Abstract
Memory for contextual fear conditioning relies upon the retrosplenial cortex (RSC) regardless of how long ago conditioning occurred, whereas areas connected to the RSC, such as the dorsal hippocampus (DH) and anterior cingulate cortex (ACC) appear to play time-limited roles. To better understand whether these brain regions functionally interact during memory processing and how the passage of time affects these interactions, we simultaneously recorded local field potentials (LFPs) from these three regions as well as anterior dorsal thalamus (ADT), which provides one of the strongest inputs to RSC, and measured coherence of oscillatory activity within the theta (4–12Hz) and gamma (30–80Hz) frequency bands. We identified changes of theta coherence related to encoding, retrieval, and extinction of context fear, whereas changes in gamma coherence were restricted to fear extinction. Specifically, exposure to a novel context and retrieval of recently acquired fear conditioning memory were associated with increased theta coherence between RSC and all three other structures. In contrast, RSC-DH and RSC-ADT theta coherence were decreased in mice that successfully retrieved, relative to mice that failed to retrieve, remote memory. Greater RSC-ADT theta and gamma coherence were observed during recent, compared to remote, extinction of freezing responses. Thus, the degree of coherence between RSC and connected brain areas may predict and contribute to context memory retrieval and retrieval-related phenomena such as fear extinction. Importantly, although theta coherence in this circuit increases during memory encoding and retrieval of recent memory, failure to decrease RSC-DH theta coherence might be linked to retrieval deficit in the long term, and possibly contribute to aberrant memory processing characteristic of neuropsychiatric disorders.
Introduction
Cognitive function is associated with the concurrent activation of a distributed network of brain regions, with the synchronization of activity between these regions often directed by one or more distinct “hubs.” Disruption of activity within such networks is associated with pathological cognitive and emotional states (Grimm et al., 2009; Hamilton et al., 2011; Yu et al., 2013), underlying the need to understand how network activity in the brain is generated and how it relates to behavior. In rodents, studies of coherent activity between brain regions has typically focused on learning, but much less is known about how network activity underlies memory retrieval or how such networks change as memories age.
One measure of network activity in the brain is the coherence of oscillatory activity across brain regions. Oscillations in the theta (4–12Hz) and gamma (30–80Hz) frequency ranges have been especially implicated in mnemonic functions in both rodents (Colgin, 2015; Fitzgerald et al., 2015; Kay, 2005; Vertes, 2005) and humans (Barr et al., 2009; Klimesch et al., 2001; Lega et al., 2012; Rutishauser et al., 2010) due to their role in synchronizing and integrating activity across distributed networks of brain regions (Kirk and Mackay, 2003). Of the many regions in which these oscillations have been observed, retrosplenial cortex (RSC) is especially interesting because it 1) provides an essential conduit for the propagation of hypothalamic-generated oscillatory activity to other brain regions (Destrade and Ott, 1982) while also generating local theta oscillations during learning (Talk et al., 2004), 2) is the cortical region in humans most consistently activated by emotionally salient stimuli (Maddock, 1999), and 3) unlike most other brain areas, plays a time-independent role in the retrieval of contextual (Corcoran et al., 2011) and spatial (Haijima and Ichitani, 2008) memories. Thus, RSC is uniquely situated to act as a hub of activity underlying the retrieval of both recently and remotely acquired memories. It is unknown, however, whether coherent activity between RSC and other regions correlates with memory retrieval, or how the patterns of such coherence change as memories age.
To address this issue, we trained mice in a contextual fear conditioning experiment and recorded simultaneous LFP activity in RSC and three regions with which it is robustly interconnected: DH, which has been implicated in the retrieval of recently acquired memories (Anagnostaras et al., 1999); ACC, which has been implicated in the retrieval of remotely acquired memories (Frankland et al., 2004); and ADT, which provides one of the largest subcortical inputs to RSC (Berger et al., 1980; van Groen and Wyss, 2003). The use of simultaneous multi-site LFP recordings allowed us to examine coherent activity between pairs of these structures prior to fear conditioning, during memory retrieval, and during fear extinction. Coherence in the theta band between RSC and the other three structures increased during memory encoding and recent memory retrieval, whereas decreased RSC-DH and RSC-ADT theta coherence were associated with successful retrieval of remote memory. Increased RSC-ADT theta coherence was observed in mice that successfully extinguished recently acquired fear memory. In contrast, RSC-ADT gamma coherence prior to fear conditioning predicted successful extinction. RSC-ACC did not show significant changes in coherent activity with memory age.
Methods
Subjects
A total of 38 nine-week-old male C57BL6/N mice obtained from a commercial supplier (Harlan, Indianapolis, IN) were used in this study. Mice were individually housed in a facility on a 12/12hr light/dark cycle (lights on at 7a.m.), and allowed free access to food and water. All procedures were approved by Northwestern University’s Animal Care and Use Committee in compliance with National Institutes of Health standards.
Surgery
Mice were anesthetized with Avertin (1.2%) and implanted with insulated silver wires (100µm diameter) aimed at RSC (1.8mm posterior, 0.4mm lateral, 0.75mm ventral to bregma), DH (1.5mm posterior, 1.0mm lateral, 1.75mm ventral), ADT (0.8mm posterior, 0.75mm lateral, 2.75mm ventral), and ACC (1.3mm anterior, 0.4mm lateral, 1.75mm ventral). All electrodes were placed in the left hemisphere. A gold screw lowered into the skull near the right parietal/occipital bone suture served as a reference and ground electrode. Two stainless steel jeweler’s screws were inserted in the skull to anchor the headcap. All wires were soldered to a 6-pin connector to which the recording devices were later attached, and the assembly was fixed to the skull with acrylic. Mice were allowed at least 72h to recover from surgery prior to behavioral procedures.
Fear conditioning
Fear conditioning took place in a 35×20×20cm Plexiglas chamber with a stainless steel rod floor (4mm diameter, 0.9cm center-to-center) in a sound-attenuating cabinet with black inner walls (TSE Inc., Bad Homburg, Germany). Mice were placed in the chamber and presented with a mild footshock (2s, 0.7mA, constant current) after 3min. The chamber was cleaned after each mouse with 70% ethanol. Subsequent 3min tests for fear to the conditioning context began 1d (Recent group) or 35d (Remote group) post-conditioning.
