 | 219 | 16.4 | 1.30 | 0.004 | 0.695 | 247 | 21.5 | 1.26 | 0.005 | 0.657 | 232 | 22.4 | 1.31 | 0.002 | 0.683 |
| 3–8 | 214 | 17.3 | 1.38 | 0.002 | 0.699 | 238 | 21.4 | 1.25 | 0.02 | 0.631 | 225 | 21.8 | 1.33 | 0.003 | 0.663 |
| 4–9 | 206 | 17.0 | 1.35 | 0.004 | 0.682 | 231 | 22.5 | 1.28 | 0.008 | 0.621 | 218 | 22.9 | 1.28 | 0.02 | 0.618 |
| 5–10 | 207 | 18.8 | 1.32 | 0.02 | 0.640 | 231 | 23.4 | 1.21 | 0.06 | 0.591 | 222 | 24.8 | 1.19 | 0.10 | 0.579 |
| Fluoride Intake Age | 2nd Molars1 | Canines/Premolars/2nd Molars3 | FRI-II Teeth3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Prev. (%) | O.R. | P | c | N | Prev. (%) | O.R. | P | c | N | Prev. (%) | O.R. | P | c | |
| 2–4 | 264 | 15.9 | 1.16 | 0.02 | 0.680 | 227 | 41.9 | 1.20 | 0.004 | 0.639 | 294 | 30.6 | 1.19 | 0.003 | 0.638 |
| 3–5 | 256 | 16.0 | 1.25 | 0.002 | 0.717 | 215 | 43.7 | 1.19 | 0.01 | 0.637 | 283 | 31.4 | 1.18 | 0.006 | 0.631 |
| 4–6 | 256 | 15.6 | 1.24 | 0.002 | 0.705 | 213 | 44.1 | 1.21 | 0.006 | 0.642 | 280 | 32.5 | 1.18 | 0.006 | 0.626 |
| 5–7 | 262 | 16.4 | 1.31 | 0.0009 | 0.696 | 223 | 45.7 | 1.24 | 0.004 | 0.644 | 287 | 34.5 | 1.23 | 0.003 | 0.625 |
| 6–8 | 245 | 15.9 | 1.42 | 0.0009 | 0.669 | 211 | 46.0 | 1.29 | 0.007 | 0.611 | 270 | 34.8 | 1.22 | 0.02 | 0.583 |
| 7–9 | 234 | 16.2 | 1.37 | 0.006 | 0.648 | 206 | 45.1 | 1.21 | 0.06 | 0.581 | 263 | 33.8 | 1.14 | 0.15 | 0.553 |
| 8–10 | 235 | 18.3 | 1.52 | 0.0009 | 0.665 | 206 | 47.1 | 1.18 | 0.14 | 0.563 | 264 | 35.2 | 1.10 | 0.32 | 0.541 |
| 2–5 | 243 | 16.0 | 1.23 | 0.004 | 0.714 | 208 | 43.3 | 1.22 | 0.005 | 0.648 | 272 | 31.3 | 1.20 | 0.004 | 0.641 |
| 3–6 | 243 | 15.6 | 1.28 | 0.002 | 0.721 | 203 | 43.3 | 1.22 | 0.008 | 0.651 | 263 | 31.9 | 1.22 | 0.004 | 0.648 |
| 4–7 | 239 | 16.3 | 1.31 | 0.001 | 0.705 | 197 | 45.2 | 1.21 | 0.02 | 0.628 | 259 | 33.6 | 1.19 | 0.02 | 0.610 |
| 5–8 | 238 | 16.0 | 1.38 | 0.002 | 0.695 | 204 | 46.1 | 1.31 | 0.004 | 0.644 | 258 | 35.3 | 1.23 | 0.01 | 0.605 |
| 6–9 | 220 | 16.8 | 1.38 | 0.006 | 0.649 | 195 | 46.7 | 1.22 | 0.048 | 0.582 | 247 | 35.6 | 1.15 | 0.12 | 0.557 |
| 7–10 | 213 | 17.4 | 1.53 | 0.002 | 0.660 | 190 | 47.4 | 1.26 | 0.06 | 0.581 | 240 | 35.8 | 1.13 | 0.24 | 0.545 |
| 2–6 | 233 | 15.5 | 1.25 | 0.006 | 0.717 | 197 | 42.6 | 1.24 | 0.007 | 0.656 | 254 | 31.5 | 1.23 | 0.004 | 0.653 |
| 3–7 | 227 | 16.3 | 1.37 | 0.0008 | 0.717 | 188 | 44.1 | 1.20 | 0.03 | 0.629 | 244 | 32.8 | 1.21 | 0.02 | 0.627 |
| 4–8 | 221 | 15.8 | 1.39 | 0.002 | 0.700 | 186 | 45.7 | 1.32 | 0.003 | 0.638 | 239 | 34.7 | 1.24 | 0.008 | 0.606 |
