Abstract
Irinotecan (CPT-11) is a first-line anti-colon cancer drug, however; CPT-11-induced toxicity remains a key factor limiting its clinical application. To search for clues to the mechanism of CPT-11-induced toxicity, metabolomics was applied using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. Intraperitoneal injection of 50 mg/kg of CPT-11 induced loss of body weight, and intestine toxicity. Changes in gallbladder morphology suggested alterations in bile acid metabolism, as revealed at the molecular level by analysis of the liver, bile, and ileum metabolomes between the vehicle-treated control group and the CPT-11-treated group. Analysis of immune cell populations further showed that CPT-11 treatment significantly decreased the IL-10-producing CD4 T cell frequency in intestinal lamina propria lymphocytes, but not in spleen or mesenteric lymph nodes. In vitro cell culture studies showed that the addition of bile acids deoxycholic acid and taurodeoxycholic acid accelerated the CPT-11-induced suppression of IL-10 secretion by activated CD4+ naive T cells isolated from mouse splenocytes. These results showed that CPT-11 treatment caused metabolic changes in the composition of bile acids that altered CPT-11-induced suppression of IL-10 expression.
Keywords: irinotecan (CPT-11), metabolomics, bile acids, interleukin-10
Graphical Abstract
Introduction
Irinotecan, also called CPT-11, is a topoisomerase 1 inhibitor used for the treatment of colon cancer (Nygard et al., 2014). However, CPT-11 treatment leads to adverse effects, including leukopenia (Umezawa et al., 2000), diarrhea (Xue et al., 2007), and mucositis (Lima-Junior et al., 2014). The mechanisms for these toxic responses were investigated, revealing a significant contribution of the intestinal drug-metabolizing enzyme UDP-glucuronosyltransferase (UGT) 1A1 towards CPT-11-induced intestinal damage (Iyer et al., 1998; Chen et al., 2013). Interleukin-33 (IL-33) was also demonstrated to mediate CPT-11-induced intestinal mucositis and severe diarrhea, and inhibition of the IL-33/ST2 pathway could limit mucositis (Guabiraba et al., 2014). Targeted inhibition of interleukin-18 (IL-18) can attenuate CPT-11-induced intestinal mucositis in mice (Lima-Junior et al., 2014). However, the mechanism by which these cytokines are altered remains to be defined.
Metabolomics is an important tool in determining the compositions and levels of metabolites in a serum, urine, cells, and tissues,. Through analyzing the biological function of altered metabolites, clues to the mechanisms of toxicity can sometimes identified. For example, an important role of lipid metabolism in trichloroethylene-induced liver toxicity was revealed using metabolomics (Fang et al., 2013). Gemfibrozil-induced disruption of lysophosphatidylcholine and bile acid homeostasis was found to be a key factor in gemfibrozil-induced hepatotoxicity (Liu et al., 2014). The present study aimed to investigate the mechanism of CPT-11-induced metabolic disorders by analysis of endogenous metabolites using metabolomics. Furthermore, the function of metabolites altered by CPT-11-induced toxicity was explored.
Methods
Chemicals, antibodies and reagents
β-MCA, ω-MCA, UDCA, HDCA, T-α-MCA, T-β-MCA, T-UDCA, and THDCA were purchased from Steraloids Inc. (Newport, RI). α-MCA, CA, DCA, CDCA, TCDCA, TDCA, and TCA were purchased from Sigma-Aldrich (St Louis, MO). Irinotecan was obtained from Sigma-Aldrich (St Louis, MO). All other reagents and solvents were of HPLC grade. Anti-mouse CD3 (clone 145-2C11), anti-mouse CD28 (clone 37.51), anti-mouse CD16/CD32 (clone 93), anti-mouse CD45 Alexa Fluor® 700 (Clone: 30-F11), anti-mouse CD4 eFluor® 450 (Clone: GK1.5), anti-mouse CD8α PE-Cyanine7 (Clone: 53-6.7), anti-mouse CD8β FITC (Clone: eBioH35-17.2), anti-mouse TCRβ PerCP-Cyanine5.5 (Clone: H57-597), anti-mouse/rat Foxp3 APC (Clone: FJK-16s), anti-mouse IL-10 APC (Clone: JES5-16E3) were from eBiosciences (San Diego, CA). Cytofix/Cytoperm Fixation/ Permeabilization Solution and Golgi Plug protein transport inhibitor were purchased from BD Biosciences (Franklin Lakes, NJ). Foxp3 Fix/Perm Buffer Set and mouse IL-10 ELISA kit were purchased from BioLegend (San Diego, CA).
