Abstract
Calcium sensitizers enhance the transduction of the Ca2+ signal into force within the heart and have found use in treating heart failure. However the mechanisms of action for most Ca2+ sensitizers remain unclear. To address this issue an efficient fluorescence based approach to Ca2+ sensitizer screening was developed which monitors cardiac troponin C’s (cTnC’s) hydrophobic cleft. This approach was tested on four common Ca2+-sensitizers, EMD 57033, levosimendan, bepridil and pimobendan with the aim of elucidating the mechanisms of action for each as well as proving the efficacy of the new screening method. Ca2+-titration experiments were employed to determine the effect on Ca2+ sensitivity and cooperativity of cTnC opening, while stopped flow experiments were used to investigate the impact on cTnC relaxation kinetics. Bepridil was shown to increase the sensitivity of cTnC for Ca2+ under all reconstitution conditions, sensitization by the other drugs was context dependent. Levosimendan and pimobendan reduced the rate of cTnC closing consistent with a stabilization of cTnC’s open conformation while bepridil increased the rate of relaxation. Experiments were also run on samples containing cTnT(T204E), a known Ca2+-desensitizing phosphorylation mimic. Levosimendan, bepridil, and pimobendan were found to elevate the Ca2+-sensitivity of cTnT(T204E) containing samples in this context.
Keywords: cardiac tropnin, drug screening, fluorescence spectroscopy
Within the heart, Ca2+-induced contraction is regulated by the thin filament (TF) which consists of actin, tropomyosin and cardiac troponin (cTn). cTn in turn consists of cardiac troponin I (cTnI), cardiac troponin C (cTnC), and cardiac troponin T (cTnT). Ca2+ binding to the N-domain of cTnC causes reorientation of alpha-helices within this region exposing the hydrophobic cleft of cTnC into which cTnI can bind. Once bound to the hydrophobic cleft, cTnI is no longer able to exert its inhibitory action on myosin’s interaction with actin. Subsequent movement of tropomyosin away from myosin-binding sites on actin enables the actomyosin interaction which generates force and drives cardiac contraction (for thin filament reviews see 1–5). Drugs which directly target and enhance this conversion of the Ca2+ signal into force show potential in the treatment of heart failure, which is characterized by an inability of the heart to meet the body’s hemodynamic requirements. These agents are collectively known as Ca2+ sensitizers as common to all is an increase in the Ca2+ sensitivity of force generation (for reviews on Ca2+ sensitizers see 6–10). Although centered around the TF, the mechanisms of action for each Ca2+ sensitizer appear to be quite varied (7). Attempts to characterize the behavior of each Ca2+ sensitizer have proceeded via two main approaches, structural and tissue. Structural studies use NMR, MS, and/or X-ray crystallography with the goal of identifying the region of drug–protein interaction and any significant changes in protein structure induced by drug binding (10–20). Despite the wealth of molecular level detail provided by structural approaches, they tend to lack functional information such as Ca2+ sensitivity of force development, cooperativity of Ca2+ binding, kinetics of contraction/relaxation, and energy consumption. Alternatively, tissue studies readily provide such functional information but rarely are they able to connect the tissue-/cellular-/or whole organ-level functional effects of Ca2+ sensitizers with their molecular-level causes (21–27).
A method for testing Ca2+ sensitizers, which bridges the gap between the molecular and tissue level, would do much to connect the underlying protein–drug interactions with their tissue- and organ-level effects. In this study, we demonstrate a fluorescence-based approach to characterize the behavior of Ca2+ sensitizers using reconstituted TF or simpler units of cTn as model systems. This approach possesses the essential features important in evaluating the effects of a Ca2+ sensitizer on contraction, namely Ca2+ sensitivity and relaxation kinetics, while utilizing only the most basic proteins involved in Ca2+ regulation of force. We demonstrate the efficacy of this new method by testing four common Ca2+ sensitizers, EMD 57033, levosimendan, bepridil, and pimobendan, for their impact on Ca2+ sensitivity and relaxation kinetics in cTnC in the presence of various combinations of cTnC’s binding partners. We also look at the effect of these four Ca2+ sensitizers on samples containing cTnT(T204E), which has been shown to significantly reduce the cooperativity and Ca2+ sensitivity in thin filaments and cardiac fiber studies (28,29). This study provides an effective approach for drug screening which offers both molecular as well as functional insight on the behavior of Ca2+ sensitizers. Through this approach, a clearer understanding of the mechanisms of action for EMD 57033, levosimendan, pimobendan, and bepridil is obtained.
Methods and Materials
Mutant generation, protein expression, and labeling
Creation of a self-quenching labeled protein in this study was achieved by generating a recombinant double-cysteine mutant of cTnC, cTnC(T13C/N51C), which was subsequently double labeled with tetramethylrhodamine-5-maleimide (TAMRA-5-maleimide or simply TAMRA, Setareh Biotech). The cTnC(T13C/N51C), wild type cTnI and cTnT(cTnI(WT) and cTnT(WT)), as well as cTnT(T204E) were generated from wild-type rat protein clones using approaches similar to those previously reported (30–33). Briefly, using the GeneTailor™ Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA, USA), rat cDNA clones of wild-type cTnI, cTnC, and cTnT subcloned into the plasmid pSBETa were used as template DNA to generate cTnI(WT), cTnC(T13C/N51C), cTnT(WT), and cTnT(T204E). Note that, the cTnC mutant’s endogenous cysteine residues Cys−35 and Cys−84 have each been substituted with serine. Recombinant cTnC, cTnI, and cTnT clones were over expressed in E. coli strain BL21(DE3) cells and purified as previously described (34–37).
Labeling of cTnC(T13C/N51C) with TAMRA-5-maleimide was accomplished by eliminating the presence of reducing agent, in this case DTT, to facilitate exposure of the sulphydryl groups in the cysteine residues. Subsequent incubation with 4–5 molar excess TAMRA-5-maleimide overnight produced a mixture of unlabeled, singly labeled, and doubly labeled cTnC(T13C/N51C). Purification of the doubly labeled cTnC was carried out by chromatographic separation using a DEAE column. The labeling ratio was determined spectroscopically using ε542 = 97 970/cm/M. Labeling ratios for all protein modification were >95%. Cardiac tropomyosin was purified from bovine heart (38), while actin was purified from rabbit skeletal muscle (39). Doubly labeled cTnC(T13C/N51C) was reconstituted into troponin in a 1:1:1 ratio and into thin filaments using a troponin: tropomyosin: actin molar ratio of 1:1:7.0 and checked by native gel electrophoresis as previously reported (33,37,40). The regulatory function of the double-cysteine cTnC mutant, used before in our laboratory, was verified by testing its ability to participate in the Ca2+-dependent regulation of acto-S1 ATPase activity (data not shown). Multiple experiments were performed on prepared samples within 5 days, and no protein degradation was observed by electrophoretic analysis over this time period.
Ca2+ sensitizer preparation
The four Ca2+ sensitizers used in this study, EMD 57033 (Merck), Levosimendan (Sigma-Aldrich, St. Louis, MO, USA), Bepridil (Sigma-Aldrich), and Pimobendan (Merck, Kenilworth, NJ, USA), were dissolved in enough DMSO to make 6 mm drug stock solutions. Stock solutions were kept at 4 °C to prevent degradation and were remade every 2 weeks.
Steady-state fluorescence measurements
Steady-state fluorescence measurements were performed at 15 ± 0.1 °C on an ISS PC1 photon-counting spectrofluorometer equipped with a microtitrator using a band pass of 3 nm on both the excitation and emission monochromators. Samples containing 1 µm protein dissolved in working buffer, which consisted of 30 mm MOPS pH 7.0, 5 mm MgCl2, 0.20 m KCl, 1 mm DTT, and 1 mm EGTA, were tested in the presence and absence of 3 mm CaCl2. Samples were excited with 495 nm light, and the emission spectra of cTnC (T13C/N51C) either singly or doubly labeled with TAMRA were collected from 500 to 600 nm using a scanning emission monochromator. Validation of distance-dependant self-quenching between residue 13 and 51 in cTnC was achieved by measuring the relative intensity change at 578 nm of the doubly labeled sample upon the addition of saturating levels of Ca2+.