LFP recordings
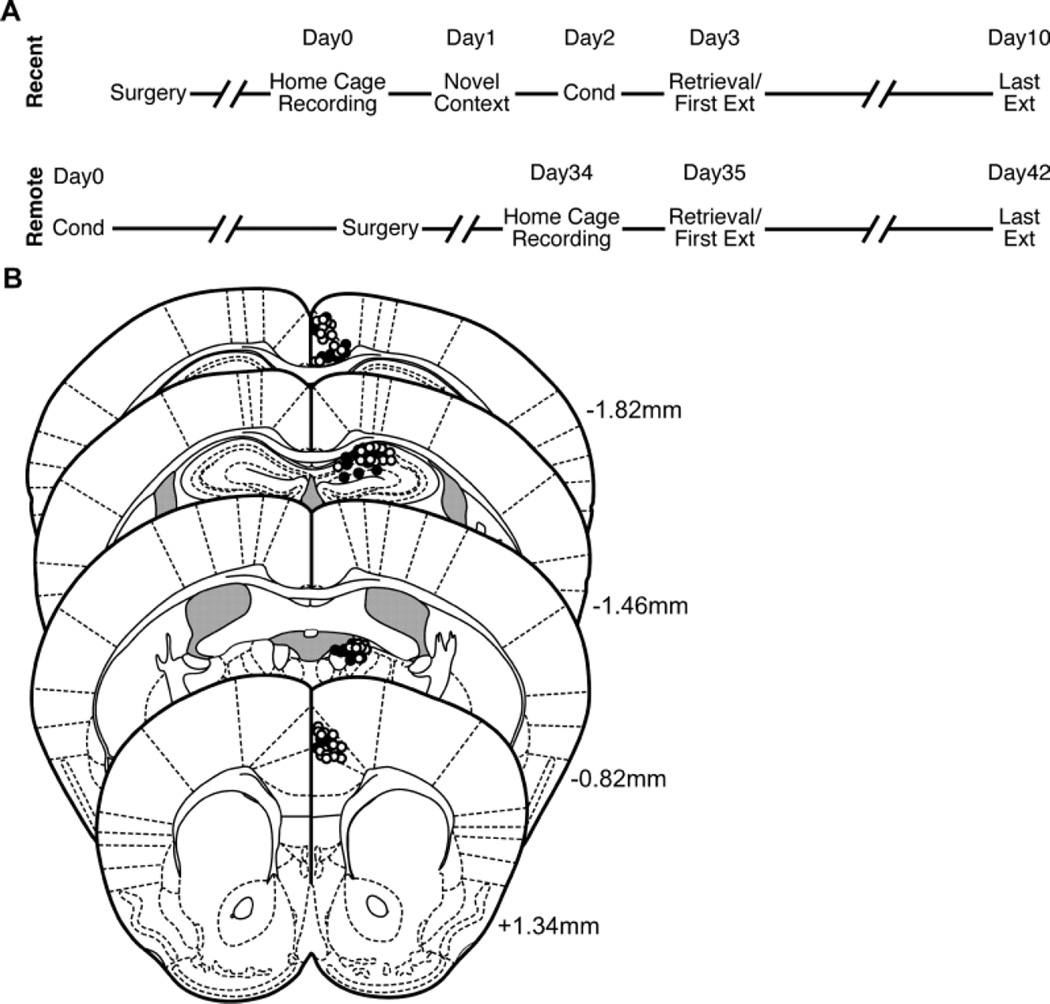
Continuous recordings were made at a sampling rate of 600Hz using wireless 4-channel recording devices (NeuroLogger, TSE Systems), which were attached to the mice prior to each session. Pre-amplification, analog-to-digital conversion (unity gain buffer, AC input range ±750µV, 1000× gain, ADC resolution 8bits), and data storage all occurred on the NeuroLogger. After each session, the NeuroLogger was removed and data were downloaded to a PC. Prior to fear conditioning, mice in the Recent group were connected to the NeuroLoggers for a recording session in their home cages to habituate them to the recording devices (home cage recording), followed 24h later by a 3min recording session in the conditioning chamber during which no shock was delivered (novel context recording). Fear conditioning occurred the following day, and retrieval/extinction tests began 24h post-conditioning. For the Remote group, fear conditioning occurred prior to electrode implantation, home cage recordings occurred on post-conditioning day 34, and retrieval/extinction tests began on post-conditioning day 35 (Figure 1A). LFPs were recorded during all 8 extinction sessions for all mice (data are only presented for the first and last extinction sessions). Mice were not connected to the recording devices during fear conditioning.
Figure 1.
(A) Timeline of experimental procedures. Mice in the Recent group went through Home Cage and Novel Context recording sessions prior to conditioning. Tests for memory retrieval began 1d post-conditioning. Mice in the Remote group were fear conditioned weeks prior to surgery. Home Cage recordings occurred 34d post-conditioning, and tests for memory retrieval began on the 35th post-conditioning day. (B) Electrode placements in RSC (Top), DH, ADT, and ACC (Bottom). Open and filled symbols represent electrode placements in mice from the Recent and Remote groups, respectively. Numbers next to illustrated sections indicate anterior/posterior distance from bregma. Atlas templates adapted from Paxinos and Franklin (2001).
Data collection and analysis
Freezing during tests for context fear was scored every 5s by a trained observer, and expressed as the percentage of the total number of observations that the mice remained motionless. During all sessions, locomotor activity in the form of infrared beam crosses was collected automatically. After fear conditioning, successful memory retrieval was defined as greater than 30% freezing during the first post-conditioning test session. LFP recordings were downloaded to a PC in compressed hexidecimal format and converted to a Matlab-compatible format for analysis. Spectral analyses were performed using open-source Chronux (http://Chronux.org) algorithms as described previously (Kay and Freeman, 1998; Rojas-Líbano et al., 2014). Coherence spectra were computed for the theta (4–12Hz) and gamma (30–80Hz) frequency bands across each 3min recording session using 35 half-overlapping 10s windows with 4 tapers, (resulting in a frequency resolution of 1.4Hz), and then transformed using the Fisher z-transform. There was no filtering. For both theta and gamma, the peak frequency within each band was taken as the center frequency, and coherence at this peak was used as the dependent measure. This z-coherence is normalized by power, allowing for direct comparison across subjects. Peak coherence in the theta and gamma bands were calculated for each mouse in each session and used for statistical analysis. To estimate the magnitude of coherence that could be expected by chance, coherence values were again calculated for each site-pair, except that data for the two structures in each site-pair were taken from separate recording sessions (e.g., RSC home cage vs. DH novel context, RSC recent retrieval vs. ADT home cage, etc.). All structure by session combinations were analyzed for each mouse at each time point for both the theta and gamma frequency bands. Significance of peak coherence values was determined by one-sample t tests against these shuffled coherence values. Group differences were determined using ANOVA or Student’s t tests, and post hoc comparisons were made using Tukey HSD tests. Pearson’s correlation coefficients were calculated to compare theta peak coherence within a site-pair to theta power in each of the individual structures that comprised that site pair. P values less than 0.05 were considered statistically significant. Verification of electrode placements was made from coronal sections through RSC, ACC, or DH (Figure 1B), and mice were only included in the final analyses if all of their electrodes were correctly placed.
