Abstract
Furosemide is the most commonly used loop diuretic in heart failure (HF) patients despite data suggesting potential pharmacologic and anti-fibrotic benefits with torsemide. We investigated HF patients in ASCEND-HF who were discharged on either torsemide or furosemide. Using inverse probability weighting to account for the non-random selection of diuretic, we assessed the relation between choice of diuretic at discharge with 30-day mortality or HF hospitalization and 180-day mortality. Of 7,141 patients in the trial, 4,177 patients were included in this analysis, of which 87% (n=3,620) received furosemide and 13% (n=557) received torsemide. Torsemide-treated patients had lower ejection fraction and blood pressure, and higher creatinine and natriuretic peptide level compared with furosemide. Torsemide was associated with similar outcomes on unadjusted analysis and nominally lower events on adjusted analysis [30–day mortality/HF hospitalization OR 0.89, 95% CI: 0.62–1.29, P=0.55 and 180-day mortality HR 0.86, 95% CI: 0.63–1.19, P=0.37]. In conclusion, these data are hypothesis-generating and randomized comparative-effectiveness trials are needed to investigate the optimal diuretic choice.
Keywords: loop diuretics, torsemide, furosemide, heart failure
Loop diuretics including furosemide and torsemide are prescribed for the majority of symptomatic heart failure (HF) patients1,2. Guidelines indicate that the optimal use of diuretics is a cornerstone of HF therapy3. However, despite preclinical and clinical data supporting benefits with torsemide, furosemide is the most commonly used loop diuretic4. Several small studies of torsemide vs. furosemide5–7 and a recent meta-analysis8 suggest a decrease in HF morbidity and potentially mortality with torsemide compared with furosemide. These previous studies were limited by modest sample sizes in cohorts of patients from more than a decade ago. In order to investigate the potential role of torsemide in contemporary clinical practice, we assessed loop diuretics in a large, international acute HF trial and evaluated the association with baseline characteristics and post-discharge outcomes.
METHODS
The design and results of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial have been reported previously9,10. Briefly, the trial was an international, double-blind, placebo-controlled study evaluating the effectiveness and safety of nesiritide in addition to standard care among 7,141 patients with acute HF. The trial was conducted from May 2007 through August 2010 at 398 centers in 30 countries throughout the world. Detailed inclusion and exclusion criteria have been described previously9. The 2 primary end points were a composite end point of all-cause mortality or HF readmission up to 30 days after randomization and the change in early dyspnea relief after study drug initiation.
Data on patient characteristics were collected during the baseline hospitalization. Loop diuretic use was documented at hospital admission and discharge. Rehospitalization and fatal events within 30 days after randomization were reviewed and categorized by an independent, blinded clinical-events committee. HF hospitalization was classified as previously described10. In brief, HF hospitalization required typical clinical manifestations of worsening HF and the addition of or increase in treatment specifically for worsening HF. All-cause mortality was assessed through 180 days.
For the primary analyses, we limited the cohort to patients discharged alive on either furosemide or torsemide. Given significant regional variation in the use of torsemide, we restricted the analyses to those countries having at least 20 patients on either torsemide or furosemide and having some patients on torsemide.
The primary outcome for the present analysis was all-cause mortality or HF hospitalization through 30-days after discharge. Secondary outcomes were 30-day all-cause mortality, 30-day HF hospitalization and 180-day all-cause mortality post-discharge. We were also interested in identifying clinical factors associated with patients being discharged on torsemide as compared with furosemide.
We summarized the patterns of loop diuretic use in ASCEND-HF patients. Demographics, physical and laboratory findings, medical history, and therapies were summarized as frequencies and percentages for categorical variables and by the medians and 25th and 75th percentiles for continuous variables in patients discharged on either torsemide or furosemide. Baseline characteristics were compared using the Student’s t-test or Wilcoxon rank sum test for continuous variables, and chi-square tests for categorical variables as appropriate. We generated a multivariable logistic regression model to determine admission variables associated with discharge torsemide use (over furosemide). We assessed the number of events for the outcomes of interest based on discharge diuretic.
