Abstract
Aim
Borderline personality disorder (BPD) is characterized by self-regulation deficits, including impulsivity and affective lability. Transference-Focused Psychotherapy (TFP) is an evidence-based treatment proven to reduce symptoms across multiple cognitive-emotional domains in BPD. This pilot study aims to investigate neural activation associated with, and predictive of, clinical improvement in emotional and behavioral regulation in BPD following TFP.
Methods
BPD subjects (N=10) were scanned pre- and post-TFP treatment using a within-subjects design. A disorder-specific emotional-linguistic go/no-go fMRI paradigm was used to probe the interaction between negative emotional processing and inhibitory control.
Results
Analyses demonstrated significant treatment-related effects with relative increased dorsal prefrontal (dorsal anterior cingulate, dorsolateral prefrontal, and frontopolar cortices) activation, and relative decreased ventrolateral prefrontal cortex and hippocampal activation following treatment. Clinical improvement in constraint correlated positively with relative increased left dorsal anterior cingulate cortex activation. Clinical improvement in affective lability correlated positively with left posterior-medial orbitofrontal cortex/ventral striatum activation, and negatively with right amygdala/parahippocampal activation. Post-treatment improvements in constraint were predicted by pre-treatment right dorsal anterior cingulate cortex hypoactivation, and pre-treatment left posterior-medial orbitofrontal cortex/ventral striatum hypoactivation predicted improvements in affective lability.
Conclusions
These preliminary findings demonstrate potential TFP-associated alterations in frontolimbic circuitry and begin to identify neural mechanisms associated with a psychodynamically-oriented psychotherapy.
Keywords: anterior cingulate cortex, borderline personality disorder, fMRI, orbitofrontal cortex, transference-focused psychotherapy
Introduction
Borderline personality disorder (BPD) is a mental illness characterized by self-regulation and interpersonal difficulties. This inability to self-regulate is manifested by rapid mood alterations and intense emotional/behavioral responses including impulsivity, aggression, and parasuicidal behaviors1,2. The mainstay of treatment is psychotherapy, while psychopharmacologic interventions have yielded mixed results. The prevalence of BPD is approximately 1.4%, and this condition utilizes disproportionally high rates of psychiatric and medical resources. Despite these statistics, both the neurobiology and treatment of BPD have received less investigative attention than other psychiatric conditions with similar morbidity. While neural substrates of symptom expression in BPD have been investigated, the mechanisms mediating symptom improvement following psychotherapy remain poorly characterized, and few studies have investigated neural changes associated with psychodynamic psychotherapy in any population.
Functional magnetic resonance imaging (fMRI) studies probing emotional processing in BPD have identified reduced top-down regulatory prefrontal cortex (PFC) and enhanced amygdala activity. Several studies in BPD demonstrated reduced anterior cingulate cortex (ACC), frontopolar cortex (FPC), and orbitofrontal cortex (OFC) activation in conjunction with increased amygdalar activation during negative emotional processing, suggesting decreased monitoring and regulation, as well as increased reactivity in the context of negative emotional stimuli. For example, BPD patients displayed reduced FPC, subgenual and rostral ACC activation in response to fearful facial emotions3, and failed dorsal and rostral ACC activation during a negatively valenced emotional word Stroop task4. Reduced OFC activation during script-driven imagery of self-injurious behavior5 and attempted emotional re-appraisal6 has also been reported. In addition to PFC dysfunction, increased amygdala activation during negatively valenced picture-viewing and fear-based tasks has been characterized in BPD3,7–10. Impaired amygdalar habituation11 and aberrant ACC-amygdala, OFC-amygdala and subgenual ACC-dorsal ACC functional connectivity have also been demonstrated in BPD10,12. Studies of BPD have also characterized behavioral response-inhibition deficits during emotionally neutral tasks13,14, impairments in cognitive control associated with decreased constraint15, and enhanced recall of salient, negatively valenced emotional information16.
Our group previously designed an emotional linguistic go/no-go fMRI study to probe the clinically salient interaction of negative affective processing and inhibitory control in BPD and healthy subjects17,18. In healthy subjects, inhibitory control in the context of negative emotional processing selectively activated the posterior-medial OFC, dorsal ACC, dorsolateral prefrontal cortex (dlPFC), amygdala and hippocampus. When comparing BPD with healthy subjects, frontolimbic dysfunction was identified in the posterior-medial OFC and the dorsal and subgenual ACC. Specific deficits in self-reported restraint of impulsive behavior correlated with decreased posterior-medial OFC activation, while negative emotion correlated with increased extended amygdala/ventral striatum activation. Recently, use of a go/no-go task following anger induction identified reduced inferior frontal cortex activation in BPD compared to controls during motor inhibition19.