Results
Increased theta coherence with RSC during novel context exploration and recent memory retrieval
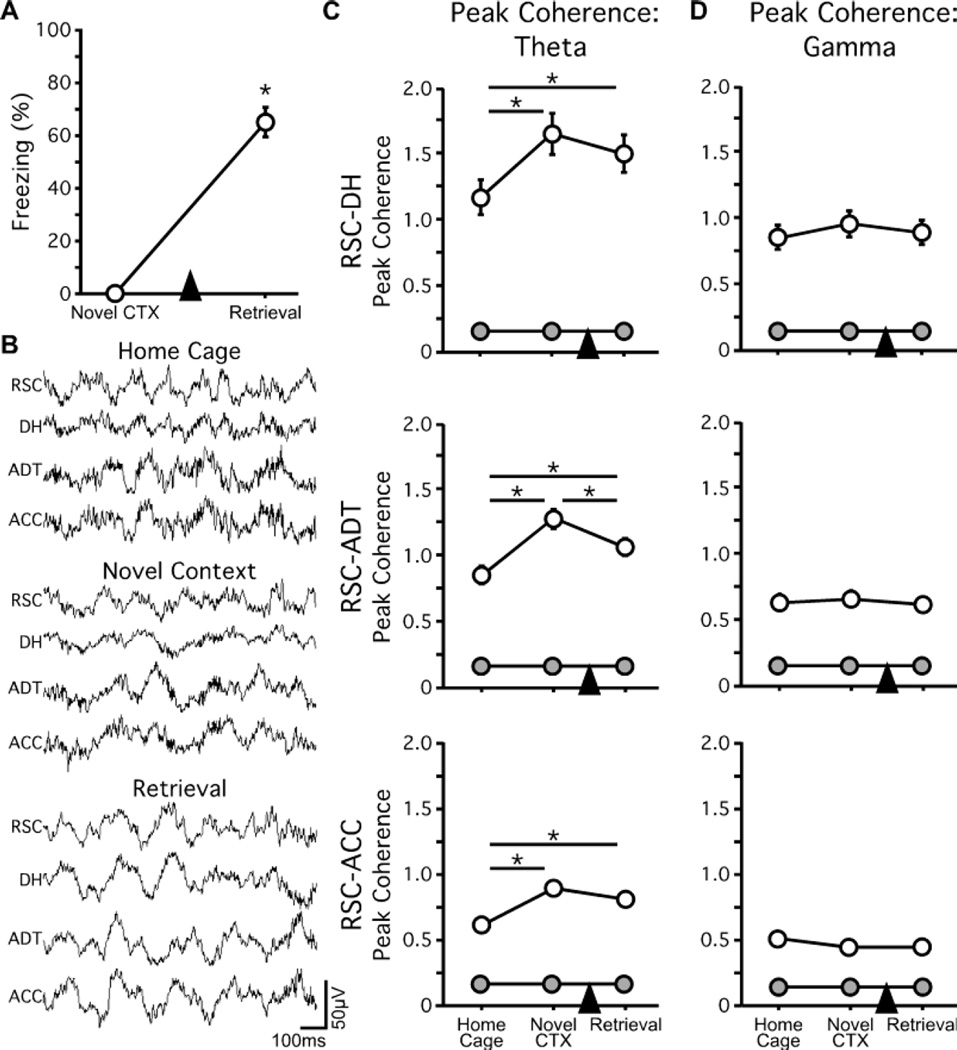
Naive mice (n = 15) were allowed to explore the novel conditioning context one day prior to fear conditioning and were tested for fear to the conditioning context one day after conditioning. All mice successfully retrieved the recently acquired conditioning memory (t14 = 11.95; p < 0.0001 versus novel context session; Figure 2A). LFPs were recorded from RSC, DH, ADT, and ACC while mice were in their home cages and exploring the novel context (prior to fear conditioning), and during memory retrieval testing (Figure 2B). Peak coherence in the theta band between RSC and the other three regions was measured for each of these three sessions, and was consistently higher than would be expected by chance (all ts ≥ 7.28; all ps < 0.0001; Figure 2C). For all three site-pairs, one-way ANOVA revealed an effect of recording session (RSC-DH: F2,28 = 11.31; p = 0.0003; RSC-ADT: F2,28 = 12.74; p = 0.0001; RSC-ACC: F2,28 = 10.63; p = 0.0004), and post hoc tests revealed that theta peak coherence was greater both during exploration of the novel context (all ps < 0.001) and during the retrieval test (all ps < 0.05), as compared to the home cage, although RSC-ADT peak coherence did decrease from the novel context to the retrieval test (p = 0.044). Despite the increased magnitude of theta peak coherence, the frequency at which the peak in each site-pair occurred remained stable across recording sessions (RSC-DH: 7.36–8.00Hz; RSC-ADT: 6.72–7.54Hz; RSC-ACC: 6.11–6.91Hz; all Fs < 1.0, all ps ≥ 0.39; data not shown). In addition, theta peak coherence increased from home cage to novel context exploration in DH-ADT (F2,28 = 7.07; p = 0.0033), DH-ACC (F2,28 = 9.95; p = 0.0005), and ADT-ACC (F2,28 = 7.23; p = 0.0029) site-pairs, and remained elevated during the retrieval test for all but the DH-ADT site-pair (data not shown). In contrast to theta peak coherence, peak coherence in the gamma band, though significantly greater than chance (all ts ≥ 6.72; all ps < 0.0001), did not change across sessions (all Fs < 2.1; all ps > 0.15; Figure 2D).
Figure 2.
Increased theta peak coherence during exploration of a novel context and recent memory retrieval. (A) All mice successfully retrieved the recently-acquired fear conditioning memory. (B) One-second sample epoch of raw LFPs simultaneously recorded from RSC, DH, ADT, and ACC from a mouse in its home cage, during novel context exploration, and during testing for memory retrieval. (C) Prior to fear conditioning, mice were allowed to explore the conditioning chamber. Peak coherence in the RSC-DH (top panel), RSC-ADT (middle panel), and RSC-ACC (bottom panel) site pairs increased during novel context exploration relative to recordings made in the home cage. This increase persisted during retrieval testing one day post-conditioning. (D) There were no differences in gamma peak coherence in any site-pair or across recording sessions. Grey symbols represent shuffled peak coherence values. Arrowheads indicate when fear conditioning occurred. *p < 0.05.