| 5–9 | 215 | 16.7 | 1.37 | 0.004 | 0.681 | 188 | 46.8 | 1.26 | 0.02 | 0.613 | 237 | 35.9 | 1.19 | 0.05 | 0.580 |
| 6–10 | 202 | 17.8 | 1.50 | 0.003 | 0.661 | 180 | 48.9 | 1.25 | 0.06 | 0.580 | 227 | 37.4 | 1.14 | 0.18 | 0.551 |
| 2–7 | 219 | 16.4 | 1.36 | 0.002 | 0.716 | 184 | 43.5 | 1.22 | 0.02 | 0.637 | 237 | 32.5 | 1.22 | 0.01 | 0.636 |
| 3–8 | 211 | 15.6 | 1.48 | 0.0007 | 0.722 | 177 | 44.6 | 1.32 | 0.006 | 0.641 | 227 | 33.5 | 1.27 | 0.008 | 0.623 |
| 4–9 | 201 | 16.4 | 1.40 | 0.003 | 0.692 | 173 | 45.7 | 1.29 | 0.02 | 0.621 | 221 | 34.8 | 1.21 | 0.03 | 0.587 |
| 5–10 | 198 | 17.7 | 1.50 | 0.002 | 0.693 | 174 | 48.9 | 1.29 | 0.03 | 0.604 | 219 | 37.4 | 1.20 | 0.07 | 0.571 |
Note: Participants in each of the tooth-type regressions not necessarily the same as those for other tooth types.
Must have all 12 zones scored (o,i,m) out of 48 to be considered no fluorosis
Odds ratio reflects change per 0.01 mg/kg increase in fluoride intake.
Must have all 48 zones scored (o,i,m) out of 48 to be considered no fluorosis.
Up to 6 missing zones permitted to be considered no fluorosis. If 1st premolars have been extracted, up to 16 missing zones permitted
Each of the individual years from ages 2 to 8 were statistically significantly related to fluorosis prevalence on the canines (Table 3), with the strongest association with fluoride intake from age 5 to 6 years (OR=1.26; C = 0.696; and p =0.0003). For both the first and second premolars considered separately, significant relationships were found for each year from ages 2 to 7 and age 5 to 6 years was most strongly related to prevalence of fluorosis. For the second molars, all the individual years from age 2 to 10 years were statistically significantly associated with dental fluorosis, with the strongest relationship with fluoride intake from age 6 to 7 years (OR=1.34; p=0.0003), although the year from age 4 to 5 years had the highest C-statistic value (0.692), and age 9 to 10 years had the highest odds ratio (1.55). When fluorosis on all four late-erupting teeth was considered together, all of the individual years from age 2 to 8 were significantly related to fluorosis, with the strongest association with intake from 5–6 years (OR=1.21; C = 0.653; and p =0.003). When the associations between fluoride intake and FRI II zones were examined, all the individual years from age 2 to 7 were statistically significantly associated with fluorosis and the intake during the age 5–6 year had the strongest association (OR=1.20; C = 0.633; and p = 0.002).
