Abstract
Adults older than 65 years undergo more than 120,000 coronary artery bypass (CAB) procedures each year in the United States. Chronological age alone, though commonly used in prediction models of outcomes after CAB, does not alone reflect variability in aging process; thus, the risk of complications in older adults. We performed a prospective study to evaluate a relationship between senescence marker p16INK4a expression in peripheral blood T-lymphocytes (p16 levels in PBTLs) with aging and with perioperative outcomes in older CAB patients. We included 55 patients age 55 and older, who underwent CAB in Johns Hopkins Hospital between September 1st, 2010 and March 25th, 2013. Demographic, clinical and laboratory data following outline of the Society of Thoracic Surgeons data collection form was collected, and p16 mRNA levels in PBTLs were measured using Taqman® qRT-PCR. Associations between p16 mRNA levels in PBTLs with length of hospital stay, frailty status, p16 protein levels in the aortic and left internal mammary artery tissue, cerebral oxygen saturation, and augmentation index as a measure of vascular stiffness were measured using regression analyses. Length of hospital stay was the primary outcome of interest, and major organ morbidity, mortality, and discharge to a skilled nursing facility were secondary outcomes. In secondary analysis, we evaluated associations between p16 mRNA levels in PBTLs and interleukin-6 levels using regression analyses. Median age of enrolled patients was 63.5 years (range 56-81 years), they were predominantly male (74.55%), of Caucasian descent (85.45%). Median log2(p16 levels in PBTLs) were 4.71 (range 1.10-6.82). P16 levels in PBTLs were significantly associated with chronological age (mean difference 0.06 for each year increase in age, 95% CI 0.01-0.11) and interleukin 6 levels (mean difference 0.09 for each pg/ml increase in IL-6 levels, 95% CI 0.01-0.18). There were no significant associations with frailty status, augmentation index, cerebral oxygenation and p16 protein levels in blood vessels. Increasing p16 levels in PBTLs did not predict length of stay in the hospital (HR 1.10, 95% CI 0.87-1.40) or intensive care unit (HR 1.02, 95% CI 0.79-1.32). Additional evaluation of p16 levels in PBTLs as predictor of perioperative outcomes is required and should include additional markers of immune system aging as well as different outcomes after CAB in addition to length of hospital stay.
Keywords: coronary artery bypass, chronological age, aging biomarker, senescence, p16INK4a levels, peripheral blood T-lymphocytes, frailty, length of hospital stay
1. Introduction
Coronary artery bypass (CAB) is among the most commonly performed surgical procedures in older adults, involving over 120,000 patients older than 65 years in the United States alone (Go 2014) 1. Models which predict length of hospital and Intensive Care Unit (ICU) stay after cardiac surgery commonly incorporate chronological age (Janssen 2004, Ghotkar 2006, 1 Messaoudi 2009) 2-4. Unfortunately, chronological age does not accurately predict an individual's aging process and decline in physiologic reserve that can be accelerated or retarded in response to environmental exposures, host experiences, and genetics (Mitnitski 2002) 5(Fried 1998) 6; therefore, identification and utilization of ‘aging biomarkers’ that accurately measure patients’ physiologic reserve is an important clinical need.
Aging is characterized by a reduction in the regenerative capacity of many tissues, and the accumulation of senescent cells appears to broadly contribute to tissue aging (Wagers 2005, Janzen 2006, Sharpless 2007, Rodier 2011) 7-10. Cellular senescence is an irreversible growth arrest (Hayflick 1965) 11 that occurs in response to a variety of noxious stimuli (e.g., DNA damage, telomere shortening, oxidative stress, and epigenetic damage) (Wright 2002, Kirkwood 2005, Kuilman 2010) 12-14. Senescent cells do not replicate, which impairs tissue homeostasis (Drummond-Barbosa 2008) 15, and secrete pro-inflammatory cytokines (Coppe 2010) 16, associated with age-associated phenotypes such as sarcopenia, immune dysfunction and delayed wound healing (Ashcroft 2002) 17. Therefore, measuring the accumulation of senescent cells in vivo has been suggested to provide a means of measuring the ‘molecular age’ of the organism.
The p16INK4a senescence marker has been suggested to serve as a biomarker of aging and predictor of physiologic reserve (Dimri 2004, Sharpless 2007) 8,18. Expression of p16INK4a is not detected in young cells, but is potently activated by stress factors that promote cellular senescence (Brenner 1998, te Poele 2002, Kim 2006, Song 2010) 19-22. Senescent cells remain in tissues indefinitely; therefore, accumulation of p16INK4a expression reflects the accumulation of senescent cells with aging and as such, expression of p16INK4a is intrinsic to the aging process. Expression of the p16INK4a transcript is highly dynamic, increasing exponentially with chronologic age in all mammalian species tested to date (Zindy 1997, Melk 2003; Krishnamurthy 2004) 23-25. The ability of p16INK4a in human kidney allograft biopsies predicts kidney function based on creatinine level at 6 month and 1 year post-transplant and performs better than telomere length (McGlynn 2009; Koppelstaetter 2008; Gingell-Littlejohn 2013) 26-28. Interestingly, in humans, expression of p16INK4a in peripheral blood T-lymphocytes (PBTL) changes >10-fold (Liu 2009) 29 throughout lifespan. Additionally, p16INK4a expression in PBTL shows a much stronger correlation with chronologic age than do other aging biomarkers (Liu 2009) 29 (r2 0.6-0.7 for p16INK4a, r2<0.2 for leukocyte telomere length or IL-6). While the role of p16INK4a as a molecular age biomarker is becoming established, the clinical utility of measuring p16INK4a levels to predict clinically-relevant outcomes is not well described, though promising as demonstrated by previous work in kidney transplantation.