Transference-Focused Psychotherapy (TFP) is an evidence-based treatment for BPD, developed by Kernberg and colleagues, that relies on techniques of clarification, confrontation, and interpretation of affect-laden themes that emerge within the transference relationship20. In a randomized, blinded one-year study, TFP reduced impulsivity, anger, irritability and suicidality, and demonstrated greater multi-symptom improvement compared to dialectical behavioral therapy (DBT) and supportive psychotherapy21. Importantly, unlike the two comparison therapies, TFP significantly reduced impulsivity. This pilot study used a within-subjects design to investigate changes in frontolimbic neural activation during the interaction of inhibitory control and negative emotional processing in BPD patients treated with TFP. Longitudinal changes in neural activation and predictors of treatment response were investigated, emphasizing a dimensional approach to study neural activity associated with symptom improvement in the clinically important domains of constraint, affective lability and aggression. We hypothesized decreased amygdalar activation and increased medial PFC activation associated with TFP-related clinical improvement, and also that baseline neural activation patterns in these regions would predict treatment response.
Methods
2.1. Participants
10 women with BPD (9 right-handed; mean age=27.8 years, range=23–32 years) were recruited from the New-York Presbyterian Hospital/Weill Cornell Medical College–Westchester Division (Supplemental Table 1). BPD diagnoses were confirmed with the International Personality Disorder Examination22 (criteria score range=5–9, dimensional score range=10–18; mean=15.00, SD=2.45). Other current diagnoses as measured by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders included panic disorder (N=1), social phobia (N=1), specific phobia (N=1), generalized anxiety disorder (N=3), alcohol abuse (N=2), and cannabis abuse (N=2). Past diagnoses included major depressive disorder (N=6), obsessive-compulsive disorder (N=1), and alcohol dependence (N=2). On the International Personality Disorder Examination, other categorical diagnoses included histrionic (N=3), avoidant (N=1), and narcissistic (N=2) personality disorders.
Five patients reported psychotropic medication during study participation (Supplemental Table 1). Written informed consent was obtained and the protocol was approved by the institutional review board of New York Presbyterian Hospital/Weill Cornell Medical College. Subject recruitment, assessments, TFP treatments, and fMRI scan acquisitions were performed at New York Presbyterian Hospital/Weill Cornell Medical College. Data analyses and manuscript preparation were approved by the Partners Human Research Committee.
Following initial assessment and pre-treatment scanning, patients participated in TFP (average number of sessions attended=76.60, SD=8.28). TFP consisted of twice weekly individual, 50-minute sessions supervised by Otto F. Kernberg M.D. and Frank Yeomans M.D., Ph.D. All therapists had advanced degrees in social work, psychology or psychiatry, with at least two years of prior experience treating BPD patients. Weekly supervision on all cases was provided for the five therapists. Rating of adherence and competence were made by the supervisors on the TFP Adherence and Competence Rating Scale23. Interrater reliability between two raters was high (intraclass correlation coefficient (ICC)=0.96). All participants received the Multidimensional Personality Questionnaire (MPQ)24, the Affective Lability Scale (ALS)25 and the Overt Aggression Scale-Modified (OAS-M)26 prior to TFP and at follow-up scanning. The MPQ was used to relate the clinically relevant factor of constraint to functional neuroimaging results. A high level of constraint reflects tendencies to inhibit and restrain impulse expression. The ALS is a 54-item self-report instrument where subjects rate the tendency of their mood to shift between normal to affectively charged domains of anger, depression, elation and anxiety, as well as their tendency to shift between depression and elation and between depression and anxiety. OAS-M is a clinician-rated scale that characterizes aggressive behavior within the past week based on observation and self-report.
2.2 fMRI task
Participants underwent pre-treatment and post-treatment scanning (average scan interval=12.1 months; range=10–14 months) while they performed an emotional linguistic go/no-go task17, with verbal stimuli containing themes salient for BPD (Supplemental Figure 1). Participants were instructed to perform a right-index-finger button-press immediately after (silently) reading a word appearing in normal font (go trial) and to inhibit this response after reading a word in italicized font (no-go trial). Button-press responses and reaction times were recorded. Following scanning, participants performed word recognition and valence rating tasks.
2.3. fMRI data acquisition, image processing and analysis
Imaging data were acquired pre- and post-TFP with a GE Signa 3Tesla MRI scanner (Supplemental Methods). The fMRI imaging data processing procedures were performed using customized Statistical Parametric Mapping software, and a two-level voxel-wise linear random-effects model was utilized to examine the effect sizes of the key Group/Condition contrasts in a three-way repeated-measures ANCOVA setting (Supplemental Methods). Based on a priori hypotheses derived from our prior studies18 as well as theoretical considerations17–19, Regions-of-Interest (ROIs) were the bilateral posterior-medial OFC, ACC and amygdala. Based on previous differential activation in BPD vs. healthy subjects18, planned Contrasts-of-Interest (COI) probing motor inhibitory control during negative versus neutral emotional processing were selected. COIs were examined (1) as a function of treatment [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (nogo vs. go)]; and (2) as predictors of treatment response [pre-treatment scan: (negative vs. neutral) × (nogo vs. go)] via correlations with TFP-related changes in MPQ-constraint, ALS-total and OAS-M. The statistical significance of the group-level comparison/interaction was assessed based on Gaussian Random Field theory as implemented in SPM. The group-level t-statistic map of a COI was initially thresholded at a voxel-wise p-value < 0.01 and a spatial extent > ¼ cc. For a ROI, the predicted peaks were considered statistically significant if their initial voxel-wise p-value was <0.001 and family-wise-error-rate (FWE) corrected p-value was < 0.05 over a sphere with a radius=6.2mm which resulted in a search volume of 1 cc.
