Figure 5. Evolution of the binding interface and hinge during the evolution of AncGKPID spindle orientation functions.
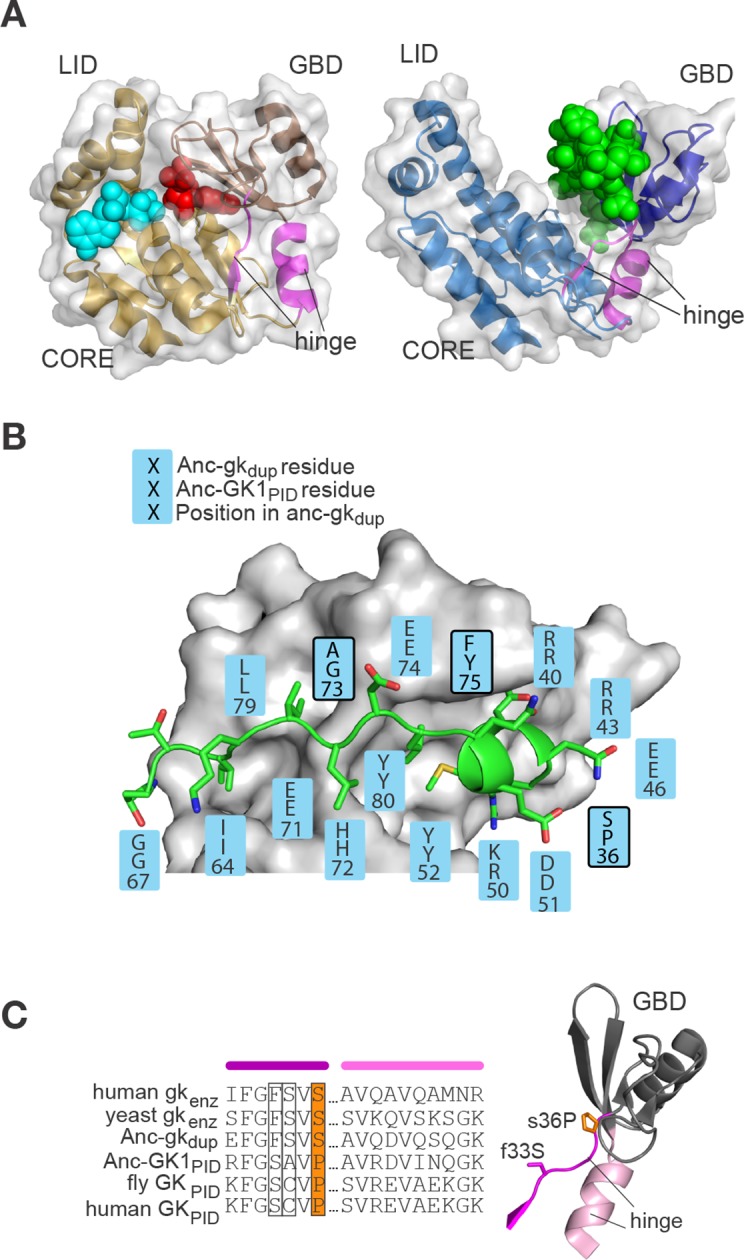
(A) All gk/GKPID family members share a common structural architecture, comprising a catalytic core, two binding lobes (the GMP-binding domain, GBD, shown in dark hue, and the ATP-binding lid), and a flexible hinge region, which connects the GBD to the core and comprises two segments of contiguous residues (magenta). Left: in gk enzymes bound to GMP (red spheres), the lobes adopt a closed conformation, bringing GMP and ATP (cyan spheres) adjacent to each other in the core. Right: the GKPID has an open conformation, in which Pins (green spheres) binds to the surface of the GBD in the cleft between the two binding lobes. Structures shown are mouse gk enzyme (brown, PDB 1LVG) and the GKPID from rat Dlg1 (blue, 3UAT). (B) Most residues in Anc-GK1PID that bind Pins (blue boxes) are unchanged from the ancestral state in Anc-gkdup. White surface, D. melanogaster Dlg GK1PID (3TVT). Green, Pins peptide. Ancestral and derived amino acid states are shown; residues with historical amino acid replacements between the two ancestral proteins are outlined. (C) In the hinge region, two historical substitutions (outlined and colored, with side-chains shown as sticks) were conserved in the ancestral state in extant enzymes and in a different state in extant GKPIDs. Colored bars above the sequence indicate position in the protein structure (right). Hinge segments are shown in pink and the GMP-binding lobe in gray.