In this prospective pilot study of older adults undergoing CAB procedure, we asked two questions: can p16INK4a expression serve as a biomarker of aging in this patient population; and can p16INK4a levels predict poor clinical outcomes. We hypothesized that older adults have higher levels of p16INK4a, and that p16INK4a mRNA levels in PBTLs correlates with other markers of aging including frailty, p16 protein levels in vascular walls, cerebral oxygen saturation and measures of vascular stiffness. We further hypothesized that patients with higher p16INK4a mRNA levels in PBTLs have a slower recovery, and therefore are more likely to have increased length of hospital stay, compared to patients with lower p16INK4a mRNA levels in PBTLs.
2. Material and Methods
2.1. Institutional Review Board
The Johns Hopkins Medicine Institutional Review Board (IRB) approved the study NA_00032660.
2.2. Study Design and Setting
We have conducted a prospective study of older adults undergoing CAB procedure in urban, academic, tertiary care hospital (Johns Hopkins Hospital). All participants were recruited between September 1st, 2010 and March 25th, 2013 and followed for 30 days after their surgical procedure.
2.3. Subjects
We have included all patients 55 years of age and older undergoing primary elective or urgent CAB surgery. We have excluded patients requiring emergency or salvage CAB surgery, being reoperated, undergoing combined, aortic or valvular surgical procedures, primary ventricular assist device implantation, having any acute illness other than coronary artery disease, or requiring preoperative inotropic or vasoactive medications. We adjusted our inclusion/ exclusion criteria after initial slow recruitment rate as follows: minimal age of participants was decreased from 65 to 55, and exclusions such as ejection fraction less than 40% and participation in other research protocols were removed.
All patients scheduled to undergo CAB surgical procedure were identified through the operating room schedule. We then screened electronic medical records of all identified patients for inclusion/ exclusion criteria. Eligible patients were approached by the research personnel either in the pre-operative clinic or at the bedside in the hospital at least one day prior to the scheduled surgical procedure. The patient's surgical attending physician was notified of the patient's enrollment into the study. All study participants provided informed consent on their participation in the study.
2.4. Study Procedures
Upon enrollment we collected the following participant's information: demographic characteristics of age, gender, race; anthropomorphic characteristics of weight, height and body mass index (BMI); smoking, exercise and alcohol consumption; organ system diseases through self-report and chart review; current medications; functional capacity including basic and instrumental activities of daily living (ADLs (Katz 1970) 30 and IADLs (Lawton 1969) 31), ability to drive; laboratory data reflecting major organ system function (white cell count, hemoglobin level, platelets, blood urea nitrogen, creatinine, albumin, alkaline phosphatase, total bilirubin and calcium levels, international normalized ratio), heart rate and myocardial ejection fraction. Comorbidities burden was summarized by Charlson index (Charlson 1987) 32We then performed following measurements: frailty assessment, vascular stiffness, p16INK4a levels in PBTLs and vascular wall, interleukin 6 (IL-6) levels in serum, and ScO2.
2.4.1. Frailty Assessment
Frailty assessment was performed pre-operatively following methodology previously described (Fried 1998) 33. Briefly, frailty was measured using a previously validated scoring system evaluating 5 domains: (1) shrinking defined as unintentional weight loss of 10 pounds or more in the last year; (2) weakness determined by a grip-strength test and adjusted for gender and body mass index (BMI); (3) exhaustion as measured by two questions from the modified 10-item Center for Epidemiological Studies-Depression scale; (4) low physical activity as measured by a version of the Minnesota Leisure Time Activities Questionnaire (Taylor 1978) 34; and (5) slowed walking speed as measured by averaging three trials of walking 15 feet at a normal pace. Each domain yielded a dichotomous score of 0 or 1. Patients were categorized based on their total score into frail (total score 3-5), prefrail (total score 1-2), and nonfrail (total score 0).
2.4.2. Vascular Stiffness
Vascular stiffness was assessed preoperatively by measuring pulse pressure (PP) based on oscillometric blood pressure measurement averaged over 3 repeated measurements and augmentation index (AI) obtained non-invasively by applanation tonometry (SPT-301, Millar, Inc., Houston, TX) from the radial artery. AI was calculated using a software package (SphygmoCor, Atcor Medical, West Ryde, NSW, Australia) to perform arterial waveform analysis, and expressed in %.
2.4.3. p16INK4a mRNA Expression Levels in PBTLs
We collected venous blood for p16INK4a expression in PBTLs either during the pre-operative visit to the clinic concomitant with routine blood draw or intraoperatively after induction of general anesthesia prior to surgical incision. Blood processing occurred in two steps. The 1st step was performed in the OAIC (Older Americans Independence Center) Molecular Genetics Core laboratory. Enriched T-cells, pelleted cells for future DNA analysis and plasma were obtained by cell sorting using the RosettSep™ kit (STEMCELL Technologies, Inc., Vancouver, BC, Canada) during this step. For complete description of the procedure please see Appendix 6.1. All fractions were stored frozen. Specimens were shipped later in bulk to the reference laboratory at the University of North Carolina, Chapel Hill, NC. RNA was prepared from PBTLs and expression of p16INK4a mRNA transcripts was determined using Taqman® qRT-PCR as per reference laboratory protocol 29. Briefly, total RNA was isolated using RNeasy Mini kit (QIAGEN, Hilden, Germany), mRNA was reverse transcribed into cDNA using ImProm-II reverse transcription system (Promega Corp., Madison, WI). Expression of p16INK4a was measured by real time qPCR using custom TaqMan® primers and normalized to two housekeeping genes. T cell isolation was assessed by measuring expression of CD3 receptor. In all cases, Ct values ≥37 were considered background.
2.4.4. IL-6 Levels
IL-6 levels in serum were analyzed in the OAIC Molecular Genetics Core laboratory by human IL-6 immunoassay (High Sensitivity Quantikine ELISA kit, R&D Systems, Minneapolis, MN).
2.4.5. ScO2
The baseline value of ScO2 while on room air was collected in the operating room as soon as INVOS™ cerebral oximeter (Somanetics Corp., Troy, MI) was placed on the patient's forehead. The lowest value between the right and left sides was recorded at this time.