In Table 4, we report results from logistic regression analyses using multi-year fluoride intakes considered as 2-, 3-, 4-, and 5-year cumulative periods, respectively, for each of the four late-erupting tooth types considered individually and together, and for FRI II zones. For canines, when fluoride intakes over 2-year cumulative periods were considered, statistically significant associations with fluorosis were found for all the periods except the age 8 to 10 year period. When fluoride intake for 3- and 4-year cumulative periods were considered, all the periods were significantly associated with fluorosis prevalence except for ages 6 to 9 years for the 3-year periods and 6 to 10 years for the 4-year periods, respectively. All the 5-year cumulative periods were significantly associated with fluorosis prevalence. The strongest relationships (based on p-values) were found with fluoride intake from age 5 to 7 (2-year), 3 to 6 (3-year), 3 to 7 (4-year) and 3 to 8 (5-year). The strongest associations consistently were found for periods which included fluoride intake from age 5 to 6 years, which in most cases, had the lowest p-values and highest C-statistic values.
The associations between periods of fluoride intake and fluorosis prevalence were mostly similar to each other for the first and second premolars. When fluoride intakes for 2-year cumulative periods were considered, all the periods except from ages 7 to 9 and 8 to 10 were statistically significant. Similarly, when 3-year periods were considered, all periods except from ages 6 to 9 and 7 to 10 were significantly associated with fluorosis prevalence. When 4-year cumulative periods were considered, only ages 6 to 10 years for the first premolars and 5 to 9 and 6 to 10 years for the second premolars were not significantly related. In the analysis that examined fluoride intake over 5-year cumulative periods, all the periods except from age 5 to 10 years were statistically significant. As with the canines, while there were significant associations with other periods, the intervals containing the age 5 to 6 year period had the strongest relationships with fluorosis risk. For the second molars, all of the multi-year periods that were examined, irrespective of the interval, were statistically significantly associated with dental fluorosis.
When fluorosis on all four late-erupting teeth was considered together, each of the two-year cumulative periods from age 2 to 8 years was significantly related to fluorosis, with the strongest association (based on p-value) with intake from 5–7 years (OR=1.24; C = 0.644; and p = 0.004). All periods from ages 2 to 9 years were significantly associated with fluorosis when 3-year and 4-year periods were considered. All the 5-year periods from age 2 to 10 years were significantly associated. The relationships between multi-year cumulative periods of fluoride intake and fluorosis on FRI II teeth were very similar to the relationships between Fluoride intake and fluorosis on all four late-erupting teeth, with all the same periods significantly related except that the 3-year cumulative period from 6–9 years and the 5-year cumulative period from 5–10 years also were not significantly associated with fluorosis on FRI II teeth.
Discussion
This analysis examined the relationships between timing of fluoride intake (from birth to age 10 years) and prevalence of dental fluorosis on late-erupting permanent teeth (canines, premolars and second molars). As the number of sources of fluoride intake, and prevalence of mild dental fluorosis have increased, it is important to refine our estimates for the relationship between timing of fluoride intake and dental fluorosis. Our findings can be useful in future efforts to adjust dosing of fluoride intake and/or for making fluoride recommendations, such as a need for additional monitoring of children, up to age 6–8 years, to minimize swallowing of fluoridated toothpaste. This can help in optimizing the benefits of fluorides and minimizing the effects of fluorosis. Our results also help in furthering the understanding of periods of susceptibility to dental fluorosis. Hence, the relationships identified could be useful/applicable in designing future research studies in regions where more severe forms are prevalent, since most fluorosis identified in this study was mild.
