Abstract
Carbonyls, especially aldehydes, are a group of harmful volatile organic compounds that are found in tobacco smoke. Seven carbonyls are listed on the FDA’s harmful and potential harmful constituents list for tobacco or tobacco smoke. Carbonyls have reactive functional groups and thus are challenging to quantitatively measure in cigarette smoke. The traditional method of measuring carbonyls in smoke involves solvent-filled impinger trapping and derivatization. This procedure is labor-intensive and generates significant volumes of hazardous waste. We have developed a new method to efficiently derivatize and trap carbonyls from mainstream smoke in situ on Cambridge filter pads. The derivatized carbonyls are extracted from the pads and subsequently quantified by ultra-high-pressure liquid chromatography coupled with tandem mass spectrometry. The new method has been validated and applied to research and commercial cigarettes. Carbonyl yields from research cigarettes are comparable to those from other published literature data. With a convenient smoke collection apparatus, a 4 min sample analysis time, and a low- or submicrogram detection limit, this new method not only simplifies and speeds the detection of an important class of chemical constituents in mainstream smoke but also reduces reactive losses and provides a more accurate assessment of carbonyl levels in smoke. Excellent accuracy (average 98%) and precision (14% average relative standard deviation in research cigarettes) ensure this new method’s sufficient fidelity to characterize conventional combusted tobacco products, with potential application toward new or emerging products.
Graphical abstract
INTRODUCTION
Tobacco smoke contains more than 4000 chemicals, including carcinogenic and toxic carbonyl compounds (e.g., formaldehyde, acetaldehyde, and acrolein).1,2 The amount of many individual carbonyl compounds in mainstream smoke is typically in the range of micrograms per cigarette.3 A risk assessment by Fowles and Dybing4 on chemical constituents in cigarette smoke suggested that mainstream smoke gas-phase constituents contribute heavily toward the cancer risk indices. In 2012, the U.S. Food and Drug Administration (FDA) published a list of 93 harmful and potentially harmful constituents (HPHC) in tobacco products and tobacco smoke.5 Seven carbonyls (formaldehyde, acetaldehyde, acrolein, acetone, propionaldehyde, crotonaldehyde, and methyl ethyl ketone) are among them. Additionally, inhaling carbonyls can cause significant short-term adverse effects such as irritation and pulmonary edema.6,7 Long-term adverse effects include cancer and respiratory congestion. Among these carbonyls, formaldehyde and acetaldehyde are classified as Group 1 and 2B carcinogens, respectively, by the International Agency for Research on Cancer.1,8 Animal studies suggest that acetaldehyde has the potential to enhance acquisition of nicotine and can further contribute to smoke addiction.9,10 Acrolein and crotonaldehyde are ciliatoxic and can inhibit lung clearance.2,11,12 Some variables in cigarette design (e.g., charcoal filtration, filter ventilation, and high-porosity wrapping paper) can affect levels of volatile organic compounds, including carbonyl emissions, in cigarette mainstream smoke.3,13 Evaluating the extent of the change in smoke levels relies on the reliability and reproducibility of the testing method. Accurate quantitation of the reactive and volatile carbonyls in cigarette smoke is essential to assessing and characterizing tobacco products, both conventional and/or new emerging ones.
Low-molecular-weight carbonyl compounds such as formaldehyde, acetaldehyde, and acrolein are difficult to accurately analyze because they are highly volatile, reactive, and water-soluble. Direct trace analyses of these reactive compounds are problematic compared with less reactive volatiles in smoke.14 Therefore, many stable derivative approaches have been developed. The most commonly used derivative is 2,4-dinitrophenylhydrazine (DNPH). However, incorporating the derivatization chemistry for carbonyl compounds from cigarette mainstream smoke poses another challenge. In conventional smoke carbonyl analysis, measurements are often performed under Health Canada method T-10415 or CORESTA recommended method No. 74.16 Under these methods, carbonyls are collected by passing the mainstream smoke of 2–5 cigarettes through impingers containing 80 or 35 mL of DNPH solution. Aliquots of the impinger solutions are injected onto a high-performance liquid chromatography (HPLC) system for quantitation. The impinger approach is labor-intensive and low-throughput and generates significant hazardous waste; therefore, scientists have been assessing alternative ways to trap and derivatize carbonyl compounds in mainstream smoke. Dong et al.17 developed a method using DNPH-treated Cambridge filter pads (CFP) to trap carbonyls in mainstream smoke generated from a rotary smoking machine and to further quantify the carbonyls by gas chromatography–mass spectrometry (GC-MS). Recently, Uchiyama et al.18 published a method using sorbent cartridges to collect carbonyls during machine smoking. The sorbent was eluted and derivatized with DNPH, and derivatized carbonyls were analyzed by HPLC coupled with ultraviolet detection (HPLC-UV).