Results
3.1. Behavioral and treatment results
There were no statistically significant treatment-related effects with in-scanner task performance as measured by reaction times and commission/omission errors. Likewise, there were no statistically significant treatment-related effects on valence ratings and word recognition. Although not powered specifically to measure TFP-related clinical changes, statistically significant improvements were found post- vs. pre-TFP in ALS-total (p=0.038; pretreatment mean 48.30± 11.97; post-treatment mean 40.50±11.28 ) and OAS-M (p=0.011; pretreatment mean 13.92±10.28; post-treatment score 6.67±3.91) using repeated measures analysis of variance (ANOVA). Change in MPQ-constraint clinical scores was not statistically significant (p=0.324; pretreatment mean 64.20±13.82; post-treatment mean 68.40±11.63).
3.2. Neuroimaging results
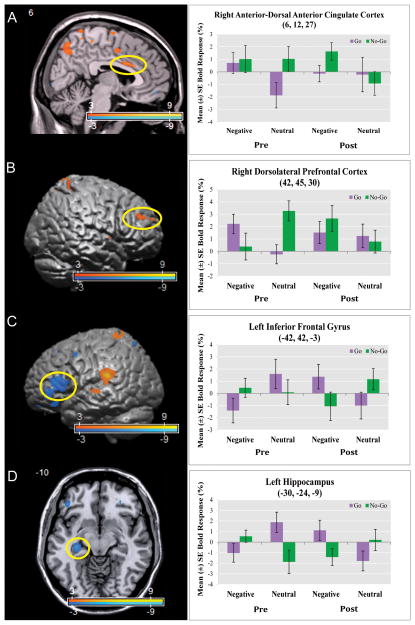
The COI probing the neural substrates of the interaction of negative (versus neutral) emotional processing and behavioral inhibition as a function of longitudinal TFP treatment was the three-way interaction term: [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (no-go vs. go)]. In comparison to pre-treatment scans, BPD patients showed relative increased activation in cognitive control regions including right anterior-dorsal ACC, dlPFC and FPC. Relative activation decreases were found in left ventrolateral PFC (vlPFC) [inferior frontal gyrus (pars orbitalis and triangularis)] and hippocampus. See Figure 1 and Supplemental Table 2 and 3.
Figure 1. Increased Dorsal Anterior Cingulate and Dorsolateral Prefrontal Cortex Activation and Decreased Inferior Frontal Gyrus and Hippocampus Activation during Behavioral Inhibition in the Context of Negative Emotional Processing Post- vs. Pre-Transference-Focused Psychotherapy.
Panels A–D depict the interaction [(post-treatment vs. pre-treatment) × (negative vs. neutral) × (no-go vs. go)] (Supplementary Table 2 and 3). Statistical parametric maps are thresholded at a voxelwise p-value of 0.01. Following treatment with Transference Focused Psychotherapy (TFP), borderline personality disorder patients demonstrated relative increased activation in the (Panel A) right anterior-dorsal anterior cingulate cortex (voxel-wise p-value=0.001; corrected p-value=0.022) and the (Panel B) right dorsolateral prefrontal cortex (voxel-wise p-value=0.001); relative activation decreases following treatment were noted in the (Panel C) left inferior frontal gyrus (voxel-wise p-value < 0.001) and the (Panel D) left hippocampus (voxel-wise p-value = 0.001).
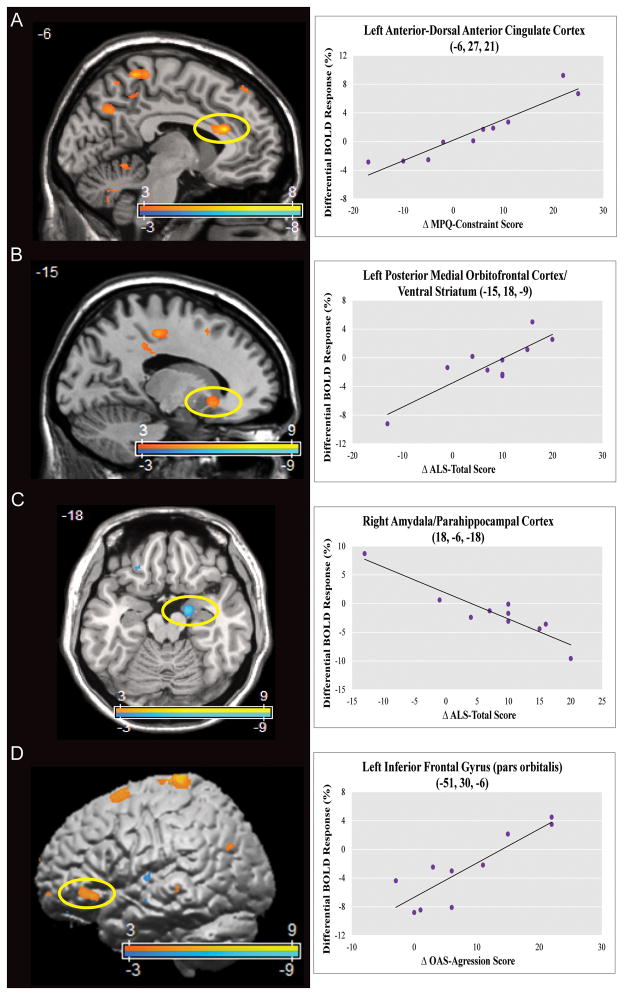
Correlational analyses assessed the association between clinical improvement in domains of interest following treatment and changes in neural activity during behavioral inhibition in the context of negative versus neutral emotional processing. With the three-way interaction contrast [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (no-go vs. go)], improvements in MPQ-constraint scores correlated positively with left anterior-dorsal ACC activation. Improvements in ALS-total correlated positively with left posterior-medial OFC/ventral striatum activation, and negatively with right amygdala/parahippocampal cortex activation. See Figure 2 and Supplemental Table 2 and 4.
