Abstract
BACKGROUND
A large body of research suggests that hospitals with intensive care units (ICUs) staffed by board certified intensivists have lower mortality rates than those that do not.
OBJECTIVE
To determine whether hospitals can reduce their mortality by adopting an intensivist staffing model.
DESIGN
Retrospective, longitudinal study using 2003–10 Medicare data and Leapfrog Group Hospital surveys.
SETTING AND PATIENTS
2,916,801 Medicare patients at 488 US hospitals.
MEASUREMENTS
We studied 30-day and in-hospital mortality among patients with several common medical and surgical conditions. We first compared risk-adjusted mortality rates of three groups of hospitals: those that were intensivist staffed throughout this time period, those that were not intensivist staffed and those that transitioned to intensivist staffing somewhere during the period. We then examined rates of mortality improvement within each of the three groups and used difference-in-differences techniques to assess the independent effect of intensivist staffing among the subset of hospitals that transitioned.
RESULTS
Hospitals with intensivist staffing at the beginning of our study period had lower mortality rates than those without. However, hospitals that adopted intensivist staffing during the study period did not substantially improve their mortality rates. In our difference-in-differences analysis, there was no significant independent improvement in mortality after transitioning to intensivist staffing either overall (relative risk [RR]: 0.96 [95% CI, 0.90–1.02]) or in the medical (RR: 0.95 [95% CI, 0.89–1.02]) or surgical populations (RR: 0.97 [95% CI, 0.84–1.10]).
LIMITATIONS
Risk adjustment was based on administrative data. Categorization of exposure was by survey response at the hospital level.
CONCLUSIONS
Adoption of an intensivist staffing model was not associated with improved mortality in Medicare beneficiaries. These findings suggest that the lower mortality rates previously observed at hospitals with intensivist staffing may be attributable to other factors.
INTRODUCTION
A large body of research suggests that hospitals with intensive care units (ICUs) staffed by board-certified intensivists have lower mortality rates than those that do not. (1–7) Possible reasons include specialist knowledge of organ support therapies, increased experience arising from increased volume of cases as well as increased compliance with evidence based protocols. (1–7) As a result, the Society of Critical Care Medicine has explicitly endorsed the value of intensivist staffing models across the United States. (8–10) More importantly, The Leapfrog Group, a large coalition of healthcare purchasers, has established the ICU Physician Staffing standard as one of its core safety practices for US hospitals. (7) A central tenet of this standard is that ICU patients are managed or co-managed by one or more physicians who are certified in critical care medicine. Many hospitals are therefore hiring more intensivists and transitioning to fully intensivist staffed ICUs.
Despite a large body of research in this regard, however, it remains uncertain whether implementing intensivist models leads directly to lower mortality. (11) Prior studies in this area fall into two main categories, each with their own limitations. Before-after studies consist largely of single-center studies evaluating outcomes following the implementation of intensivist staffing and these suffer from secular trends and lack of a control group. (4) The cross-sectional studies, which constitute the preponderance of research in this area compare intensivist staffed hospitals to non-intensivist staffed hospitals. These studies are prone to confounding as there may be many factors that explain differences in mortality other than intensivist staffing. (12–14) The limitations of prior studies make it difficult to understand the true impact of transitioning to an intensivist staffing model.
In this context, we used national Medicare data to evaluate the extent to which hospitals transitioning to intensivist staffing reduced their mortality. We compared risk-adjusted mortality rates over an eight year period between three groups of hospitals: those that were intensivist-staffed throughout this time period, those that were never intensivist-staffed and those that transitioned to intensivist staffing sometime during the period. We then examined rates of improvement in mortality within each of the three groups and used difference-in-differences techniques to assess the independent effect of intensivist staffing among the subset of hospitals that transitioned.