To quantify derivatized carbonyls, HPLC-UV is often used; however, it requires long column separation times and has limited selectivity, especially for complex matrices such as tobacco smoke. Both the Health Canada and CORESTA methods require a 40 min HPLC run time to separate structurally similar carbonyl compounds and/or their isomers. Miller et al.19 developed a method using ultrapressure liquid chromatography (UPLC) and mass spectrometry to quantify derivatized carbonyl compounds. This method greatly reduced run time (4.5 min) while providing higher sensitivity and selectivity.
We have recently developed a new method to efficiently trap and derivatize carbonyls from mainstream cigarette smoke on CFPs in-situ. By treating CFPs with DNPH in batches, we reduce the usage of DNPH solution to about 3 mL per sample, compared to 35 or 80 mL used for conventional methods. Smoking was performed using a linear smoking machine. The derivatized carbonyls were extracted from the pads and subsequently quantified by UPLC coupled with tandem mass spectrometry (UPLC-MS/MS). This fully validated method includes the analysis of seven carbonyl compounds listed on the FDA’s HPHC list.
EXPERIMENTAL PROCEDURES
Standards, Reagents, and Materials
2,4-Dinitrophenyl-hydrazine (DNPH), perchloric acid (70%), and pyridine were purchased from Sigma-Aldrich (St. Louis, MO). Solvents used including acetonitrile and water were obtained from Fisher Scientific and are HPLC-grade. Calibration standard solution (formaldehyde, acetaldehyde, acetone, acrolein, propionaldehyde, crotonaldehyde, and 2-butanone) were formulated at various concentrations (o2Si smart solutions, Charleston, SC). Their purities were ≥96% except formaldehyde (≥37%). Isotopically labeled analogues (formaldehyde-d2-DNPH, acetaldehyde-d4-DNPH, acetone-d6-DNPH, acrolein 2,4-dinitrophenlhydrazone-3,5,6-d3, propionaldehyde-2,2,3,3,3-d5-DNPH, crotonaldehyde 2,4-dinitrophenlhydrazone-3,5,6-d3, and 2-butanone-4,4,4-d3-DNPH) were also synthesized and formulated at a concentration of 1000 mg/L and at ≥99% purity (o2Si smart solutions, Charleston, SC). Aliquots of the calibration standard and internal standard mix were stored in flame-sealed ampules at −70 °C for long-term storage or at −20 °C for a maximum of 2 months. DNPH solution was prepared by dissolving 1.5 g of DNPH and 200 µL of 70% perchloric acid into 100 mL of acetonitrile. Extraction solution was prepared by adding pyridine into acetonitrile to make a final concentration of 2% pyridine.
Cambridge filter pads (CFP, 44 mm glass fiber) were obtained from Whatman (Maidstone, UK). Unfiltered custom-blended cigarettes were purchased from Murty Pharmaceuticals, Inc. (Lexington, KY). Four different types of nonfiltered cigarettes were selected. They each contained a single type of tobacco (burley, bright, oriental, or reconstituted). Research cigarettes (3R4F) were from the University of Kentucky (Lexington, KY). Commercial cigarettes were purchased from various retail sources in Atlanta, GA.
Carbonyl Calibration Curve Preparation
First, 3 mL of DNPH solution was aliquoted to a 2 oz amber vial for each calibration point. Then, the designated volume (ranging from 5 to 300 µL) of carbonyl standard solution was spiked into the vial to create each calibration point. Derivatization happens instantly. After that, 27 mL of extraction solution was added to neutralize the DNPH-carbonyl reaction. Each calibration standard is then spiked with 50 µL of internal standard solution and subjected to the same preparation procedure used for smoke samples.
Machine-Smoke Regimens and Smoke Sample Collection
Cigarettes were conditioned at 22 °C and 60% relative humidity for at least 48 h prior to smoking, according to ISO 3308. Prior to smoking, one set of CFPs (pretreated CFP weights about 0.34 g) was soaked in DNPH solution and dried under vacuum for 2 h at room temperature. After drying, one DNPH-treated CFP weighs about 0.37 g (dry pad). Another set of an equal number of CFPs was soaked in DNPH solution and dried in a chemical fume hood for 6–7 min or until they were about 1.3 g in weight (wet pad). The conditioned pads were assembled so that the mainstream smoke was pulled through the wet pad first and then the dry one. Cigarettes were smoked to the marked length of the filter overwrap (tipping) plus 3 mm using a Borgwaldt 20-port smoking machine. Mainstream carbonyls generated under either the ISO (35 mL puff volume, 60 s puff interval, 2 s puff duration, and filter-tip vent open) or Canadian intense (CI, 55 mL puff volume, 30 s puff interval, 2 s puff duration, and 100% vent block) regimen were derivatized and collected on DNPH-treated CFPs.
Smoke Sample Preparation
After smoking, the CFPs were allowed to rest for 3–4 min for the reaction to come to completion. CFPs were removed and each CFP pair was placed with the tar side rolled inward into a 2 oz amber vial. A 50 µL of internal standard solution was placed onto each pad. Pads were extraced with 30 mL of extraction solution and shaken at 160 rpm for 5 min. Five microliters of the extracted sample was diluted into 1.2 mL of dilution solution (50:50 10 mM ammonium acetate and acetonitrile), and 5 µL was injected into an ultra-high-pressure liquid chromatography (UPLC) system.