Figure 2. Dorsal Anterior Cingulate, Posterior-Medial Orbitofrontal, Amygdala, and Inferior Frontal Gyrus Activation Changes Post- vs. Pre-Transference-Focused Psychotherapy Correlated With Clinical Improvement.
Panels A–D depict correlational analyses of post vs. pre-treatment related effects on constraint, affective lability, and aggression for the interaction [(post-treatment vs. pre-treatment) × (negative vs. neutral) × (no-go vs. go)] (Supplementary Table 2 and 4). Statistical parametric maps are thresholded at a voxelwise p-value of 0.01. Panel A shows a positive correlation between improvements in Multidimensional Personality Questionnaire (MPQ) – Constraint score and relative increased activation in the left anterior-dorsal anterior cingulate cortex (voxel-wise p-value < 0.001, corrected p-value=0.002). Panel B shows a positive correlation between improvements in Affective Lability Scale (ALS) – Total score and relative increased activation in the left posterior-medial orbitofrontal cortex/ventral striatum (voxel-wise p-value=0.001, corrected p-value=0.028). Panel C shows a negative correlation between improvements in ALS-Total score and relative decreased activation in the right amygdala/parahippocampal cortex (voxel-wise p-value < 0.001, corrected p-value=0.005). Panel D shows a positive correlation between improvements in Overt Aggression Scale-Modified (OAS-M) aggression score and relative increased activation in the left inferior frontal gyrus (voxel-wise p-value=0.001). X-axes formatted so that increasing values reflect clinical improvement.
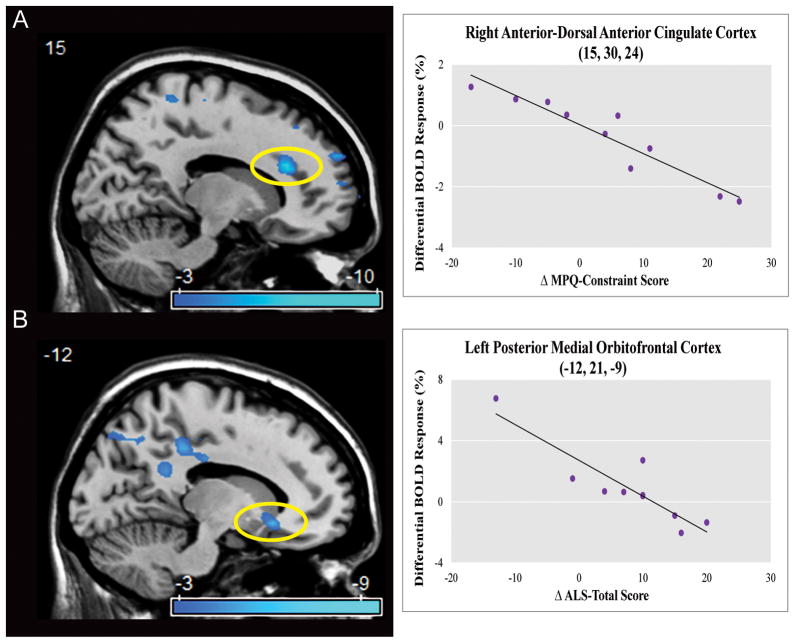
Neural predictors of treatment response were examined by correlating pre-treatment neural activation to changes in clinical scores using the two-way contrast: [pre-treatment scan: (negative vs. neutral) × (no-go vs. go)]. Improvements in MPQ-constraint negatively correlated with pre-treatment right anterior-dorsal ACC activation. Improvement in ALS-total negatively correlated with left posterior-medial OFC/ventral striatum activation. See Figure 3 and Supplemental Table 2 and 4.