METHODS
Data source and study population
We used the Medicare Provider Analysis and Review (MedPAR) files from 2003–10. The Centers for Medicare and Medicaid Services (CMS) maintains this administrative database using all claims submitted by hospitals for services provided to Medicare beneficiaries. Each patient record includes age, gender, race, admission and discharge dates, principal diagnosis codes, secondary diagnosis codes, procedure codes, and 30-day mortality. The University of Michigan Institutional Review Board approved this protocol and waived the requirement for informed consent.
Using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes, we identified all patients aged 65 to 99 admitted for 1 of 3 medical conditions (congestive heart failure, acute myocardial infarction (MI), pneumonia) or undergoing 1 of 3 major operations (hip fracture repair, abdominal aortic aneurysm repair, colectomy). We selected these conditions prospectively, given their prevalence and substantial morbidity and mortality, i.e., most likely to receive care in the intensive care unit for complication and therefore outcomes are dependent on the quality of care in this setting.15 Comorbid conditions were defined with the Elixhauser method, which uses ICD-9-CM codes to classify secondary diagnoses into 29 different comorbid conditions. (16) For sensitivity analyses, ICU admission was defined as having a MedPAR defined intensive care unit day count of at least 1.
Characterizing staffing in hospital intensive care units
Intensivist staffing was characterized using The Leapfrog Group’s voluntary, publicly, self-reported data. They report on various safety standards in an effort to reduce variation in healthcare and reward hospitals that have made successful quality improvement ‘leaps’. (17)
Intensivist staffing was captured by the answer to the following question: “Are all patients in these ICUs managed or co-managed by one or more physicians who are certified in critical care medicine?”. Hospitals had three possible answers: (1) No, (2) Yes or (3) Yes using an expanded definition.17 This third group included physicians who: (a) completed training prior to availability of subspecialty certification in critical care in their specialty (1987 for (Internal) Medicine, Anesthesiology, Pediatrics and Surgery), who were board-certified in one of these four specialties, and who had provided at least six weeks of full-time ICU care annually since 1987; or (b) were board-certified in (Internal) Medicine, Anesthesiology, Pediatrics or Surgery and had completed training programs required for certification in the subspecialty of Critical Care Medicine but were not yet certified in this subspecialty. For this study, both groups 2 and 3 were considered as intensivist-staffed. Hospitals that answered “no” were deemed not to have met the Leapfrog Group’s ICU Physician Staffing Leap. Hospitals were then classified into three groups—1) those that did not adopt intensivist staffing (“no-intensivist” group), 2) those that had been intensivist staffed throughout 2003–10 (“always-intensivist” group), and 3) those that transitioned to intensivist staffing during the 2003–10 period (“transition” group). The date on which the hospital became intensivist staffed was used to construct a time-dependent hospital status variable.
Primary outcome
The primary outcome for this study was risk-adjusted 30-day mortality. This was defined as death before hospital discharge or within 30-days of admission. For risk-adjustment, we used standard logistic regression to account for patient characteristics and risk factors (age, gender, race, admission cause, admission acuity, 29 Elixhauser comorbidities) and year of admission. (18)
Statistical analysis
We first conducted a cross-sectional analysis comparing mortality at hospitals that were always-intensivist staffed during the study period with those that were never intensivist staffed. To explore the degree to which hospital factors explain this relationship, we repeated this analysis adding hospital characteristics available from the American Hospital Association Annual Survey (hospital geography, teaching status, financial status, bed size and government status).
To enhance causal inference, we then conducted a longitudinal difference-indifferences regression analysis focusing on hospitals that transitioned to this model during the study period. This is an econometric technique commonly used to assess the impact of policy changes by isolating the effect of the policy from trends affecting both control and intervention groups. (19–22) The control group was hospitals that were not intensivist-staffed throughout 2003–10. The intervention group was hospitals that transitioned to intensivist staffing (according to their response on the Leapfrog Hospital Survey) between 2003–10. Patients were categorized by the intensivist transition status of their hospital. A continuous time variable was created consisting of the time difference between admission and the transition date of the hospital for the intervention group.