Because the choice of diuretic at discharge was not randomized, we developed a propensity score model to predict the use of furosemide vs. torsemide. The propensity score model was fitted using a logistic regression model with baseline covariates identified in previous ASCEND-HF models11. The covariates were country of randomization, age, previous hospitalization for HF, baseline systolic blood pressure (SBP), baseline sodium, baseline BUN, and having a qualifying episode with jugular venous distension (JVD). The estimated propensity scores were used in inverse propensity weighted (IPW) models12 to assess the association of furosemide or torsemide use with clinical endpoints. IPW methods are a set of statistical techniques that re-weight the data to create a pseudo-population where patient characteristics are independent of treatment received13. This condition where there is independence of observed baseline factors and treatment is similar to what would be expected in a randomized study. Thus, IPW is a powerful method for using observational data to compare the effectiveness of 2 or more treatments13. This technique offers methodology to address concerns that any observed difference in outcome between 2 groups may reflect inherent differences between the groups (e.g., one group has an increased severity of disease) rather than effects caused by the 2 treatments. In the present analysis, covariate balance was assessed using standardized differences14. Logistic regression analyses (for 30-day endpoints) and Cox proportional hazards (for the 180-day endpoint) models were generated to assess the association between loop diuretics and clinical outcomes, weighted by the inverse of the estimated probability of the choice of a particular diuretic. Multivariate regression analysis adjusting for covariates above was also performed as a secondary analysis. Hazard ratios (HRs) for 180-day mortality and Odds Ratios (ORs) for other endpoints were calculated with corresponding 95% confidence intervals (CIs) relative to discharge diuretic. Event rate curves were shown using unadjusted and IPW adjusted Kaplan-Meier estimates. Statistical significance was assessed using 2-sided P values. A P value <0.05 was considered statistically significant. All statistical computations were generated using SAS version 9.4 (SAS Institute Inc., Cary, NC). No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
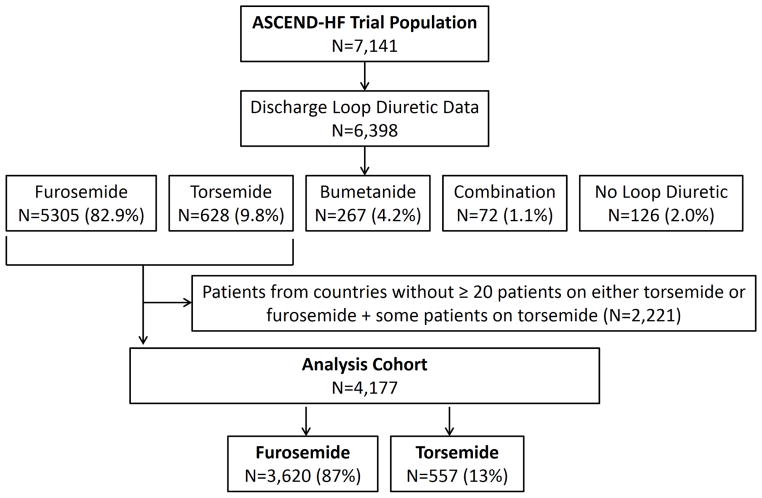
Figure 1 presents the patients included in this analysis. Of the 7,141 patients in ASCEND, there was discharge diuretic information on 6,398. The majority of patients discharged on a loop diuretic received furosemide (N=5,305; 82.9%). Torsemide was the next most common loop diuretic (N=628; 9.8%) and 267 patients (4.2%) were prescribed bumetanide. There were 72 patients discharged on a combination of loop diuretics and 126 patients (2.0%) who were not discharged on a loop diuretic. The analysis cohort restricted to patients from countries with at least 20 participants on either torsemide or furosemide and having some patients on torsemide consisted of 4,177 patients from 7 countries: China, India, Korea, Poland, Russia, Ukraine and the United States (Table 1). Of the 4,177 patients in the outcomes analysis cohort, 87% (n=3,620) received furosemide and 13% (n=557) received torsemide. Patients enrolled in the United States constituted nearly half of the overall torsemide cohort (N=265, 47.6%), where there was 11.4% use of torsemide compared to 88.6% use of furosemide.
Figure 1.
Study population.
Table 1.
Baseline characteristics of the study population by discharge diuretic.