2.4.6. Vascular Wall p16INK4a Protein Levels
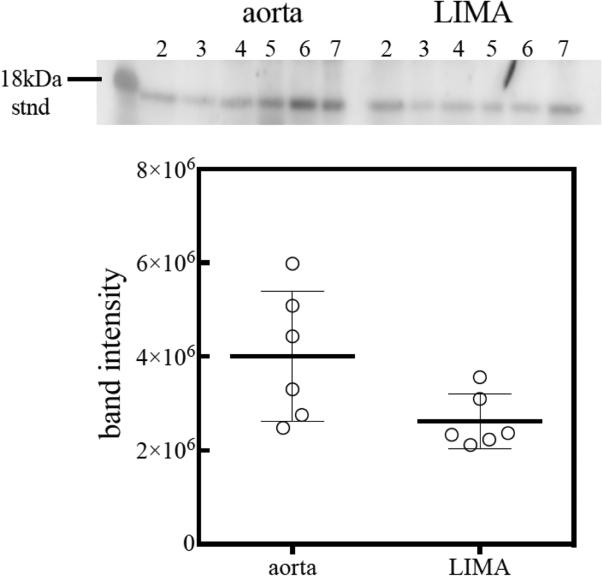
In patients requiring left internal mammary artery (LIMA) for revascularization, the portion of the artery discarded during trimming of the vessel was collected for p16INK4a quantification in the vessel wall. Aortic punches, removed and discarded during anastomoses of the venous grafts to the aorta, were also collected for p16INK4a quantification in the aortic wall. The OAIC Molecular Genetics Core laboratory analyzed p16INK4a protein levels in the vascular wall by Western blotting following previously described method (Werner 2009) 35. Briefly, flash frozen tissues were homogenized in Tris/Glycerol lysis buffer containing 4% SDS and protease inhibitors. Soluble fraction was resolved on SDS-PAGE gel and transferred to nitrocellulose membrane. Following the transfer of proteins to the nitrocellulose, the membrane was blocked for two hours in SuperBlock blocking reagent (Thermo Scientific, Inc.). Two hour primary and secondary antibody incubations were performed in 10% SuperBlock in Tris buffered saline containing 0.05% Tween-20. The dilution of the p16 (F-12) primary antibody (Santa Cruz Biotechnology, Inc., Dallas, TX) was 1:250 while a 1:10,000 secondary antibody (goat anti-mouse IgG conjugated to HRP) was employed. Chemiluminescent signal was developed using SuperSignal West Dura reagents following the manufacturer's protocol (Thermo Fisher Scientific, Inc., Waltham, MA). The bands were digitally captured using a Gel Logic 220 Imaging System (CareStream Health, Inc., Rochester, NY). Band intensity was quantified using NIH Image version 1.46, where background levels were subtracted from band signals using integrated adjacent pixels. The representative blot is demonstrated in Figure 1. For complete description of methodology please see Appendix 6.2.
Figure 1.
Western Blot analysis of p16 protein levels in aortic tissue (labeled as aorta) and left internal mammary artery (labeled as LIMA). The internal standard is marked at 18 kDa and corresponds to recombinant human protein p16, Novus Biologicals, LLC (Littleton CO); the lines under corresponding numbers represent p16 protein, with darker lines corresponding to higher levels of p16 protein. Expression of p16 protein in the aortic tissue is substantially higher compared to LIMA.
2.5. Patient Care
Anesthesia, surgical procedures, intensive care unit (ICU) and hospital care of the patients were conducted according to current hospital protocols. We had no specific requirements as to the conduct of anesthesia, surgical procedure and postoperative care. Briefly, intraoperative care was conducted as follows: all patients received general anesthesia and were placed on cardiopulmonary bypass (CPB) for CAB procedure. Induction of anesthesia was performed with a combination of midazolam and fentanyl; vecuronium was given for muscle relaxation. Isoflurane was used for anesthesia maintenance during the case. Mechanical ventilation goal was to maintain normocapnia during surgery. Heparin (350 IU/kg) was used for anticoagulation, which was monitored by the kaolin-activated clotting time and maintained at a level of >480 seconds during CPB. The extracorporeal circuit consisted of roller pumps (Terumo, Tokyo, Japan), a hollow fiber membrane oxygenator (Sorin, Milan, Italy), and a standard arterial line filter (Sorin, Milan, Italy). The priming consisted of 1600 mL of lactated Ringer's solution and a retrograde autologous prime of 700 mL. CPB pump flow was adjusted to 1.8-2.2 L/m2/min. Nasopharyngeal temperature during CPB was maintained at 32°C, followed by rewarming to a urinary bladder temperature >=35°C. After the termination of CPB, heparin was neutralized by protamine. Propofol infusion was initiated at the end of the case and used for sedation during transport and in ICU until extubation.
2.6. Outcome Measures
Information about intraoperative course, major morbidities and mortality, length of ICU and hospital stay were collected following Society of Thoracic Surgeons (STS) adult cardiac surgery database worksheet outline 36. Patients were followed for the duration of hospital stay; if they were discharged prior to day 30 they received a follow-up phone call on day 30. The hospital follow-up was provided by daily evaluation of patients’ electronic medical records and interviews with care providers whenever insufficient documentation was present. Our primary outcome of interest was time to hospital discharge. We also followed patients for secondary outcomes: time to ICU discharge, hospital and 30-day all cause mortality, neurologic, pulmonary, cardiac, renal and composite major morbidity, functional recovery expressed as time to extubation, time to out-of-bed and need for physical therapy, need for reoperation, infection, blood use in perioperative period, and mesenteric and acute limb ischemia. We additionally collected data on discharge disposition. All data were entered in a database developed by using online tool ASP.NET (Microsoft Corp., Redmond, WA), and maintained using SQL Server (Microsoft Corp., Redmond, WA) by the Division of Geriatric Medicine and Gerontology in the Department of Medicine, Johns Hopkins School of Medicine.