For the control group, there was no change in hospital intensivist status. Therefore, the time variable was set as the difference between the mid point of the time period under investigation (2003–10) and the admission date (see supplementary appendix for further details). For the main difference-in-differences analysis, we used logistic regression models to assess the relationship between mortality and the change in hospital status. Two key dummy variables were included: intervention group (0 for control, 1 for the intervention group) and the hospital pre-post change status (0 for before the change, 1 for after). We also included an interaction term between intervention and pre-post change status. The coefficient of this term gives an estimate of the independent effect of transitioning to intensivist staffing on mortality. (23,24) All models included additional variables to adjust for patient characteristics and comorbidities.
We used the coefficient of the interaction term to predict marginal effects from the logistic regression model. We then calculated relative risk ratios and 95% confidence intervals. Our results are presented as relative risk (RR) ratios because odds ratios (OR) may not accurately reflect outcome rates which are more common than 10%. (25,26) Our results are reported with standard errors clustered by hospital to account for the non-independence of patients within hospitals.
All reported p-values are two sided with the statistical significance threshold set to a p-value of less than 0.05. All analyses were performed using STATA statistical software version 12.1 (College Station, TX). This study had no external funding source.
RESULTS
A total of 488 hospitals were included for this study. There were 234 hospitals that did not adopt intensivist staffing (no-intensivist group), 129 hospitals that had been intensivist staffed throughout 2003–10 (always-intensivist group), and 125 hospitals that transitioned to intensivist staffing during the 2003–10 period (transition group). Table 1 presents the characteristics of patients and hospitals in these three groups. In the transition group, the median month for transition was June 2005 (interquartile range September 2004 to January 2007). Table 2 highlights the difference in characteristics in Leapfrog survey respondent and non-respondent hospitals.
Table 1.
Patient and hospital characteristics by intensivist staffing status.
| No intensivist staffing | Always intensivist staffed | Transitioned to intensivist staffing | |
|---|---|---|---|
| No. (%) of patients * | |||
| n = 1,086,286 | n = 1,036,470 | n = 794,045 | |
| Age | |||
| 65–69 | 167,429 (15.4) | 164,296 (15.9) | 121,821 (15.3) |
| 70–74 | 170,242 (15.7) | 162,295 (15.7) | 123,967 (15.6) |
| 75–79 | 204,986 (18.9) | 195,772 (18.9) | 150,350 (18.9) |
| 80–84 | 223,241 (20.6) | 213,656 (20.6) | 164,860 (20.8) |
| 85–89 | 185,734 (17.1) | 173,296 (16.7) | 135,936 (17.1) |
| 90+ | 135,014 (12.4) | 127,155 (12.3) | 97,111 (12.2) |
| Male sex | 484,037 (44.6) | 473,647 (45.7) | 355,740 (44.8) |
| Non-white race | 130,627 (12.0) | 179,452 (17.3) | 121,971 (15.4) |
| Comorbidities, mean (SD) | 2.5 (1.4) | 2.5 (1.3) | 2.5 (1.4) |
| Cause of admission | |||
| Congestive heart failure | 458,549 (42.2) | 444,839 (42.9) | 337,366 (42.5) |
| Acute MI | 174,212 (16.0) | 191,736 (18.5) | 141,323 (17.8) |
| Pneumonia | 287,361 (26.5) | 229,910 (22.2) | 192,708 (24.3) |
| Hip fracture repair | 80,275 (7.4) | 71,599 (6.9) | 57,447 (7.2) |
| AAA repair | 21,178 (2.0) | 33,023 (3.2) | 18,940 (2.4) |
| Colectomy | 64,711 (6.0) | 65,363 (6.3) | 46,261 (5.8) |