| Characteristic | Furosemide (N=3620) | Torsemide (N=557) | P-Value |
|---|---|---|---|
| Age (years) | 65 (54–75) | 65 (54–74) | 0.34 |
| Women | 1255 (34.7%) | 179 (32.1%) | 0.24 |
| Race | <.001 | ||
| White | 1750 (48.3%) | 257 (46.1%) | |
| Black | 814 (22.5%) | 86 (15.4%) | |
| Asian | 1027 (28.4%) | 207 (37.2%) | |
| Other | 29 (0.8%) | 7 (1.3%) | |
| Country* | <.001 | ||
| China | 182 (90.6%) | 19 (9.4%) | |
| India | 730 (81.3%) | 168 (18.7%) | |
| Korea | 99 (84.6%) | 18 (15.4%) | |
| Poland | 224 (93.7%) | 15 (6.3%) | |
| Russia | 211 (86.8%) | 32 (13.2%) | |
| Ukraine | 117 (74.5%) | 40 (25.5%) | |
| United States | 2057 (88.6%) | 265 (11.4%) | |
| Heart failure history | |||
| HF hospitalization within prior year | 1476 (40.8%) | 239 (42.9%) | 0.35 |
| Ischemic etiology | 2228 (61.5%) | 38 (69.5%) | <.001 |
| Ejection fraction (%) | 30 (20–35) | 25 (20–35) | 0.025 |
| Ejection fraction ≥50% | 346 (9.6%) | 52 (9.3%) | 0.87 |
| NYHA Class | 0.12 | ||
| I | 77 (2.6%) | 8 (1.7%) | |
| II | 501 (17.0%) | 79 (16.6%) | |
| III | 1572 (53.5%) | 238 (50.0%) | |
| IV | 791 (26.9%) | 151 (31.7%) | |
| Past medical history | |||
| Hypertension | 2667 (73.7%) | 383 (68.8%) | 0.015 |
| Diabetes mellitus | 1537 (42.5%) | 271 (48.7%) | 0.006 |
| Hyperlipidemia | 1473 (40.7%) | 233 (41.8%) | 0.62 |
| Coronary artery disease | 2025 (56.0%) | 361 (64.8%) | <.001 |
| Myocardial infarction | 1267 (35.0%) | 223 (40.0%) | 0.021 |
| Atrial fibrillation or flutter | 1206 (33.3%) | 214 (38.4%) | 0.018 |
| Ventricular tachycardia | 340 (9.4%) | 73 (13.1%) | 0.006 |
| Cerebrovascular disease | 445 (12.3%) | 65 (11.7%) | 0.68 |
| Peripheral vascular disease | 393 (10.9%) | 65 (11.7%) | 0.57 |
| Chronic respiratory disease | 595 (16.4%) | 101 (18.1%) | 0.32 |
| Prior percutaneous coronary intervention | 604 (16.7%) | 101 (18.1%) | 0.40 |
| Prior coronary bypass | 643 (17.8%) | 129 (23.2%) | 0.002 |
| Qualifying Episode Symptoms | |||
| Dyspnea | 0.50 | ||
| At Rest | 2148 (59.3%) | 339/557 (60.9%) | |
| With Minimal Activity | 1472 (40.7%) | 218/557 (39.1%) | |
| Orthopnea | 2723 (75.3%) | 424/556 (76.3%) | 0.63 |
| Weight Gain | 2333 (64.6%) | 387/557 (69.5%) | 0.026 |
| Physical Examination | |||
| BMI (kg/m2) | 28 (24–33) | 28 (24–34) | 0.70 |
| Systolic BP (mmHg) | 125 (110–140) | 120 (110–131) | <.001 |
| Diastolic BP (mmHg) | 76 (68–85) | 72 (66–80) | <.001 |
| Heart rate (bpm) | 82 (72–95) | 82 (72–94) | 0.36 |
| Elevated JVP documented | 1968 (54.4%) | 340 (61.0%) | 0.003 |
| S3 gallop | 971 (26.8%) | 144 (25.9%) | 0.63 |
| Mitral regurgitation | 983 (27.2%) | 170 (30.5%) | 0.098 |
| Pulmonary edema | 3072 (84.9%) | 450 (80.9%) | 0.018 |
| Peripheral edema | 2641 (73.0%) | 431 (77.4%) | 0.028 |
| Laboratories and imaging | |||
| Sodium (mmol/L) | 139 (136–141) | 138 (135–141) | <.001 |
| Creatinine (mg/dL) | 1.2 (1.0–1.5) | 1.3 (1.1–1.6) | 0.002 |
| Blood urea nitrogen (mg/dL) | 23 (17–34) | 28 (19–39) | <.001 |
| Hemoglobin (g/dL) | 12.6 (11.3–13.9) | 12.5 (11.2–14.0) | 0.54 |
| NT-proBNP (pg/mL) | 4307 (2112–8770) | 5345 (2661–9315) | 0.006 |
| X-ray indicating pulmonary congestion | 2560 (78.1%) | 329 (70.0%) | <.001 |
| Baseline medications and devices | |||
| Furosemide-equivalent dose (mg) | 40 (40, 80) | 40 (20, 80) | 0.0013 |
| ACE-inhibitor or ARB | 2145 (59.3%) | 332 (59.6%) | 0.88 |
| Beta blocker | 2133 (58.9%) | 336 (60.3%) | 0.54 |
| Aldosterone antagonists | 969 (26.8%) | 199 (35.7%) | <.001 |
| Oral or Topical Nitrates | 843 (23.3%) | 170 (30.5%) | <.001 |
| Digoxin or Digitalis Glycoside | 973 (26.9%) | 195 (35.0%) | <.001 |
| Hydralazine | 311 (8.6%) | 55 (9.9%) | 0.32 |
| Implantable Cardioverter Defibrillator | 685 (18.9%) | 143 (25.7%) | <.001 |
| Biventricular pacemaker | 325 (9.0%) | 74 (13.3%) | 0.001 |
Values presented as median (IQR), mean ± SD or N (percentage); the denominator is noted when different from the overall number of individuals in the group.