2.7. Power Calculation
For the purpose of power calculation we arbitrarily assumed low versus high levels of log2(p16INK4a mRNA in PBTLs) as being separated at the median, since we did not know what the precise values of p16INK4a in PBTLs would be in our population. For the primary outcome of total hospital length of stay, assuming alpha=0.05, power=80%, and a 20% increase in length of stay, 29 patients would be needed in each group, i.e. those who have low vs. high levels of p16 INK4a in PBTLs. This was based on a theoretical and conservative estimate, with a length of stay of 7 days in the group with low levels of p16 INK4a in PBTLs (S.D.=2 days) versus the group with high levels of p16 INK4a in PBTLs, with a length of stay of 8.5 days (20% greater, S.D.=2 days). Ultimately our primary analyses included 47 patients on whom p16INK4a mRNA in PBTLs were successfully measured: for this sample size, a 24% increase in length of stay is detectable with power=80%.
2.8. Statistical Analyses
We performed exploratory data analyses by constructing stem and leaf plots for continuous variables, and frequency tabulation for categorical variables. We then created a scatter plot matrix for all continuous variables and side-by-side box plots of the p16INK4a levels in PBTLs by all the categorical variables. P16INK4a level in PBTLs were log2(p16INK4a)-transformed. We then conducted a linear regression of a) p16INK4a protein levels in LIMA and b) aorta, c) vascular stiffness as expressed by AI, and d) ScO2 on p16INK4a mRNA levels in PBTLs (each separately), and a multinomial logistic regression of frailty status on p16INK4a mRNA levels in PBTLs, as well as these analyses adjusted for age. For the age-adjusted analyses, we evaluated adjusted variable plots for the main predictors to ensure that that the findings were consistent with the data. In secondary analyses we conducted a linear regression of IL-6 levels on p16INK4a mRNA levels in PBTLs and performed multivariable regression based on previously described associations of p16INK4a mRNA levels in PBTLs with chronologic age, smoking, exercise and IL-6 levels as covariates29. Finally, we conducted a Cox regression of length of hospital stay (days) and ICU stay (hours) on p16INK4a levels in PBTLs, adjusting for chronologic age, frailty status and Charlson comorbidity index. For length of hospital stay (days) and ICU stay (hours) we additionally performed sensitivity analyses: by categorizing p16INK4a levels in PBTLs into 2 groups – less than 4.71 (median level) and equal or greater than 4.71 relative levels; by censoring the longest hospital discharge day at 15 days and ICU discharge hour at 122 hours (to assess influence of the outlying points).
3. Results
3.1. Study Flow
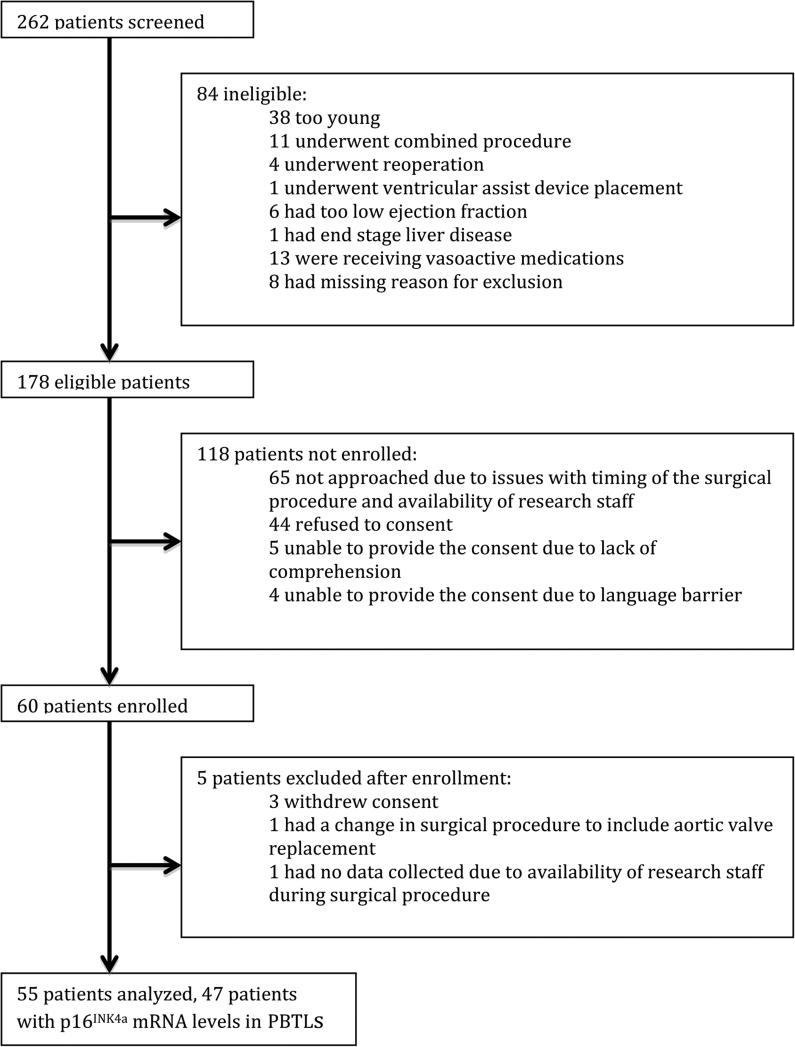
262 patients were screened for inclusion into the study, 178 were qualified to participate based on initial screening, 60 patients were enrolled, and 55 patients were analyzed (figure 1). Among 55 patients, 47 patients had completed analysis of p16INK4a levels in PBTLs. 2 patients were lost to 30 days follow-up. 45 out of 55 CAB procedures were performed by the same surgeon.