| Admission type | |||
| Emergency | 670,959 (61.8) | 750,837 (72.4) | 523,861 (66.0) |
| Urgent | 291,531 (26.8) | 158,230 (15.3) | 178,101 (22.4) |
| Elective | 123,796 (11.4) | 127,403 (12.3) | 92,083 (11.6) |
| No. (%) of hospitals * † | |||
| n = 234 | n = 129 | n = 125 | |
| Geography | |||
| Urban | 174 (78.4) | 125 (99.2) | 118 (95.9) |
| Rural | 32 (14.4) | 1 (0.8) | 3 (2.4) |
| Teaching status ‡ | |||
| No teaching | 185 (83.3) | 35 (27.6) | 80 (64.5) |
| Minor teaching | 31 (14.0) | 33 (26.0) | 28 (22.6) |
| Major teaching | 6 (2.7) | 59 (46.5) | 16 (12.9) |
| Financial status | |||
| Not for profit | 112 (50.5) | 97 (76.3) | 90 (72.6) |
| For profit | 100 (45.1) | 7 (5.5) | 28 (22.6) |
| Bed size | |||
| 6–49 beds | 14 (6.3) | 0 (0) | 1 (0.8) |
| 50–299 beds | 157 (70.7) | 46 (36.2) | 72 (58.1) |
| 300+ beds | 51 (23.0) | 81 (63.8) | 51 (41.1) |
| Registered nurse hours per patient day, mean (SD) | 7.9 (2.5) | 8.9 (2.8) | 8.3 (2.7) |
All comparisons across the three groups had p-values of <0.01 except minor teaching (0.02) and RN hours per patient day (0.25).
Data were not available for every hospital. Thus the total number of hospitals in each category (e.g. beds) does not necessarily add to the total number of hospitals of that intensivist staffing type.
Major teaching included those hospitals that were members of the Council of Teaching Hospitals (CTH). Minor hospitals included those that were not CTH members but still had a residency program certified by Accreditation Council for Graduate Medical Education. The remaining hospitals were classified as ‘no teaching’.
Abbreviations: AAA, abdominal aortic aneurysm; MI, myocardial infarction; SD, standard deviation.
Table 2.
Characteristics in Leapfrog survey respondent and non-respondent hospitals.
| No. (%) of hospitals * † | ||
|---|---|---|
| Leapfrog survey respondent | Leapfrog survey non- respondent | |
| n = 488 | n = 5,407 | |
| Geography | ||
| Urban | 417 (89) | 2,942 (55) |
| Rural | 36 (8) | 826 (15) |
| Teaching status ‡ | ||
| No teaching | 300 (63) | 4,645 (86) |
| Minor teaching | 92 (19) | 538 (10) |
| Major teaching | 81 (17) | 224 (4) |
| Financial status | ||
| Not for profit | 299 (63) | 2,751 (51) |
| For profit | 135 (29) | 1,330 (25) |
| Bed size | ||
| 6–49 beds | 15 (3) | 1,924 (36) |
| 50–299 beds | 275 (58) | 2,793 (52) |
| 300+ beds | 183 (39) | 690 (13) |
| Registered nurse hours per patient day, mean (SD) | 8.3 (2.7) | 6.4 (3.3) |
All comparisons across the three groups had p-values of <0.05 except for profit status (0.057)
Data were not available for every hospital. Thus the total number of hospitals in each category (e.g. beds) does not necessarily add to the total number of hospitals of that intensivist staffing type.
Major teaching included those hospitals that were members of the Council of Teaching Hospitals (CTH). Minor hospitals included those that were not CTH members but still had a residency program certified by Accreditation Council for Graduate Medical Education. The remaining hospitals were classified as ‘no teaching’.
The cross-sectional analysis compared the no-intensivist group to the always-intensivist group. There was a greater proportion of non-white and emergency admissions in the always-intensivist group. The longitudinal analysis compared the no-intensivist group to the transition group. In both analyses, there was a higher proportion of hospitals that were urban, not-for-profit, teaching, and with 300+ beds in the transition and always-intensivist groups versus the no-intensivist group (p<0.001) (table 1).