Steady-state fluorescence Ca2+ titrations
To characterize the effect of each drug on the Ca2+ sensitivity of the N-domain opening of cTnC, steady-state fluorescence was measured during Ca2+ titrations performed using the micro-titrator as described previously (33). Because of the position of residues 13 and 51 in cTnC the distance between these two residues increases significantly as Ca2+ binds to site II in cTnC and induces opening of the hydrophobic cleft which initiates the structural changes necessary for generation of force (36). As both residues are labeled with TAMRA, the fluorescence intensity at 578 nm is relatively low while the N-domain of cTnC is closed because both TAMRA molecules are in close proximity allowing for significant self-quenching. As the N-domain of cTnC binds to Ca2+ and opens the two TAMRA molecules move further apart reducing the amount of self-quenching, resulting in a higher fluorescence intensity at 578 nm. In a typical titration experiment up to 100 data points were acquired after successively injecting aliquots of 3 µL of a 14 mm Ca2+ solution into 1.2 mL of 1 µm cTnC-cTnI, cTnC-cTnI –cTnT, or TF(cTnC-cTnI – cTnT + actin-tropomyosin) in a titration buffer containing 50 mm MOPS, 1.6 mm EGTA, 4 mm nitrilotriacetic acid (NTA), 200 mm KCl, 5 mm MgCl2, and 1 mm DTT at pH 7.10 with 3.3% DMSO by volume and 100 µm drug (or no drug in the same vehicle solution). Fluorescence versus free Ca2+ concentrations data were acquired by monitoring total fluorescence intensity of samples at 578 nm with excitation at 545 nm. Data from Ca2+ titrations were fit using the Hill equation:
| (1) |
where I represents steady-state TAMRA intensity, Imin is TAMRA intensity under Ca2+-free conditions, Imax is TAMRA intensity under Ca2+-saturated conditions, pCa50 is the −Log10 ([Ca2+]) at which apparent half occupancy of cTnC Ca2+-binding sites occurs, and nH, the Hill coefficient which represents the steepness of the Ca2+-dependent increase in TAMRA fluorescence.
Stopped-flow measurements
Stopped-flow experiments were performed to examine the kinetics of closing of the N-domain of cTnC by Ca2+ dissociation using a T format KinTek stopped-flow spectrometer with a 1.8 ms dead time. Ca2+-saturated cTnI –cTnC, cTn, or TF samples were rapidly mixed with an equal volume of buffer containing an 10 mm of the Ca2+ chelator 1,2-bis (2-aminophenoxy) ethane-N,N,N′N′-tetraacetic acid (BAPTA) (41). The change in TAMRA emission intensity as a function of time after mixing was recorded for each sample. For each experiment, 15–20 of these kinetic traces were collected for every Ca2+ sensitizer, for each level of reconstitution (cTnI –cTnC, cTn, and TF). The set of kinetic traces for each sample was averaged, and nonlinear regression used to fit the averaged trace with a mono-exponential function from which the rate of conformation change was obtained.
Statistical analysis
To determine the statistical significance between control-and Ca2+ sensitizer-treated samples, one-tailed paired t-tests were performed on the titration parameters of pCa50 while two-tailed paired t-tests were performed on all other parameters reported. The justification for the use of a one-tailed t-test for the parameter of pCa50 is that we are only interested in identifying the sensitization as would be the case in an industrial drug screening aimed at identifying the effective Ca2+ sensitizers. Agents that are unable to prove significant by this test would be eliminated as potential inotropes. Tabulated parameter values are given as averages over n trials as indicated in each table, with standard deviations obtained shown next to each parameter. Significance at the 95% level is indicated by an asterisk. Unless otherwise mentioned, parameter values were obtained through nonlinear regression using the least squares fitting method to the appropriate model.
Results
Self-quenching as fluorescence marker for cTnC opening
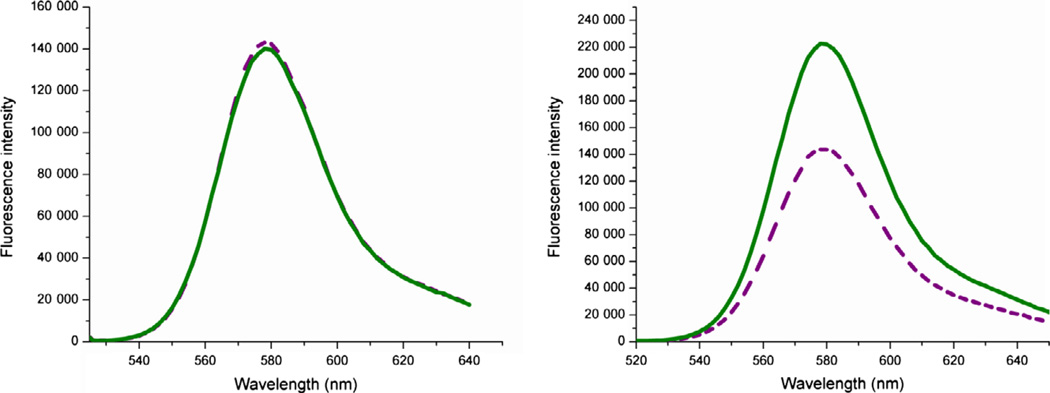
Residues cTnC(13T) and cTnC(51N) are known to move 10 Å further apart (19.21–29.06 Å) upon transitioning from cTnCs closed to open conformation (30). This structural transition requires Ca2+ co-ordination by site II of N-cTnC and subsequent binding by cTnI for stability (42). Opening of cTnC’s hydrophobic cleft then initiates the cascade of events leading to cross-bridge cycling and thereby cardiac contraction. Residues 13 and 51 in cTnC are ideal labeling sites as these residues lie in relatively flexible regions of cTnC and are not part of any alpha-helices or Ca2+ co-ordination sites. Monitoring the proximity between these residues would thus enable determination of cTnC’s activation state and deactivation rate as well as any changes induced in these parameters by Ca2+ sensitizers. To obtain this proximity information, both residues 13 and 51 in cTnC were labeled with TAMRA, a fluorophore known to undergo self-quenching to a significant extent (see Figure 1). To establish the existence of significant distance-dependent self-quenching within our doubly labeled cTnC, we measured the emission spectra of both doubly and singly TAMRA labeled cTnI –cTnC(13C/51C)-cTnT either in the absence or presence of Ca2+ (see Figure 2). For singly labeled cTnC-containing troponin, no significant intensity change resulted from the addition of Ca2+ while a large intensity change (~30%) resulted for doubly labeled cTnC samples. Because no increase in intensity is observed for the singly labeled cTnI–cTnC (13C/51C)-cTnT in the presence of Ca2+, we can rule out environmentally dependent effects underlying double-labeled cTnC’s intensity increase upon Ca2+ binding. Thus, the double-labeled cTnI–cTnC (13C/51C)-cTnT must undergo intensity changes arising from a change in distance between cTnC residues 13 and 51 whereon the TAMRA molecules have been labeled. This Ca2+-induced opening of cTnC’s hydrophobic cleft increases the distance between residues 13 and 51 resulting in reduced self-quenching and increased fluorescence intensity. Having linked the observable fluorescence intensity change with the opening of cTnC’s hydrophobic cleft allows for the measurement of Ca2+ sensitizer-induced alterations on this key structural transition.