For country, the data are presented as the number of patients (%) on each medication such that the row adds up to 100% for each country.
Abbreviations: NYHA indicates New York Heart Association; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; ACE, angiotensin converting enzyme and ARB, angiotensin receptor blocker.
The baseline patient characteristics by discharge diuretic are presented in Table 1. Patients receiving torsemide had more comorbidities than those receiving furosemide. For instance, torsemide-treated patients had more diabetes mellitus, and atrial and ventricular arrhythmias compared with furosemide-treated patients. The median ejection fraction (EF) was lower in those receiving torsemide. Ischemic etiology was more common in torsemide patients. Torsemide-treated patients had lower systolic blood pressure, and greater creatinine, BUN, and natriuretic peptide level. Patients discharged on torsemide more often received mineralocorticoid receptor antagonists (MRAs) and digoxin on admission compared with furosemide patients. They also had greater baseline use of implantable cardiac devices compared with furosemide-treated patients.
Factors associated with torsemide use at discharge as presented in Table 2. Country was the variable most strongly predictive of baseline torsemide use. Clinical factors associated with torsemide use were higher BUN, lower SBP, and JVD noted during the qualifying episode.
Table 2.
Variables associated with torsemide use at discharge.
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Country (reference=US) | <.001 | |
| China | 0.94 (0.57 – 1.56) | |
| Korea | 1.62 (0.92 – 2.84) | |
| Poland | 0.44 (0.23 – 0.86) | |
| Russia | 1.22 (0.80 – 1.85) | |
| Ukraine | 3.74 (2.48 – 5.63) | |
| India | 1.61 (1.28 – 2.03) | |
| BUN (per doubling) | 1.37 (1.21 – 1.55) | <.001 |
| Baseline systolic BP (per 10 mmHg increase) | 0.87 (0.83 – 0.92) | <.001 |
| Prior HF hospitalization | 1.15 (0.94 – 1.40) | 0.175 |
| Qualifying episode JVD | 1.29 (1.05 – 1.59) | 0.015 |
Abbreviations: BUN indicates blood urea nitrogen; BP, blood pressure; HF, heart failure; JVD, jugular venous distension.
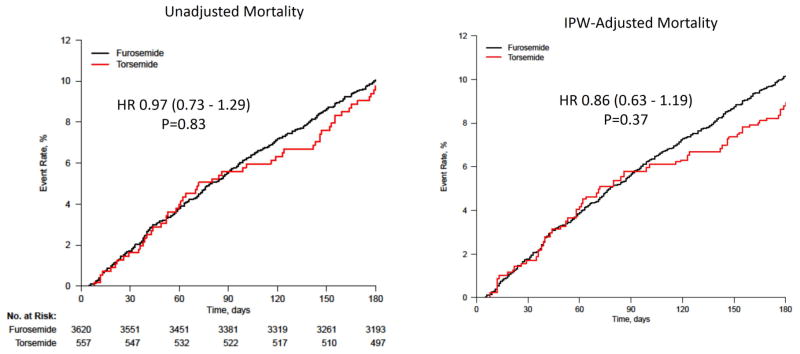