Mouse treatment and samples preparation
Twenty male C57BL/6J (6- to 8-week-old) mice were divided into a vehicle-treated control group (n=10) and a CPT-11-treated group (n=10). All studies involving mice were carried out under protocols approved by the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines. Mouse handling was performed in accordance with an animal study protocol approved by the National Cancer Institute Animal Care and Use Committee. The mice were maintained under a standard 12 h light/12 h dark cycle with water and chow provided ad libitum. CPT-11 was dissolved in dimethyl sulfoxide (DMSO), and then diluted in PBS buffer (v/v=1:1000). CPT-11 (50 mg/kg body weight) was given to mice by a single intraperitoneal injection once per day for eight days. After killing by CO2 asphyxiation, the gallbladder, liver, duodenum, jejunum, ileum, and colon were harvested, and a small section of each tissue was excised for histological analysis. All the samples were stored at −80°C until analysis. Bile was taken from the gallbladder, and 1 μl of bile diluted with 199 μl of 66% aqueous acetonitrile containing 5 μM chlorpropamide. The homogenates of liver and ileum tissue were prepared and diluted 20 fold with 50% aqueous acetonitrile (5 μM chlorpropamide). All samples were centrifuged at 14,000×g for 20 min, and 5-μl aliquots of the supernatants injected into a Waters UPLC-ESI-QTOFMS system (Waters Corporation, Milford, MA).
Histology analysis
Analysis of liver histology was performed as previously described (Cheng et al., 2014). In brief, small blocks of liver tissues from all mice were immediately fixed in 10% neutral formalin and embedded in paraffin. Sections (4 μm thick) were stained by the hematoxylin and eosin or Sirius red dyes. Histology of duodenum, jejunum, ileum, and colon tissues was performed as described in a previous study (Kim et al., 2013).
UPLC-ESI-QTOFMS analysis
UPLC-ESI-QTOFMS analysis in full-scan mode at m/z 100–1,000, was performed in the positive and negative modes, as described in a previous study (Li et al., 2013). The liquid chromatography system was an ACQUITY UPLC (Waters Corp., Milford, MA) consisting of a reverse-phase 2.1×50mm ACQUITY UPLC BEH C18 1.7 mm column (Waters Corp.) with a gradient mobile phase consisting of 0.1% formic-acid (solution A) and acetonitrile containing 0.1% formic acid (solution B). The mobile phase composition was maintained at 100% A for 0.5 min, increased to 100% B over the next 7.5 min and returned to 100% A in last 2 min. Nitrogen was used as both cone gas (50 l/h) and desolvation gas (600 l/h). Source and desolvation temperatures were set at 120°C and 350°C, respectively. The capillary and cone voltages were 3000 and 20 V, respectively. Structures of metabolites were elucidated using tandem MS fragmentography with collision energies ranging from 15 to 40 eV.
Data processing and multivariate data analysis (MDA)
MarkerLynx software (Waters Corp.) was used to deconvolute the chromatographic and mass spectrometric data. A multivariate data matrix containing information on sample identity, ion identity (Rt and m/z), and ion abundance was generated through centroiding, deisotoping, filtering, peak recognition, and integration. The data matrix was further analyzed using SIMCA-13.0 software (Umetrics, Kinnelon, NJ). Principle components analysis (PCA) was used to examine the separation of control group and MRS5980-treated group. Potential metabolites were identified by analyzing the ions contributing to the separation of sample groups in the loading scatter plots.
Cell isolation and cell culture
Spleen and mesenteric lymph nodes (MLN) were removed from mice and gently dispersed in DMEM containing 10% FBS to prepare single cell suspensions. The intraepithelial lymphocyte (IEL) and lamina propria lymphocyte (LPL) were isolated from the small intestine by mechanical separation and enzymatic digestion as described (Hall et al., 2008). IL-10 protein in CD4+ T cells was determined by intracellular IL-10 staining by flow cytometry analysis. CD4+CD25− T cells to be used for cell culture studies were separated by use of a EasySep™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit from Stemcell Technologies, and cultured with plate-bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (2 μg/ml) antibodies in the presence or absence of CPT-11 (20 μg/ml), DCA (20 μg/ml) or TDCA (20 μg/ml) in complete medium for 48 hours. Cells were then collected to determine IL-10 mRNA by quantitative PCR (qPCR), and IL-10 protein measured in culture supernatants by ELISA analysis.