At both the beginning and end of our study period we found that hospitals with intensivist staffing had lower mortality than those without intensivist staffing. The relative reduction in mortality over the eight-year period was comparable for both groups (table 3). Specifically, intensivist staffed hospitals did not improve at a faster rate to non-intensivist staffed hospitals. Both these findings were consistent across medical and surgical admissions. A risk-adjusted cross-sectional analysis over the entire study period suggested lower mortality for patients admitted to an intensivist staffed hospital versus a non-intensivist staffed hospital (RR: 0.89 [95% CI, 0.86–0.93]). This reduced mortality remained after adjusting for hospital characteristics (RR: 0.93 [95% CI, 0.89–0.97]).
Table 3.
Risk-adjusted mortality rate by hospital intensivist staffing status and time period.
| Risk-adjusted mortality rate (%) | Relative risk ratio (95% CI)a | |||
|---|---|---|---|---|
| 2003–2004 | 2009–2010 | Difference (95% CI) | ||
| All admissions | ||||
| No intensivist staffing | 10.8 | 9.6 | −1.2 (−1.1 to −1.2) | 1.0 (index) |
| Intensivist staffed | 10.0 | 9.0 | −1.0 (−1.0 to −1.1) | - |
| Transitioned to intensivist staffing | 10.5 | 9.6 | −0.9 (−0.8 to −0.9) | 0.96 (0.89 to 1.02) |
| Medical admissions | ||||
| No intensivist staffing | 11.2 | 10.1 | −1.1 (−1.0 to −1.1) | 1.0 (index) |
| Intensivist staffed | 10.4 | 9.5 | −1.0 (−0.9 to −1.0) | - |
| Transitioned to intensivist staffing | 10.9 | 10.2 | −0.8 (−0.7 to −0.8) | 0.95 (0.89 to 1.02) |
| Surgical admissions | ||||
| No intensivist staffing | 8.4 | 7.2 | −1.3 (−1.2 to −1.4) | 1.0 (index) |
| Intensivist staffed | 7.6 | 6.5 | −1.1 (−1.0 to −1.2) | - |
| Transitioned to intensivist staffing | 8.1 | 7.0 | −1.0 (−0.9 to −1.2) | 0.97 (0.84 to 1.10) |
Abbreviations: CI, confidence interval.
The relative risk ratio is derived from a difference-in-differences regression model and represents the independent effect of transition to intensivist staffing on mortality. Results are presented relative to a control group of hospitals that did not transition to intensivist staffing during the study time period.
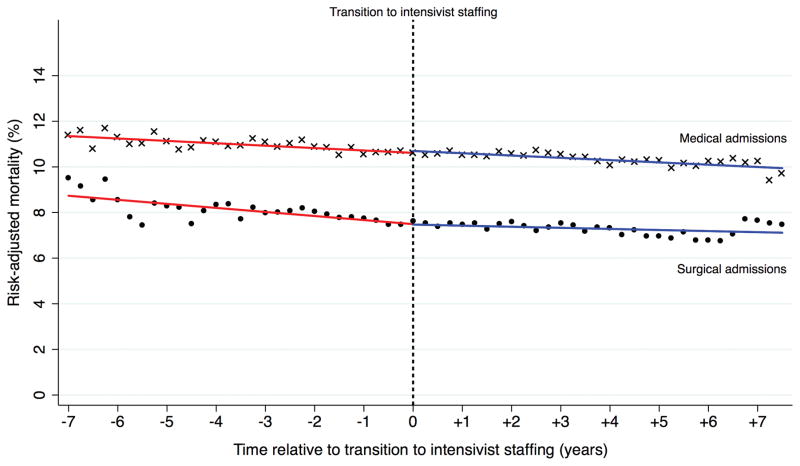
We then examined mortality in hospitals that transitioned to intensivist staffing. We did not find a significant association between transitioning to intensivist staffing and decreasing mortality, above and beyond pre-existing trends. After adjusting for patient factors and pre-existing trends, there was no significant mortality improvement as a result of transitioning to intensivist staffing (RR: 0.96 [95% CI, 0.89–1.02]). This lack of significant improvement remained when admissions were stratified as medical (RR: 0.95 [95% CI, 0.89–1.02]) or surgical (RR: 0.97 [95% CI, 0.84–1.10]) (figure 1).