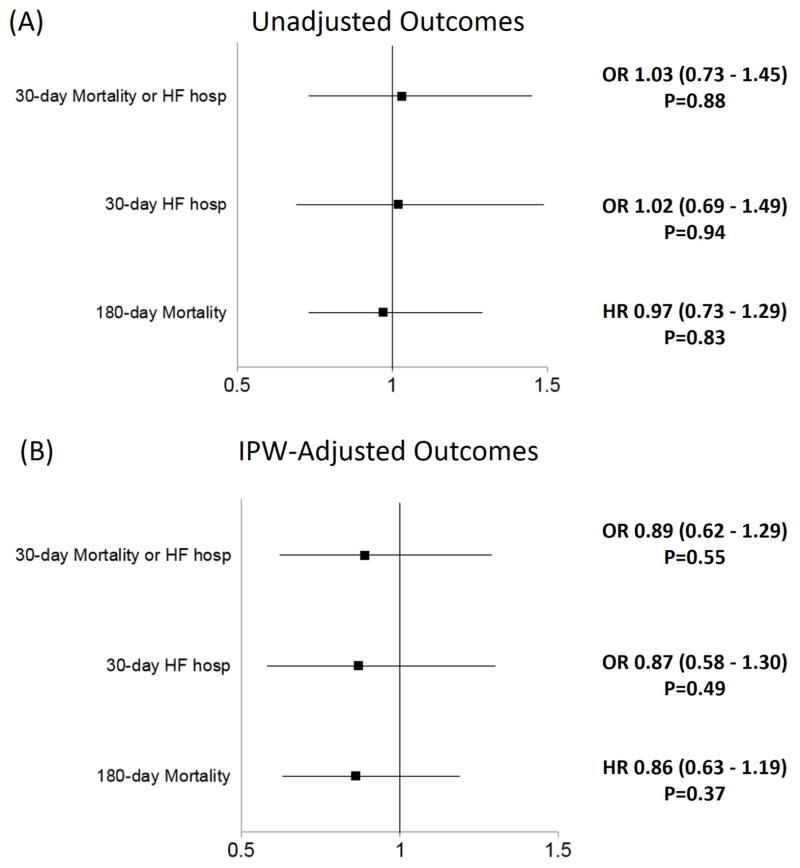
Table 3 presents the outcomes data in patients treated with furosemide or torsemide. Torsemide was associated with similar outcomes on unadjusted analysis [30-day mortality/HF hospitalization Odds Ratio (OR) 1.03, 95% Confidence Interval (CI): 0.73–1.45, P=0.88 and 180-day mortality Hazard Ratio (HR) 0.97, 95% CI: 0.73–1.29, P=0.83]. On IPW-adjusted analysis, torsemide use was associated with nominally lower 30-day mortality or HF hospitalization (OR 0.89, 95% CI: 0.62–1.29, P=0.55), 30-day mortality (OR 0.89, 95% CI: 0.40–1.97, P=0.78), 30-day HF hospitalization (OR 0.87; 95% CI: 0.58–1.30, P=0.49) and 180-day mortality (HR 0.86, 95% CI: 0.63–1.19; P=0.37) compared with furosemide. Figures 2 and 3 present the unadjusted and IPW-adjusted event rate curves and Forest Plots, respectively.
Table 3.
Unadjusted and Adjusted* Outcomes associated with torsemide use at discharge (reference=furosemide).
| Endpoint | Number of Events/Sample Size (%) | Odds Ratio or Hazard Ratio (95% Confidence Interval) | P-value | |
|---|---|---|---|---|
| Furosemide | Torsemide | |||
| 30-day Mortality or HF hospitalization | 262/3526 (7.4%) | 41/538 (7.6%) | ||
| Unadjusted | 1.03 (0.73 – 1.45) | 0.88 | ||
| Adjusted IPW | 0.89 (0.62 – 1.29) | 0.55 | ||
| Covariate Adjustment | 0.86 (0.60 – 1.24) | 0.43 | ||
| 30-day Mortality | 62/3613 (1.7%) | 9/556 (1.6%) | ||
| Unadjusted | 0.94 (0.47 – 1.91) | 0.87 | ||
| Adjusted IPW | 0.89 (0.40 – 1.97) | 0.78 | ||
| Covariate Adjustment | 0.78 (0.38 – 1.62) | 0.51 | ||
| 30-day HF hospitalization | 207/3525 (5.9%) | 32/537 (6.0%) | ||
| Unadjusted | 1.02 (0.69 – 1.49) | 0.94 | ||
| Adjusted IPW | 0.87 (0.58 – 1.30) | 0.49 | ||
| Covariate Adjustment | 0.87 (0.58 – 1.31) | 0.49 | ||
| 180-day Mortality | 360/3620 (9.9%) | 54/557 (9.7%) | ||
| Unadjusted | 0.97 (0.73 – 1.29) | 0.83 | ||
| Adjusted IPW | 0.86 (0.63 – 1.19) | 0.37 | ||
| Covariate Adjustment | 0.86 (0.64 – 1.15) | 0.30 | ||
Adjustment variables: country of randomization, age, hospitalization for heart failure within the prior year, qualifying episode of JVP, baseline systolic BP, baseline sodium, and BUN (log).