In our study sample, fluoride intakes across individual years of life were substantially correlated. Hence, determining specific proportions of etiology to be attributed to the most influential ages of fluoride ingestion is challenging. However, our results indicate that fluoride intake from age 2 to 8 years plays an important role in determining the risk of dental fluorosis for most late-erupting permanent teeth. The strongest association for fluorosis on the late-erupting permanent teeth was with fluoride intake during the sixth year of life. The periods of elevated risk identified in this study are generally consistent with the findings from previous studies and mostly encompass the periods identified in those studies. Nonetheless, this study differs from some of the previous studies in the fluorosis index that was used, the sources of fluoride that were assessed and use of prospective estimates of fluoride intake. Furthermore, few previous studies considered the actual dose of fluoride intake. Instead, they mostly used the age of onset or frequency or duration of certain behaviors such as tooth-brushing with fluoridated dentifrice, infant formula, or fluoride supplement use to study the relationships between timing of fluoride intake and fluorosis (12, 16–18)
Larsen et al. (17) reported that the periods of increased risk for fluorosis of maxillary and mandibular canines were from 3.5 to 6.5 years and 3.5 to 5.5 years, respectively, and for maxillary and mandibular premolars were age 3.5 to 8 years and 3.5 to 6.5 years, respectively The high risk periods identified in this study overlap with the periods identified by Larsen et al. (17). We found that fluoride intakes during each of the individual years from the ages 2 to 8 years were associated with increased risk of fluorosis on the canines and during each of the individual years from age 2 to 7 years were significantly associated with increased risk of fluorosis on both first and second premolars. Ishii et al. (18) identified two patterns for higher risk of fluorosis on maxillary first premolars: when high fluoride intake occurred during the first two years of life, and when exposure began before the age of four years and continued until age 7 years. The second period identified by Ishii et al. (18) does overlap with the time periods we found for premolars. However, we did not find significant association between fluoride intake before 2 years of age and dental fluorosis on any of the late-erupting teeth. Among both the maxillary and mandibular second molars, Larsen et al. (17) found that the periods of higher risk were from age 5.5 to 8 years. On the other hand, we found that fluoride intake during each of the individual years from age 2 to 10 years was statistically significantly associated with fluorosis.
We also examined the relationship between timing of fluoride intake and fluorosis on all the late-erupting teeth considered together, as well as on the FRI-II zones considered together. Each of the individual years from age 2 to 9 years was significantly associated with fluorosis on all late-erupting teeth considered together. When FRI II zones were considered together, the years from age 2 to 7 years were all significantly associated with fluorosis and the strongest association was with fluoride intake during the sixth year of life. These findings are somewhat similar to, and overlap with, the periods that were identified by Pendrys et al. (12). They reported that the first 4 years of life are the most important periods for development of fluorosis on FRI-II zones. For children living in fluoridated communities, they reported that dietary fluoride supplementation during the first six years of life and frequent brushing from birth to 8 years were important risk factors for fluorosis. Our study is similar to the Pendrys et al. (12) study in that both studies examined fluoride intake from multiple sources and both used the Fluorosis Risk Index to record dental fluorosis and most participants in our study lived in areas with community water fluoridation. However, Pendrys et al. (16) used retrospective estimates and did not estimate the actual amount of fluoride intake.
Our analyses examining the relationships between cumulative multi-year fluoride intake and dental fluorosis identified periods of elevated risk that were similar to the periods identified in our analysis using individual years of fluoride intake. The cumulative periods which included fluoride intake during the sixth year of life had stronger associations when compared to periods which did not include the sixth year. Also, we found stronger associations with fluorosis when fluoride intake over longer periods was examined. In the analysis examining the correlation between FRI sum-scores and timing of fluoride intake, we found that the correlations were higher and the results smoothed when fluoride intake over longer periods of time was considered. This finding is similar to what was reported by Bardsen (13), in a meta-analysis of 10 studies on risk periods for fluorosis of maxillary central incisors. That analysis found that the teeth which were exposed to high fluorides for two of the first four years of life were at much higher risk of developing fluorosis and concluded that individual phases of tooth development cannot be singled out as being periods of higher risk for fluorosis and duration of exposure is a better predictor of fluorosis risk. Furthermore, we found that there was more variation in the correlations when 1- and 2- year cumulative periods were considered. For most tooth types, we found a period of increased correlation at an early age (2–3 years) and a second peak at a later age (5–6 years). This is supported by Ishii et al. (18).who also found two clear peaks when the risk of fluorosis increased. For most tooth types, there was less correlation with fluoride intake during the fourth year of life.