UPLC-MS/MS Analysis
All samples were analyzed using an Agilent 1260 liquid chromatography (Agilent Technologies, Wilmington, DE) system coupled with an API 5500 triple quadruple mass spectrometer (UPLC-MS/MS) (AB Sciex, Foster City, CA). Samples were injected onto a Water Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 µm particle size, Waters Corp., Milford, MA). Solvent A was 10 mM ammonium acetate in water, and solvent B was acetonitrile. The column oven temperature was set at 25 °C. The flow rate was 0.5 mL/min. The gradient used the following settings: 0–0.75 min, 50–30% A; 0.75–1.5 min, 30–50% A; and 1.5–4.0 min, 50% A. The mass spectrometer was operated in negative ion electrospray mode. The instrument settings were as follows: curtain gas (N2) at 40 psi; ion source: nebulizer gas and heater gas were both at 40 psi; source temperature at 300 °C; ion transfer voltage at −4500 V; collision gas at 7 when vacuum gauge pressure was at 0.6 × 10−5 Torr; mass spectral data on precursor and product ions were collected in multiple reaction monitoring mode. The quantitation/confirmation ion pairs, delustering potential, entrance potential, collision energy, and cell exit potential were optimized for each analyte.
Data Analysis
Analyst software, version 1.5 (AB Sciex, Foster City, CA), was used to process peak area determinations for all samples, blanks, standards, and quality control (QC) materials. Each ion of interest in the reconstructed ion chromatogram was automatically selected and integrated. The peak integrations were manually inspected for errors (e.g., wrong retention time) and, if necessary, reintegrated. For each analyte, two pairs of transition ions, one for quantification and one for confirmation, were collected to verify analyte identity. The acceptable ratio of peak areas for quantifying and confirming transition ions of unknown samples was within 30% of that for QC materials.
Safety Consideration
Personnel involved in weighing, diluting, or otherwise manipulating the compounds used were instructed in the safe handling of chemicals. These instructions included the wearing of personal protection items and proper laboratory practices. All compounds were handled in a fume hood, and personnel used appropriate protective safety glasses, gloves, and lab coats.
RESULTS AND DISCUSSION
Method Development
To quantify the reactive carbonyl compounds, we derivatized carbonyls into relatively stable DNPH-carbonyls for measurement. The traditional method of trapping carbonyls from cigarette mainstream smoke is through impinger traps. To increase throughput and reduce hazardous waste, we assessed the DNPH-treated pad method that Dong et al.17 published previously. We modified the method so that DNPH-treated CFPs can be used in conjunction with linear smoking machines. This modification greatly increased the sample throughput. Up to 20 cigarette samples can be smoked and the samples can be prepared and analyzed in the same batch. During the experimental trial, we observed a low yield of smoke acrolein compared to published values. Further testing revealed that maintaining the moisture of the DNPH-treated CFPs was crucial to trap acrolein effectively. However, if we used a semidry DNPH-treated CFP for smoking, then the pad’s structural integrity could fail around the central area, causing breakthrough losses. To avoid breakthrough, we added a second CFP behind the treated to pad to provide structural support. The addition of the second pad did not impede smoking or trapping. Smoke carbonyl delivery results of 3R4F under the ISO regimen were comparable to previously published values.16,17,19,20 To further assess whether this double-pad assembly (one DNPH-treated semidry CFP and one untreated CFP) has enough capacity to trap smoke carbonyls generated from the intense regimen, we assembled a second double-pad set with both CFPs treated with DNPH: one completely dry and one semidry. We then assessed these two double-pad sets using two smoking regimens, ISO and CI. Results indicated that both pads need to be treated with DNPH to adequately trap smoke carbonyls generated from the intense regimen. Puff profiles on the smoking machine were not perturbed by the double-pad setup. Our stability tests indicate that DNPH-treated CFPs (dry pads) can be stored in the desiccator under vacuum for up to 6 h. Overnight storage showed increasing formaldehyde levels. However, DNPH-treated CFPs (wet pads) need to be assembled with the dry ones and smoked right after preparation to maintain a semidry status.