Abbreviations: HF indicated heart failure; IPW, inverse probability weighted.
Figure 2.
Unadjusted (A) and IPW adjusted (B) event rate curves by discharge loop diuretic*.
*Hazard Ratio (95% Confidence Interval) is for torsemide compared with furosemide.
Figure 3.
(A) Unadjusted and (B) IPW-Adjusted Outcomes associated with torsemide use at discharge (reference=furosemide).
DISCUSSION
In a large international acute HF trial, we found that furosemide was the primary loop diuretic used for volume management. There was significant regional variation in the use of other loop diuretics such as torsemide with no use in most countries and comparatively greater use in the Ukraine, Korea, Russia, India and the United States. Patients treated with torsemide tended to have features of more severe disease compared with furosemide-treated patients. After risk-adjustment, torsemide was associated with a non-significant reduction in 30 and 180-day events. These findings support prior data suggesting potential benefits with torsemide and extend the data to the contemporary population hospitalized with acute HF.
A major finding of our analysis was that torsemide-treated patients tended to have features of more severe disease. The clinical factors most strongly associated with torsemide use beyond geographic region were higher BUN, lower SBP, and JVD. These findings suggest that clinicians tend to use torsemide in the setting of refractory volume overload and renal dysfunction. The preferential use of torsemide in these circumstances may be related to torsemide’s increased bioavailability, longer half-life (particularly in the setting of renal dysfunction), and maintained absorption in the setting of oral intake and intestinal edema15. In fact, the median dose of furosemide equivalents was the same between groups. Importantly, the cause and effect relationship between variables associated with torsemide use is uncertain and requires further investigation. For instance, patients with lower SBP may have more advanced disease leading clinicians to preferentially select torsemide. Alternatively or in parallel, torsemide’s pharmacologic profile may lead to more potent diuresis with resultant lower SBP. Similar relationships may be observed with torsemide use and renal function. Torsemide-treated patients also more often received MRA therapy, which influences renal function, but could also be a marker of worse cardiac dysfunction and resultant underlying cardiorenal syndrome. Thus, it is only through prospective, randomized trials that these issues can be adequately assessed.
A primary finding of our analysis was that despite increased severity of disease in the torsemide-treated patients, they had similar outcomes compared to those treated with furosemide. Following risk adjustment, torsemide use was associated with a nominal reduction in 30-day and 180-day events. Interestingly, the adjusted event curves begin to diverge fairly late (i.e., after 90-days post-discharge). One possible explanation is that those patients with more advanced HF die during this early period and are unable to experience any potential beneficial effect of torsemide on underlying fibrosis. On the other hand, those patients who survive the early vulnerable period and have a longer exposure to torsemide may benefit from underlying anti-fibrotic effects16–21.
Previous studies suggested that torsemide may be associated with better outcomes than furosemide in chronic HF patients22. A small (N=234) open-label, randomized study by Murray et al compared chronic HF with reduced EF patients receiving torsemide vs. furosemide5. All-cause mortality at 12 months was lower with torsemide vs. furosemide (18 vs. 25 deaths), but the difference was not statistically significant (risk ratio 0.77, 95% CI: 0.45–1.33). The TOrasemide In Congestive HF Study investigated torsemide compared with furosemide/other loop diuretics in 1,377 chronic HF patients in an open-label, observational study6. Mortality through 12 months was significantly lower in the torsemide group (2.2% vs. 4.5%, P<0.005). These study results as well as another small (N=237) chronic HF study7 were the focus of a recent meta-analysis by Bikdeli et al, which suggested a non-significant trend toward reduced mortality with torsemide compared with furosemide (risk ratio 0.68, 95% CI: 0.39, 1.18)4. These prior studies were limited by smaller sample sizes and low event counts (101 total deaths). They also were limited to chronic HF patients and were published between 2001 and 2003. Thus, we provide the first contemporary data related to outcomes in HF patients treated with torsemide vs. furosemide following acute HF hospitalization. These data suggest potential benefits associated with torsemide use even in the context of current HF therapies. Importantly, these results should be recognized as hypothesis-generating given the observational nature of this analysis.