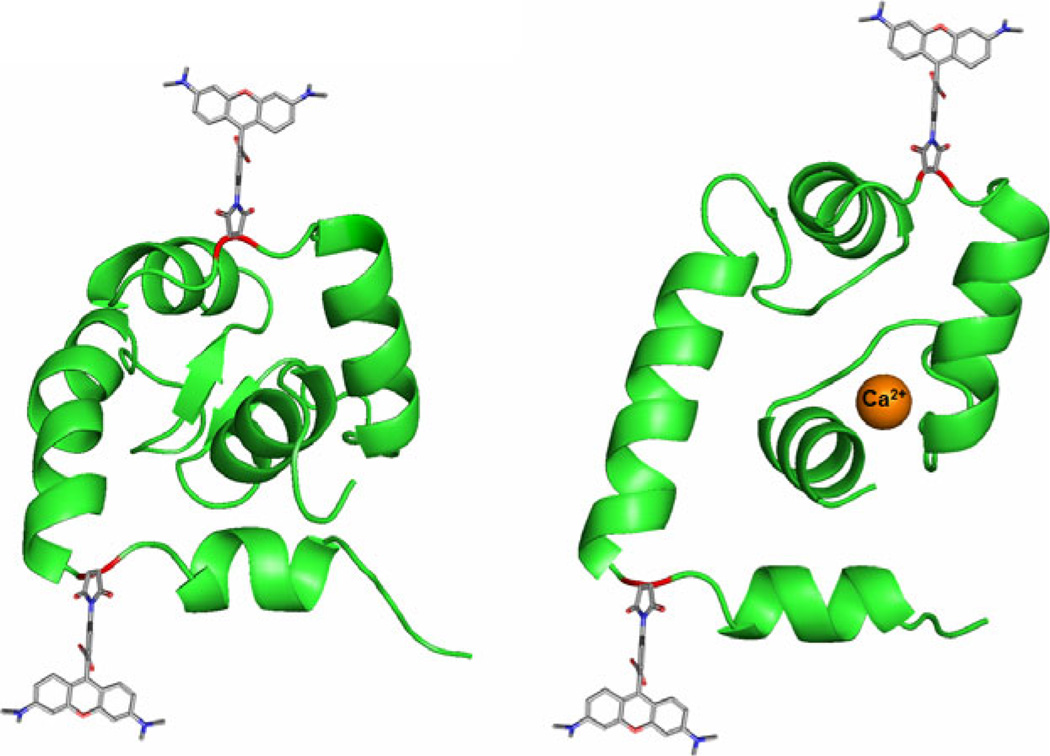
Figure 1.
Labeling scheme for the detection of hydrophobic cleft opening via self-quenching. (Left) The N-domain of cTnC is shown in the closed conformation with residues 13 (lower site) and 51 (upper site) shown in red. Both residues 13 and 51 are shown bound to TAMRA-5-maleimide. (Right) Upon binding to Ca2+, the N-domain of cTnC is able to adopt an open conformation which allows for interaction with cTnI. Ca2+ is shown in orange coordinated in cTnC site II. The residues 13 and 51 can be seen to move further apart in the open conformation, taking the attached TAMRA molecules with them. As the two TAMRA molecules separate, the ability for TAMRA to self-quench is greatly reduced resulting in higher observed fluorescence intensity (structures adapted from PDBID structures 1SPY, left, and 1J1E, right) (42,53).
Figure 2.
(Left) Singly TAMRA labeled cTnC showing no intensity change upon addition of Ca2+. Purple dashed trace shows fluorescence intensity in the Mg2+ state, while the green solid trace shows the intensity in the presence of saturating Ca2+. (Right) Doubly TAMRA labeled cTnC showing a large Ca2+-induced fluorescence intensity change. Dashed purple trace shows the Mg2+− state intensity, while the solid green trace shows the increased Ca2+ state intensity. This figure demonstrates that TAMRA’s fluorescence does not change non-specifically upon the addition of Ca2+ but requires TAMRA molecules at both residues 13 and 51 in cTnC to undergo Ca2+-dependent changes in intensity. Specifically, as residues 13 and 51 move further apart upon Ca2+ addition, this shows that TAMRA’s fluorescence is greatly diminished when in close proximity to another TAMRA molecule but increases significantly when the inter-TAMRA distance is increased. Thus, the state of cTnC’s hydrophobic cleft can be monitored via TAMRA’s fluorescence intensity.
Ca2+ titrations
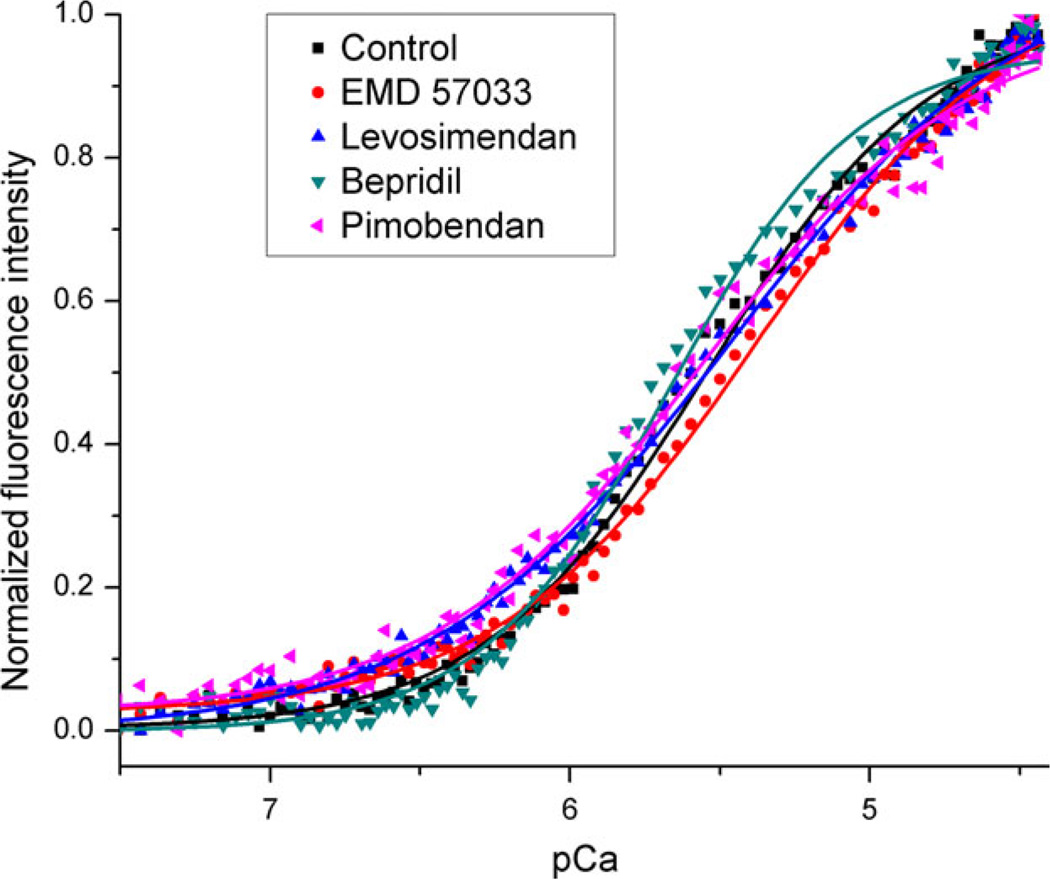
The effect of each Ca2+ sensitizer on cTnC’s Ca2+-induced hydrophobic cleft opening was assessed via Ca2+ titration in the presence of various combinations of cTnC’s binding partners. The protein combinations used were cTnC–cTnI, cTnC–cTnI–cTnT, cTnC–cTnI-cTnT + actin– tropomyosin, and cTnC–cTnI–cTnT(T204E) + actin–tropomyosin. Ca2+ titrations were carried out via controlled addition of a 14 mM Ca2+ solution to continuously stirred and temperature controlled protein samples. Subsequent fitting of the normalized fluorescence intensity versus pCa curve (see Figure 3) by the Hill equation enabled determination of the pCa50 and Hill coefficient for cTnC’s opening in the presence of each Ca2+ sensitizer and for each combination of protein-binding partners. We hypothesized that the presence of cTnC’s binding partners would alter the efficacy of each Ca2+ sensitizer according to that Ca2+ sensitizer’s mechanism of action, confirming or contesting the suggested functionality of each and establishing an efficient method for future Ca2+ sensitizer development and testing.