Figure 3. Pre-Treatment Dorsal Anterior Cingulate and Posterior Medial Orbitofrontal Activation Negatively Correlated With Clinical Improvement.<.
br>Panels A–B depict correlational analyses of pre-treatment related effects on constraint and affective lability for the interaction [pre-treatment: (negative vs. neutral) × (no-go vs. go)] (Supplementary Table 2 and 4). Statistical parametric maps are thresholded at a voxelwise p-value of 0.01. Panel A shows an inverse correlation between pre-treatment activation in the right anterior-dorsal anterior cingulate cortex and post-treatment improvements in Multidimensional Personality Questionnaire (MPQ) – Constraint score (voxel-wise p-value < 0.001, corrected p-value=0.002). Panel B shows an inverse correlation between pre-treatment activation in the left posterior-medial orbitofrontal cortex/ventral striatum and post-treatment improvements in Affective Lability Scale (ALS) – Total score (voxel-wise p-value < 0.001, corrected p-value=0.013). X-axes formatted so that increasing values reflect clinical improvement.
Discussion
This initial study examined changes in frontolimbic neural activity associated with TFP treatment in BPD patients while probing behavioral inhibition in the context of negative emotional processing. Based on our previously published neuroimaging findings in healthy17 and BPD subjects18, along with psychological and neurobiological models of BPD27,28, we hypothesized treatment-related changes in prefrontal and limbic regions as neural mechanisms associated with TFP-mediated clinical improvement.
Treatment with TFP was associated with relative activation increases in emotional and cognitive control areas and relative decreases in areas associated with emotional reactivity and semantic-based memory retrieval. These findings suggest that TFP may potentially facilitate symptom improvement in BPD, in part, by improving cognitive-emotional control via increased dorsal ACC, posterior-medial OFC, frontopolar, and dlPFC engagement. Baseline ACC dysfunction has been characterized in BPD across a number of affectively valenced paradigms3,4,9,10,12,18,19. The subgenual, perigenual and anterior-dorsal ACC subregions are heavily interconnected with limbic regions including the amygdala and hippocampus, while the anterior and posterior dorsal ACC subregions are interconnected to lateral prefrontal and premotor regions involved in higher-order executive and behavioral functions29. Based on structural connectivity, the anterior-dorsal ACC may be conceptualized as a critical node for the convergence of emotional regulation, cognitive control and behavioral expression. The anterior-dorsal ACC and dlPFC have been described to have regulatory efferent connections to the amygdala. TFP treatment was associated with relative increases in dorsal ACC and dlPFC activation following treatment. Post vs. pre-treatment anterior-dorsal ACC activation correlated positively with improvements in constraint, while reduced pre-treatment anterior-dorsal ACC activation predicted clinical improvement in constraint. The association between clinical improvement, low pre-treatment, and relatively elevated post-treatment anterior-dorsal ACC activation suggests that TFP may potentially modulate neural activity in this region to improve behavioral restraint.
Enhanced post vs. pre-treatment and blunted pre-treatment posterior-medial OFC activation positively correlated with improvement in affective lability. The medial OFC is implicated in emotion and value-based decision making, behavioral flexibility and choice maintenance, with medial/lateral functional distinctions based on anatomical connectivity suggesting that the medial OFC (and its ventral striatum connections) subserves behavioral responses in the context of viscerosomatic function, while lateral OFC mediates more sensory-based evaluations30. The posterior-medial OFC is particularly implicated in emotion regulation given its ACC, lateral PFC, amygdala, and hypothalamic connections.
In addition to modulation of medial PFC, TFP-associated amygdalar effects were also observed. Improvements in affective lability inversely correlated with post vs. pre-treatment right amygdala activation during behavioral inhibition in the context of negative emotional processing. The amygdala is critical for negative emotion and fear expression, salience, and emotional memory. Consistent with models of emotion and behavioral regulation, response to TFP was associated with increased dorsal ACC and posterior-medial OFC activation, along with reduced amygdala activation. These frontolimbic activation patterns suggest that BPD patients were potentially able to engage in task demands with reduced negative emotional interference post-treatment.
Apart from hypothesized frontolimbic regions, potentially important treatment-related changes in the vlPFC and hippocampus were also noted (Supplemental Table 3 and 4). Decreased left vlPFC (pars orbitalis and pars triangularis) activation (post- vs. pre-treatment) was observed during the interaction between negative emotional processing and behavioral inhibition. Post-treatment, a positive correlation was observed between increased left vlPFC (pars orbitalis) activation and improvements in aggression; pre-treatment activation in the pars orbitalis and triangularis portions of the vlPFC predicted improvements in constraint and affective lability, respectively. The ventral and anterior portions of the left vlPFC (pars orbitalis) are interconnected with the medial temporal lobe and are implicated in cognitive control processes that guide access to relevant semantic memories by facilitating flexibility and integration between contextually meaningful representations of perceptual, mnemonic, and behavioral responses31. Given the observed role of the vlPFC with the current task and the recall bias for salient, negatively valenced information in BPD16, it might be hypothesized that treatment enabled a decreased need for goal-directed access to semantic information and that negative emotion was detected and controlled rapidly. Neutral stimuli were potentially interpreted more ambiguously and required additional evaluation. The observed positive correlation between pars orbitalis activation and improved aggression supports a relationship between cognitive control mechanisms and anger regulation. Posterior and dorsal portions of the vlPFC (pars triangularis) are implicated more in controlled semantic disambiguation en route to a behavioral response, facilitating controlled post-retrieval selection31. Following treatment, this portion of the vlPFC was also relatively less active in response to negative versus neutral words, suggesting automatic forms of semantic-level conflict resolution in the context of negative emotion. The association of pre-treatment activity in the pars orbitalis and triangularis with improved constraint and affective lability may further suggest these regions of vlPFC are associated with improvements in impulsivity and mood lability following TFP.