Flow cytometry analysis
The cells were washed using PBS containing 0.5% BSA for 10 min at 300×g, and then incubated with antibodies against CD4, CD8α, CD8β, CD45, TCR-β in staining buffer (PBS containing 0.5% BSA) for 20 minutes, and then fixed with Foxp3 Fix/Perm Buffer Set and stained with Foxp3, or fixed with Cytofix/Cytoperm Fixation/Permeabilization solution and stained with intracellular IL-10. For intracellular IL-10 staining, the cells were stimulated with PMA, an ionomycin plus protein transport inhibitor (Golgi Plug), for 4 hours in culture medium before staining of the surface markers. Stained cells were analyzed by Fortessa flow cytometry.
Quantitative polymerase chain reaction analysis
Total liver RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), and cDNA was generated from 1 μg RNA with a SuperScript IITM Reverse Transcriptase kit and random oligonucleotides (Invitrogen, Grand Island, NY). qPCR was performed using SYBR green PCR master mix and ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The primer pairs were designed using qPrimerDepot, and messenger RNA quantitation was performed using the comparative cycle threshold (CT) method. The expression of mRNA was normalized to mouse b β-actin or Hprt mRNA.
Statistical analysis
The experimental data were presented as mean±S.E.M. Comparisons between two groups were performed using a two-tailed unpaired student’s t test or Tukey analysis. For all the tests, a value of P<0.05 was considered to be statistically significant.
Results
CPT-11 induced phenotype alteration of mice
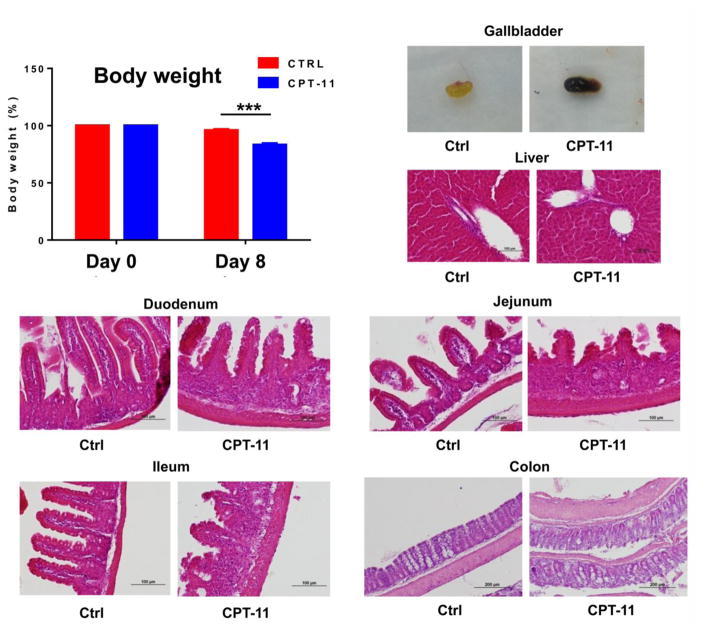
Treatment with CPT-11 for eight days significantly induced body weight loss (p<0.001, Fig. 1A), and altered gallbladder appearance (Fig. 1B). Liver histology appeared normal (Fig. 1C) and there was no elevation in the liver toxicity markers ALT and AST. However, histology analysis revealed that the intestine (including duodenum, jejunum, ileum) and colon had a dramatic remodeling of the mucosa, with different degrees of submucosal edema, submucosal hemorrhage, venous congestion, submucosal leukocyte infiltration, exfoliation of epithelial cells, and inflammatory submucosal leukocyte infiltrates, and shortening of villi (Fig. 1D–G).
Fig. 1.
Phenotype of mice after intraperitoneal (i.p.) injection of CPT-11 (50 mg/kg). (A) Body weight loss, (B) gallbladder color change, (C) liver histology, (D) duodenum histology, (E) jejunum histology, (F) ileum histology, and (G) colon histology ***, p<0.001.
