Abstract
Introduction
We evaluated the response to immunosuppression in a case of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR)-autoantibody myopathy.
Methods
T- and B-cell subsets were determined by flow cytometry pre- and post-therapy.
Results
Baseline immune profiling demonstrated strikingly elevated T-follicular helper (Tfh) cells and plasmablasts. Immunosuppression resulted in clinical improvement and decreased Tfh cells, plasmablasts, and autoantibodies.
Discussion
Immune profiling in HMGCR-autoantibody myopathy suggests a B-cell-mediated disease. Tfh cells and plasmablasts may be therapeutic biomarkers.
Keywords: inflammatory myopathy, HMGCR protein, human, T-lymphocytes, autoimmunity, immune suppression, B-lymphocytes, B-cells, T-cells
Introduction
Statin exposure rarely causes a progressive necrotizing myopathy with predominantly proximal weakness.1,2 This type of myopathy has been recently associated with the presence of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) autoantibodies.3 HMGCR autoantibody myopathy patients often respond to immunosuppressive therapy, and treatment usually results in lower autoantibody and creatine kinase (CK) levels.2,4
The role of HMGCR autoantibodies in disease pathogenesis is not clear. However, membrane attack complex deposition and the absence of a prominent cellular immune response in muscle biopsy specimens have suggested a humoral immune mechanism.2 We describe a patient with HMGCR autoantibody myopathy treated with immunosuppressive therapy and the results of T- and B-cell immune profiling before and up to 1 year after treatment. Our assays included profiles of Tfh cells, which are a subset of CD4 T-cells critical for supporting B-cell maturation to memory B-cells and plasmablasts in the germinal center.5
Case Report
A 52 year old man presented with insidious onset, painless right leg weakness. He also reported difficulty opening jars, poor balance, and difficulty climbing stairs, and he later developed difficulty rolling over in bed. Within several months he had progressive difficulty walking, getting out of a car, stepping up on curbs, negotiating stairs, and standing from a chair, and he began using a cane. He had no difficulty breathing or swallowing and had no myalgias. There was no history of dark urine, anesthesia difficulties, prior rhabdomyolysis, or family history of neuromuscular disease. He had taken atorvastatin for 1-2 years, but it was discontinued approximately 1 year prior to onset of symptoms. On initial examination 1 year after symptom onset, there was no clinical myotonia, and he had marked proximal upper and lower extremity weakness (Medical Research Council 2/5 hip flexion) with truncal instability. Manual muscle testing (MMT) performed according to established methods6 was 116 (maximum 140), and the inclusion body myositis functional rating score (IBM FRS)7 was 28 (maximum 40).
Nerve conduction studies of upper and lower extremity motor and sensory nerves were normal. Electromyography showed abnormal spontaneous activity with fibrillation potentials and positive sharp waves in all muscles examined, and prominent waxing and waning myotonic discharges in the tibialis anterior, first dorsal interosseous, and thoracic paraspinal muscles. Voluntary activation in all muscles examined demonstrated short duration, polyphasic motor unit potentials, and an early recruitment pattern. Laboratory testing, including a dried blood spot test for Pompe disease, genetic testing for myotonic dystrophy types 1 and 2, and myositis autoantibody panel (anti-Jo1, MI-2, PL-7, PL-12, EJ, OJ, SRP, KU, PM/SCL, U2 SNRNP; RDL Reference Laboratory) were negative. Thyroid function, paraneoplastic antibody panel (Mayo Medical Laboratory), anti-nuclear antibody, and angiotensin converting enzyme levels were normal. Muscle biopsy of the clinically involved right vastus lateralis muscle that included electron microscopy demonstrated mild nonspecific myopathic changes, but no strong features of necrotizing myopathy. There were rare CD3 positive T-cells associated with blood vessels, rare CD20 positive B-cells, and occasional degenerating fibers undergoing myophagocytosis. There was no MHC I upregulation. HMGCR autoantibodies determined by enzyme immunoassay (RDL Reference Laboratory) were strongly positive at 201 units (normal <20 units).