Theta peak coherence changes do not follow theta power changes
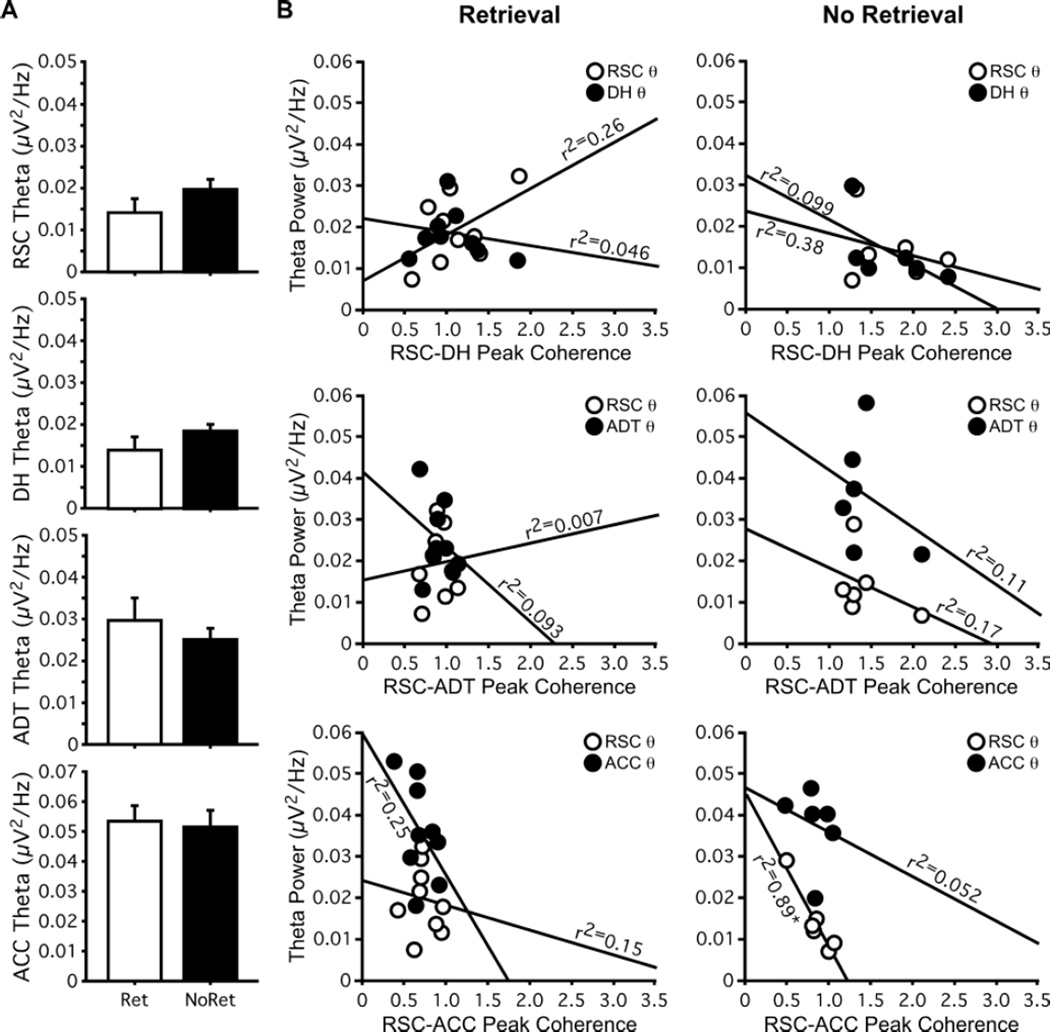
Theta rhythms in rodents are strongly influenced by ongoing motor activity, especially during exploration and spatial learning (Buszaki, 2002). Memory retrieval in our experiments reflects a robust change in motor activity (zero freezing during novel context exploration and ~70% average freezing during retrieval testing; Figure 2A), and is therefore expected to correlate with changes in theta power. During recent memory retrieval, we found significant decreases in theta power during retrieval testing compared to novel context exploration in RSC (t14 = 3.32; p = 0.0051), DH (t14 = 2.18; p = 0.048); and ACC (t14 = 5.11; p = 0.0002; Figure 3A), but no decrease in ADT theta power (p = 0.08), suggesting that theta power and theta peak coherence are not correlated.
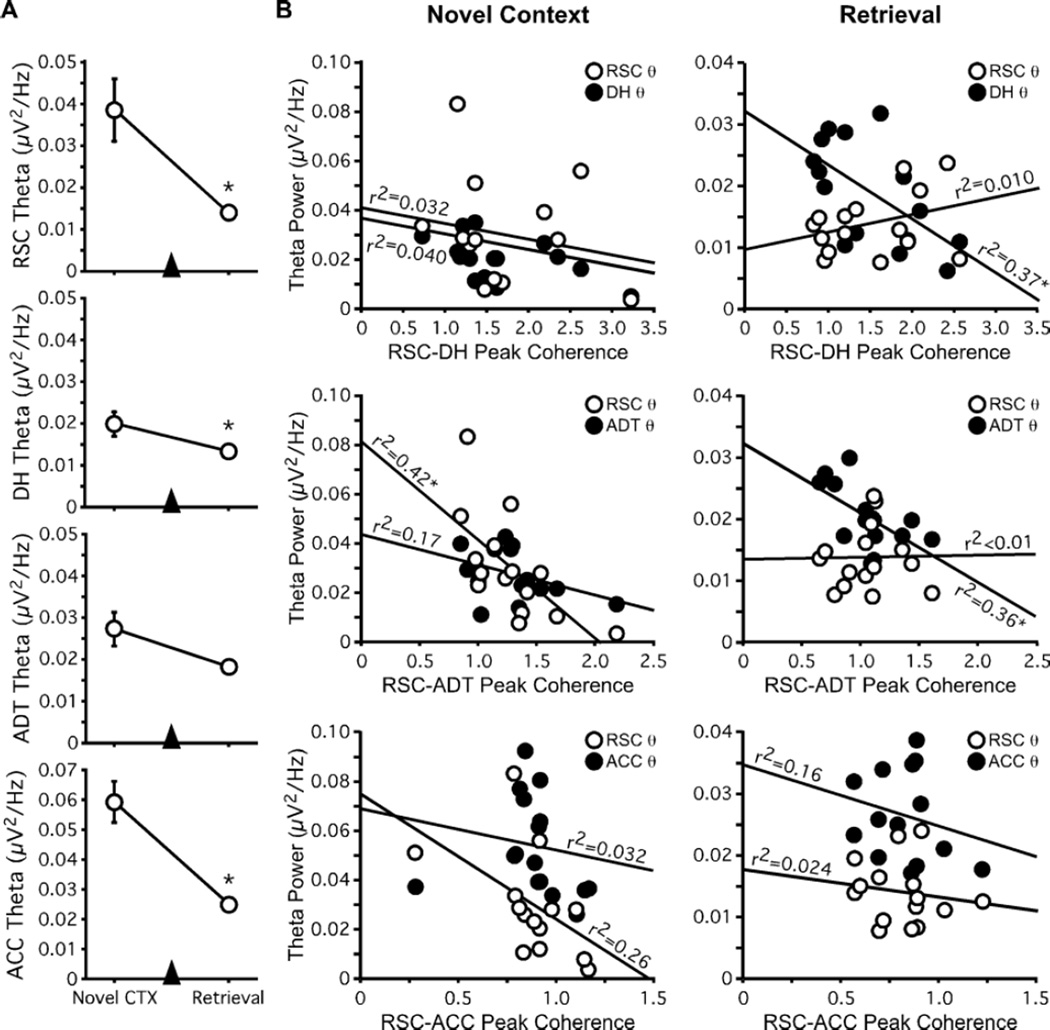
Figure 3.