CPT-11 treatment resulted in altered of bile acids composition
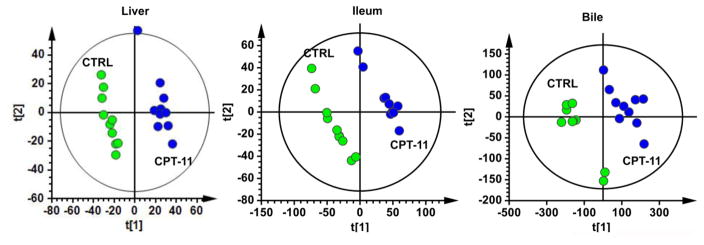
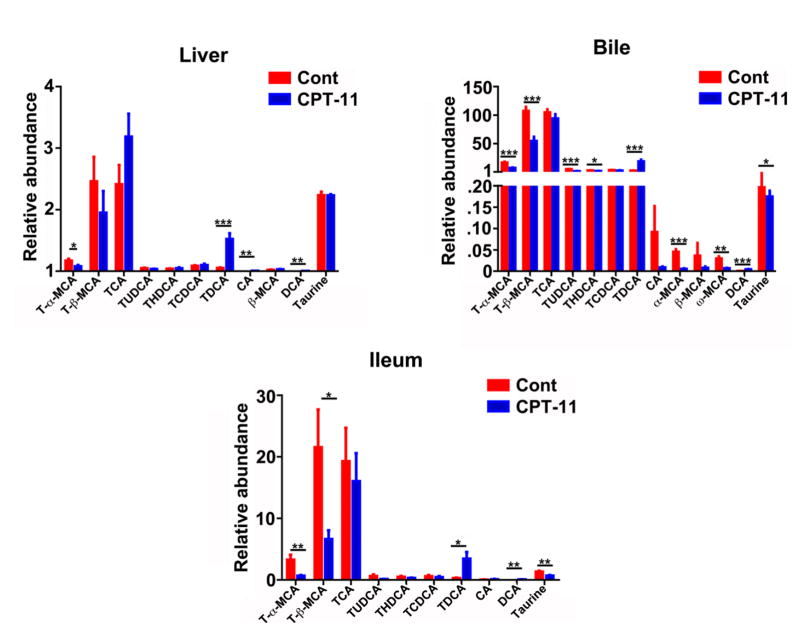
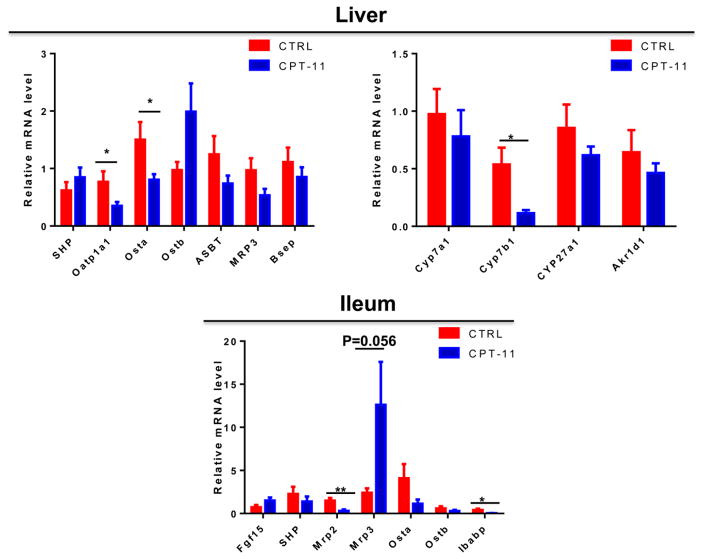
UPLC-ESI-QTOFMS analysis coupled with multivariate data analysis was employed to profile the metabolomes of liver, ileum and bile. Unsupervised principal components analysis separated the CPT-11-treated group from the control vehicle-treated group (Fig. 2). Through searching metabolomic databases (Madison Metabolomics Consortium Database and METLIN) and comparing with authentic standard standards by column retention times, molecular masses and fragmentation patterns (Supplemental figures 1–4), the major compounds contributing the group separation were identified as bile acid metabolites. CPT-11 treatment induced a decrease in T-α-MCA (p<0.05), and an elevation of TDCA (p<0.001), CA (p<0.01) and DCA (p<0.01) in liver (Fig. 3). Levels of many bile acid metabolites were changed in bile by treatment with CPT-11. The bile acid metabolites that were decreased upon CPT-11 administration included T-α-MCA (p<0.001), T-β-MCA (p<0.001), TUDCA (p<0.001), THDCA (p<0.05), α-MCA (p<0.001), w-MCA (p<0.01). Additionally, taurine was also decreased (p<0.05). Compared with the control group, the levels of DCA and TDCA were increased in the CPT-11-treated group (p<0.001). Notably, in ileum, CPT-11 treatment induced a reduction of T-α-MCA (p<0.01), T-β-MCA (p<0.05), and taurine (p<0.01). In contrast, an elevation of DCA (p<0.01) and TDCA (p<0.05) was observed. The mRNAs encoded by genes involved in the metabolism and transport of bile acids were also changed (Figure 4). In liver, CPT-11 treatment significantly decreased the expression of Oatp1a1, Ostα and Cyp7b1 mRNAs. in ileum, treatment with CPT-11 resulted in decreased expression of Mrp2 and Ibabp mRNAs.
Fig. 2.