Figure 3.
Representative Ca2+ titration data from cTnC–cTnI–cTnT + actin–tropomyosin-containing samples. Samples are either in the presence of 100 µM Ca2+-sensitizer or vehicle (control). At high pCa (low Ca2+) TAMRA’s fluorescence intensity is at a minimum indicating maximum self-quenching, as the pCa decreases (Ca2+ increases) more cTnC molecules are bound to Ca2+ separating the two TAMRA molecules, which in turn reduces self-quenching and increases fluorescence intensity. The value of pCa at which the curves cross 0.5 fluorescence intensity is the pCa50 and gives a measure of the Ca2+ affinity for that sample. In this case, only bepridil shows significant Ca2+ sensitization.
Ca2+ sensitizer impact on cTnC–cTnI’s pCa50 and cooperativity
In samples containing cTnC–cTnI, all Ca2+ sensitizers produced an apparent increase in Ca2+ sensitivity; however, the sensitization by levosimendan is not significant by t-test (Table 1). Bepridil had the most noticeable sensitization under these conditions. There was no significant alteration in cooperativity induced by the Ca2+ sensitizers at this level of protein complexity.
Table 1.
Numerical results from fitting of the Hill equation (eqn 1) to Ca2+ titration curves for cTnC–cTnI samples treated with Ca2+ sensitizer or control
| cTnC–cTnI (n = 11) | pCa50 | nH |
|---|---|---|
| Control | 5.11 ± 0.25 | 1.31 ± 0.26 |
| EMD 57033 | 5.28 ± 0.13* | 1.42 ± 0.22 |
| Levosimendan | 5.18 ± 0.33 | 1.26 ± 0.38 |
| Bepridil | 5.38 ± 0.13* | 1.40 ± 0.23 |
| Pimobendan | 5.31 ± 0.17* | 1.21 ± 0.39 |
Parameter standard deviation is given to the right of each parameter value.
Indicates a significant difference from wild type at the 95% level.
Ca2+ sensitizer impact on cTnC–cTnI–cTnT’s pCa50 and cooperativity
Samples reconstituted with cTnC–cTnI–cTnT showed a significant increase in Ca2+ sensitivity compared to samples containing only cTnC–cTnI (control from Table 1 compared to control from Table 2). This cTnT-based sensitization may result from cTnT acting as a scaffold bringing cTnI and cTnC into proper alignment for physiological functioning as it is known that cTnT makes contacts with both cTnI and cTnC. The Ca2+-sensitizing effect of EMD 57033 and levosimendan is insignificant at this level of reconstitution, while bepridil and pimobendan maintain a significant increase in pCa50 again with bepridil showing the most marked sensitization (Table 2). This implies that the mechanism behind EMD 57033’s sensitization on cTnC–cTnI samples is either partially saturated or blocked by cTnT, while bepridil and pimobendan must enhance Ca2+ sensitivity by a distinctly different mechanism from that by which cTnT increased Ca2+ sensitivity. Again no significant alteration in cooperativity was induced by the Ca2+ sensitizers.
Table 2.
Numerical results from fitting of the Hill equation (eqn 1) to Ca2+ titration curves for cTnC–cTnI–cTnT samples treated with Ca2+ sensitizer or control
| cTnC–cTnI–cTnT (n = 10) | pCa50 | nH |
|---|---|---|
| Control | 5.27 ± 0.25 | 1.27 ± 0.28 |
| EMD 57033 | 5.32 ± 0.23 | 1.37 ± 0.28 |
| Levosimendan | 5.35 ± 0.19 | 1.40 ± 0.28 |
| Bepridil | 5.48 ± 0.12* | 1.40 ± 0.25 |
| Pimobendan | 5.43 ± 0.10* | 1.24 ± 0.20 |
Parameter standard deviation is given to the right of each parameter value.
Indicates a significant difference from wild type at the 95% level.
Ca2+ sensitizer impact on cTnC–cTnI–cTnT’s pCa50 and cooperativity in TF
Samples reconstituted with actin and tropomyosin present showed an additional increase in Ca2+ sensitivity over samples containing only cTnC–cTnI–cTnT, as well as a significant increase in Hill slope (control from Table 2 compared to control from Table 3). These effects likely result from additional protein contacts, particularly between cTnT and tropomyosin, which allow for structural transitions between cTnC and cTnI to be sensed by neighboring troponin units through the physical connection of tropomyosin. EMD 57033, bepridil, and pimobendan all appeared to elevate the Ca2+ sensitivity of the TF; however. only bepridil’s sensitization was statistically significant at this level of reconstitution. As these samples most closely resemble the TF found in healthy heart, it appears that most Ca2+ sensitizers would likely impart only a moderate sensitization in healthy patients. Figure 3 shows the titration curves for the samples tested at this level of protein complexity.
Table 3.
Numerical results from fitting of the Hill equation (eqn 1) to Ca2+ titration curves for thin filament samples treated with Ca2+ sensitizer or control
| cTnC–cTnI–cTnT +Actin– Tropomyosin (n = 8) |
pCa50 | nH |
|---|---|---|
| Control | 5.49 ± 0.15 | 1.59 ± 0.33 |
| EMD 57033 | 5.52 ± 0.14 | 1.65 ± 0.58 |
| Levosimendan | 5.44 ± 0.12 | 1.27 ± 0.35 |
| Bepridil | 5.57 ± 0.14* | 1.51 ± 0.26 |
| Pimobendan | 5.55 ± 0.20 | 1.27 ± 0.22 |
Parameter standard deviation is given to the right of each parameter value.
Indicates a significant difference from wild type at the 95% level.
Ca2+ sensitizer impact on cTnC–cTnI–cTnT (T204E)’s pCa50 and cooperativity in TF
Compared to wild-type cTnT-containing samples cTnC– cTnI–cTnT(T204E) + actin–tropomyosin samples showed both lower Ca2+ sensitivity and Hill slope, this general trend is in accordance with previous studies; however, the shifts in pCa50 and Hill slope in this study were not found to be significant at the 95% level (control from Table 3 compared to control from Table 4) (28,29). Under these conditions, levosimendan, bepridil, and pimobendan increased the Ca2+ sensitivity of cTnC to a statistically significant extent (Table 4). This leads to the notion that the mechanism underlying cTnT(T204E)’s effects on the TF is opposed by those of levosimendan and pimobendan which increased the pCa50 and Hill slope significantly. It is also noteworthy that these are the only conditions in which levosimendan’s sensitization was shown to be statistically significant, implying that in healthy heart, levosimendan may have only a moderate effect on Ca2+ sensitivity but that at least for the case of cTnT(T204E), it is able to remedy desensitization.
Table 4.
Numerical results from fitting of the Hill equation (eqn 1) to Ca2+ titration curves for cTnT(T204E)-containing thin filament samples treated with Ca2+ sensitizer or control
| cTnC–cTnI–cTnT(T204E) + Actin– Tropomyosin (n = 5) |
pCa50 | nH |
|---|---|---|
| Control | 5.42 ± 0.10 | 1.36 ± 0.16 |
| EMD 57033 | 5.45 ± 0.15 | 1.42 ± 0.28 |
| Levosimendan | 5.53 ± 0.10* | 1.51 ± 0.12* |
| Bepridil | 5.52 ± 0.13* | 1.44 ± 0.14 |
| Pimobendan | 5.51 ± 0.16* | 1.28 ± 0.35 |
Parameter standard deviation is given to the right of each parameter value.
Indicates a significant difference from wild type at the 95% level.