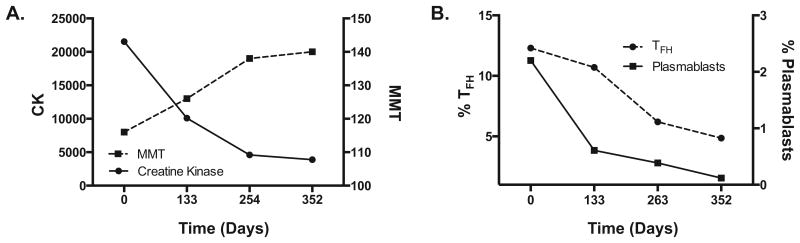
He received 1,000 mg intravenous methylprednisolone daily for 3 days followed by methotrexate 15mg weekly and prednisone 60mg daily for 12 months. He became gradually stronger, and 1 year after starting immunosuppressive therapy had no clinically detectable weakness (MMT 140), was able to step up on stairs with either leg without assistance, and the IBM FRS was normal. Along with the improvement in his exam, his CK trended downward from a maximum of 21,539 IU/L prior to therapy to 3,890 IU/L after 1 year of therapy, at which time HMGCR autoantibodies were 80 units (Figure 1A).
Figure 1. Clinical improvement is associated with a decrease in the frequency of circulating Tfh and plasmablasts.
Clinical and immunological profiles (y-axis) were measured before immunosuppression (T1) and three timepoints post immunosuppression (x-axis). A) MMT scores improved and CK levels decreased with immunosuppressive therapy. B) PFC analysis was performed on PBMCs to identify circulating Tfh cells (CD4+CD45RA-CXCR5+) and plasmablasts (CD19+CD27+CD20-CD38+). Highly elevated percentages of Tfh and plasmablasts were present prior to therapy and decreased dramatically with immunosupppresion.
Methods
Sample collection
Blood samples were collected, and peripheral blood mononuclear cells (PBMCs) were cryopreserved according to established methods8 prior to immunosuppressive therapy and after 4, 8, and 12 months of therapy. PBMCs from 11 healthy individuals served as controls.
Tfh and B-cell staining
106 PBMCs were plated in 96-well round bottom plates in RPMI +10% FBS. Cells were stained with Zombie Violet, CD14 Pacific Blue, CD16 Pacific Blue, CD4 FITC, CD27 APC, CD20 BV510, ICOS BV650, CD38 BV711, CD3 BV785, IgD PE, CXCR5 PE-Cy7, and CD45RA AF700 conjugates for 30 minutes at 4°C. After incubation, cells were washed, then fixed with 1% paraformaldehyde (PFA) prior to acquisition on an LSRII flow cytometer (BD Biosciences (San Jose, CA). CD45RA and ICOS fluorescent antibodies were obtained from BD Biosciences, while the rest of the antibody conjugates were purchased from Biolegend (San Diego, CA).
Intracellular cytokine staining
106 PBMCs were plated in 96-well round bottom plates in RPMI +10% FBS. Cells were left untreated, stimulated with either α-CD3 and α-CD28 or phorbol 12-myristate 13-acetate (PMA) and ionomycin (ION) in the presence of brefeldin A (BD Biosciences).9 Cells were incubated for 5 hours at 37°C in 6% CO2 in a humidified incubator. After this period, cells were stained with Zombie Violet, CD14 Pacific Blue, CD4 FITC, and CD8 APC-Cy7 conjugates for 30 minutes at 4°C. CD14, CD3, CD4, and CD8 fluorescent antibodies were obtained from Biolegend. Following cell surface staining, cells were treated with cytofix/cytoperm (BD Biosciences) in accordance with the manufacturer's recommendations. Intracellular staining was then performed for 30 mins at 4°C using IFN-γ PE-Cy7, TNF-α Alexa Fluor 700, IL-2 APC, IL-17 PcP Cy5.5, and IL-4 PE conjugates. All cytokine fluorescent antibodies were purchased from Biolegend. Cells were fixed with 1% PFA and acquired on a LSRII flow cytometer (BD Biosciences).
Data analysis
Data analysis was performed using Flowjo software (Tree Star, Ashland, OR). Student t-tests were used to determine statistical significance between 2 groups. The P-values were calculated using Prism software (Graph Pad, LaJolla, CA).