Changes in theta power are not correlated with changes in theta peak coherence. (A) Theta power recorded in RSC, DH, and ACC decreased during retrieval testing relative to novel context exploration. (B) Regression plots of theta peak coherence in each site pair versus theta power recorded in the individual structures in each site pair during both novel context exploration and retrieval testing. Theta peak coherence was not consistently correlated with theta power within individual structures or across recording sessions. Open circles represent values for RSC theta power; filled circles represent values for theta power in the other region making up each site-pair. *p < 0.05.
We next performed a more direct test of whether peak coherence correlated with theta power within either of the individual structures that made up our site-pairs (Figure 3B). Strong positive correlations would indicate that peak coherence measured in a given site-pair provided only an indirect measure of theta power, rather than a distinct index of memory processing. During novel context exploration, RSC-ADT peak coherence was inversely correlated with theta power in RSC (r2 = 0.42; p = 0.0087). During retrieval testing, RSC-DH peak coherence was inversely correlated with theta power in DH (r2 = 0.37; p =0.016) and RSC-ADT peak coherence was inversely correlated with theta power in ADT (r2 = 0.36; p =0.019). No other peak coherence/power correlations were statistically significant. Thus, peak coherence measured between RSC and connected structures does not simply reflect changes in theta power, and is therefore not unduly affected by changes in movement (or lack thereof) between testing sessions. Rather, increased theta peak coherence between these regions may reflect the sensory and mnemonic processes that underlie learning about new environments and support retrieval of recently acquired contextual memories.
Decreased RSC-DH theta coherence predicts remote memory retrieval
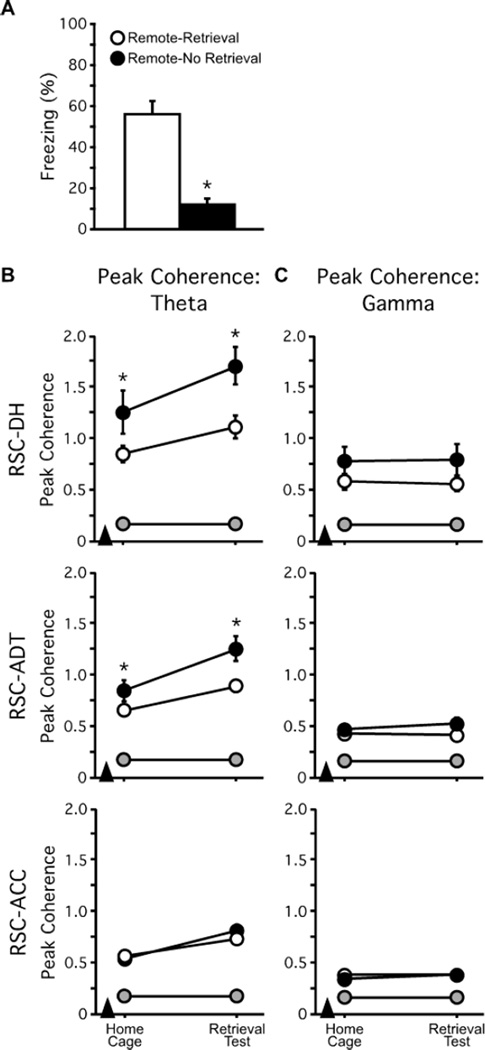
Mice were tested for memory retrieval 35d post-conditioning. During this remote memory test, just over half of the mice froze for more than 30% of the retrieval session (i.e., successful memory retrieval; n = 9; Remote-Retrieval), whereas the rest did not (i.e., retrieval failure; n = 7; Remote-No Retrieval; t1,15 = 5.32; p < 0.0001; Figure 4A). Peak coherence was greater than chance during both recording sessions for both Remote-Retrieval (ts ≥ 7.05; ps ≤ 0.0001) and Remote-No Retrieval (ts ≥ 5.75; ps ≤ 0.0012) groups. ANOVA revealed no group differences in theta peak coherence between RSC and ACC during either of the recording sessions (F1,13 = 0.69; p = 0.16; Figure 4B, Bottom). For theta peak coherence between RSC and DH, however, ANOVA revealed a main effect of group (F1,13 = 5.64; p = 0.034) and an interaction of group by recording session (F1,13 = 4.76; p = 0.048; Figure 4B, Top). Post hoc comparisons showed that theta peak coherence was lower during home cage recording in the Remote-Retrieval group compared to the Remote-No Retrieval group (p = 0.00026), and that this difference increased during the memory retrieval test (p = 0.0002). Similarly, for RSC-ADT theta peak coherence, ANOVA revealed a significant main effect of group (F1,13 = 7.21; p = 0.019) and a group by recording session interaction (F1,13 = 4.87; p = 0.046; Figure 4B, Middle). Post hoc comparisons showed that theta peak coherence was lower in the Remote-Retrieval group relative to the Remote-No Retrieval group during the home cage session (p = 0.023) and that this difference increased during the retrieval test (p = 0.00028). Despite these differences in RSC-DH and RSC-ACC theta peak coherence, the frequency at which these peaks occurred were similar between groups and across recording sessions (RSC-DH: 6.85–7.83Hz; RSC-ADT: 5.88–7.77Hz; all Fs < 3.67, all ps > 0.075; data not shown). In contrast to RSC and connected structures, there were no differences between the Remote-Retrieval and Remote-No Retrieval groups in DH-ADT, DH-ACC, or ADT-ACC theta or gamma peak coherence (data not shown). Additionally, there were no between-group or between-session differences in gamma peak coherence for any site-pair (all Fs < 2.7; all ps > 0.11; Figure 4C), although all peak coherence values were significantly greater than chance (all ts ≥ 4.13; all ps ≤ 0.0021).
Figure 4.
Pre-test RSC-DH and RSC-ADT theta peak coherence predict remote memory retrieval. (A) Freezing in the conditioning context 35d post-conditioning. During this retrieval test, mice self-selected into two subgroups: mice either succeeded (Retrieval; open symbols) or failed (No Retrieval; filled symbols) to retrieve the fear memory, as indicated by high and low levels or freezing, respectively. (B) Theta peak coherence prior to and during memory retrieval. RSC-DH (Top panel) and RSC-ADT (Middle panel) theta peak coherence were decreased in the home cage, prior to retrieval testing, in the Remote-Retrieval group. These differences persisted during the retrieval test the following day. There were no group differences in RSC-ACC theta peak coherence during either of the recording sessions (Bottom panel). (C) There were no differences in gamma peak coherence in any site-pair or across recording sessions. Grey symbols represent shuffled peak coherence values. Arrowheads indicate when fear conditioning occurred. *p < 0.05.