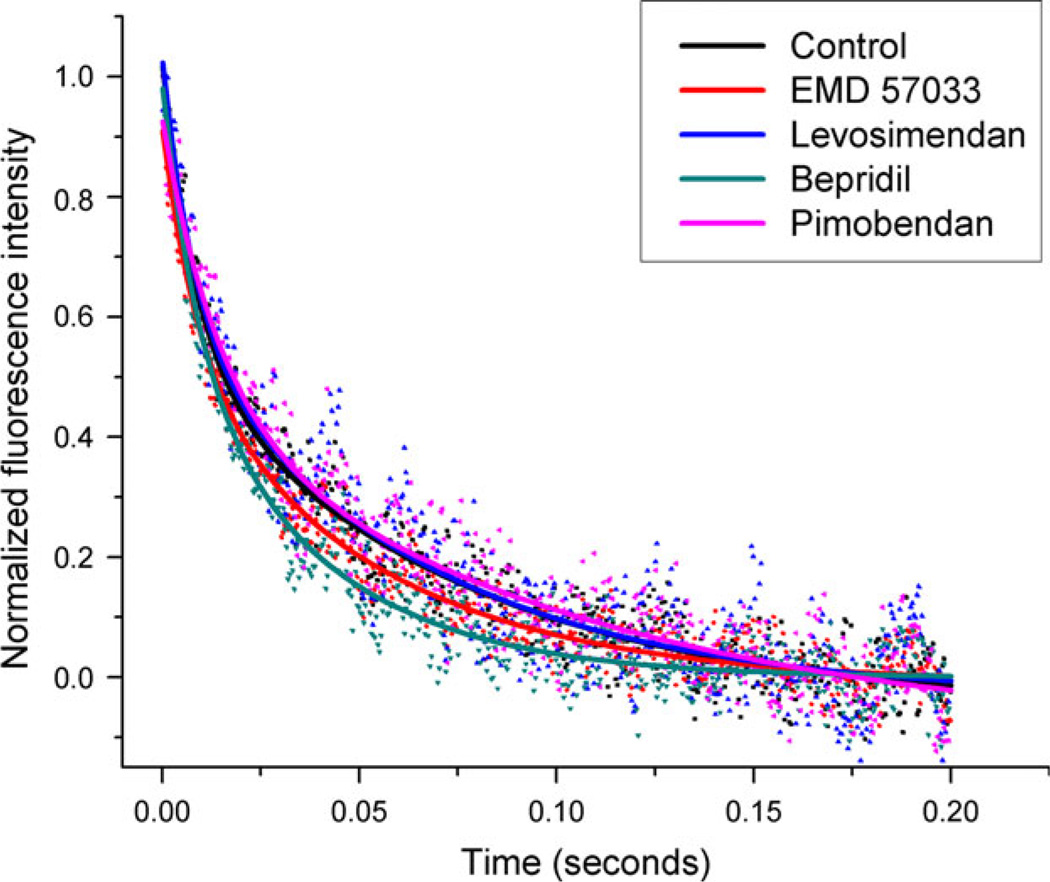
Kinetic impact of Ca2+ sensitizers and cTnC-binding partners on cTnC deactivation
The effect on relaxation is a critical consideration for drugs which impact the Ca2+ sensitivity of the sarcomere as an increased Ca2+ sensitivity is often associated with slowed deactivation which may impair diastolic function in the heart. By performing the rapid removal of Ca2+ from cTnC by mixing protein sample with BAPTA in a stopped-flow setup and monitoring the change in fluorescence intensity with respect to time, the rate of closing of cTnC’s hydrophobic cleft can be computed by fitting this fluorescence decay with an exponential function and extracting the rate constant (see Figure 4). This allows for the elucidation of the effects of each Ca2+ sensitizer and binding partner on cTnC’s rate of relaxation. The impact on deactivation rate by the various Ca2+ sensitizers showed little dependence on the presence of binding partners (Table 5). EMD 57033 did not display a significant effect on deactivation rate. This is not surprising as its impact on Ca2+ sensitivity was the most modest of all the drugs tested, only influencing the cTnC-/cTnI-containing samples. Thus, it could be surmised that EMD 57033 does not exert a strong influence on the deactivation rate within reconstituted troponin or reconstituted thin filament because its mechanism of action lies outside the thin filament. For levosimendan, an overall tendency to slow the rate of deactivation, particularly pronounced in the cTnT(T204E)-containing samples, indicates a stabilization of the troponin conformation in which cTnI is bound to Ca2+-saturated cTnC. Bepridil, which, in the titration experiments, proved to be the most potent direct Ca2+ sensitizer, leads to a consistent increase in the rate of deactivation under all combinations of binding partners (despite this often being just outside of the range of statistical significance). This is counter to the usual reasoning which says an increase in Ca2+ sensitivity tends to result from a more stable interaction between cTnC’s hydrophobic cleft and cTnI and that this stronger cTnC–cTnI interaction leads to slower deactivation. This could be explained by a steric clash between bepridil and cTnI which both bind in similar regions within cTnC’s hydrophobic cleft increasing the rate of cTnI dissociation upon rapid removal of Ca2+. Pimobendan, on the other hand, which also sensitizes the thin filament to Ca2+, results in a decrease in the rate of deactivation, the hallmark of enhanced cTnI–cTnC–Ca2+ stability.
Figure 4.
Representative stopped-flow data showing BAPTA-induced Ca2+ dissociation curves in cTnC–cTnI–cTnT + actin–tropomyosin samples. Rapid mixing of 10 mM BAPTA with Ca2+-saturated samples either with Ca2+ sensitizer or with vehicle (control) induces the removal of Ca2+ from the N-domain of cTnC. This in turn leads to dissociation of cTnI from cTnC and closure of cTnC’s hydrophobic cleft. The exponential decrease in fluorescence intensity with respect to time indicates the conformational change from open to closed in cTnC’s hydrophobic cleft. This figure shows the increased rate of relaxation for cTnC in the presence of bepridil and EMD 57033, and the slight reduction in this rate caused by pimobendan and levosimendan.
Table 5.
Kinetic parameters for Ca2+ dissociation-induced structural changes in cTnC for all levels of reconstitution
| Kinetic rate (seconds−1) |
||||
|---|---|---|---|---|
| Ca2+ sensitizer | cTnC-cTnI (n = 5) | cTn (n = 5) | cTn + TF (n = 6) | cTn(T204E) + TF (n = 3) |
| Control | 29.4 ± 11.0 | 34.5 ± 7.6 | 32.0 ± 4.2 | 30.6 ± 2.8 |
| EMD 57033 | 27.5 ± 12.7 | 34.7 ± 11.0 | 32.4 ± 3.2 | 29.2 ± 2.0 |
| Levosimendan | 29.8 ± 7.3 | 32.1 ± 5.9 | 30.5 ± 3.8 | 28.3 ± 1.7 |
| Bepridil | 37.4 ± 10.3* | 42.6 ± 14.1 | 38.0 ± 5.2 | 37.1 ± 5.1 |
| Pimobendan | 25.8 ± 5.1 | 29.2 ± 5.0* | 27.5 ± 3.3* | 25.4 ± 1.6* |
Parameters are given plus or minus one standard deviation.
Indicates a significant difference from wild type at the 95% level.