3.2. Participants
Baseline patient characteristics, clinical and laboratory data as well as length of stay in the ICU and hospital postoperatively are presented in Table 1. The median age of participants was 63.5 years, with range 56-81 years. They were predominantly male (74.55%) of Caucasian origin (85.45%), representative of CAB patient population (Go 2014) 1. The median p16INK4a mRNA level in PBTLs was 4.71 (range 1.10-6.82). The levels of p16 protein in LIMA were significantly lower than in aorta as reflected by ratio of p16 protein in LIMA/ p16 protein in aorta 0.49 (range 0.21-1.05), p<0.0001 for the difference. Median IL-6 levels were 2.66 (range 0.59-18.48) pg/ml. 10 (18.18%) of patients were not frail, 28 (50.91%) were pre-frail and 17 (30.91%) were frail. Median duration of hospital stay was 6 (range 3-27) days, and median duration of ICU stay was 33 (range 7-174) hours.
Table 1.
Characteristics of Analyzed Patients Undergoing Coronary Artery Bypass Surgery during the Study Period
| Variable | Value* |
|---|---|
| Age, in years | 63.5 (56-81) |
| Gender, n (%) | |
| Males | 41 (74.55) |
| Females | 14 (25.45) |
| Race, n (%) | |
| Caucasian | 47 (85.45) |
| African-American | 5 (9.09) |
| Other | 3 (5.46) |
| P16 mRNA levels in PBTLs (log2-transformed) | 4.71 (1.10-6.82) |
| Baseline P16 mRNA levels in PBTLs, n (%) | |
| < 4.71 log2-transformed p16INK4a levels | 23 (41.82) |
| ≥ 4.71 log2-transformed p16INK4a levels | 24 (43.64) |
| Missing | 8 (14.55) |
| Baseline p16 protein level in LIMA/ p16 protein level in aorta | 0.49 (0.26-1.05) |
| IL-6 levels, in pg/ml | 2.66 (0.59-18.48) |
| Cerebral oximetry, in % | 63.5 (39-80) |
| Augmentation index, in % | 26 (5-79) |
| Charlson comorbidity index | 2 (0-12) |
| Myocardial infarction, n (%) | 12 (21.82) |
| Congestive heart failure, n (%) | 2 (3.64) |
| Chronic pulmonary disease, n (%) | 9 (16.36) |
| Diabetes mellitus, n (%) | 19 (34.55) |
| Moderate or severe renal disease, n (%) | 3 (5.45) |
| Baseline Frailty, n (%) | |
| Non-frail | 10 (18.18) |
| Prefrail | 28 (50.91) |
| Frail | 17 (30.91) |
| Smoking, n (%) | |
| ≤ 5 pack-years | 24 (43.64) |
| > 5 pack-years | 31 (56.36) |
| Exercise, n (%) | |
| ≤ 240 minutes/ month | 32 (58.18) |
| > 240 minutes/ month | 23 (41.82) |
| Duration of hospital stay, days | 6 (3-27) |
| Duration of ICU stay, hours | 33 (7-174) |
Continuous variables are presented as median (range), and categorical variables are presented as number of participants (%)
Abbreviations: PBTLs Peripheral Blood T-Lymphocytes; LIMA Left Internal Mammary Artery; IL-6 Interleukin 6; ICU Intensive Care Unit
3.3. Baseline expression of p16INK4a mRNA levels in PBTLs and aging
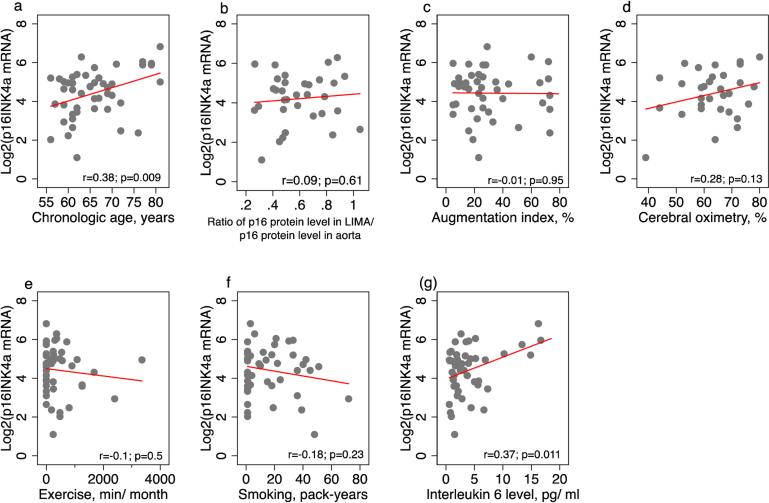
To test our hypothesis that p16INK4a mRNA levels in PBTLs is an aging biomarker in this patient population, we explored association of p16INK4a mRNA levels in PBTLs with markers of aging identified for a priori analyses (chronologic age, p16 protein level in LIMA/ p16 protein level in aorta ratio, AI and ScO2) and secondary analyses (interleukin 6, smoking and exercise). In a priori analyses expression of p16 in PBTL was associated with chronological age only (for every year increase in age p16INK4a mRNA levels in PBTLs increase by 0.07, 95% CI 0.02-0.12, p<0.05) and was not associated with p16 protein level in LIMA/ p16 protein level in aorta ratio, AI and ScO2. In secondary analyses p16INK4a mRNA levels in PBTLs were associated with IL-6 protein levels (for every pg/ml increase in IL-6 protein level p16INK4a mRNA levels in PBTLs increase by 0.11, 95% CI 0.03-0.20, p<0.05 on univariate analysis, and increase by 0.09, 95% CI 0.01-0.18, p<0.05 on multivariable analysis). P16INK4a mRNA levels in PBTLs were not associated with smoking or exercise. Univariate and multivariate analyses data are summarized in Table 2.
Table 2.