Decreased left hippocampal activation was also noted pre-to-post TFP treatment. The hippocampus is critical for rapid encoding, consolidation, and retrieval of contextual features. Consistent with relative decreased vlPFC activation, relative hippocampal activation decreases suggest a reduced need for semantic memory retrieval in the context of negative versus neutral emotion. In response to TFP, activation of the left hippocampus negatively correlated with improvement in affect lability and constraint, while pre-treatment hippocampal activation positively correlated with improvement in these symptom-domains. In contrast, relative decreased hippocampal activation pre-treatment was predictive of improvements in measures of aggressive behavior. These results suggest potential differences in processing semantic memories associated with affective lability and constraint versus aggression that require further exploration.
We postulate that TFP treatment effects are primarily associated with top-down prefrontal control over limbic emotional reactivity and semantic memory processing systems. Another potential mechanism includes a cognitive form of semantic-linguistic modulation (many left-lateralized findings). This possible interpretation is consistent with a therapeutic model of TFP, which describes an engagement of the patient’s “observing ego” that leads to improved awareness of potentially threatening negative emotions and a renewed ability to integrate realistic representations of self and other20. The transference process is relational and may also engage social-cognitive systems. In addition, one may consider that TFP may potentially facilitate mechanisms of exposure, extinction, and reconsolidation in relation to challenging emotions and behavior enabling more adaptive associations and behaviors. These findings suggest that the dorsal ACC, posterior-medial OFC, vlPFC, amygdala and hippocampus warrant further investigation as potential biomarkers associated with clinical improvement following TFP treatment.
In a prior study of six BPD patients scanned before and after 12-weeks of DBT32, four individuals improved following DBT and displayed reduced amygdalar and hippocampal activation consistent with our findings. In a more recent 12-month post vs. pre DBT neuroimaging study in 11 BPD patients and 11 healthy subjects, ROI analyses showed decreased amygdalar activation, and an association between post-treatment decreased amygdala activation and improvements in emotion regulation33. These studies suggest some shared mechanisms of treatment and/or correlates of symptom reductions. In addition, two other studies have probed neural activation changes related to psychodynamic psychotherapy (neither in BPD). Following 15 months of psychodynamic psychotherapy, 16 subjects with depression demonstrated reduced left amygdala and anterior hippocampal activation34. Similar normalization of pre-treatment elevated amygdalar and hippocampal activation following short-term, psychodynamic inpatient psychotherapy in panic disorder has also been characterized35. Furthermore, pre-treatment relative increases in dorsal ACC, medial OFC and dlPFC activation have predicted clinical response to cognitive-behavioral therapy36.
There are several limitations of this study. Our BPD cohort had multiple axis I and II psychiatric co-morbidities and five subjects were on psychotropic medications which were not held constant throughout the TFP treatment intervention (including four subjects discontinuing anxiolytic medications). These confounds were only partially controlled for using a within-subjects design. While holding medications constant would have strengthened our ability to attribute activation changes to TFP, this is particularly challenging in BPD patients. The current study nonetheless advances our understanding of brain-symptom relationships related to links between neural activation changes and improvements in constraint and affective lability. The lack of a matched healthy control group also limited the ability to account for time-related scanner and other non-specific effects. While there is significant variability in the shape of BOLD responses collected across single subjects, it is important to note that the longitudinal stability of group activation maps in similarly robust cognitive tasks has been found to be reproducible and suitable for within-subjects designs37. Although the current findings are also limited by the small number of subjects and the interval range between scans (10–14 months), the delineation of clinically relevant neural activation changes related to psychodynamic psychotherapy have been scarcely studied to date.
In conclusion, this study provides preliminary empirical support for systems-level frontolimbic neural mechanisms and potential biomarkers associated with clinical improvements in patients with BPD following TFP. These results advance our currently limited understanding of neural mechanisms associated with psychodynamically-oriented psychotherapy. Activation in the anterior-dorsal ACC, posterior-medial OFC, amygdala-hippocampus, and vlPFC was associated with improvements in behavioral constraint, emotional regulation and/or aggression in patients with BPD. Future research should seek to replicate these findings in a larger, controlled sample, and investigate hypoactivation of the anterior-dorsal ACC and posterior-medial OFC as possible endophenotypes linked to impulsivity and affective lability, respectively, in BPD and individuals at increased risk for developing BPD.