PCA scores scatter plots of liver, ileum and bile from the vehicle-treated control group and the CPT-11-treated mice (10 mice/group). The green circles represent the vehicle-treated control group, and the blue represent the CPT-11-treated group.
Fig. 3.
Comparisons of the relative abundance of bile acid components between the vehicle-treated control group and the CPT-11-treated group. The red bar represents control group, and the blue bar represents CPT-11-treated group. The value are given as mean plus S.E.M. (n=10). *, p<0.05; **, p<0.01; ***, p<0.001.
Fig. 4.
The influence of CPT-11 on the expression of mRNAs encoded by genes related involved in the metabolism and transport of bile acids. The value are given as mean plus S.E.M. (n=10).*, p<0.05.
CPT-11 treatment impacts the intestinal immune tolerance
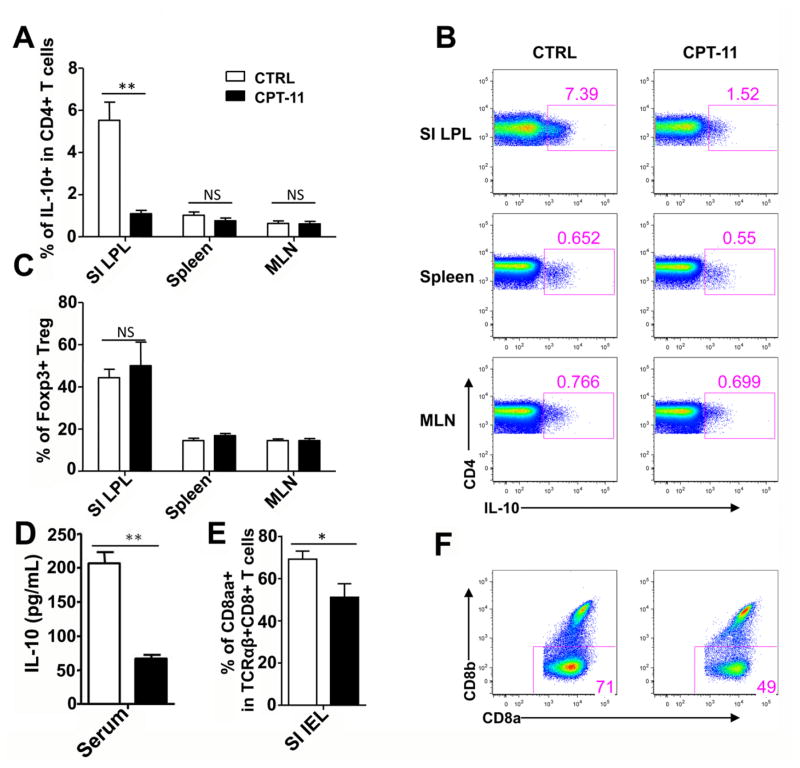
It is well established that CD4+Foxp3+ regulatory T cells (Treg cells) and IL-10 producing CD4+ T cells play critical roles in maintaining mucosal immune tolerance (Konkel et al., 2011; Wilson et al., 2011; Li et al., 2014). Furthermore, TCRαβ+CD8αα+ unconventional T cells in intraepithelial lymphocyte (IEL) can maintain immune balance (van Wijk et al., 2009; Konkel et al., 2011). This suggests that CPT-11 could alter cell populations influencing immune tolerance in the small intestine and these cells may have a role in CPT-11-induced gut toxicity. To verify this hypothesis, lymphocytes were isolated from the small intestine, Treg cells, IL-10 producing CD4+ T cells, and TCRαβ+CD8αα+ unconventional T cells, were examined in small intestine. The IL-10-producing CD4 T cell frequency was significant decreased in the lamina propria lymphocytes (LPL) (Fig. 5A and 5B), while Treg cell frequency was not altered after CPT-11 treatment (Fig. 5C). Moreover, the IL-10 concentrations in the serum was significant decreased after CPT-11 treatment (Fig. 5D), suggesting that IL-10 was systematically effected, although the reduction of IL-10 producing CD4+ T cells occurred in the small intestine, and not in spleen or mesenteric lymph nodes (MLN) (Fig. 5A and 5B). In addition, the TCRαβ+CD8aa+CD8β− unconventional T cell frequency in intraepithelial lymphocytes (IEL) was also significant decreased (Fig. 5E and 5F). Together these data indicated that CPT-11 treatment impacts intestinal immune tolerance by suppressing IL-10-producing CD4+ T cells and decreasing the frequency of TCRαβ+CD8αα+ unconventional T cells.
Fig. 5.