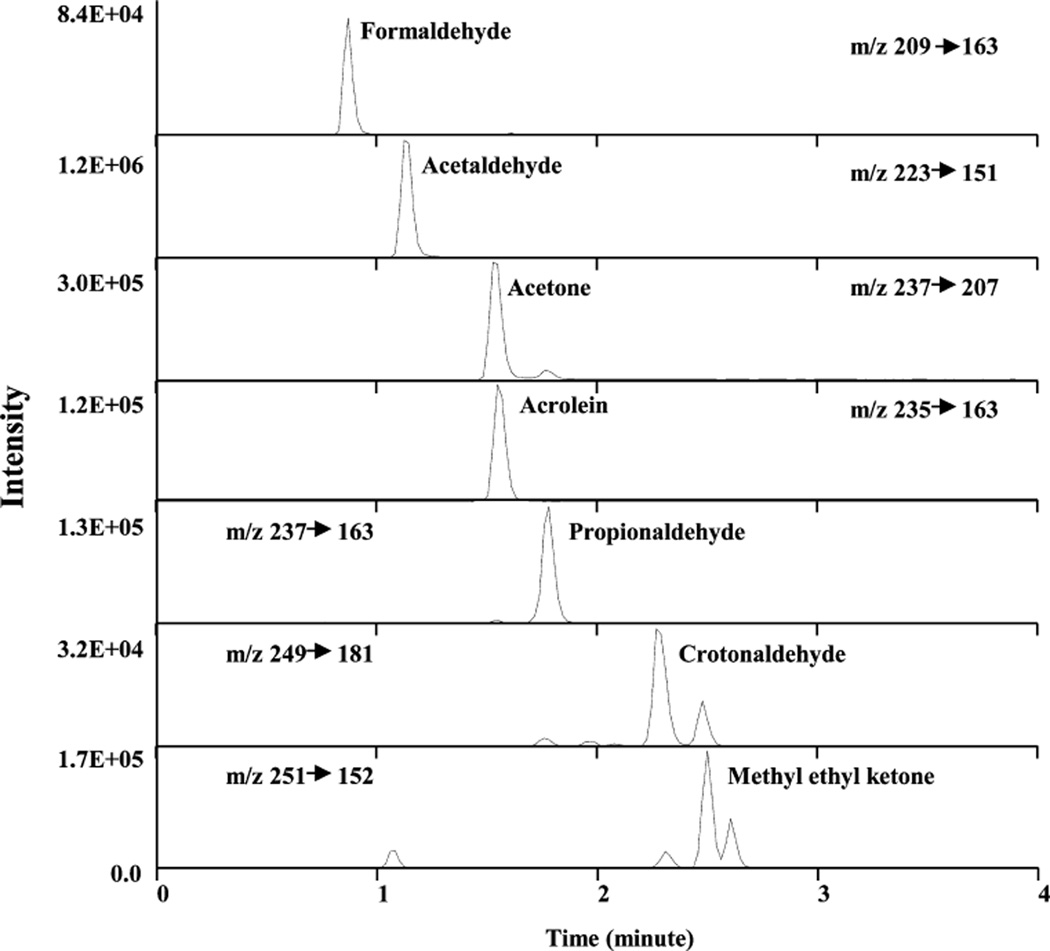
After smoking, DNPH-carbonyls were extracted from both pads and injected into UPLC-MS/MS for quantitation. Because of the complexity of smoke, we used MS/MS to monitor precursor and product ions to increase sensitivity (Table 1). We also monitored a second precursor/product ion pair to confirm analyte identity and maintain high specificity with the simple sample preparation (Table 1). Reconstructed ion chromatograms of DNPH-carbonyls from 3R4F mainstream smoke showed excellent sensitivity and chromatographic resolution (Figure 1). Even with only 4 min total run time (compared to 40 min HPLC run time), all seven DNPH-carbonyls were baseline-separated on column except for DNPH-acetone and DNPH-acrolein. These two analytes were separated by the two different precursor/product pairs monitored (Figure 1). To ensure selectivity, we chose isotopically labeled DNPH-carbonyls as internal standards. Each DNPH-carbonyl used its own labeled internal standard for analyte identification and concentration calculation (Table 1).
Table 1.
Multiple Reaction Monitoring Analysis of DNPH-Carbonyls and Their Internal Standardsa
| analyte | precursor ion | product ion | CEd | internal standard | precursor ion | precursor ion | CEd |
|---|---|---|---|---|---|---|---|
| DNPH-formaldehyde | 209.1 | 163.1b | −12 | formaldehyde-d2-DNPH | 211.1 | 151.0 | −13 |
| 151.0c | −12 | ||||||
| DNPH-acetaldehyde | 223.1 | 151.0b | −13 | acetaldehyde-d4-DNPH | 227.1 | 151.0 | −13 |
| 163.0c | −13 | ||||||
| DNPH-acetone | 237.2 | 207.0b | −17 | acetone-d6-DNPH | 243.2 | 213.0 | −16 |
| 178.0c | −26 | ||||||
| DNPH-acrolein | 235.1 | 163.0b | −20 | acrolein-DNPH-d3 | 238.1 | 161.0 | −20 |
| 158.0c | −20 | ||||||
| DNPH-propionaldehyde | 237.2 | 163.0b | −15 | propionaldehyde-2,2,3,3,3-d5-DNPH | 242.2 | 163.0 | −18 |
| 122.1c | −30 | ||||||
| DNPH-crotonaldehyde | 249.1 | 181.0b | −27 | crotonaldehyde-DNPH-d3 | 252.1 | 175.0 | −17 |
| 172.0c | −17 | ||||||
| DNPH-methyl ethyl ketone | 251.0 | 152.0b | −26 | methyl ethyl ketone-4,4,4-d3-DNPH | 254.0 | 153.0 | −26 |
| 122.1c | −30 |
Other parameters (V): declustering potential, −10; entrance potential, −10; and collision exit potential, −9.