Pre-clinical and clinical data suggest potential mechanisms for improved outcomes with torsemide compared with furosemide as recently reviewed22. In brief, torsemide also has beneficial effects on aldosterone production, myocardial fibrosis, sympathetic activation, and ventricular remodeling16–21. Animal studies of torsemide demonstrated reduced aldosterone production and inhibition of aldosterone receptor binding that were not seen with furosemide17,18. Since aldosterone blockade is a primary treatment for reduced EF, these observations are highly relevant. In chronic HF patients, torsemide has been shown to decrease myocardial fibrosis as a result of reduced collagen synthesis and cross-linking, whereas furosemide has not20,23,24. Of note, none of the patients included in these earlier studies received MRAs. Nonetheless, data suggest that torsemide acts at a different level than MRAs to interfere with the myocardial fibrotic process25. Importantly, conflicting data have recently been presented suggesting that torsemide may not block aldosterone receptor binding26 such that co-administration of torsemide and an MRA may be warranted. A small randomized trial of torsemide vs. furosemide in patients with renal failure found that the diuretics had similar effects on fluid and sodium excretion, but torsemide led to less renin-angiotensin-aldosterone system activation16. A study by Yamato et al compared torsemide and furosemide therapy in 50 chronic HF patients27. At 6 months, study parameters were unchanged in the furosemide group, whereas the torsemide group had a smaller left ventricular (LV) diameter, improved LV filling parameters, and decreased natriuretic peptide levels. Additional benefits with torsemide over furosemide include less urinary potassium loss28,29 with the potential for reduced arrhythmia burden and improved compliance due to less frequent dosing, and decreased urinary urgency and micturition episodes7.
We did not find that torsemide use was associated with significantly improved outcomes. However, the point estimates for the adjusted association between torsemide and outcomes favored torsemide for each endpoint. The lack of a significant association may be due to type 2 error. Alternatively, the lack of a significant association may have been related, in part, due to the complex interaction of a number of factors that influence early post-discharge outcomes. For instance, HF rehospitalization rates are subject to significant regional variation30.
In sum, outside the context of a randomized trial, we do not believe these findings warrant a change in current clinical practice regarding loop diuretic selection. However, these data provide further rationale for such a trial and suggest potential clinical advantages to using torsemide in acute HF patients.
This was a retrospective analysis from a clinical trial. Given the significant regional variation in the use of torsemide, our sample size was reduced for the present analysis and results should be viewed as hypothesis-generating. We used adjustment covariates that have been used in prior ASCEND-HF analyses as one strategy to minimize bias via a consistent variable selection process. Despite IPW adjustment, other measured and unmeasured variables may have influenced these results. Nonetheless, it is interesting to note that the torsemide-treated patients tended to have features of more severe disease, and despite this, had similar outcomes on unadjusted analysis and a nominal reduction in events following adjustment with the ASCEND risk model variables. However, outside of the context of a randomized clinical trial, the effect of torsemide as compared with furosemide cannot be established. The analysis population was not a new-user design given the common use of both diuretics and a requirement for the care of symptomatic heart failure patients. Furthermore, data were not available regarding post-discharge diuretic changes (e.g., crossover between furosemide and torsemide). These data should be viewed as exploratory.
Highlights.
Furosemide is the most commonly used loop diuretic in heart failure (HF) patients despite data suggesting potential pharmacologic and anti-fibrotic benefits with torsemide.
In this large international acute HF trial, a minority of patients received torsemide and commonly had indicators of more severe disease.
After risk-adjustment, torsemide was associated with a non-significant reduction in 30 and 180-day events.
Acknowledgments
Funding: Dr. Mentz was supported by grant U10HL110312 from the National Institute of Health.