Results of linear regression analyses of p16INK4a mRNA levels in PBTLs on other markers of age
| Predictors | Univariate analyses, estimate (95% CI) | *Multivariable analyses, estimate (95% CI) |
|---|---|---|
| A priori analyses | ||
| Chronologic age, years | 0.07 (0.02-0.12)** | - |
| P16 protein level in LIMA/ p16 protein level in aorta | 0.56 (-1.64-2.77) | 0.31 (−1.87-2.49) |
| Augmentation index, % | 0.00 (−0.02-0.02) | −0.01 (−0.02-0.01) |
| Cerebral oximetry, % | 0.03 (−0.01-0.08) | 0.04 (0.00-0.07) |
| Secondary analyses | ||
| Chronologic age, years | 0.07 (0.02-0.12)** | 0.06 (0.01-0.11)** |
| Interleukin 6 levels, pg/ml | 0.11 (0.03-0.20)** | 0.09 (0.01-0.18)** |
| Exercise | ||
| > 240 min/month | REF | - |
| ≤ 240 min/month | 0.01 (−0.74-0.76) | 0.11 (−0.58-0.79) |
| Smoking | ||
| ≤ 5 pack-years | REF | - |
| > 5 pack-years | 0.06 (−0.68-0.80) | 0.01 (−0.67-0.68) |
In a priori multivariable analyses adjustments were made for chronologic age; in secondary multivariable analyses variables included interleukin 6 levels, exercise, smoking and chronologic age
Indicates significant results based on evaluation of CI
Abbreviations: CI, Confidence Interval; LIMA, Left Internal Mammary Artery; REF, reference value
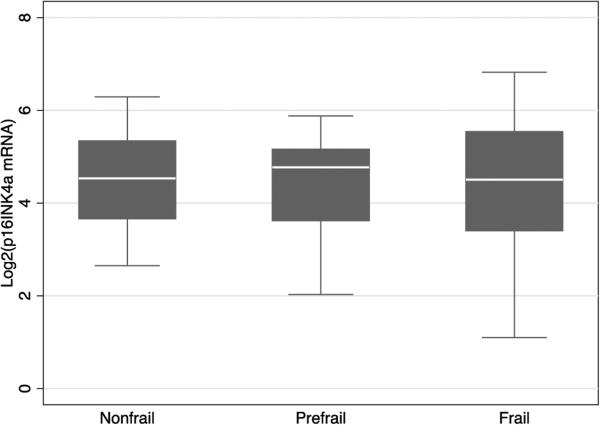
3.4. P16INK4a mRNA levels in PBTLs and frailty
There were no significant associations between p16INK4a mRNA levels in PBTLs and frailty (Figure 3). The lack of association between p16INK4a mRNA levels in PBTLs and frailty remained after adjustment for chronological age (RRR 1.06, 95% CI 0.53-2.12, p=0.87 when comparing pre-frail to non frail; RRR 0.78, 95% CI 0.38-1.60, p=0.51 when comparing frail to non frail) (Table 3).
Figure 3.
Association of p16INK4a mRNA levels in PBTLs (peripheral blood T-lymphocytes) with other markers of aging: (a) Chronologic age; (b) Ratio of p16 protein level in LIMA (left internal mammary artery) versus p16 protein level in aorta; (c) Augmentation index; (d) Cerebral oximetry; (e) Exercise intensity; (f) Smoking; (g) Interleukin 6 levels. Dots indicate individual data, red line is a line from univariate linear regression of p16INK4a mRNA levels in PBTLs on variables mentioned in (a)-(f). Figures (a)-(d) reflect a priori analyses, figures (e)-(g) reflect secondary analyses. Correlation coefficients (r) and p-values are provided for each marker of aging in association with p16INK4a mRNA levels in PBTLs; p-value<0.05 was considered significant.
Table 3.
Frailty status according to p16 levels in PBTLs
| Phenotype | *Univariate analysis, RRR (95% CI) | **Multivariable analysis, RRR (95% CI) |
|---|---|---|
| Nonfrail | REF | REF |
| Prefrail | 0.97 (0.52-1.80) | 1.06 (0.53-2.12) |
| Frail | 0.92 (0.48-1.76) | 0.78 (0.38-1.60) |
Multinomial regression was performed, results are reported as RRR (95% CI)
Adjustments were made for chronologic age
Abbreviations: RRR, Relative Risk Ratio; CI, Confidence Interval; REF, Reference value
3.5. P16INK4a mRNA levels in PBTLs and length of hospital and ICU stay
Median length of stay in the hospital was 6 days with the range 3-27 days, and median length of stay in the ICU was 33 hours with the range 7-174 hours. There was no association between p16INK4a mRNA levels in PBTLs and length of hospital stay (HR 1.10 for each one unit increase in p16INK4a mRNA levels in PBTLs, 95% CI 0.87-1.40, p=0.49), or length of ICU stay (HR 0.98 for each unit increase in p16INK4a mRNA levels in PBTLs, 95% CI 0.74-1.28, p=0.87) as further described in table 4.
Table 4.
Duration of hospital and ICU length of stay according to increasing p16 levels in PBTLs
| *Univariate analysis, HR (95% CI) | **Multivariable analysis, HR (95% CI) | |
|---|---|---|
| Duration of stay in the hospital | 1.07 (0.87-1.32) | 1.10 (0.87-1.40) |
| Duration of stay in ICU | 1.13 (0.90-1.43) | 0.98 (0.74-1.28) |
Cox proportional hazards regression was performed, with results reported as HR (95% CI) of discharge from the hospital or ICU
Adjustments were made for chronologic age, frailty status and Charlson comorbidity index
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; ICU, Intensive Care Unit
3.6. Sensitivity analyses
When we categorized patients according to their p16INK4a mRNA levels in PBTLs into 2 groups based on median levels – less then 4.71 (low level of p16INK4a mRNA levels in PBTLs) and equal or greater than 4.71 relative levels (p16INK4a mRNA levels in PBTLs), the inference has not changed for length of hospital stay (HR 1.39, 95% CI 0.72-2.68, p=0.33) or length of ICU stay (HR 0.76, 95% CI 0.37-1.56, p=0.46) comparing group with high level of p16INK4a mRNA levels in PBTLs to a group with low levels p16INK4a mRNA levels in PBTLs.