CPT-11 treatment affects the intestinal immune tolerance in vivo. (A) Bar graph showing the frequency of IL-10+ CD4+ T cells in the small intestine lamina propria lymphocyte (SI LPL, left), spleen (middle) and mesenteric lymph nodes (MLN, right) of the vehicle-treated control and CPT-11-treated mice. (B) Representative FACS plots showing IL-10 staining from TCRαβ+CD4+ T cells isolated from SI LPL (upper), spleen (middle) and MLN (lower) of vehicle-treated control and CPT-11-treated mice. (C) Bar graph showing frequency of Foxp3+ Treg cells in SI LPL (left), spleen (middle) and MLN (right) of the vehicle-treated control and CPT-11-treated mice. (D) Bar graph shows IL-10 concentration in serum of vehicle-treated control and CPT-11-treated mice. (E) Bar graph showing the frequency of TCRαβ+CD8aa+CD8b-unconventional T cells in the small intestine intraepithelial lymphocyte (SI IEL) of the vehicle-treated control and CPT-11-treated mice. (F) Representative FACS plots showing CD8α against CD8β staining from TCRαβ+CD8+ T cells isolated from SI IEL of vehicle-treated control and CPT-11-treated mice. Data shown are representative of two independent experiments. n=4 (control) or 5 (CPT-11-treated). Unpaired t-test. *p<0.05, **p<0.01.
DCA and TDCA aggravate IL-10 reduction caused by CPT-11 in CD4+ T cells
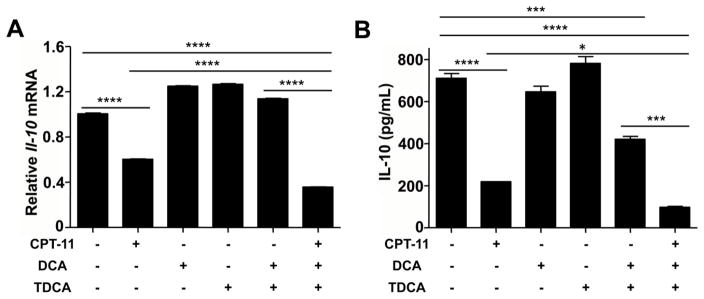
As noted earlier, in mice were treated with CPT-11, the alteration in bile acids mainly occurs mainly in the digestive tract. CPT-11 treatment also reduced IL-10 producing CD4+ T cells in the small intestine, but not in other lymphoid organs (Fig. 5A and 5B). Thus, altered bile acid compositions may contribute to the reduction of IL-10 producing CD4+ T cells and the development of mucositis. To investigate whether the changes of bile acids are involved in IL-10 reduction, taurocholic acid (DCA) and taurodeoxycholic acid (TDCA), the most significantly changed bile acids in vivo after CPT-11 treatment were used to test their influence to CD4+ T cells. CD4+ Naive T cells were isolated from splenocytes of mice and cultured under TCR stimulation (anti-CD3 plus anti-CD28) for 48 h, with or without CPT-11, DCA and/or TDCA treatment. After culturing, both Il-10 mRNA expression and IL-10 protein were reduced in CPT-11-treated cells (Fig. 6A and 6B), and DCA and TDCA treatment suppressed IL-10 production (Fig. 6B). Additionally, CPT-11, together with DCA and TDCA treatment, rapidly reduced IL-10 production (Fig. 6A and 6B). These data indicate that CPT-11 can reduce IL-10 production in CD4+ T cells, and the bile acid-induced changes may intensify the reduction of IL-10 production.
Fig. 6.
DCA and TDCA influence reduction of IL-10 caused by CPT-11 in CD4+ T cells. CD4+ Naive T cells were isolated from mice splenocytes and cultured with anti-CD3 plus anti-CD28 for 48hrs. (A) Bar graph showing Il-10 mRNA expression in T cells cultured with or without CPT-11, DCA and/or TDCA. Data were presented relative to Hprt mRNA expression. (B) Bar graph showing IL-10 production in the culture media of (A). Data shown are representative of three independent experiments. One-way ANOVA followed by Tukey analysis. *p<0.05, ***p<0.001, ****p<0.0001.