Figure 1.
Risk-adjusted mortality rate in hospitals that transitioned to intensivist staffing. Abbreviations: CI, confidence interval.
We conducted several sensitivity analyses to assess the robustness of our findings (full results reported in supplementary appendix). First, we limited our longitudinal analysis to only patients officially admitted to an ICU. In hospitals that did not transition to intensivist staffing, 39% of patients were admitted to an ICU (423,932 patients). In hospitals that did transition, 37% of patients were admitted to an ICU (291,165 patients). There was no effect on our results (overall RR: 0.98 [95% CI, 0.87–1.08]). Second, we reclassified intensivist staffing using the stricter Leapfrog definition rather than including the expanded definition (see methods). Admissions in hospitals transitioning to intensivist staffing under the expanded definition were therefore considered with the non-intensivist staffed group. The effect estimate under this scenario was 0.94 [95% CI, 0.87–1.00] in the total population and 0.92 [95% CI, 0.80–1.05] in the ICU population. Third, we carried out an isolated analysis of only those patients admitted with pneumonia. Of the three medical conditions we included, pneumonia is thought to be most sensitive to the effect of an intensivist presence. There was no effect on our results either in the full pneumonia population (RR: 0.97 [95% CI, 0.88–1.06]) or in those admitted to the ICU (RR: 1.03 [95% CI, 0.88 to 1.17]).
DISCUSSION
In this study, we found hospitals with intensivist staffing tended to have lower mortality rates with both medical and surgical admissions compared to those without intensivists. Nonetheless, both groups of hospitals are improving at the same rate over time and more importantly, hospitals transitioning to intensivist staffing do not accelerate their improvement or catch up with intensivist staffed hospitals.
Our first finding is consistent with a large number of studies showing that hospitals with intensivist staffing have lower mortality than those without. Pronovost and colleagues published a highly cited systematic review reporting a hospital relative risk of mortality of 0.71 [95% CI 0.62–0.82] for intensivist staffed hospitals. (4) An updated review in 2013 found a weaker but still significant estimate of 0.83 [95% CI 0.70–0.99]. (27) Our cross-sectional analysis yielded a comparable mortality benefit (RR: 0.89 [95% CI, 0.86–0.93]). However, both reviews were predominated by small cross-sectional and pre-post implementation studies. Pronovost’s review included 27 studies of which only four assessed more than two ICUs. (4) Additionally, only two of the 22 cohort studies made use of contemporary rather than historic controls. This is problematic as ICU mortality has been the subject of several major quality improvement efforts such as the Surviving Sepsis Campaign. (13) Consequently, pre-post implementation studies tend to over estimate the benefits of intensivist staffing due to a background trend towards better outcomes over time. (28,29)
Our second finding that hospitals transitioning to intensivist staffing do not accelerate their rate of improvement sheds new light on the importance of adequate implementation of intensivist staffing. The difference-in-differences study design takes into account pre-existing time trends and utilizes a concurrent control group for isolating the effect of transitioning. To our knowledge, our study is the first to longitudinally assess the mortality benefit of transitioning to intensivist staffing. The results of our study suggest that the lower mortality at intensivist-staffed hospitals may be attributable to other unmeasured factors.
Although these factors remain largely unknown, they may include quality improvement strategies such as improved adherence to evidence based protocols (e.g. Surviving Sepsis Campaign), volume-outcome effects, or multidisciplinary staffing models. (13,30–34) Always-intensivist staffed hospitals tended to be large, urban, teaching institutions that may suggest a greater likelihood of evidence based protocols or more effective implementation of multi-disciplinary care team rounds. A state-wide cohort study suggested that the mortality benefit from such teams may partly explain the observed survival advantage of intensivist staffing. (32) Alternatively, smaller hospitals transitioning to intensivist staffing may struggle to provide the appropriate volume of patients to ensure proficient and expert ICU care. (30,31,34) Ultimately, the overriding theme is the hospital context in which intensivist staffing is implemented may be just as, or more, important than the transition itself.