Footnotes
Disclosures: The authors report no relevant conflicts of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med. 1994;154:1905–1914. [PubMed] [Google Scholar]
- 2.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, Casey DE, Jr, McMurray JJ, Drazner MH, Mitchell JE, Fonarow GC, Peterson PN, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Bikdeli B, Strait KM, Dharmarajan K, Partovian C, Coca SG, Kim N, Li SX, Testani JM, Khan U, Krumholz HM. Dominance of furosemide for loop diuretic therapy in heart failure: time to revisit the alternatives? J Am Coll Cardiol. 2013;61:1549–1550. doi: 10.1016/j.jacc.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray MD, Deer MM, Ferguson JA, Dexter PR, Bennett SJ, Perkins SM, Smith FE, Lane KA, Adams LD, Tierney WM, Brater DC. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513–520. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 6.Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–513. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 7.Muller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV--efficacy and quality of life. Eur J Heart Fail. 2003;5:793–801. doi: 10.1016/s1388-9842(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 8.Dinicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol. 2012;8:707–728. doi: 10.2217/fca.12.54. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez AF, O’Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF) Am Heart J. 2009;157:271–277. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 11.Kaul P, Reed SD, Hernandez AF, Howlett JG, Ezekowitz JA, Li Y, Zheng Y, Rouleau JL, Starling RC, O’Connor CM, Califf RM, Armstrong PW. Differences in Treatment, Outcomes, and Quality of Life Among Patients With Heart Failure in Canada and the United States. JACC: Heart Fail. 2013;1:523–530. doi: 10.1016/j.jchf.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 13.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using Inverse Probability-Weighted Estimators in Comparative Effectiveness Analyses With Observational Databases. Medical Care. 2007;45:S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 16.Clasen W, Khartabil T, Imm S, Kindler J. Torasemide for diuretic treatment of advanced chronic renal failure. Arzneimittel-Forschung. 1988;38:209–211. [PubMed] [Google Scholar]
- 17.Uchida T, Yamanaga K, Nishikawa M, Ohtaki Y, Kido H, Watanabe M. Anti-aldosteronergic effect of torasemide. Eur J Pharmacol. 1991;205:145–150. doi: 10.1016/0014-2999(91)90812-5. [DOI] [PubMed] [Google Scholar]
- 18.Goodfriend TL, Ball DL, Oelkers W, Bahr V. Torsemide inhibits aldosterone secretion in vitro. Life Sci. 1998;63:PL45–50. doi: 10.1016/s0024-3205(98)00265-3. [DOI] [PubMed] [Google Scholar]
- 19.Harada K, Izawa H, Nishizawa T, Hirashiki A, Murase Y, Kobayashi M, Isobe S, Cheng XW, Noda A, Nagata K, Yokota M, Murohara T. Beneficial effects of torasemide on systolic wall stress and sympathetic nervous activity in asymptomatic or mildly symptomatic patients with heart failure: comparison with azosemide. J Cardiovasc Pharmacol. 2009;53:468–473. doi: 10.1097/FJC.0b013e3181a717f7. [DOI] [PubMed] [Google Scholar]
- 20.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–2035. doi: 10.1016/j.jacc.2003.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart. 2006;92:1434–1440. doi: 10.1136/hrt.2005.079764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buggey J, Mentz RJ, Pitt B, Eisenstein EL, Anstrom KJ, Velazquez EJ, O’Connor CM. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323–333. doi: 10.1016/j.ahj.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez B, Querejeta R, Gonzalez A, Beaumont J, Larman M, Diez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009;53:236–242. doi: 10.1161/HYPERTENSIONAHA.108.125278. [DOI] [PubMed] [Google Scholar]
- 24.Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–867. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 25.Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physio Heart Circ Physio. 2010;299:H1–9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 26.Gravez B, Tarjus A, Jimenez-Canino R, El Moghrabi S, Messaoudi S, Alvarez de la Rosa D, Jaisser F. The diuretic torasemide does not prevent aldosterone-mediated mineralocorticoid receptor activation in cardiomyocytes. PloS One. 2013;8:e73737. doi: 10.1371/journal.pone.0073737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamato M, Sasaki T, Honda K, Fukuda M, Akutagawa O, Okamoto M, Hayashi T. Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circ J. 2003;67:384–390. doi: 10.1253/circj.67.384. [DOI] [PubMed] [Google Scholar]
- 28.Broekhuysen J, Deger F, Douchamps J, Ducarne H, Herchuelz A. Torasemide, a new potent diuretic. Double-blind comparison with furosemide. Eur J Clin Pharmacol. 1986;31(Suppl):29–34. doi: 10.1007/BF00541464. [DOI] [PubMed] [Google Scholar]
- 29.Herchuelz A, Deger F, Douchamps J, Ducarne H, Broekhuysen J. Comparative pharmacodynamics of torasemide and furosemide in patients with oedema. Arzneimittel-Forschung. 1988;38:180–183. [PubMed] [Google Scholar]
- 30.Mentz RJ, Cotter G, Cleland JGF, Stevens SR, Chiswell K, Davison BA, Teerlink JR, Metra M, Voors AA, Grinfeld L, Ruda M, Mareev V, Lotan C, Bloomfield DM, Fiuzat M, Givertz MM, Ponikowski P, Massie BM, O’Connor CM. International differences in clinical characteristics, management, and outcomes in acute heart failure patients: better short-term outcomes in patients enrolled in Eastern Europe and Russia in the PROTECT trial. Eur J Heart Fail. 2014;16:614–624. doi: 10.1002/ejhf.92. [DOI] [PMC free article] [PubMed] [Google Scholar]