Despite the different freezing levels between Remote Retrieval and Remote No Retrieval groups, there were no differences in theta power recorded in any structure (all ts ≤ 1.263; all ps ≥ 0.23; Figure 5A). As with recent retrieval, theta peak coherence in each site-pair was not reliably correlated with theta power in either of the structures that made up those site pairs; the only significant correlation was between RSC-ACC theta peak coherence and RSC theta power in the Remote-No Retrieval group (r2 = 0.89; p =0.0049; Figure 5B).
Figure 5.
Theta power and theta peak coherence are not correlated during remote memory retrieval. (A) Theta power recorded in RSC, DH, and ACC was similar for Remote-Retrieval and Remote-No Retrieval groups. (B) Regression plots of theta peak coherence in each site pair versus theta power recorded in the individual structures in each site pair for Remote-Retrieval (left) and Remote-No Retrieval (right) groups. Theta peak coherence was not consistently correlated with theta power within individual structures or between groups. Open circles represent values for RSC theta power; filled circles represent values for theta power in the other region making up each site-pair. *p < 0.05.
Decreased theta peak coherence could have existed in the Remote-Retrieval group prior to fear conditioning, or could reflect pre-existing differences in locomotor behavior or responses to shock among these mice. During fear conditioning, however, mice in both the Remote-Retrieval and Remote-No Retrieval groups had similar levels of locomotor activity both prior to (t15 = 0.022; p = 0.98) and in response to (t15 = 0.90; p = 0.38) footshock (data not shown), suggesting that the groups were behaviorally indistinct. Thus, retrieval of remote memory is predicted by and associated with decreased RSC-DH and RSC-ADT theta peak coherence prior to and during testing.
Increased RSC-ADT theta and gamma peak coherence are associated with extinction of recent memory
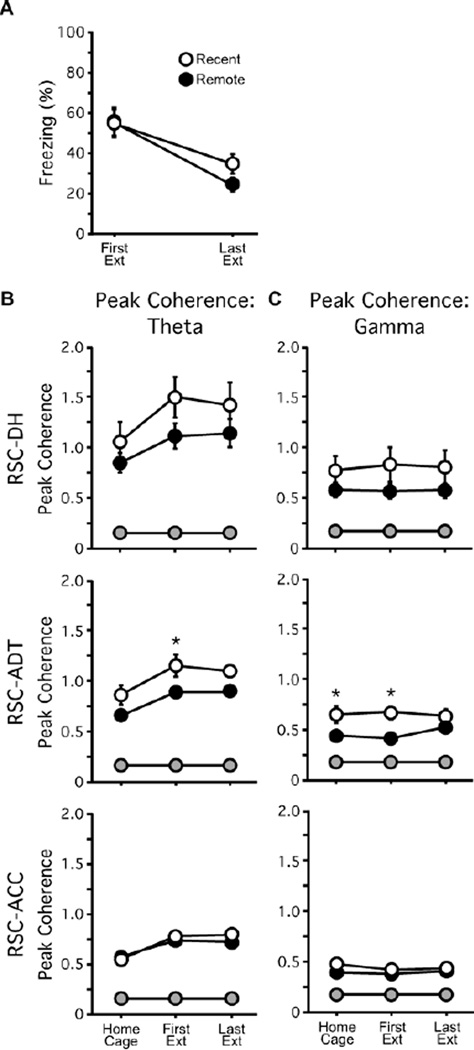
After either recent or remote memory retrieval tests, all mice were given an additional 7 consecutive days of extinction training in the conditioning context. Mice were only included in the subsequent analysis if they 1) successfully retrieved the conditioning memory on the first test day and 2) significantly reduced their freezing responses over the course of extinction training (Recent: n = 9; Remote: n = 8). There was no group difference in freezing across the first and last extinction sessions (F = 0.63; p = 0.44; Fig. 6A), but there was a significant effect of extinction session (F1,15 = 26.15; p = 0.0001), indicating that both groups successfully reduced their freezing responses over the course of extinction training. Theta peak coherence in the RSC-DH and RSC-ACC site pairs was no different between the Recent and Remote groups (Fs ≤ 1.80; ps ≥ 0.20; Figure 6B, Top and Bottom). In contrast, for the RSC-ADT site pair, ANOVA revealed a main effect of group (F1,15 = 7.66; p = 0.015; Figure 6B, Middle). Post hoc analyses showed that RSC-ADT theta peak coherence was increased in the Recent group relative to the Remote group during the first extinction session (p = 0.011). The frequency at which RSC-ADT theta peak coherence occurred was similar between groups and remained stable across testing sessions (5.72–7.17Hz; all Fs < 0.65, all ps ≥ 0.55; data not shown). Similarly, gamma peak coherence in the RSC-DH and RSC-ACC site pairs was no different between the Recent and Remote groups (Fs ≤ 1.60; ps ≥ 0.20; Figure 6C, Top and Bottom), but there was a main effect of group in the RSC-ADT site-pair (F1,15 = 4.40; p = 0.049; Figure 6C, Middle). Post hoc analyses showed that RSC-ADT gamma peak coherence was increased in the Recent group relative to the Remote group in the home cage (p = 0.0002) and during the first extinction session (p = 0.00014). There were no differences between Recent and Remote groups in DH-ADT, DH-ACC, or ADT-ACC theta or gamma peak coherence (data not shown). Thus, extinction of fear responses evoked by memories of recent events is associated with increased RSC-ADT theta and gamma peak coherence.
Figure 6.
Peak coherence during extinction of recently- versus remotely-acquired fear responses. (A) Freezing responses during the first and last of 8 daily extinction sessions. Extinction sessions began 1 (Recent; open symbols) or 35 (Remote; filled symbols) days post-conditioning. (B) RSC-ADT theta peak coherence was greater in the Recent versus the Remote group, especially during the first extinction session. (C) RSC-ADT gamma peak coherence was also greater in the Recent versus the Remote group, in both the home cage and first extinction session. There were no group differences in RSC-DH or RSC-ACC theta or gamma peak coherence. For the Recent group, fear conditioning occurred two days after the home cage recording session and one day prior to the first extinction session; for the Remote group, conditioning occurred 34d prior to the home cage recording session. Grey symbols represent shuffled peak coherence values. *p <0.05.