Quantitation.
Confirmation.
CE (V), collision energy.
Figure 1.
Multiple reaction monitoring chromatograms of DNPH-carbonyls in 3R4F mainstream smoke.
Method Validation
To mimic carbonyl derivatization during smoking, we derivatized parent carbonyls to DNPH-carbonyls in situ to generate each calibration point. The calibration curve was prepared by spiking different amounts of carbonyls into DNPH solution. The derivatization reaction happened instantly. The dynamic range of each carbonyl was set to cover smoke carbonyl deliveries (Table 2). A linear regression fit with 1/x concentration weighting was used for each analyte’s calibration curve. The detection limit (LOD) for each carbonyl was estimated from calibration curves as three times the standard deviation extrapolated to zero concentration; LODs were in the low- or submicrogram range (Table 2). The method’s accuracy was assessed by spiking three concentrations of known amounts (low, medium, and high) of the carbonyls into DNPH solution. Accuracy was calculated as the mean of the experimentally determined concentration from replicate analysis divided by the nominal concentration. Good accuracies were achieved for all analytes and ranged from 83 to 106% (Table 2). Matrix effects were assessed by comparing slopes of calibration curves prepared from two set of standards. One set was prepared neat, and the second set was prepared with smoke matrices present. The percentage difference (neat vs smoke matrix) ranged from −7.8 to 3.1% (Table 2), which indicates the minimum matrix-suppressed or -enhanced effect. The precision of the method was determined by calculating the relative standard deviations of 30 replicate measurements of 3R4F smoked under the ISO and CI regimens during a 4 month interval. Relative standard deviations for all analytes were less than 20% (Table 2).
Table 2.
Method Validation Parameters for Measuring Carbonyls in Tobacco Smoke
| accuracy (n = 3) | relative standard deviation (n = 30) |
|||||||
|---|---|---|---|---|---|---|---|---|
| analyte | calibration standard range (µg) | low | medium | high | smoke matrix effecta (n = 3) | LOD (µg) (n = 7) | 3R4F ISOb | 3R4F CIc |
| formaldehyde | 5–300 | 106% | 102% | 99% | 0% | 2.4 | 19% | 19% |
| acetaldehyde | 50–3000 | 90% | 100% | 98% | −1.3% | 2.7 | 10% | 12% |
| acetone | 25–1500 | 102% | 99% | 95% | 3.1% | 6.4 | 14% | 14% |
| acrolein | 5–300 | 100% | 103% | 95% | −7.5% | 0.1 | 10% | 13% |
| propionaldehyde | 5–300 | 83% | 102% | 103% | −2.4% | 0.6 | 13% | 16% |
| crotonaldehyde | 2.5–150 | 101% | 101% | 96% | −3.6% | 0.2 | 12% | 13% |
| methyl ethyl ketone | 5–300 | 104% | 93% | 96% | −7.8% | 0.3 | 14% | 13% |
Matrix effects were assessed by comparing slopes of calibration curves prepared from two set of standards. One set was prepared neat, and the second set was prepared with smoke matrices present. “+” or “−” indicates a matrix-enhanced or -suppressed effect, respectively.
ISO (35 mL puff volume, 60 s puff interval, 2 s puff duration, and filter-tip vent open).
CI (55 mL puff volume, 30 s puff interval, 2 s puff duration, and 100% vent block).
Because of airborne formaldehyde, we observed a low level (background) formaldehyde (<15 µg) from extracts prepared from a set of blank DNPH-treated CFPs. Because the blank level varies from day to day, we analyzed blank DNPH-treated CFPs with every batch of smoking samples. Background formaldehyde was subtracted from the formaldehyde measurement of each sample before the result was reported.
The validity of our method was also tested by additional experiments. Analyte pad recovery was performed by comparing the amount of carbonyls before and after the pad extraction process. Results showed a 93% average pad recovery rate. Extraction time was also assessed, and 5 min was found to be enough to extract all analytes from the pads. To ensure two DNPH-treated CFPs are enough to capture all smoke carbonyls, we attached a Tedlar bag behind the cigarette holder to trap the mainstream smoke after it passed through DNPH-treated CFPs. After smoking, DNPH solution was added into the Tedlar bag. The extracted solution was measured for any residual carbonyls. Residual carbonyls were below 5% for both the ISO and CI smoking regimens.