Results
Tfh cells
5,10 At baseline, the HMGCR patient had high levels of CD45RA-CXCR5+ circulating Tfh cell population (12.3% of CD4 T-cells), which subsequently decreased with immunosuppressive therapy to 4.86% at the last time point (Figure 1B). By comparison, the mean percentage of Tfh cells from healthy control subjects was 6.75%. The decrease in Tfh cells was associated with improved clinical outcome scores (MMT and IBM FRS) and decreased CK and HMGCR antibody levels (Figure 1A).
B-cell panel
To determine whether the high frequency of circulating Tfh cells correlated with circulating plasmablasts capable of producing antibodies, we used polychromatic flow cytometry (PFC) to phenotype circulating B-cells. We identified 2.84% of CD19+CD27+ memory B-cells to be CD20-CD38+ plasmablasts (Figure 1B). In 11 healthy controls, we observed a mean of 0.16% of memory B-cells to be plasmablasts (data not shown). Similar to Tfh cells, the frequency of plasmablasts decreased after therapy to 0.12% at the final time point. This stepwise decrease in the frequency of plasmablasts parallels the clinical improvement and decreased HMGCR autoantibody levels.
T-cell panel with intracellular cytokine analysis
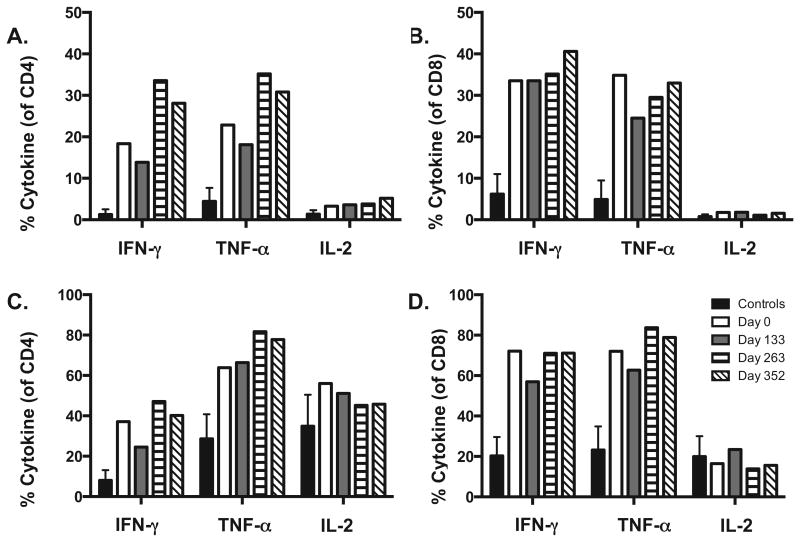
We performed PFC to measure the capacity of CD4 and CD8 T-cells to produce IFN-γ, TNF-α, IL-17, IL-4, and IL-2. Cytokines were detected following stimulations with PMA and ION or α-CD3 and α-CD28. For both stimulations, a Th1 response was the dominant response as evidenced by the enhanced production of IFN-γ, TNF-α, and IL-2 (Figure 2). The capacity of CD4 and CD8 T-cells to produce IFN-γ and TNF-α is significantly higher in the HMGCR patient compared with the controls. We did not detect significant production of IL-4 or IL-17, further supporting a Th1 response (data not shown). After initiation of therapy, the percentages of IFN-γ, TNF-α, and IL-2 producing CD4 and CD8 T-cells remained stable following both PMA/ION and α-CD3/α-CD28 stimulations (Figure 2).
Figure 2. Increased production of Th1 associated cytokines in a HMGCR patient.
PBMCs were stimulated for 5 hours with (A and B) α-CD3/α-CD28 and (C and D) PMA/ION and cytokines were detected by intracellular cytokine staining for IFN-γ, TNF-α, and IL-2. A stable Th1 pattern of IFN-γ and TNF-α intracellular cytokine production was observed in CD4 and CD8 T cells.