When we censored the longest hospital discharge day at 15 days and ICU discharge hour at 122 hours (to assess influence of the outlying points), the inference has not changed either for hospital length of stay (HR 1.20, 95% CI 0.92-1.54, p=0.18) or ICU length of stay (HR 0.86, 95% CI 0.64-1.15, p=0.31).
4. Discussion
Our study is the first one to evaluate clinical utility of p16INK4a mRNA levels in PBTLs in cardiac surgical patient population. It showed that p16INK4a mRNA levels in PBTLs in patients undergoing CAB are associated with chronologic age. P16INK4a mRNA levels in PBTLs did not associate with frailty score and with p16 protein levels in blood vessels, vascular stiffness expressed by augmentation index, ScO2. In secondary analyses, p16INK4a mRNA levels in PBTLs were associated with serum levels of IL-6, a marker of aging and frailty (Taaffe 2000, Cesari 2004, Ferrucci 2005, Maggio 2006, Singh 2011) 37-41Finally, there were no association between p16INK4a mRNA levels in PBTLs and postoperative length of stay in the hospital or ICU after CAB in older adults.
Our results are consistent with previous report showing association of p16INK4a mRNA levels in PBTLs with chronologic age and IL-6 levels 29; however, we were unable to demonstrate association with smoking or exercise history in our patient population. Our results are different from previous reports of using pretransplant p16INK4a mRNA levels from donor kidney biopsies to predict clinical outcomes (McGlynn 2009; Koppelstaetter 2008; Gingell-Littlejohn 2013) 26-28. In kidney transplantation, p16INK4a mRNA levels turned to be one of the best predictors of kidney function at 1 year. Importantly, telomere length performed not as well compared to p16INK4a mRNA levels, emphasizing that p16INK4a mRNA levels in pretransplant kidney biopsies is a better predictor of outcomes. Compared to these reports, we evaluated p16INK4a mRNA levels in PBTLs, focusing rather on the entire organism; thus, evaluating more complex clinical outcomes such as length of stay in the hospital and in ICU. The lack of apparent associations of p16INK4a mRNA levels in PBTLs with frailty, vascular stiffness and p16 protein levels in blood vessels and duration of stay in the hospital and ICU in our study could be partially explained by insufficient power to detect differences in our patient population, as well by very complex physiology of aging and influence of multiple factors on p16INK4a mRNA levels in PBTLs, which are not entirely established. Furthermore, medical management of acute coronary artery disease limits measuring frailty in this patient population, when patients are prescribed strict bed rest. Even though our sample size provided adequate power to detect moderately extended length of stay among persons with higher p16INK4a mRNA levels, the direction of the association of p16INK4a mRNA levels in PBTLs with length of hospital stay was the opposite to the expected (8.1±4.8 days for the group with low p16INK4a mRNA levels in PBTLs versus 6.8±2.8 days for the group with high p16INK4a mRNA levels in PBTLs). More importantly, this pilot study achieved our main goals: it confirmed that we can successfully recruit patients with based on our inclusion/ exclusion criteria, confirmed that they would consent to an aging biomarker study, learned how to isolate T-cells successfully, learned what p16INK4a in PBTLs will be like in this population (since they are not healthy donors and we did not know how p16 INK4a in PBTLs is affected in this population).
Of note, in this report, a 70-year-old patient has on average a p16INK4a mRNA levels in PBTLs of ~4; while p16 level in PBTLs for the same age individual was on average ~7 in the original report 29. The difference in reported values is because of a different equation that was used to convert Ct values into reported log2 p16INK4a mRNA levels in PBTLs in this report and the values are otherwise consistent with previous studies (Sharpless, personal communications).
We described differential expression of p16 protein in aorta and LIMA arterial vessels. We observed significantly higher levels of p16 in aortic tissue compared to LIMA. This result could reflect different rate of aging across different arterial vascular beds, and could explain why results of coronary revascularization with LIMA are successful. When taken together with p16INK4a mRNA levels in PBTLs, these results highlight heterogeneity of senescence across tissues. The main limitation of our methodology is that we measured p16 protein in the entire vascular tissue, and we are unable to tell whether endothelium or smooth muscle is responsible for the reported result. Interpretation of findings is further complicated by lack of control group without CAD. This is justifiable, however, due to invasiveness of vascular tissue collection, whereas CAB provides with the unique opportunity to harvest vascular tissue. Whether differential levels of senescence in vascular beds play role in vascular pathology associated with aging deserves additional investigation.
Our study has several limitations. First, limited number of patients undergoing CAB procedure compared to the entire population of older adults may threaten generalizability of our findings. However, the design of our study evaluating performance of p16INK4a mRNA levels in PBTLs using baseline measurements prior to surgical insult is robust to represent older adults with the burden of CAD. Second, we focused only on one biomarker of senescence, p16INK4a mRNA levels in PBTLs, which reflects telomere-independent pathway of senescence in lymphocytes, as due to insufficient number of viable PBTLs in stored samples we were unable to process our samples for telomere length. Evaluation of telomere length and telomerase activity in T-lymphocytes, as well as changes in cellular composition of the immune system with aging would allow more comprehensive picture of the immune system involvement in determining outcomes of surgical procedures. Third, our study has low power to detect difference between groups with levels of p16INK4a mRNA levels in PBTLs above and below the median. Finally, we evaluated length of hospital stay as the primary outcome of interest. It is a complex outcome, which depends on multiple clinical variables as well as administrative decisions, and future investigation will need to focus on outcomes more directly related to function of the immune system in perioperative period: infectious complications, deep venous thrombosis, and renal failure.
5. Conclusions
Although p16INK4a mRNA levels in PBTLs were significantly correlated with chronologic age in older adults undergoing CAB, these levels did not predict any adverse outcomes in this population. In addition, p16INK4a mRNA levels in PBTLs did not correlate with p16 levels in LIMA or in aorta tissue within the same individual, possibly suggesting a tissue specific difference in rate of senescence. Given this, evaluation of p16INK4a mRNA levels in PBTLs and other tissues as a predictor of outcomes after surgical procedures may warrant further investigation in conjunction with other markers of senescence.