Our findings must be borne in light of several study limitations. First, our assessment of outcomes did not extend beyond mortality. It may be that the benefits of intensivist staffing are more apparent in other domains such as length of stay, adherence to evidence based protocols or patient and family satisfaction with care. (11,35) This is especially true if mortality at Leapfrog participating hospitals is already near a minimum thereby reducing the sensitivity of mortality as a measure of staffing change. Second, we relied on data from The Leapfrog Group. While this data provides one of the richest sources of information currently available on ICU staffing, it nonetheless gives a relatively imprecise estimate of the quantity and quality of ICU staffing. For example, some hospitals may have multiple ICUs each with different staffing models. Such hospitals are only considered to adhere to the Leapfrog Group definition if all general medical and surgical ICUs are intensivist staffed. Thus the no intensivist group (controls) may include hospitals where some ICUs are intensivist staffed and some are not. The net result would be to bias results towards the null hypothesis of no difference. Hence, we acknowledge that the accuracy of categorization is a limitation of our study. Third, the sample of hospitals may not be random, but instead self-selected on the basis of their participation in the Leapfrog Hospital Survey. Such hospitals may be more motivated to improve their outcomes in general than the average US hospital. However, it is unlikely that they would have selected into leapfrog participation based on how they decide on ICU staffing. As demonstrated in table 2, Leapfrog hospitals were more likely to be urban, more involved with teaching and larger by bed size. However, if such hospitals were more motivated to improve outcomes, it would only strengthen the null finding in our analysis that transition to intensivist staffing did not improve mortality. Fourth, we used administrative data and its limitations with regards to risk adjustment have been well characterized. (36) In particular, the lack of ICU specific risk adjustment scores (e.g. Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE)) may mean that non-intensivist staffed hospitals are fielding sicker patients that are not apparent from non-physiological administrative data. Fifth, although, the difference-in-differences approach controls to a large extent for secular trends that may confound cross-sectional and pre-post implementation analyses, the possibility of other unmeasured differences in patient factors remains a weakness of any observational research. Sixth, there is reduced generalizability to younger patients as we used administrative MedPAR data that limits our sample to patients aged over 65. However, as the majority of ICU mortality is concentrated in this demographic, we would argue that this is a high priority group for study. (37) Finally, we cannot discount the possibility that the small proportion of hospitals that transitioned late in the 8 year period did so before any impact on mortality might reasonably be expected. For the 90th percentile of transition hospitals, transition month was December 2007 allowing only 3 years of post transition data to be assessed.
Our study has two major sets of policy implications. First, regardless of causal mechanisms, choosing an intensivist staffed hospital will likely lead patients to a lower mortality hospital. In this regard, selecting an intensivist-staffed hospital may provide analogous information about the quality of a hospital to other proxy measures such as hospital volume in selecting a hospital for surgery. Second, our findings may influence how hospitals seek to improve their outcomes. There is a growing recruitment shortfall in certified intensivists and an increasing demand projected for an aging US population. (10) We acknowledge that there may be other benefits associated with staffing ICUs with board-certified intensivists. Nonetheless, our findings suggest that hospitals should not necessarily expect to reduce their mortality rate or catch up with their peers with established ICUs by transitioning to intensivist staffing. Consequently, if intensivist staffing serves primarily as a proxy for other hospital attributes, quality improvement will require identifying the precise factors that directly affect mortality. We described earlier several potential mechanisms or hospital attributes—some modifiable, some not—that may contribute to the observed differences in outcomes. However, there is a growing interest and recognition that modifiable organizational dynamics, such as teamwork, leadership, culture, and communication practices are integral to improving ICU outcomes. (38–40) The Comprehensive Unit-based Safety Program (CUSP), designed to reduce preventable ICU morbidity, is an example of the intersection of these concepts. (41) It has demonstrated significant efficacy in reducing central-line associated blood stream infections and is now expanding nationally to increase coverage of other preventable morbidity. (42–44) Such an example strengthens the case for investment in areas of organizational development and growth that were historically overlooked.