Discussion
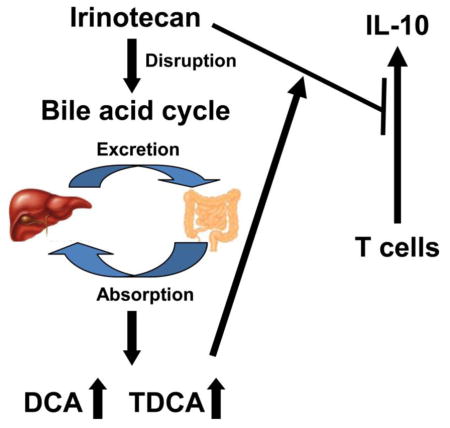
Disruption of the bile acid cycle has been observed in various toxic and disease models. Thus, elucidation of the function of bile acid metabolites in the intestine could facilitate our understanding of the mechanisms associated with toxicity and disease states. For example, lithocholic acid (LCA)-induced hepatotoxicity can disrupt the bile acids homeostasis, and therapeutic targets were found based on the alteration of bile acids, including the TGF-β-SMAD3 signaling pathway (Matsubara et al., 2012) and intestinal cytochrome CYP3A4 (Cheng et al., 2014). The present study demonstrated CPT-11 induced disruption of the bile acids cycle, including altered levels of DCA and TDCA, the most significantly changed bile acids in liver, bile and ileum. This change of bile acid metabolites might be induced by altered expression of genes involved in the metabolism and transport of bile acids. For example, Oatp1a1-null mice were found to have increased concentrations of TDCA (Zhang et al., 2012), and the expression of Oatp1a1 mRNA was decreased in CPT-11-treated mice. Therefore, the role of CPT-11-induced alteration of Oatp1a1, Osta, and Cyp7b1 gene expression in liver, and Mrp2 and Ibabp gene expression in ileum in CPT-11-induced intestinal toxicity should be investigated.
Earlier studies focused on the role of immunoregulation in CPT-11-induced intestinal damage (Guabiraba et al., 2014; Lima-Junior et al., 2014). A correlation was noted between the toxicity of CPT-11 and the elevation of pro-inflammation cytokines, but the impact of immune tolerance was not investigated in this study. It is known that the CD4+Foxp3+ regulatory T cells (Treg cells) and IL-10 producing CD4+ T cells play a critical role in maintaining immune tolerance in the mucosa (Annacker et al., 2003; Konkel et al., 2011; Harrison et al., 2013). TCRαβ+CD8αα+ unconventional T cells in intraepithelial lymphocytes can also maintain the immune balance in intestine (Poussier et al., 2002). Therefore, the variation of populations influencing immune tolerance in the small intestine during CPT-11-induced toxicity was investigated, and the results showed that IL-10-producing CD4+ T cells were significantly suppressed. IL-10 is an essential immunoregulator and plays a key role in modulating intestinal inflammation, as mice genetically deficient in IL-10 spontaneously develop severe intestinal inflammation (Rennick et al., 1995; Scheinin et al., 2003). As IL-10 is one of the critical anti-inflammatory cytokines, the present work and previous studies (Lima-Junior et al., 2014) suggest that IL-10 treatment could be a therapeutic approach to alleviate CPT-11-induced mucositis. The correlation between elevation of DCA and TDCA and suppressed production of IL-10 was further investigated using in vitro cell experimentation and potentiation of CPT-11-induced decreased IL-10 by these bile acids was demonstrated. In conclusion, the present studies demonstrated that CPT-11-induced metabolic disorders of bile acids cycle potentiate CPT-11-induced suppression of IL-10.
Supplementary Material
Highlights.
CPT-11 is an effective anticancer drug, but induced toxicity limits its application in the clinic.
CPT-11 decreased IL-10-producing CD4 T cell frequency in intestinal lamina propria lymphocytes.
CPT-11 altered the composition of bile acid metabolites, notably DCA and TDCA in liver, bile and intestine.
DCA and TDCA potentiated CPT-11-induced suppression of IL-10 secretion by active CD4+ naive T cells.
Acknowledgments
This study was funded by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Natural Science Foundation of China (No. 81202586), Tianjin Project of Thousand Youth Talents, and Shandong Natural Science Foundation of China (No. BS2013YY054 and ZR2010HL023).