Discussion
We have demonstrated here that learning about novel environments, retrieval of recently and remotely acquired context memories, and extinction of fear triggered by these memories are associated with different patterns of coherent theta and gamma band activity between RSC and DH, ADT, and ACC (Fig. 6). Exploration of a novel context was associated with increased theta coherence in each of these site-pairs; this increase was maintained during memory retrieval one day after fear conditioning in that context, independent of changes in theta power within each structure. In contrast, decreased RSC-DH and RSC-ADT theta peak coherence during post-conditioning recordings in the home cage were predictive of successful retrieval of remotely acquired memory. Extinction of a recently learned fear response was associated with greater theta and gamma peak coherence between RSC and ADT than extinction of a remotely learned fear response. These findings suggest that, as memories for aversive events age, different interactions among brain regions may be required for the successful retrieval of those memories and the subsequent extinction of associated fear responses.
Based on previous studies (Anagnostaras et al., 1999; Frankland et al., 2004), we expected to find increased coherence in the RSC-DH and RSC-ACC site pairs during retrieval of recently and remotely acquired memories, respectively, as well as differences in RSC-DH peak coherence relevant to recent vs. remote fear extinction (Inda et al., 2011; Corcoran et al., 2013; Gräff et al., 2014). Our findings provided only partial support for these hypotheses, in that increased coherence between RSC and DH was only observed during exposure to a novel context and re-exposure to that context one day after fear conditioning. Contrary to our expectation, ACC did not show coherent activity with RSC during retrieval of remote memory, and neither DH nor ACC was coherent with RSC during fear extinction. These results do not negate potential roles for DH or ACC in retrieval or extinction at the expected time points, however, because our measurements were only indicative of the degree to which their activity was coherent. Thus, although DH and ACC activity may still be required for recent and remote retrieval and extinction, respectively, their contributions were most likely unrelated to their interactions with RSC.
When exposed to the conditioning context for the first time, theta coherence increased in all three of the site-pairs. RSC receives a robust input from visual cortex (Vogt, 1985), as well as highly processed sensory information from other cortical regions (Kobayashi and Amaral, 2003). These connections are hypothesized to support a role for RSC in spatial navigation and reconciling allocentric environmental cues with egocentric states (Vann et al., 2009). Connections between RSC and subcortical structures such as ADT and DH support a variety of learning-related functions, including cue-elicited neuronal activity, immediate early gene responses, and synaptic plasticity, whereas cortical RSC connections such as ACC are necessary for long-term memory storage (reviewed in Miller et al., 2015). Thus, increased coherence between RSC and DH, ADT, and ACC during novel context exploration might reflect the integration of sensory and mnemonic information across this network of structures as the mice formed a representation of the conditioning chamber. Similarly, the maintenance of increased RSC-DH and RSC-ADT peak coherence during recent retrieval testing may reflect the recall of that contextual representation.
Our data suggest that hippocampal and retrosplenial activity are uncoupled when retrieving memories long after they are formed, as successful retrieval of remote memory was associated with decreased RSC-DH peak coherence. Because it is difficult to keep electrode implants viable for the 5+ weeks of our study, we were unfortunately unable to record pre-conditioning LFPs in the home cage of mice that would later be tested for remote memory. It therefore remains to be seen whether the decreased RSC-DH peak coherence in mice that successfully retrieved the remote memory existed prior to, or was induced by, contextual fear conditioning. Nevertheless, decreased cortico-hippocampal coherence during remote memory retrieval is consistent with previous research, in which synchronization between entorhinal cortex and hippocampus in an eyeblink conditioning task decreased as the post-conditioning interval increased (Takehara-Nishiuchi et al., 2012). Our findings are also consistent with the standard model of systems consolidation of memory, which posits that as memories age, their storage and retrieval become increasingly independent of hippocampal activity (Frankland and Bontempi, 2005). This uncoupling may serve to limit hippocampus-mediated interference with cortical memory traces, which would otherwise lead to decontextualization, generalization, and ultimately poorer retrieval of remote memory (Yassa and Reagh, 2013).
The interplay between hippocampus and RSC supports both initial learning of contextual information as well as the updating of well-consolidated long-term memory (Miller et al., 2015). Consistent with this, extinction of fear responses requires extensive processing by DH (Tronson et al., 2012) and RSC (Corcoran at al., 2013). However, we found that extinction was only associated with increased RSC-ADT, and not RSC-DH, peak coherence during initial retrieval of a recent, relative to a remote, fear conditioning memory. These findings do not rule out a role for DH in extinction, but suggest that whereas RSC and DH act independently of one another, RSC and ADT work in concert. Given that inputs from the mediodorsal thalamic nucleus to the medial prefrontal cortex are also required for fear extinction (Hugues, Garcia, 2007), these findings collectively suggest that thalamo-cortical interactions play an important role in the reduction of learned fear.
Time-dependent reorganization of activity coherent with RSC may reflect the time-dependent reorganization of cellular activity and signaling pathways in RSC that are associated with memory consolidation and retrieval, and extinction of conditioned fear responses. Cue-elicited activity in RSC was found to be greater during retrieval testing 48 hours post-conditioning than during testing immediately after conditioning in rabbits performing an aversive avoidance task (Freeman and Gabriel, 1999). This delayed increase suggests a time-limited role of RSC in memory consolidation; we unfortunately did not record activity immediately post-conditioning, or in the home cage days to weeks after conditioning, so it remains to be seen whether consolidation-related changes occur in coherence between RSC and connected structures. At a molecular level, retrieval of remote memory for context fear conditioning and subsequent extinction of freezing behavior require protein kinase A-dependent signaling in RSC. This pathway is not necessary for retrieval of recently acquired memory or the extinction of recently acquired freezing responses (Corcoran et al., 2013). A key question for future studies is how time-dependent activity in the intracellular signaling cascades necessary for retrieval and extinction contributes to the functional connectivity between brain regions that make up the networks of recent and remote memory.
In summary, time-dependent changes in RSC-DH and RSC-ADT coherence suggest that RSC may serve as a hub that coordinates the activity of distinct brain regions mediating recent and remote memory. Such a role has been hinted at by human and rodent imaging studies which have identified RSC as a node of connectivity between regions of the Default Mode Network (Vann et al., 2009; Upadhyay et al., 2011; White et al., 2011), in which activity is associated with emotional regulation and autobiographical memory retrieval (Bressler and Menon 2002), and dysfunction is thought to underlie a host of mental disorders, including major depression (Hamilton et al., 2011) and anxiety (Boyd et al., 2009). Disruptions in RSC-dependent interregional connectivity may yield susceptibility to extinction failure, which has been posited as a mechanism underlying the persistence of symptoms in post-traumatic stress disorder (Rothbaum and Davis, 2003), or to aging-related impairments in memory retrieval, as RSC is among the first areas of the brain to show metabolic decline (Minoshima et al., 1997), and functional connectivity with RSC is disrupted (Andrews-Hanna et al., 2007; Jones et al., 2011), in both mild cognitive impairment and Alzheimer’s disease. Treatment for disorders that reflect altered RSC functional connectivity will therefore need to account for time-dependent engagement of distinct neural networks.