Discussion
New Ca2+-sensitizing agents that target the thin filament directly offer promise in the treatment of heart failure. Development of such drugs benefits from a clear understanding of their mechanism of action. The more complete the molecular-level picture of drug action, the more easily these and future agents can be tailored to fit a specific need. Traditional methods of characterizing Ca2+ sensitizers rely on techniques such as NMR, MS, X-ray crystallography, and cardiac myocyte or fiber studies (10–27). In this study, we have demonstrated a new fluorescence-based approach to characterize four important Ca2+ sensitizers in vitro. One benefit of this technique is that many drugs can be tested with only a marginal increase in time, thus allowing for faster development and iteration of drug design. In addition the model being used represents the simplest units involved in the Ca2+ regulation of force under physiological conditions and thus reduces the number of confounding variables compared to tissue studies. Through the use of standard in vitro fluorescence equipment, that is, a spectrofluorometer and stopped-flow device, we were able to quantitate the effect of each Ca2+ sensitizer on cTnC’s Ca2+ sensitivity and relaxation kinetics in the presence of various combinations of cTnC’s binding partners as well as in the presence of a known Ca2+ sensitivity and cooperativity reducing cTnT mutant, cTnT (T204E). Interestingly, by examining the parameters of pCa50, nH, and krelaxation, within cTnC–cTnI, cTnC–cTnI– cTnT, and TF levels of reconstitution and in the presence of cTnT(T204E), we obtained a picture of how these parameters depend on the presence of cTnT, actin, and tropomyosin. In the following sections, we discuss how our data confirm or contest the prevailing understanding of each Ca2+ sensitizer’s mechanism of action, and we also discuss the role of protein–protein interactions in cTn and the TF on Ca2+ sensitivity and relaxation kinetics.
Dependence of cTnC’s Ca2+-induced opening on the presence of binding partners
Several important observations can be made by examining the non-drug-treated samples at the various levels of reconstitution and in the presence of cTnT(T204E) (controls from Table 1–Table 5). First is the importance of cTnT on Ca2+ sensitivity. Going from cTnC–cTnI to cTnC–cTnI– cTnT, we see a significant increase in Ca2+ sensitivity. The presence of cTnT allows for formation of the ‘IT’ arm, an important structural interaction between cTnI and cTnT which may play a scaffolding role bringing cTnI and cTnC into proper alignment facilitating binding of cTnI to cTnC’s hydrophobic cleft in the presence of Ca2+ (43). The presence of cTnT may also enhance the Ca2+ sensitivity by positioning cTnI’s cardiac-specific N-terminal extension into the appropriate conformation with respect to cTnC. This region of cTnI has been implicated as playing a key role in the enhancement of cTnC’s Ca2+ sensitivity (44). In addition, there is a possibility that the N-domain of cTnT directly stabilizes cTnC’s Ca2+-bound conformation (45).
Going from cTn to the full TF means inclusion of actin and tropomyosin. These two filamentous proteins are thought to be essential for the cooperativity of the thin filament by acting as physical contacts between cTn units and conducting conformational changes longitudinally to neighboring cTn units, thus enhancing their Ca2+ sensitivity (46). Support for this is seen in the increased Hill coefficient for TF control as compared to cTn control (1.59 compared to 1.27). The additional increase in Ca2+ sensitivity seen when going from cTn to full TF likely results from the enhanced cooperativity and thus does not imply a true increase in cTnC Ca2+ affinity, but represents an apparent increase in Ca2+ binding due to enhanced cooperativity. Incorporation of cTnT(T204E) into TF shifted the pCa50 from 5.49 to 5.42 and the Hill coefficient from 1.59 to 1.36; however, these shifts were insignificant at the 95% level. The sensitization effects of levosimendan and pimobendan became more pronounced in the presence of cTnT(T204E), both becoming significant at the 95% level.
Insights into EMD 57033’s mechanism of action
Through an NMR study, it was found that EMD 57033 appears to bind the C-domain of cTnC, although the importance of this binding site is unclear (7,19). It is thought that the primary mechanism of action for EMD 57033 to directly affect the sarcomere is through enhancement of the force per cross-bridge (47). Our data are consistent with this notion of a ‘downstream’ mechanism of action for EMD 57033 as both kinetic and Ca2+ sensitivity data showed little change compared to control at most levels of reconstitution. This implies there is no direct effect on the cTnC–cTnI switch event. The most widely accepted mechanism of action for EMD 57033 is through the actin– myosin interface. As our model does not incorporate cross-bridges or the dynamic process of cross-bridge feedback, there would be no way for EMD 57033 to impact the thin filament via this process. Only Ca2+ sensitivity at the cTnC–cTnI level of reconstitution showed a significant increase upon the addition of EMD 57033 (Table 1). The cTnC–cTnI level of reconstitution showed lower Ca2+ sensitivity than either the cTn or full TF levels of reconstitution indicating that the additional protein–protein interactions conferred additional stability to cTnC’s open conformation. Perhaps at the cTnC–cTnI level, EMD 57033 was able to bind non-specifically to some of the exposed hydrophobic surface area of the cTnC–cTnI complex not yet involved in a protein–protein interaction and impart some additional stability to the open conformation of cTnC.
Insights into levosimendan’s mechanism of action
Levosimendan has been shown to bind both to C-domain and N-domain of cTnC, although the binding of C-domain is not considered physiologically important, and levosimendan’s action as a Ca2+ sensitizer is attributed to its Ca2+-dependant N-domain binding (16,48,49). The stabilization of cTnI binding to cTnC’s hydrophobic patch has been claimed as a likely mechanism of action for this drug (50). A trend of slight sensitization was seen in the titration data (Table 1–Table 4); however, this sensitization was only statistically significant in TF with cTnT (T204E) present. Our Ca2+ titrations also showed an increase in nH with this phosphomimic mutation. The rate of Ca2+ dissociation-induced cTnC relaxation showed a trend toward slower deactivation; however, this was only statistically significant in the presence of cTnT(T204E). Taken together, our findings show that levosimendan does likely enhance cTnI’s affinity for cTnC’s hydrophobic cleft, but that this effect is much more noticeable when the normal Ca2+ sensitivity/cooperativity of the TF is disrupted. This is an ideal behavior for a Ca2+ sensitizer, working to enhance Ca2+ sensitivity and cooperativity but only under conditions where the normal Ca2+ sensitivity is impaired. From our data, it is unlikely that levosimendan would have any negative impact on diastole, a common issue among Ca2+ sensitizers. It should be noted that levosimendan’s function in the presence of other physiologically relevant cTn or TF mutations/ post-translational modifications would offer more specific understanding of how this drug would work in different disease models.
Insights into bepridil’s mechanism of action
Bepridil is known to bind directly to the hydrophobic cleft of cTnC and stabilize its open conformation (13). Unlike levosimendan, this binding to cTnC is not Ca2+ dependant. In addition it has been proposed that bepridil sterically inhibits the binding of cTnI to cTnC’s hydrophobic patch (51). Our data show a marked Ca2+ sensitization at all levels of reconstitution (Table 1–Table 4) implying increased stability of cTnC’s activated state. In most cases, bepridil was clearly the most potent Ca2+ sensitizer, likely owing to its ability to bind cTnC in the presence or absence of Ca2+, thus having a stronger effect on Ca2+ sensitivity at lower levels of free Ca2+ where this sensitivity is most noticeable. Based on the results from the titration experiments with bepridil, one might anticipate a slowed rate of closing for cTnC’s hydrophobic cleft upon Ca2+ chelation by BAPTA; however, our data show an increase in cTnC’s relaxation rate. Remembering that bepridil appears to sterically interfere with cTnI’s binding to cTnC’s hydrophobic cleft helps to clear up this apparent contradiction. As the triggering event for cTnC closing in the stopped-flow experiment is the removal of Ca2+ from cTnC by BAPTA, it becomes apparent that the rate of cTnI ejection from cTnC’s hydrophobic cleft should be faster in the presence of bepridil owing to the steric destabilization. And as the total relaxation time for cTnC can be considered as the sum of the Ca2+ removal time, the cTnI ejection time, and the hydrophobic pocket closing time, it makes sense for bepridil to increase deactivation rate under these conditions as Ca2+ chelation time and cTnC closing time should be minimally affected. Thus, based on our in vitro data, bepridil would likely be a powerful enhancer of systolic force and ironically an enhancer of relaxation rate. It is important to note that simply because the rate of Ca2+ dissociation-induced relaxation is enhanced does not imply that diastole in vivo will be preserved. In fact, seeing how potently bepridil enhances Ca2+ sensitivity in a non-Ca2+-specific manner may result in incomplete relaxation at diastolic Ca2+ levels in the sarcomere despite the faster kinetics of cTnC closing.