Previous studies have found that both timing and cumulative duration of higher levels of fluoride intake play a role in the etiology of dental fluorosis. Ishii et al. (21) found that children who had high fluoride intake from birth throughout the period of tooth development had much higher risk of moderate to severe fluorosis. Similarly, Larsen et al. (17) reported that the age periods of elevated risk of dental fluorosis extended beyond the age when crowns become detectable on radiographs. On the other hand, studies have also reported that certain periods during enamel formation, such as early maturation (10) and the secretory (10, 13) phases, are critical in uptake and distribution of fluoride and consequently the development of fluorosis. The periods of susceptibility we identified in this study are much longer than the periods reported in previous studies (10, 13, 17 and 21). The longer periods identified in this study confirm findings from previous reports (17, 21) that teeth are susceptible to development of fluorosis outside the critical periods of tooth development. However, we found the highest correlations for fluorosis in most teeth was with higher fluoride intake from about age 6 to 8 years. This period generally corresponds with the pre-eruptive maturation phase of the teeth which occurs after crown completion. Hence, the periods identified in this study suggest that the teeth could be susceptible to fluorosis until after the completion of calcification, which is generally completed by the ages of 5 to 6, 6 to 7, 6 to 7, and 7 to 8 years for the first premolars, canines, second premolars, and second molars, respectively (32).
Strengths and Limitations
Most of the previous studies which examined the relationship between timing of fluoride intake and fluorosis prevalence used retrospective and cross-sectional fluoride intake estimates. We used data from the longitudinal Iowa Fluoride Study that was collected using period-specific fluoride intake estimates from multiple sources such as water, other beverages, dietary fluoride supplements and dentifrices, which makes this study unique. Also, the IFS had rigorous training and calibration protocols for examiners, including initial calibration with the researcher who developed the FRI. This certainly increases the validity of the findings from our dental examinations. However, there are certain limitations. First, fluoride intake data were collected using self-administered questionnaires and was not directly validated. Second, ingested fluoride which is not excreted tends to be deposited in calcifying/calcified tissues, such as bone, and can be released from bone at later periods (10). The IFS assessed fluoride intake only from dietary and non-dietary sources and did not collect blood or urinary samples. This precludes the possibility of estimating the quantity and timing of fluoride levels released from bone. However, this physiological mechanism probably did not have a significant impact on our findings, as the amounts of fluoride released from bones of individuals with stable fluoride intake levels are considered to be low (33). Third, we used a conservative definition for fluorosis and only included participants who had definitive or severe fluorosis on two or more teeth. We assigned scores of 0 to incompletely erupted cervical zones. Individuals with FRI scores of 1 on all surfaces were included as non-fluorosis cases. An FRI score of 1 indicates Questionable fluorosis when <50% of the zone had white striations (FRI Score =1). Hence, we probably underestimated the prevalence of very mild fluorosis in this study.
The dental fluorosis identified in this study was mostly mild and less than 1% of participants had severe fluorosis. Also, the relationships between timing of fluoride intake and fluorosis identified in this study are associations based on probability of having 2 or more teeth with fluorosis or the fluorosis sum scores and none of the fluoride intake amounts in this study were associated with absolute fluorosis outcomes. Hence, comparisons of results to those with other populations, where severe forms of fluorosis are prevalent or populations with high fluoride intake must be made with caution. Additionally, variation in the timing of formation of tooth zones across individuals could have had an impact on our study findings. Hence, the results must be interpreted with caution. Finally, Larsen et al. (17) reported some variations in high risk periods for fluorosis between the maxillary and mandibular teeth, which they examined separately. In this study, we examined all the teeth of a given tooth type in one category as the calcification periods for the various tooth types in the upper and lower arches are generally similar to each other (32).
Conclusions
This study’s findings suggest that the sixth year of life is the most important period for development of dental fluorosis in late-erupting permanent teeth, but the teeth are susceptible for an extended period from about age 2 to 8 years. Although not as visually prominent as the maxillary central incisors, some of the late-erupting teeth are esthetically important and this should be taken into consideration when making recommendations about fluoride intake.
Acknowledgments
This work was supported in part by NIH grants R01- DE09551, P30-DE10126, R01-DE12101, and M01- RR00059.