Discussion
Clinical features
The clinical features in this patient with HMGCR autoantibody myopathy are consistent with prior reports. He experienced chronically progressive, proximal weakness that accelerated over time. This was associated with myopathic changes and myotonic discharges on electromyography. Features of an irritative myopathy with myotonic discharges have been reported in HMGCR autoantibody myopathy,2 and clinicians should be aware of myotonic discharges in this disorder. Prior reports emphasized the presence of MHC-I upregulation in HMGCR autoantibody myopathy,1 although our case, similar to some other published studies, demonstrates that this biopsy feature may not be present.11
Response to immunosuppression
The patient responded very well to immunosuppressive treatment with prednisone and methotrexate, as has been reported previously.1,2 Our patient was also treated with intravenous corticosteroids prior to oral therapy due to his rapid clinical deterioration. Whether this approach has benefit beyond oral immunosuppressives is uncertain, and the optimal therapeutic regimen for HMGCR autoantibody myopathy is unclear. Many immunomodulatory therapies, such as mycophenolate mofetil, intravenous immunoglobulins, rituximab, methotrexate, azathioprine, and cyclophosphamide have also been used successfully.2,12 It has been noted that patients often relapse when discontinuing therapy, so caution should be used when attempting to do so.
Immunological studies
Prior studies demonstrated that anti-HMGCR antibody levels in patients exposed to statins remit with clinical improvement, and remained stable in statin-unexposed patients.4 This raised the possibility that the antibodies may not be directly pathogenic, and the immunological pathway driving autoimmunity in this disease is uncertain.
In our patient, PFC analysis at baseline revealed high levels of circulating Tfh and plasmablasts, which is observed in several autoimmune diseases.13-15 Germinal center Tfh cells are known to drive B-cell maturation, and the discovery of Tfh cells in circulation with a phenotype similar to germinal center Tfh cells allows for more comprehensive immune profiling studies without the need for invasive techniques to collect lymphoid tissue.5,14 In general, Tfh cells are closely associated with B-cell responses, and we observed this association in which we demonstrate a step-wise decrease in the frequency of Tfh cells and plasmablasts that correlated with improved clinical outcome and lower HMGCR autoantibodies. One theory is that increased frequencies of Tfh cells enhance the maturation of B-cells into plasmablasts, which in turn, drive the generation of HMGCR-specific plasmablasts and subsequent production of HMGCR-specific autoantibodies. Interestingly, by the final time point, the proportion of Tfh cells and plasmablasts reached normal levels.
Analysis of cytokine production by CD4 and CD8 T-cells revealed a dominant Th1 response. Although the percentages of IFN-γ, TNF-α, and IL-2 producing T-cells remained steady from baseline to the last time point, the IFN-γ and TNF-α producing T-cells were significantly elevated in the patient compared to controls. In the patient, the maturational subset of CD4 and CD8 T-cells were skewed to the terminal effector subset (CD45RA+CCR7-), and the terminal effector cells produced a majority of the inflammatory cytokines (data not shown). Compared to other maturational subsets, including naïve, central memory, and effector memory cells, terminal effector cells have the lowest capacity to proliferate, self-renew, survive, and function.16,17 Intracellular cytokine staining measures a cell's potential to produce cytokines following ex vivo stimulation, and enhanced intracellular inflammatory cytokine responses do not directly reflect cytokines in circulation at each time point. Therefore, immunosuppressive therapy could be effective at blocking the T-cell's ability to produce cytokines. However, if patients are tapered off immunosuppression and relapse, these Th1 cytokines will likely be a significant factor in the immunopathogenesis of the disease.
With successful immunotherapy, plasmablasts and Tfh cell subsets progressively decreased, eventually normalizing, concomitant with a reduction in HMGCR autoantibodies. This trend further supports a B-cell mediated disease, since these immunological changes were observed in parallel with gradual normalization of measured clinical outcomes, such as MMT and the IBM FRS, and decreased CK. CK levels, which are easy to obtain and inexpensive, may be good markers of therapeutic response.4 Immunologically, Tfh and plasmablasts may provide additional information and could be explored in the future as a biomarker of disease status.
Statin unexposed patients with HMGCR autoantibody myopathy tend to be younger, African American, seem to be less responsive to immunosuppressive therapy, present with more inflammation on muscle biopsy, and have high CK levels that remain unresponsive with immunotherapy.4,18 The Identification of a population subset susceptible to HMGCR autoantibody myopathy in the absence of statin exposure suggests that the pathogenesis of disease may be associated with additional genetic and environmental factors. Currently, the immunobiology of HMGCR associated autoimmune myopathy is undefined, and the most important immune signature is the presence of anti-HMGCR antibodies. Our results suggest that immunological subsets and the functionality of these cells are important factors in understanding the underlying immunopathology of statin-exposed HMGCR associated autoimmune myopathy and responses to treatment. Therefore, the combination of anti-HMGCR antibody levels with additional immune profiling will likely be valuable for a better understanding of the disease. Although this report is based on 1 patient, the impressive change in the immunological profile over the course of a year provides support for a cohort study incorporating longitudinal immune profiling of the cellular immune response in order to identify additional biomarkers/immune signatures between statin-exposed and unexposed patients, in addition to therapy responsive and refractory disease.