Insights into pimobendan’s mechanism of action
Pimobendan appears to enhance the affinity of cTnC for Ca2+ and cTnI, thus sensitizing troponin to the Ca2+ signal. As pimobendan increases cTnC’s affinity for Ca2+ even at the lowest levels of Ca2+, it has been thought this drug may slow relaxation kinetics and disrupt diastole. In human cardiac muscle strips, the rate of relaxation indeed appeared to be reduced (52). Our results appear to confirm that not only does pimobendan have a direct and powerful sensitizing effect on the contractile proteins from the level of cTnI–cTnC up to full TF but that a reduction in relaxation of cTn/TF may underlie the reduced relaxation rate seen in human tissue samples. Unlike bepridil, which appears to reduce cTnI’s affinity for cTnC’s hydrophobic cleft, pimobendan is not thought to sterically inhibit this interaction and the reduced cTnC relaxation rates from this study support this notion. Pimobendan appears to act most similarly to levosimendan but with greater potency both at sensitizing cTnC to Ca2+ and in reducing the rate of relaxation, likely by increasing the affinity of cTnI for cTnC’s hydrophobic cleft. This increased potency likely results from pimobendan’s non-Ca2+-dependant interaction with cTnC making it more effective at the lower concentrations of Ca2+. Taken together, these findings show that pimobendan would be a good Ca2+ sensitizer to use only when an improvement of systolic function much outweighs the risk of a reduction in diastolic function.
Conclusion
The central hypothesis of this study appears to have been validated, namely that the level of reconstitution of the troponin complex affects the efficacy of a given Ca2+ sensitizer in a manner dependant on its mechanism of action. The fluorescence-based approach used in this study independently confirms the mechanisms of action for four well-known Ca2+ sensitizers while adding new insights on the efficacy of these sensitizers in the presence of Ca2+ desensitizing phosphomimic mutation, cTnT(T204E). The effect on relaxation of each Ca2+ sensitizer was also determined, something which has previously been difficult to obtain but which is important in assessing potential diastolic interference. Thus, this study offers several new insights on thin filament function and Ca2+ sensitizer action which were obtained using a new and efficient fluorescence-based method, amenable to drug screening and design.
Acknowledgments
This work was partially supported by the National Institutes of Health Grant HL80186 (W.-J. D.) and IR21HL109693 (W.-J. D.), and Instrument Usage grant (ID: 34731 to W.-J. D. and D. H.) from EMSL, PNNL, and by the M. J. Murdock Charitable Trust (W.-J. D.). Partial support for this publication came from the NIH/NIGMS through an institutional training grant award T32-GM008336. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
References
- 1.Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- 2.Filatov VL, Katrukha AG, Bulargina TV, Gusev NB. Troponin: structure, properties, and mechanism of functioning. Biochemistry. 1999;64:969–985. [PubMed] [Google Scholar]
- 3.Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002;54:323–333. doi: 10.1080/15216540216037. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 5.Tardiff JC. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res. 2011;108:765–782. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endoh M. Cardiac Ca2+ signaling and Ca2+ sensitizers. Circ J. 2008;72:1915–1925. doi: 10.1253/circj.cj-08-0838. [DOI] [PubMed] [Google Scholar]
- 7.Endoh M. Mechanism of action of Ca2+ sensitizers-update 2001. Cardiovasc Drugs Ther. 2001;15:397–403. doi: 10.1023/a:1013385305567. [DOI] [PubMed] [Google Scholar]
- 8.Robertson IM, Sun YB, Li MX, Sykes BD. A structural and functional perspective into the mechanism of Ca2+-sensitizers that target the cardiac troponin complex. J Mol Cell Cardiol. 2010;49:1031–1041. doi: 10.1016/j.yjmcc.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 10.Li MX, Robertson IM, Sykes BD. Interaction of cardiac troponin with cardiotonic drugs: a structural perspective. Biochem Biophys Res Commun. 2008;369:88–99. doi: 10.1016/j.bbrc.2007.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollesello P, Ovaska M, Kaivola J, Tilgmann C, Lundstrom K, Kalkkinen N, Ulmanen I, Nissinen E, Taskinen J. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J Biol Chem. 1994;269:28584–28590. [PubMed] [Google Scholar]
- 12.MacLachlan LK, Reid DG, Mitchell RC, Salter CJ, Smith SJ. Binding of a calcium sensitizer, bepridil, to cardiac troponin C. A fluorescence stopped-flow kinetic, circular dichroism, and proton nuclear magnetic resonance study. J Biol Chem. 1990;265:9764–9770. [PubMed] [Google Scholar]
- 13.Li Y, Love ML, Putkey JA, Cohen C. Bepridil opens the regulatory N-terminal lobe of cardiac troponin C. Proc Natl Acad Sci USA. 2000;97:5140–5145. doi: 10.1073/pnas.090098997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abusamhadneh E, Abbott MB, Dvoretsky A, Finley N, Sasi S, Rosevear PR. Interaction of bepridil with the cardiac troponin C/troponin I complex. FEBS Lett. 2001;506:51–54. doi: 10.1016/s0014-5793(01)02790-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li MX, Sykes BD. Structure of the regulatory N-domain of human cardiac troponin C in complex with human cardiac troponin I147–163 and bepridil. J Biol Chem. 2002;277:31124–31133. doi: 10.1074/jbc.M203896200. [DOI] [PubMed] [Google Scholar]
- 16.Sorsa T, Heikkinen S, Abbott MB, Abusamhadneh E, Laakso T, Tilgmann C, Serimaa R, Annila A, Rosevear PR, Drakenberg T, Pollesello P, Kilpelainen I. Binding of levosimendan, a calcium sensitizer, to cardiac troponin C. J Biol Chem. 2001;276:9337–9343. doi: 10.1074/jbc.M007484200. [DOI] [PubMed] [Google Scholar]
- 17.Kleerekoper Q, Putkey JA. Drug binding to cardiac troponin C. J Biol Chem. 1999;274:23932–23939. doi: 10.1074/jbc.274.34.23932. [DOI] [PubMed] [Google Scholar]
- 18.Sorsa T, Pollesello P, Permi P, Drakenberg T, Kilpelainen I. Interaction of levosimendan with cardiac troponin C in the presence of cardiac troponin I peptides. J Mol Cell Cardiol. 2003;35:1055–1061. doi: 10.1016/s0022-2828(03)00178-0. [DOI] [PubMed] [Google Scholar]
- 19.Li MX, Spyracopoulos L, Beier N, Putkey JA, Sykes BD. Interaction of cardiac troponin C with Ca(2+) sensitizer EMD 57033 and cardiac troponin I inhibitory peptide. Biochemistry. 2000;39:8782–8790. doi: 10.1021/bi000473i. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Li MX, Spyracopoulos L, Beier N, Chandra M, Solaro RJ, Sykes BD. Structure of the C-domain of human cardiac troponin C in complex with the Ca2+ sensitizing drug EMD 57033. J Biol Chem. 2001;276:25456–25466. doi: 10.1074/jbc.M102418200. [DOI] [PubMed] [Google Scholar]
- 21.Kolseth SM, Rolim NP, Salvesen O, Nordhaug DO, Wahba A, Hoydal MA. Levosimendan improves contractility in vivo and in vitro in a rodent model of post-myocardial infarction heart failure. Acta Physiol. 2014;210:865–874. doi: 10.1111/apha.12248. [DOI] [PubMed] [Google Scholar]
- 22.Fujino K, Sperelakis N, Solaro RJ. Sensitization of dog and guinea pig heart myofilaments to Ca2+ activation and the inotropic effect of pimobendan: comparison with milrinone. Circ Res. 1988;63:911–922. doi: 10.1161/01.res.63.5.911. [DOI] [PubMed] [Google Scholar]