Carbonyl Levels in Mainstream Smoke from Research Cigarettes
We measured seven carbonyl levels in mainstream smoke from 3R4F using the ISO and CI regimens and compared the results with literature values (Table 3). Kentucky research cigarettes 2R4F and 3R4F are examples of American blended cigarettes with filler consisting of bright, burley, oriental, and reconstituted tobaccos. In addition to 3R4F values, we also listed the literature 2R4F values because a previous study suggested that 2R4F and 3R4F have equivalent smoke chemistry.21 Results from our work were similar to literature reported values (Table 3). This further proved that our method is accurate and robust. In addition to 3R4F, we also measured mainstream carbonyls from CORESTA reference cigarette CM6 (Table 3). Again, our results were comparable to literature reported values and CORESTA 2012 collaborative study values (Table 3). We observed differences in carbonyl profiles between 3R4F and CM6 under the CI smoking regimen. Mainstream smoke delivery of formaldehyde from CM6 was statistically higher than that from 3R4F. Although mainstream smoke deliveries of acetaldehyde, acetone, and methyl ethyl ketone from CM6 were lower than those from 3R4F, these differences were not significant. The difference in formaldehyde delivery is possibly due to the difference in the tobacco blend between these two research cigarettes. Tobacco in 3R4F cigarettes is a typical American blend with a mixture of bright, burley, oriental, and reconstituted tobaccos. Whereas, tobacco in CM6 is exclusively bright. To gain insight on carbonyl formation as a function of tobacco blend, we measured the levels of carbonyls in a series of custom-blended unfiltered cigarettes. These cigarettes were divided into four groups on the basis of the blend composition of the different tobaccos: 100% bright (flue-cured) cigarettes, 100% burley (air-cured) cigarettes, 100% oriental (sun-cured) cigarettes, and 100% reconstituted cigarettes. Because these single-blend cigarettes were unfiltered and had different weights from one type to the other, we weighed the cigarettes and their corresponding butts before and after smoking to estimate tobacco weights consumed during smoking. Mainstream smoke deliveries of carbonyls were displayed as micrograms per gram of tobacco consumed (Table 4). Cigarettes made from pure bright, oriental, or reconstituted tobacco delivered a statistically higher amount of formaldehyde than those made from burley tobacco. Cigarettes made from pure burley tobacco delivered statistically higher amounts of acetone and methyl ethyl ketone than those made from the other three types of tobacco. Cigarettes made from pure bright, burley, or reconstituted tobacco delivered a statistically higher amount of acetaldehyde than those made from oriental tobacco. These results indicate that the tobacco blend mix can influence deliveries, which contribute to the different delivery profiles of carbonyls in mainstream smoke. Furthermore, since the majority of U.S. smokers smoke filtered cigarettes, the physical design of the cigarette filter (e.g., ventilation) can also influence the delivery of mainstream smoke carbonyls.
Table 3.
Comparison of Mainstream Smoke Carbonyls (µg per Cigarette Mean ± Standard Deviation) with Literature Valuesa
| 3R4F | 2R4F | CM6 | ||||||
|---|---|---|---|---|---|---|---|---|
| ISOb | this work (n = 30) |
Sampson 2014 (n = 137) |
Uchiyama 2013 (n = 5) |
CORESTA 2012 |
Miller 2010 (n = 6) |
this work (n = 4) |
Uchiyama 2013 (n = 5) |
CORESTA 2012d |
| formaldehyde | 26 ± 4.1 | N/A | N/A | 21 | 24 ± 3.2 | 102 ± 10.5 | N/A | 47 |
| acetaldehyde | 659 ± 68.1 | 620 ± 127 | 570 | 552 | 601 ± 41.6 | 1194 ± 300.5 | 670 | 694 |
| acetone | 256 ± 35.3 | N/A | 250 | 210 | 356 ± 22.1 | 286 ± 26.1 | 320 | 269 |
| acrolein | 58 ± 6.2 | N/A | 56 | 48 | 65 ± 6.4 | 78 ± 9.9 | 72 | 69 |
| propionaldehyde | 62 ± 7.9 | N/A | 49 | 42 | 48 ± 3.5 | 94 ± 13.9 | 62 | 53 |
| crotonaldehyde | 14 ± 1.7 | 10 ± 1.3 | 15 | 11 | 18 ± 2.3 | 24 ± 6.4 | 25 | 21 |
| methyl ethyl ketone | 77 ± 10.1 | 56 ± 6.6 | 97 | 52 | 83 ± 6.7 | 98 ± 18.5 | 130 | 62 |
| CIc | this work (n = 30) |
Sampson 2014 (n = 106) |
Uchiyama 2013 (n = 5) |
CORESTA 2012 |
Miller 2010 (n = 6) |
this work (n = 4) |
Uchiyama 2013 (n = 5) |
CORESTA 2012d |
| formaldehyde | 79 ± 14.6 | N/A | N/A | 76 | 74 ± 6.5 | 127 ± 14.8 | N/A | 104 |
| acetaldehyde | 1705 ± 190.8 | 1740 ± 212 | 1400 | 1606 | 1369 ± 59.1 | 1333 ± 152.6 | 1100 | 1309 |
| acetone | 628 ± 86.2 | N/A | 600 | 597 | 773 ± 34.9 | 506 ± 87.3 | 580 | 517 |
| acrolein | 138 ± 17.9 | N/A | 140 | 155 | 168 ± 17.4 | 122 ± 16.7 | 120 | 133 |
| propionaldehyde | 157 ± 23.2 | N/A | 130 | 123 | 120 ± 11.2 | 141 ± 21.9 | 120 | 105 |
| crotonaldehyde | 49 ± 6.0 | 42 ± 4.2 | 51 | 50 | 73 ± 6.3 | 41 ± 7.9 | 53 | 48 |
| methyl ethyl ketone | 183 ± 23.0 | 170 ± 16.1 | 220 | 147 | 206 ± 9.1 | 145 ± 34.8 | 240 | 131 |
All results are reported in µg/cigarette.