Summary
We demonstrated a strong relationship between Tfh and B-cell subsets that supports an antibody mediated disease. Therapeutic strategies successful for treating other antibody mediated diseases may be more efficacious than T-cell targeted therapies for treating HMGCR autoantibody myopathy. In addition, Tfh cells and plasmablasts could potentially be an important immune signature of disease status in HMGCR myopathy and a useful tool to determine efficacy of treatment or relapses. Studies in additional patients are needed to confirm our observations.
Acknowledgments
This study was supported by 1K23NS085049-01A1 (Dr. Guptill). This publication was made possible with the help from the Duke University Center for AIDS Research (CFAR), a NIH funded program (P30 AI 64518) and the Duke Immune Profiling Core (DIPC).
Abbreviations
- ACE
angiotensin-converting enzyme
- ANA
antinuclear antibody
- CK
creatine kinase
- HMGCR
3-hydroxy-3-methylglutaryl-coenzyme A reductase
- IBM FRS
inclusion body myositis functional rating score
- IFN-γ
interferon-gamma
- IL-2
Interleukin-2
- ION
ionomycin
- IV
intravenous
- MHC I
major histocompatibility complex I
- MMT
manual muscle testing
- PBMCs
Peripheral blood mononuclear cells
- PFC
polychromatic flow cytometry
- PMA
phorbol 12-myristate 13-acetate
- Tfh
T follicular helper
- Th1
Type 1 T helper
- Th17
Type 17 T helper
- TNF-α
tumor necrosis factor-alpha
References
- 1.Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscular disorders : NMD. 2007;17(2):194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41(2):185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 3.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, Casciola-Rosen LA. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis and rheumatism. 2011;63(3):713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner JL, Christopher-Stine L, Ghazarian SR, Pak KS, Kus JE, Daya NR, Lloyd TE, Mammen AL. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis and rheumatism. 2012;64(12):4087–4093. doi: 10.1002/art.34673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkove SB, Parker RA, Nardin RA, Connolly CE, Felice KJ, Raynor EM. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology. 2002;58(7):1081–1087. doi: 10.1212/wnl.58.7.1081. [DOI] [PubMed] [Google Scholar]
- 6.Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L, Muscle Study G. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve. 2008;37(4):473–476. doi: 10.1002/mus.20958. [DOI] [PubMed] [Google Scholar]
- 7.Guptill JT, Yi JS, Sanders DB, Guidon AC, Juel VC, Massey JM, Howard JF, Jr, Scuderi F, Bartoccioni E, Evoli A, Weinhold KJ. Characterization of B cells in muscle-specific kinase antibody myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e77. doi: 10.1212/NXI.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi JS, Guidon A, Sparks S, Osborne R, Juel VC, Massey JM, Sanders DB, Weinhold KJ, Guptill JT. Characterization of CD4 and CD8 T cell responses in MuSK myasthenia gravis. Journal of autoimmunity. 2014;52:130–138. doi: 10.1016/j.jaut.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis and rheumatism. 2010;62(9):2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanathan S, Langguth D, Hardy TA, Garg N, Bundell C, Rojana-Udomsart A, Dale RC, Robertson T, Mammen AL, Reddel SW. Clinical course and treatment of anti-HMGCR antibody-associated necrotizing autoimmune myopathy. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e96. doi: 10.1212/NXI.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavele KM, Merry E, Ehrenstein MR. Cutting edge: circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. Journal of immunology. 2015;194(6):2482–2485. doi: 10.4049/jimmunol.1401190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2014;92(1):64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 15.Dorner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. Journal of immunological methods. 2011;363(2):187–197. doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nature reviews Cancer. 2012;12(10):671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. Journal of immunology. 2008;180(3):1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 18.Mohassel P, Mammen AL. Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies. Muscle Nerve. 2013;48(4):477–483. doi: 10.1002/mus.23854. [DOI] [PubMed] [Google Scholar]