Highlights.
Retrosplenial cortex (RSC) plays a time-independent role in the retrieval of memory for contextual conditioning and extinction of freezing responses
RSC is interconnected with cortical and subcortical regions also involved in context fear memory, including dorsal hippocampus (DH), anterior dorsal thalamus (ADT), and anterior cingulate cortex (ACC)
Coherent theta activity between these regions increased during exploration of a novel context and remained elevated during re-exposure to that context one day after fear conditioning
Successful remote memory retrieval was associated with decreased theta coherence between RSC-DH and RSC-ADT
Increased RSC-ADT theta and gamma activity were associated with extinction of recently-acquired freezing responses
Acknowledgments
This work was supported by NIMH grant MH078064 and Dunbar Funds to JR and NIDCD grant DC014367 to LMK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare no competing financial interests.
References
- Aggleton JP. Understanding anterograde amnesia: Disconnections and hidden lesions. Q J Exp Psychol. 2008;61:1441–1471. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ. Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharm. 2009;34:2359–2367. doi: 10.1038/npp.2009.79. [DOI] [PubMed] [Google Scholar]
- Berger TW, Milner TA, Swanson GW, Thompson RF. Reciprocal anatomical connections between anterior thalamus and cingulate—retrosplenial cortex in the rabbit. Brain Res. 1980;201:411–417. doi: 10.1016/0006-8993(80)91044-6. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Boyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cog Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Do slow and fast gamma rhythms correspond to distinct functional states in the hippocampal network? Brain Res. 2015 doi: 10.1016/j.brainres.2015.01.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, Guedea AL, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Radulovic J. Extinction of remotely acquired fear depends on an inhibitory NR2B/PKA pathway in the retrosplenial cortex. J Neurosci. 2013;33:19492–19498. doi: 10.1523/JNEUROSCI.3338-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrade C, Ott T. Is a retrosplenial (cingulate) pathway involved in the mediation of high frequency hippocampal rhythmical slow activity (theta)? Bran Res. 1982;252:29–37. doi: 10.1016/0006-8993(82)90975-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Pinard CR, Camp MC, Feyder M, Sah A, Bergstrom HC, Graybeal C, Liu Y, Schlüter OM, Grant SG, Singewald N, Xu W, Holmes A. Durable fear memories require PSD-95. Mol Psychiat. 2015 doi: 10.1038/mp.2014.161. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Gabriel M. Changes of cingulothalamic topographic excitation patterns and avoidance response incubation over time following initial discriminative conditioning in rabbits. Neurobiol Learn Mem. 1999;72:259–272. doi: 10.1006/nlme.1998.3896. [DOI] [PubMed] [Google Scholar]
- Garden DLF, Massey PV, Caruana DA, Johnson B, Clea Warburton E, Aggleton JP, Bashir ZI. Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: Expanding the pathology of diencephalic amnesia. Brain. 2009;132:1847–1857. doi: 10.1093/brain/awp090. [DOI] [PubMed] [Google Scholar]
- Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, Rei D, Bero AW, Phan TX, Wagner F, Holson E, Xu J, Sun J, Neve RL, Mach RH, Haggarty SJ, Tsai LH. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156:261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharm. 2009;34:932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Haijima A, Ichitani Y. Anterograde and retrograde amnesia of place discrimination in retrosplenial cortex and hippocampal lesioned rats. Learn Mem. 2008;15:477–482. doi: 10.1101/lm.862308. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiat. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland BC, Collings DA, McNaughton N, Abraham WC, Dalrymple-Alford JC. Anterior thalamic lesions reduce spine density in both hippocampal CA1 and retrosplenial cortex, but enrichment rescues CA1 spines only. Hippocampus. 2014;24:1232–1247. doi: 10.1002/hipo.22309. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Machulda MM, Vemuri P, McDad EM, Zeng G, Senjem ML, Gunter JL, Przybelski SA, Avula RT, Knopman DS, Boeve BF, Petersen RC, Jack CR. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Theta oscillations and sensorimotor performance. Proc Nat Acad Sci. 2005;102:3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 1998;112:541–553. doi: 10.1037//0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, Mackay JC. The role of theta-range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex. 2003;39:993–1008. doi: 10.1016/s0010-9452(08)70874-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Röhm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Cog Brain Res. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Marchand A, Faugère A, Coutureau E, Wolff M. A role for anterior thalamic nuclei in contextual fear memory. Brain Struct Funct. 2014;219:1575–1586. doi: 10.1007/s00429-013-0586-7. [DOI] [PubMed] [Google Scholar]
- Miller AM, Vedder LC, Law LM, Smith DM. Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. Front Hum Neurosci. 2014 doi: 10.3389/fnhum.2014.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Rojas-Líbano D, Frederick DE, Egaña JI, Kay LM. The olfactory theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci. 2014;8:2014. doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis ME. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Maal-Bared G, Morrissey MD. Increased entorhinal-prefrontal theta synchronization parallels decreased entorhinal-hippocampal theta synchronization during learning and consolidation of associative memory. Front Beh Neurosci. 2012;5:1–13. doi: 10.3389/fnbeh.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talk A, Kang E, Gabriel M. Independent generation of theta rhythm in the hippocampus and posterior cingulate cortex. Bran Res. 2004;1015:15–24. doi: 10.1016/j.brainres.2004.04.051. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci. 2012;35:145–155. [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Baker SJ, Chandran P, Miller L, Lee Y, Marek GJ, Sakoglu U, Chin CL, Luo F, Fox GB, Day M. Default-mode-like network activation in awake rodents. PLoS One. 2011;6:e27839. doi: 10.1371/journal.pone.0027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:192–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: A tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vogt BA. In: Cerebral Cortex: Association and Auditory Cortices. Peters A, Jones E, editors. Vol. 4. New York: Plenum; 1985. pp. 88–149. [Google Scholar]
- White BR, Bauer AQ, Snyder AZ, Schlaggar BL, Lee JM, Culver JP. Imaging of functional connectivity in the mouse brain. PLoS One. 2011;6:e16322. doi: 10.1371/journal.pone.0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Reagh ZM. Competitive trace theory: A role for the hippocampus in contextual interference during retrieval. Front Behav Neurosci. 2013;7:107. doi: 10.3389/fnbeh.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Shen H, Zeng LL, Ma Q, Hu D. Convergent and divergent functional connectivity patterns in schizophrenia and depression. PLoS One. 2013;8:e98250. doi: 10.1371/journal.pone.0068250. [DOI] [PMC free article] [PubMed] [Google Scholar]