References
- 1.Burt BA. The changing patterns of systemic fluoride intake. J Dent Res. 1992;71:1228–37. doi: 10.1177/00220345920710051601. [DOI] [PubMed] [Google Scholar]
- 2.Pendrys DG, Stamm JW. Relationship of total fluoride intake to beneficial effects and enamel fluorosis. J Dent Res. 1990;69:556–7. doi: 10.1177/00220345900690S107. [DOI] [PubMed] [Google Scholar]
- 3.Kumar JV, Green EL, Wallace W, Carnahan T. Trends in dental fluorosis and dental caries prevalences in Newburgh and Kingston, NY. Am J Public Health. 1989;79:565–9. doi: 10.2105/ajph.79.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltran-Aguilar ED, Griffin SO, Lockwood SA. Prevalence and trends in enamel fluorosis in the united states from the 1930s to the 1980s. J Am Dent Assoc. 2002;133:157–65. doi: 10.14219/jada.archive.2002.0139. [DOI] [PubMed] [Google Scholar]
- 5.Fejerskov O, Manji F, Baelum V. The nature and mechanisms of dental fluorosis in man. J Dent Res. 1990;69:692–700. doi: 10.1177/00220345900690S135. [DOI] [PubMed] [Google Scholar]
- 6.Kroon J. The relation between toothpaste usage and fluorosis: A cause for concern? SADJ. 2001;56:20–7. [PubMed] [Google Scholar]
- 7.Hong L, Levy SM, Broffitt B, Warren JJ, Kanellis MJ, Wefel JS, et al. Timing of fluoride intake in relation to development of fluorosis on maxillary central incisors. Community Dent Oral Epidemiol. 2006;34:299–309. doi: 10.1111/j.1600-0528.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 8.Evans RW, Stamm JW. An epidemiologic estimate of the critical period during which human maxillary central incisors are most susceptible to fluorosis. J Public Health Dent. 1991;51:251–9. doi: 10.1111/j.1752-7325.1991.tb02223.x. [DOI] [PubMed] [Google Scholar]
- 9.Hong L, Levy SM, Warren JJ, Broffitt B, Cavanaugh J. Fluoride intake levels in relation to fluorosis development in permanent maxillary central incisors and first molars. Caries Res. 2006;40:494–500. doi: 10.1159/000095648. [DOI] [PubMed] [Google Scholar]
- 10.DenBesten PK, Thariani H. Biological mechanisms of fluorosis and level and timing of systemic exposure to fluoride with respect to fluorosis. J Dent Res. 1992;71:1238–43. doi: 10.1177/00220345920710051701. [DOI] [PubMed] [Google Scholar]
- 11.Pendrys DG, Katz RV. Risk of enamel fluorosis associated with fluoride supplementation, infant formula, and fluoride dentifrice use. Am J Epidemiol. 1989;130:1199–208. doi: 10.1093/oxfordjournals.aje.a115448. [DOI] [PubMed] [Google Scholar]
- 12.Pendrys DG, Katz RV, Morse DE. Risk factors for enamel fluorosis in a fluoridated population. Am J Epidemiol. 1994;140:461–71. doi: 10.1093/oxfordjournals.aje.a117268. [DOI] [PubMed] [Google Scholar]
- 13.Bardsen A. “Risk periods” Associated with the development of dental fluorosis in maxillary permanent central incisors: A meta-analysis. Acta Odontol Scand. 1999;57:247–56. doi: 10.1080/000163599428652. [DOI] [PubMed] [Google Scholar]
- 14.Evans RW, Darvell BW. Refining the estimate of the critical period for susceptibility to enamel fluorosis in human maxillary central incisors. J Public Health Dent. 1995;55:238–49. doi: 10.1111/j.1752-7325.1995.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 15.Bardsen A, Bjorvatn K. Risk periods in the development of dental fluorosis. Clin Oral Investig. 1998;2:155–60. doi: 10.1007/s007840050063. [DOI] [PubMed] [Google Scholar]
- 16.Pendrys DG, Katz RV, Morse DE. Risk factors for enamel fluorosis in a nonfluoridated population. Am J Epidemiol. 1996;143:808–15. doi: 10.1093/oxfordjournals.aje.a008819. [DOI] [PubMed] [Google Scholar]