- 23.Gambassi G, Capogrossi MC, Klockow M, Lakatta EG. Enantiomeric dissection of the effects of the inotropic agent, EMD 53998, in single cardiac myocytes. Am J Physiol. 1993;264(3 Pt 2):H728–H738. doi: 10.1152/ajpheart.1993.264.3.H728. [DOI] [PubMed] [Google Scholar]
- 24.Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, Solaro RJ. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995;77:107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- 25.Hasenfuss G, Pieske B, Castell M, Kretschmann B, Maier LS, Just H. Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation. 1998;98:2141–2147. doi: 10.1161/01.cir.98.20.2141. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi R, Endoh M. Effects of OR-1896, a metabolite of levosimendan, on force of contraction and Ca2+ transients under acidotic condition in aequorin-loaded canine ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:440–448. doi: 10.1007/s00210-002-0627-x. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi R, Talukder MA, Endoh M. Inotropic effects of OR-1896, an active metabolite of levosimendan, on canine ventricular myocardium. Eur J Pharmacol. 2000;400:103–112. doi: 10.1016/s0014-2999(00)00385-x. [DOI] [PubMed] [Google Scholar]
- 28.Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 29.Schlecht W, Zhou Z, Li KL, Rieck D, Ouyang Y, Dong WJ. FRET study of the structural and kinetic effects of PKC phosphomimetic cardiac troponin T mutants on thin filament regulation. Arch Biochem Biophys. 2014;550–551:1–11. doi: 10.1016/j.abb.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong WJ, Jayasundar JJ, An J, Xing J, Cheung HC. Effects of PKA phosphorylation of cardiac troponin I and strong crossbridge on conformational transitions of the N-domain of cardiac troponin C in regulated thin filaments. Biochemistry. 2007;46:9752–9761. doi: 10.1021/bi700574n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong WJ, Robinson JM, Stagg S, Xing J, Cheung HC. Ca2+-induced conformational transition in the inhibitory and regulatory regions of cardiac troponin I. J Biol Chem. 2003;278:8686–8692. doi: 10.1074/jbc.M212886200. [DOI] [PubMed] [Google Scholar]
- 32.Xing J, Jayasundar JJ, Ouyang Y, Dong WJ. Forster resonance energy transfer structural kinetic studies of cardiac thin filament deactivation. J Biol Chem. 2009;284:16432–16441. doi: 10.1074/jbc.M808075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Z, Li KL, Rieck D, Ouyang Y, Chandra M, Dong WJ. Structural dynamics of C-domain of cardiac troponin I protein in reconstituted thin filament. J Biol Chem. 2012;287:7661–7674. doi: 10.1074/jbc.M111.281600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong WJ, Chandra M, Xing J, Solaro RJ, Cheung HC. Conformation of the N-terminal segment of a monocysteine mutant of troponin I from cardiac muscle. Biochemistry. 1997;36:6745–6753. doi: 10.1021/bi962226d. [DOI] [PubMed] [Google Scholar]
- 35.Dong WJ, Xing J, Robinson JM, Cheung HC. Ca2+ induces an extended conformation of the inhibitory region of troponin I in cardiac muscle troponin. J Mol Biol. 2001;314:51–61. doi: 10.1006/jmbi.2001.5118. [DOI] [PubMed] [Google Scholar]
- 36.Dong WJ, Xing J, Villain M, Hellinger M, Robinson JM, Chandra M, Solaro RJ, Umeda PK, Cheung HC. Conformation of the regulatory domain of cardiac muscle troponin C in its complex with cardiac troponin I. J Biol Chem. 1999;274:31382–31390. doi: 10.1074/jbc.274.44.31382. [DOI] [PubMed] [Google Scholar]
- 37.Robinson JM, Dong WJ, Xing J, Cheung HC. Switching of troponin I: Ca(2+) and myosin-induced activation of heart muscle. J Mol Biol. 2004;340:295–305. doi: 10.1016/j.jmb.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Smillie LB. Preparation and identification of α-and β-Tropomyosins. Methods Enzymol. 1982;85:234–241. doi: 10.1016/0076-6879(82)85023-4. [DOI] [PubMed] [Google Scholar]
- 39.Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- 40.Dong WJ, Xing J, Ouyang Y, An J, Cheung HC. Structural kinetics of cardiac troponin C mutants linked to familial hypertrophic and dilated cardiomyopathy in troponin complexes. J Biol Chem. 2008;283:3424–3432. doi: 10.1074/jbc.M703822200. [DOI] [PubMed] [Google Scholar]
- 41.Dong WJ, Robinson JM, Xing J, Cheung HC. Kinetics of conformational transitions in cardiac troponin induced by Ca2+ dissociation determined by Forster resonance energy transfer. J Biol Chem. 2003;278:42394–42402. doi: 10.1074/jbc.M304858200. [DOI] [PubMed] [Google Scholar]
- 42.Spyracopoulos L, Li MX, Sia SK, Gagne SM, Chandra M, Solaro RJ, Sykes BD. Calcium-induced structural transition in the regulatory domain of human cardiac troponin C. Biochemistry. 1997;36:12138–12146. doi: 10.1021/bi971223d. [DOI] [PubMed] [Google Scholar]
- 43.Knowles AC, Irving M, Sun YB. Conformation of the troponin core complex in the thin filaments of skeletal muscle during relaxation and active contraction. J Mol Biol. 2012;421:125–137. doi: 10.1016/j.jmb.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Biesiadecki BJ, Tachampa K, Yuan C, Jin JP, de Tombe PP, Solaro RJ. Removal of the cardiac troponin I N-terminal extension improves cardiac function in aged mice. J Biol Chem. 2010;285:19688–19698. doi: 10.1074/jbc.M109.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stelzer JE, Patel JR, Olsson MC, Fitzsimons DP, Leinwand LA, Moss RL. Expression of cardiac troponin T with COOH-terminal truncation accelerates cross-bridge interaction kinetics in mouse myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1756–H1761. doi: 10.1152/ajpheart.00172.2004. [DOI] [PubMed] [Google Scholar]
- 46.Campbell SG, Lionetti FV, Campbell KS, McCulloch AD. Coupling of adjacent tropomyosins enhances cross-bridge-mediated cooperative activation in a markov model of the cardiac thin filament. Biophys J. 2010;98:2254–2264. doi: 10.1016/j.bpj.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brixius K, Mehlhorn U, Bloch W, Schwinger RH. Different effect of the Ca(2+) sensitizers EMD 57033 and CGP 48506 on cross-bridge cycling in human myocardium. J Pharmacol Exp Ther Dec. 2000;295:1284–1290. [PubMed] [Google Scholar]
- 48.Sorsa T, Pollesello P, Rosevear PR, Drakenberg T, Kilpelainen I. Stereoselective binding of levosimendan to cardiac troponin C causes Ca2+-sensitization. Eur J Pharmacol. 2004;486:1–8. doi: 10.1016/j.ejphar.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Robertson IM, Baryshnikova OK, Li MX, Sykes BD. Defining the binding site of levosimendan and its analogues in a regulatory cardiac troponin C-troponin I complex. Biochemistry. 2008;47:7485–7495. doi: 10.1021/bi800438k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, Mebazaa A, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 2012;159:82–87. doi: 10.1016/j.ijcard.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Varughese JF, Baxley T, Chalovich JM, Li Y. A computational and experimental approach to investigate bepridil binding with cardiac troponin. J Phys Chem B. 2011;115:2392–2400. doi: 10.1021/jp1094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honerjager P, Heiss A, Schafer-Korting M, Schonsteiner G, Reiter M. UD-CG 115-a cardiotonic pyridazinone which elevates cyclic AMP and prolongs the action potential in guinea-pig papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:259–269. doi: 10.1007/BF00495953. [DOI] [PubMed] [Google Scholar]
- 53.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]