ISO (35 mL puff volume, 60 s puff interval, 2 s puff duration, and filter-tip vent open).
CI (55 mL puff volume, 30 s puff interval, 2 s puff duration, and 100% vent block).
Ref 16.
Table 4.
Mainstream Smoke Carbonyls (µg/g Tobacco Mean ± Standard Deviation) from Single-Blend Unfiltered Research Cigarettesa
| analyte | bright | burley | oriental | reconstituted |
|---|---|---|---|---|
| formaldehyde | 118 ± 33.7 | 27 ± 14.0 | 82 ± 14.7 | 85 ± 22.9 |
| acetaldehyde | 1147 ± 41.0 | 1157 ± 198.5 | 843 ± 26.8 | 1178 ± 146.0 |
| acetone | 346 ± 19.9 | 563 ± 62.8 | 367 ± 54.7 | 380 ± 82.7 |
| acrolein | 115 ± 17.1 | 90 ± 12.2 | 77 ± 6.2 | 88 ± 17.1 |
| propionaldehyde | 113 ± 12.9 | 107 ± 5.8 | 105 ± 3.1 | 115 ± 19.0 |
| crotonaldehyde | 37 ± 2.6 | 32 ± 3.2 | 28 ± 3.0 | 35 ± 3.6 |
| methyl ethyl ketone | 119 ± 10.3 | 172 ± 16.9 | 125 ± 20.8 | 135 ± 4.1 |
n = 3; ISO regimen (35 mL puff volume, 60 s puff interval, 2 s puff duration, and filter-tip vent open).
Carbonyl Levels in Mainstream Smoke from Domestic Cigarettes
In addition to research cigarettes, we analyzed carbonyls in 10 American blended cigarettes that are representative of the current marketplace (Table 5). These cigarettes are among the market share leaders for the king and 100 sizes. Since filter ventilation is a key parameter influencing smoke delivery, cigarettes were smoked under the CI regimen to minimize any artifacts associated with air dilution of mainstream smoke in cigarette varieties with high levels of filter ventilation. Brands A–E are king size cigarettes, whereas brands F–J are 100s. Despite the cigarette rod length difference between king size and 100s, mainstream deliveries of carbonyls were similar among these 10 brands.
Table 5.
Mainstream Carbonyls (µg per Cigarette Mean ± Standard Deviation) in Domestic United States Cigarettesa
| formaldehyde | acetaldehyde | acetone | acrolein | propionaldehyde | crotonaldehyde | methyl ethyl ketone | |
|---|---|---|---|---|---|---|---|
| brand A | 108 ± 10.4 | 1947 ± 128.6 | 724 ± 33.1 | 130 ± 6.4 | 232 ± 21.9 | 72 ± 12.7 | 215 ± 8.1 |
| brand B | 67 ± 7.14 | 1825 ± 7.1 | 657 ± 213.6 | 144 ± 21.2 | 208 ± 17.0 | 60 ± 7.6 | 193 ± 40.3 |
| brand C | 108 ± 6.27 | 1483 ± 40.4 | 635 ± 45.9 | 128 ± 13.6 | 173 ± 17.0 | 50 ± 2.5 | 156 ± 20.8 |
| brand D | 55 ± 6.0 | 1198 ± 273.2 | 418 ± 110.0 | 106 ± 33.5 | 116 ± 33.3 | 25 ± 4.7 | 102 ± 14.3 |
| brand E | 56 ± 14.7 | 1310 ± 376.7 | 385 ± 107.3 | 113 ± 50.3 | 136 ± 32.3 | 31 ± 7.3 | 118 ± 29.9 |
| brand F | 82 ± 12.3 | 1680 ± 180.8 | 553 ± 93.3 | 143 ± 14.0 | 179 ± 14.0 | 46 ± 3.8 | 149 ± 12.0 |
| brand G | 55 ± 11.6 | 1293 ± 117.2 | 450 ± 32.1 | 107 ± 11.6 | 141 ± 18.2 | 35 ± 2.3 | 128 ± 13.4 |
| brand H | 99 ± 7.5 | 1517 ± 158.9 | 544 ± 45.2 | 127 ± 8.1 | 167 ± 6.5 | 42 ± 3.0 | 153 ± 8.1 |
| brand I | 76 ± 14.6 | 1270 ± 228.7 | 511 ± 51.2 | 108 ± 10.5 | 140 ± 8.0 | 39 ± 2.9 | 136 ± 9.3 |
| brand J | 72 ± 25.7 | 1897 ± 474.4 | 596 ± 157.7 | 169 ± 42.7 | 200 ± 34.1 | 53 ± 10.4 | 151 ± 38.7 |
n = 3; CI regimen (55 mL puff volume, 30 s puff interval, 2 s puff duration, and 100% vent block); Brand A–J are representative of the current marketplace; brands A–E are king size cigarettes; brands F–J are 100s.