- 17.Larsen MJ, Richards A, Fejerskov O. Development of dental fluorosis according to age at start of fluoride administration. Caries Res. 1985;19:519–27. doi: 10.1159/000260892. [DOI] [PubMed] [Google Scholar]
- 18.Ishii T, Suckling G. The severity of dental fluorosis in children exposed to water with a high fluoride content for various periods of time. J Dent Res. 1991;70:952–6. doi: 10.1177/00220345910700060801. [DOI] [PubMed] [Google Scholar]
- 19.Sudhir KM, Suresh S, Prashant GM, Reddy VV, Shafiulla M, Chandu GN. Distribution patterns of enamel fluorosis in permanent dentition. Oral Health Prev Dent. 2012;10:167–74. [PubMed] [Google Scholar]
- 20.Ramires I, Pessan JP, Levy FM, Rodrigues MH, de Almeida BS, Kato MT, et al. Prevalence of dental fluorosis in Bauru, Sao Paulo, Brazil. J Appl Oral Sci. 2007;15:140–3. doi: 10.1590/S1678-77572007000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha-Cruz J, Nadanovsky P. Dental fluorosis increases caries risk. J Evid Based Dent Pract. 2005;5(3):170–1. doi: 10.1016/j.jebdp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Cortes DF, Ellwood RP, O’Mullane DM, Bastos JR. Drinking water fluoride levels, dental fluorosis, and caries experience in Brazil. J Public Health Dent. 1996;56:226–8. doi: 10.1111/j.1752-7325.1996.tb02441.x. [DOI] [PubMed] [Google Scholar]
- 23.Levy SM, Warren JJ, Broffitt B, Nielsen B. Factors associated with parents’ esthetic perceptions of children’s mixed dentition fluorosis and demarcated opacities. Pediatr Dent. 2005;27:486–92. [PubMed] [Google Scholar]
- 24.Chankanka O, Levy SM, Warren JJ, Chalmers JM. A literature review of aesthetic perceptions of dental fluorosis and relationships with psychosocial aspects/oral health-related quality of life. Community Dent Oral Epidemiol. 2010;38:97–109. doi: 10.1111/j.1600-0528.2009.00507.x. [DOI] [PubMed] [Google Scholar]
- 25.Do LG, Spencer A. Oral health-related quality of life of children by dental caries and fluorosis experience. J Public Health Dent. 2007;67:132–9. doi: 10.1111/j.1752-7325.2007.00036.x. [DOI] [PubMed] [Google Scholar]
- 26.Pendrys DG. The fluorosis risk index: A method for investigating risk factors. J Public Health Dent. 1990;50:291–8. doi: 10.1111/j.1752-7325.1990.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 27.Levy SM, Kiritsy MC, Slager SL, Warren JJ. Patterns of dietary fluoride supplement use during infancy. J Public Health Dent. 1998;58:228–33. doi: 10.1111/j.1752-7325.1998.tb02998.x. [DOI] [PubMed] [Google Scholar]
- 28.Levy SM, Warren JJ, Davis CS, Kirchner HL, Kanellis MJ, Wefel JS. Patterns of fluoride intake from birth to 36 months. J Public Health Dent. 2001;61:70–7. doi: 10.1111/j.1752-7325.2001.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 29.Levy SM, Warren JJ, Broffitt B. Patterns of fluoride intake from 36 to 72 months of age. J Public Health Dent. 2003;63:211–20. doi: 10.1111/j.1752-7325.2003.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 30.Russell AL. The differential diagnosis of fluoride and nonfluoride enamel opacities. J Public Health Dent. 1961;21:143–46. [Google Scholar]
- 31.Slayton RL, Warren JJ, Kanellis MJ, Levy SM, Islam M. Prevalence of enamel hypoplasia and isolated opacities in the primary dentition. Pediatr Dent. 2001;23:32–6. [PubMed] [Google Scholar]
- 32.Nelson SJ. Wheeler’s Dental anatomy, physiology and occlusion. 9th. St Louis: Saunders Elsevier; 2010. [Google Scholar]
- 33.Whitford GM. Fluoride metabolism and excretion in children. J Public Health Dent. 1999;59:224–8. doi: 10.1111/j.1752-7325.1999.tb03273.x. [DOI] [PubMed] [Google Scholar]