Supplementary Material
Figure 2.
Study Flow. The consort diagram for the study shows number of evaluable patients at each step. 262 patients were screened for study participation, 178 were identified as eligible based on inclusion/exclusion criteria, 60 patients consented and 55 were analyzed. Results from the baseline p16INK4a mRNA levels in PBTLs (peripheral blood T-lymphocytes) were not available for 8 out of 55 patients. These 8 patients were excluded from the paired analysis of p16 expression and other aging markers or hospital and ICU (Intensive Care Unit) stay.
Figure 4.
Box and whiskers plot of p16INK4a mRNA levels in PBTLs among nonfrail, prefrail and frail older adults undergoing CAB (coronary artery bypass). The box demonstrates the median value and interquartile range, whereas whiskers demonstrate upper and lower limits of p16INK4a mRNA levels in PBTLs (peripheral blood T-lymphocytes) among groups within calculated fences.
Highlights.
CAB is a commonly performed surgical procedure in older adults
First prospective study of senescence marker p16INK4a in patients undergoing coronary artery bypass (CAB)
Preoperative mRNA levels of p16INK4a in peripheral blood T-lymphocytes (PBTL) correlate with chronological age and interleukin-6 levels in older adults with coronary artery disease
p16INK4a mRNA levels in PBTLs are not associated with frailty, cerebral oxygen saturation, aortic and left internal mammary artery p16INK4a protein levels, and augmentation index
p16INK4a mRNA levels in PBTLs do not predict duration of hospital or ICU stay after CAB
Acknowledgements
We would like to acknowledge efforts of Michelle A. Parish, BSN, Mala Gurbani, DO and Karen Miller, MD in enrolling participants and collecting data throughout the study; Jack Yang, MS in processing blood samples; and Neal S. Fedarko, PhD in processing vascular tissue. We would also like to thank Long He for creating and maintaining database for the study and Jing Tian for her assistance with biostatistical analysis of data. Finally, we would like to acknowledge Natalia Mitin, PhD for her thoughtful review of the manuscript.
Funding:
This work was supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, National Institute on Aging, NIA P30AG021334.
The funding sources had no involvement in the study design or in the collection, analysis and interpretation of data. The funding sources also had no involvement in the writing of this report or in the decision to submit the report for publication.
Abbreviations
- AI
Augmentation index
- BMI
Body mass index
- CAB
Coronary artery bypass
- CAD
Coronary artery disease
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- IL-6
Interleukin 6
- ICU
Intensive Care Unit
- LIMA
Left internal mammary artery
- OAIC
Older Americans Independence Center
- P16 levels in PBTLs
p16INK4a levels in peripheral blood T-lymphocytes
- RNA
ribonucleic acid
- ScO2
Cerebral oxygen saturation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
N. E. Sharpless is a co-inventor on a University of North Carolina–owned patent related to this work (US PCT/US2005/034542 “Determination of Molecular Age by Detection of INK4a/ARF Expression”).
Bibliography
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen DP, Noyez L, Wouters C, Brouwer RM. Preoperative prediction of prolonged stay in the intensive care unit for coronary bypass surgery. Eur J Cardiothorac Surg. 2004;25(2):203–207. doi: 10.1016/j.ejcts.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Ghotkar SV, Grayson AD, Fabri BM, Dihmis WC, Pullan DM. Preoperative calculation of risk for prolonged intensive care unit stay following coronary artery bypass grafting. J Cardiothorac Surg. 2006;1:14. doi: 10.1186/1749-8090-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messaoudi N, De Cocker J, Stockman BA, Bossaert LL, Rodrigus IE. Is EuroSCORE useful in the prediction of extended intensive care unit stay after cardiac surgery? Eur J Cardiothorac Surg. 2009 doi: 10.1016/j.ejcts.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The cardiovascular health study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 7.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myogenesis. Cell. 2005;122(5):659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 9.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443(7110):421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 10.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HAYFLICK L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 12.Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20(7):682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond-Barbosa D. Stem cells, their niches and the systemic environment: An aging network. Genetics. 2008;180(4):1787–1797. doi: 10.1534/genetics.108.098244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3(6):337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 18.Dimri GP. The search for biomarkers of aging: Next stop INK4a/ARF locus. Sci Aging Knowledge Environ. 2004;2004(44):pe40. doi: 10.1126/sageke.2004.44.pe40. [DOI] [PubMed] [Google Scholar]
- 19.Brenner AJ, Stampfer MR, Aldaz CM. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene. 1998;17(2):199–205. doi: 10.1038/sj.onc.1201919. [DOI] [PubMed] [Google Scholar]
- 20.te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62(6):1876–1883. [PubMed] [Google Scholar]
- 21.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127(2):265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Song Z, von Figura G, Liu Y, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9(4):607–615. doi: 10.1111/j.1474-9726.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15(2):203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 24.Melk A, Kittikowit W, Sandhu I, et al. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63(6):2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGlynn LM, Stevenson K, Lamb K, et al. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell. 2009;8(1):45–51. doi: 10.1111/j.1474-9726.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 27.Koppelstaetter C, Schratzberger G, Perco P, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell. 2008;7(4):491–497. doi: 10.1111/j.1474-9726.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- 28.Gingell-Littlejohn M, McGuinness D, McGlynn LM, et al. Pre-transplant CDKN2A expression in kidney biopsies predicts renal function and is a future component of donor scoring criteria. PLoS One. 2013;8(7):e68133. doi: 10.1371/journal.pone.0068133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 31.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 35.Werner C, Furster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 36.Society of Thoracic Surgeons [02/15, 2011];Adult cardiac surgery database data collection form version 2.61. http://www.sts.org/documents/pdf/AdultCV2.61DCF.pdf.
- 37.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55(12):M709–15. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 38.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 39.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.