CONCLUSIONS
This improved smoke carbonyl method allows for the rapid and accurate determination of seven carbonyls in mainstream cigarette smoke. Previously, it had been difficult to quantitatively analyze the constituents of this important class of cigarette mainstream smoke due to their reactivity. With the new method, the machine smoking and sample preparation procedures are much less labor-intensive and generate much less hazardous waste compared to that by the traditional impinger trapping approach. By using a linear 20-port smoking machine, one can easily survey 20 cigarettes in 6 h from pad preparation to result output. The excellent accuracy, precision, and high throughput demonstrated for this method make it applicable to the surveys of carbonyl levels in cigarette smoke, and it was developed with sufficient capacity to investigate newly emerging tobacco products.
Acknowledgments
Funding
This study was funded through an interagency agreement by the U.S. Food and Drug Administration Center for Tobacco Products. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
ABBREVIATIONS
- HPHC
harmful and potentially harmful constituents
- DNPH
2,4-dinitrophenylhydrazine
- HPLC
high-performance liquid chromatography
- GC-MS
gas chromatography–mass spectrometry
- UV
ultraviolet
- CFP
Cambridge filter pad
- UPLC
ultrapressure liquid chromatography
- UPLC-MS/MS
UPLC coupled with tandem mass spectrometry
- CI
Canadian intense smoking regimen
- LOD
detection limit
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, France: IARC; 2004. Tobacco Smoke and Involuntary Smoking. [PMC free article] [PubMed] [Google Scholar]
- 2.Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Bethesda, MD: U.S. Department of Health and Human Services, National Instituties of Health, National Cancer Institute; 2001. [Google Scholar]
- 3.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J. Toxicol. Environ. Health. 1997;50:307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 4.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke. Rockville, MD: U.S. FDA; 2012. http://www.fda.gov/downloads/TobaccoProducts/Labeling/RulesRegulationsGuidance/UCM297981.pdf. [Google Scholar]
- 6.Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- 7.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol. Appl. Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 88. Lyon, France: IARC; 2006. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. [PMC free article] [PubMed] [Google Scholar]
- 9.Talhout R, Opperhuizen A, van Amsterdam JG. Role of acetaldehyde in tobacco smoke addiction. Eur. Neuro-psychopharmacol. 2007;17:627–636. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 11.Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol. Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 38. Lyon, France: IARC; 1986. Tobacco Smoking. [PMC free article] [PubMed] [Google Scholar]
- 13.Xue L, Thomas CE, Koller KB. Mainstream Smoke Gas Phase Filtration Performance of Adsorption Materials Evaluated With A Puff-by-Puff Multiplex GC-MS Method. Beitr. Tabakforsch. Int. 2002;20:251–256. [Google Scholar]
- 14.Sampson MM, Chambers DM, Pazo DY, Moliere F, Blount BC, Watson CH. Simultaneous analysis of 22 volatile organic compounds in cigarette smoke using gas sampling bags for high-throughput solid-phase microextraction. Anal. Chem. 2014;86:7088–7095. doi: 10.1021/ac5015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Determination of Selected Carbonyls in Mainstream Tobacco Smoke. Ottawa, Canada: Health Canada; 1999. [Google Scholar]
- 16.CORESTA Recommended Method N° 74: Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC. Paris, France: CORESTA; 2014. http://www.coresta.org/Recommended_Methods/CRM_74-update(July14).pdf. [Google Scholar]
- 17.Dong JZ, Moldoveanu SC. Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. J. Chromatogr. A. 2004;1027:25–35. doi: 10.1016/j.chroma.2003.08.104. [DOI] [PubMed] [Google Scholar]
- 18.Uchiyama S, Tomizawa T, Inaba Y, Kunugita N. Simultaneous determination of volatile organic compounds and carbonyls in mainstream cigarette smoke using a sorbent cartridge followed by two-step elution. J. Chromatogr. A. 2013;1314:31–37. doi: 10.1016/j.chroma.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Miller JH, Gardner WP, Gonzalez RR. UHPLC separation with MS analysis for eight carbonyl compounds in mainstream tobacco smoke. J. Chromatogr. Sci. 2010;48:12–17. doi: 10.1093/chromsci/48.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Roemer E, Schramke H, Weiler H, Buettner A, Kausche S, Weber S, Berges A, Stueber M, Muench M, Trelles-Sticken E, Pype J, Kohlgrueber K, Voelkel H, Wittke S. Mainstream Smoke Chemistry and in Vitro and In Vivo Toxicity of the Reference Cigarettes 3R4F and 2R4F. Beitr. Tabakforsch. Int./Contrib. Tob. Res. 2014;25:316–335. [Google Scholar]