Abbreviations
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CPT-11
irinotecan
- DCA
deoxycholic acid
- HDCA
hydroxyursodeoxycholic acid
- IL-10
interleukin-10
- MCA
muricholic acid
- α-MCA
α-muricholic acid
- β-MCA
β-muricholic acid
- ω-MCA
ω-muricholic acid
- T-α-MCA
tauro-α-muricholic acid
- T-β-MCA
tauro-β-muricholic acid
- TUDCA
tauro-ursodeoxycholic acid
- THDCA
tauro-hydroxyursodeoxycholic acid
- T
tauro-chenodeoxycholic acid
- TDCA
tauro-deoxycholic acid
- TCA
tauro-cholic acid
- TCT
T cell receptor
- UGT1A1
UDP-glucuronosyltransferase 1A1
- UPLC-ESI-QTOFMS
ultra-performance liquid chromatography, electrospray ionization, quadrupole time-of-flight, spectrometry mass spectrometry
- UDCA
ursodeoxycholic acid
Footnotes
Conflicts of Interest
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annacker O, Asseman C, Read S, Powrie F. Interleukin-10 in the regulation of T cell-induced colitis. J Autoimmun. 2003;20:277–279. doi: 10.1016/s0896-8411(03)00045-3. [DOI] [PubMed] [Google Scholar]
- Chen S, Yueh MF, Bigo C, Barbier O, Wang K, Karin M, et al. Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11) Proc Natl Acad Sci U S A. 2013;110:19143–19148. doi: 10.1073/pnas.1319123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Fang ZZ, Kim JH, Krausz KW, Tanaka N, Chiang JY, et al. Intestinal CYP3A4 protects against lithocholic acid-induced hepatotoxicity in intestine-specific VDR-deficient mice. J Lipid Res. 2014;55:455–465. doi: 10.1194/jlr.M044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Tanaka N, Li F, Qu A, Idle JR, et al. Metabolomics reveals trichloroacetate as a major contributor to trichloroethylene-induced metabolic alterations in mouse urine and serum. Arch Toxicol. 2013;87:1975–1987. doi: 10.1007/s00204-013-1053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, et al. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol. 2014;7:1079–1093. doi: 10.1038/mi.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Powrie FM. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. The Journal of clinical investigation. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yamaori S, Tanabe T, Johnson CH, Krausz KW, Kato S, et al. Implication of intestinal VDR deficiency in inflammatory bowel disease. Biochim Biophys Acta. 2013;1830:2118–2128. doi: 10.1016/j.bbagen.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel JE, Chen W. Balancing acts: the role of TGF-beta in the mucosal immune system. Trends Mol Med. 2011;17:668–676. doi: 10.1016/j.molmed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Alli R, Vogel P, Geiger TL. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014;7:869–878. doi: 10.1038/mi.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior RC, Freitas HC, Wong DV, Wanderley CW, Nunes LG, Leite LL, et al. Targeted inhibition of IL-18 attenuates irinotecan-induced intestinal mucositis in mice. Br J Pharmacol. 2014;171:2335–2350. doi: 10.1111/bph.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Krausz KW, Fang ZZ, Brocker C, Qu A, Gonzalez FJ. Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARalpha and its relevance to hepatotoxicity. Arch Toxicol. 2014;88:983–996. doi: 10.1007/s00204-013-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Tanaka N, Sato M, Kang DW, Krausz KW, Flanders KC, et al. TGF-beta-SMAD3 signaling mediates hepatic bile acid and phospholipid metabolism following lithocholic acid-induced liver injury. J Lipid Res. 2012;53:2698–2707. doi: 10.1194/jlr.M031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard SB, Christensen IJ, Nielsen SL, Nielsen HJ, Brunner N, Spindler KL. Assessment of the topoisomerase I gene copy number as a predictive biomarker of objective response to irinotecan in metastatic colorectal cancer. Scand J Gastroenterol. 2014;49:84–91. doi: 10.3109/00365521.2013.856464. [DOI] [PubMed] [Google Scholar]
- Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin Immunol Immunopathol. 1995;76:S174–178. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol. 2003;133:38–43. doi: 10.1046/j.1365-2249.2003.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Kiba T, Numata K, Saito T, Nakaoka M, Shintani S, et al. Comparisons of the pharmacokinetics and the leukopenia and thrombocytopenia grade after administration of irinotecan and 5-fluorouracil in combination to rats. Anticancer Res. 2000;20:4235–4242. [PubMed] [Google Scholar]
- van Wijk F, Cheroutre H. Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity. Semin Immunol. 2009;21:130–138. doi: 10.1016/j.smim.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Ramalingam TR, Rivollier A, Shenderov K, Mentink-Kane MM, Madala SK, et al. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Ralpha2-mediated attenuation of IL-13 activity. Gastroenterology. 2011;140:254–264. doi: 10.1053/j.gastro.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Sawyer MB, Field CJ, Dieleman LA, Baracos VE. Nutritional modulation of antitumor efficacy and diarrhea toxicity related to irinotecan chemotherapy in rats bearing the ward colon tumor. Clin Cancer Res. 2007;13:7146–7154. doi: 10.1158/1078-0432.CCR-07-0823. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Limaye PB, Lehman-McKeeman LD, Klaassen CD. Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. PLoS One. 2012;7:e34522. doi: 10.1371/journal.pone.0034522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.