Supplementary Material
Supplemental Figure 1. Schematic figure of the emotional-linguistic go/no-go task and timing parameters.
Supplemental Table 1. Demographic and clinical information for the 10 recruited patients with borderline personality disorder.
Supplemental Table 2. Region of interest (ROI) analyses of brain regions showing differential blood-oxygen-level-dependent (BOLD) neural activation in borderline personality disorder patients post vs. pre-treatment for the interaction effect between negative (versus neutral) emotional words and nogo (versus go) conditions [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (nogo vs. go)] shown in Figure 1. ROI analyses showing post vs. pre-treatment and pre-treatment effects correlated to clinical improvement in constraint (Multidimensional Personality Questionnaire-Constraint), and affective lability (Affective Lability Scale-Total) shown in Figures 2 and 3.
Supplemental Table 3. Brain regions showing differential blood-oxygen-level-dependent (BOLD) neural activation in borderline personality disorder patients post vs. pre-treatment for the interaction effect between negative (versus neutral) emotional words and nogo (versus go) conditions [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (nogo vs. go)] (See also Supplemental Table 2 and Figure 1).
Supplemental Table 4. Brain regions showing post vs. pre-treatment and pre-treatment effects correlated to clinical improvement in constraint (Multidimensional Personality Questionnaire-Constraint), affective lability (Affective Lability Scale-Total) and aggression (Overt Aggression Scale-Modified) (See also Supplemental Table 2 and Figures 2 and 3).
Acknowledgments
This work was supported by NINDS R25NS065743-05S1 grant to Dr. Perez and partial support from the DeWitt Wallace Fund.
Footnotes
All authors report no conflicts of interest.
Author contributions:
Drs. Pan, Cain, Clarkin, Lenzenweger, Kernberg, Levy, Epstein, Silbersweig, and Stern contributed to the concept/design; Drs. Pan, Root, Tuescher, Cain, Clarkin, Lenzenweger, Kernberg, and Levy contributed to the data acquisition; Drs. Perez, Vago, Pan, Silbersweig, and Stern, and Mr. Fuchs and Ms. Leung contributed to the analysis/data interpretation. Drs. Perez, Vago, Pan, Silbersweig, and Stern drafted the article and all authors revised the manuscript.
References
- 1.Clarkin JF, Levy KN, Lenzenweger MF, Kernberg OF. The Personality Disorders Institute/Borderline Personality Disorder Research Foundation randomized control trial for borderline personality disorder: rationale, methods, and patient characteristics. J Pers Disord. 2004;18:52–72. doi: 10.1521/pedi.18.1.52.32769. [DOI] [PubMed] [Google Scholar]
- 2.Levy KN, Clarkin JF, Yeomans FE, Scott LN, Wasserman RH, Kernberg OF. The mechanisms of change in the treatment of borderline personality disorder with transference focused psychotherapy. J Clin Psychol. 2006;62:481–501. doi: 10.1002/jclp.20239. [DOI] [PubMed] [Google Scholar]
- 3.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155:231–43. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingenfeld K, Rullkoetter N, Mensebach C, et al. Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology. 2009;34:571–86. doi: 10.1016/j.psyneuen.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Kraus A, Valerius G, Seifritz E, et al. Script-driven imagery of self-injurious behavior in patients with borderline personality disorder: a pilot FMRI study. Acta Psychiatr Scand. 2010;121:41–51. doi: 10.1111/j.1600-0447.2009.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze L, Domes G, Kruger A, et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry. 2011;69:564–73. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Donegan NH, Sanislow CA, Blumberg HP, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–93. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 8.Herpertz SC, Dietrich TM, Wenning B, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–8. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 9.Niedtfeld I, Schulze L, Kirsch P, Herpertz SC, Bohus M, Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol Psychiatry. 2010;68:383–91. doi: 10.1016/j.biopsych.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Kamphausen S, Schroder P, Maier S, et al. Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. World J Biol Psychiatry. 2013;14:307–18. S1–4. doi: 10.3109/15622975.2012.665174. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett EA, Zhang J, New AS, et al. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol Psychiatry. 2012;72:448–56. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen KR, Vizueta N, Thomas KM, et al. Amygdala functional connectivity in young women with borderline personality disorder. Brain Connect. 2011;1:61–71. doi: 10.1089/brain.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCloskey MS, New AS, Siever LJ, et al. Evaluation of behavioral impulsivity and aggression tasks as endophenotypes for borderline personality disorder. J Psychiatr Res. 2009;43:1036–48. doi: 10.1016/j.jpsychires.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rentrop M, Backenstrass M, Jaentsch B, et al. Response inhibition in borderline personality disorder: Performance in a Go/Nogo task. Psychopathology. 2008;41:50–7. doi: 10.1159/000110626. [DOI] [PubMed] [Google Scholar]
- 15.Lenzenweger MF, Clarkin JF, Fertuck EA, Kernberg OF. Executive neurocognitive functioning and neurobehavioral systems indicators in borderline personality disorder: a preliminary study. J Pers Disord. 2004;18:421–38. doi: 10.1521/pedi.18.5.421.51323. [DOI] [PubMed] [Google Scholar]
- 16.Korfine L, Hooley JM. Directed forgetting of emotional stimuli in borderline personality disorder. J Abnorm Psychol. 2000;109:214–21. [PubMed] [Google Scholar]
- 17.Goldstein M, Brendel G, Tuescher O, et al. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36:1026–40. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Silbersweig D, Clarkin JF, Goldstein M, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–41. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- 19.Jacob GA, Zvonik K, Kamphausen S, et al. Emotional modulation of motor response inhibition in women with borderline personality disorder: an fMRI study. J Psychiatry Neurosci. 2013;38:164–72. doi: 10.1503/jpn.120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernberg OF, Yeomans FE, Clarkin JF, Levy KN. Transference focused psychotherapy: overview and update. Int J Psychoanal. 2008;89:601–20. doi: 10.1111/j.1745-8315.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 21.Clarkin JF, Levy KN, Lenzenweger MF, Kernberg OF. Evaluating Three Treatments for Borderline Personality Disorder: A Multiwave Study. Am J Psychiatry. 2007;164:922–8. doi: 10.1176/ajp.2007.164.6.922. [DOI] [PubMed] [Google Scholar]
- 22.Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215–24. doi: 10.1001/archpsyc.1994.03950030051005. [DOI] [PubMed] [Google Scholar]
- 23.Clarkin JF, Yeoman FE, Kernberg OF. Psychotherapy for Borderline Personality. John Wiley & Sons; New York: 1999. [Google Scholar]
- 24.Tellegen A. Brief manual for the Multidimensional Personality Questionnaire. Minneapolis, MN: University of Minnesota; 1982. [Google Scholar]
- 25.Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. J Clin Psychology. 1989;45:786–93. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Coccaro EF, Harvey PD, Kupsaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3:S44–51. [PubMed] [Google Scholar]
- 27.Clarkin JF, De Panfilis C. Developing conceptualization of borderline personality disorder. J Nerv Ment Dis. 2013;201:88–93. doi: 10.1097/NMD.0b013e31827f61f6. [DOI] [PubMed] [Google Scholar]
- 28.Brendel GR, Stern E, Silbersweig DA. Defining the neurocircuitry of borderline personality disorder: functional neuroimaging approaches. Dev Psychopathol. 2005;17:1197–206. doi: 10.1017/s095457940505056x. [DOI] [PubMed] [Google Scholar]
- 29.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 31.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Schnell K, Herpertz SC. Effects of dialectic-behavioral-therapy on the neural correlates of affective hyperarousal in borderline personality disorder. J Psychiatr Res. 2007;41:837–47. doi: 10.1016/j.jpsychires.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Goodman M, Carpenter D, Tang CY, et al. Dialectical behavior therapy alters emotion regulation and amygdala activity in patients with borderline personality disorder. J Psychiatr Res. 2014;57:108–16. doi: 10.1016/j.jpsychires.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchheim A, Viviani R, Kessler H, et al. Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy. PLoS One. 2012;7:e33745. doi: 10.1371/journal.pone.0033745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beutel ME, Stark R, Pan H, Silbersweig D, Dietrich S. Changes of brain activation pre- post short-term psychodynamic inpatient psychotherapy: an fMRI study of panic disorder patients. Psychiatry Res. 2010;184:96–104. doi: 10.1016/j.pscychresns.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Gabrieli JD, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 2015;85:11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plichta MM, Schwarz AJ, Grimm O, et al. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. Neuroimage. 2012;60:1746–58. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Schematic figure of the emotional-linguistic go/no-go task and timing parameters.
Supplemental Table 1. Demographic and clinical information for the 10 recruited patients with borderline personality disorder.
Supplemental Table 2. Region of interest (ROI) analyses of brain regions showing differential blood-oxygen-level-dependent (BOLD) neural activation in borderline personality disorder patients post vs. pre-treatment for the interaction effect between negative (versus neutral) emotional words and nogo (versus go) conditions [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (nogo vs. go)] shown in Figure 1. ROI analyses showing post vs. pre-treatment and pre-treatment effects correlated to clinical improvement in constraint (Multidimensional Personality Questionnaire-Constraint), and affective lability (Affective Lability Scale-Total) shown in Figures 2 and 3.
Supplemental Table 3. Brain regions showing differential blood-oxygen-level-dependent (BOLD) neural activation in borderline personality disorder patients post vs. pre-treatment for the interaction effect between negative (versus neutral) emotional words and nogo (versus go) conditions [(post-treatment scan vs. pre-treatment scan) × (negative vs. neutral) × (nogo vs. go)] (See also Supplemental Table 2 and Figure 1).
Supplemental Table 4. Brain regions showing post vs. pre-treatment and pre-treatment effects correlated to clinical improvement in constraint (Multidimensional Personality Questionnaire-Constraint), affective lability (Affective Lability Scale-Total) and aggression (Overt Aggression Scale-Modified) (See also Supplemental Table 2 and Figures 2 and 3).