In summary, taking into account the aforementioned limitations, our results indicate that transitioning to an intensivist staffing model was not associated with improved mortality in Medicare beneficiaries. These findings suggest that the lower mortality rate previously observed at hospitals with intensivist staffing may be attributable to other factors.
Supplementary Material
Footnotes
Disclosures
No other conflicts of interest reported for any authors other than those disclosed above.
conflicts of interest:
Drs. Ghaferi, Birkmeyer, and Dimick are supported by a grant from the National Institute on Aging (R01 AG042340). Dr. Birkmeyer also receives support from the National Institute of Aging grant P01 AG019783. Drs. Gonzalez and Nagendran report no disclosures.
References
- 1.Iyegha UP, Asghar JI, Habermann EB, Broccard A, Weinert C, Beilman G. Intensivists improve outcomes and compliance with process measures in critically ill patients. J Am Coll Surg. 2013;216(3):363–72. doi: 10.1016/j.jamcollsurg.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Lwin AK, Shepard DS. Estimating Lives and Dollars Saved from Universal Adoption of the Leapfrog Safety and Quality Standards: 2008 update. Washington, DC: The Leapfrog Group; 2008. [Google Scholar]
- 3.Parikh A, Huang SA, Murthy P, et al. Quality improvement and cost savings after implementation of the Leapfrog intensive care unit physician staffing standard at a community teaching hospital. Crit Care Med. 2012;40(10):2754–9. doi: 10.1097/CCM.0b013e31825b26ef. [DOI] [PubMed] [Google Scholar]
- 4.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288(17):2151–62. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 5.Pronovost PJ, Jenckes MW, Dorman T, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281(14):1310–7. doi: 10.1001/jama.281.14.1310. [DOI] [PubMed] [Google Scholar]
- 6.Pronovost PJ, Needham DM, Waters H, et al. Intensive care unit physician staffing: financial modeling of the Leapfrog standard. Crit Care Med. 2004;32(6):1247–53. doi: 10.1097/01.ccm.0000128609.98470.8b. [DOI] [PubMed] [Google Scholar]
- 7.The Leapfrog Group. [accessed 10 August 2015];ICU Physician Staffing Factsheet. 2011 http://www.leapfroggroup.org/media/file/FactSheet_IPS.pdf.
- 8.Brilli RJ, Spevetz A, Branson RD, et al. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29(10):2007–19. doi: 10.1097/00003246-200110000-00026. [DOI] [PubMed] [Google Scholar]
- 9.NQF. Safe Practices for Better Healthcare: A Consensus Report. Washington DC: The National Quality Forum; 2003. [Google Scholar]
- 10.Ward NS, Afessa B, Kleinpell R, et al. Intensivist/patient ratios in closed ICUs: a statement from the society of critical care medicine taskforce on ICU staffing. Crit Care Med. 2013;41(2):638–45. doi: 10.1097/CCM.0b013e3182741478. [DOI] [PubMed] [Google Scholar]
- 11.Rubenfeld GD, Angus DC. Are intensivists safe? Ann Intern Med. 2008;148(11):877–9. doi: 10.7326/0003-4819-148-11-200806030-00010. [DOI] [PubMed] [Google Scholar]
- 12.Afessa B, Gajic O, Keegan MT, Seferian EG, Hubmayr RD, Peters SG. Impact of introducing multiple evidence-based clinical practice protocols in a medical intensive care unit: a retrospective cohort study. BMC Emerg Med. 2007;7:10. doi: 10.1186/1471-227X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–74. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 14.Palomar M, Alvarez-Lerma F, Riera A, et al. Impact of a National Multimodal Intervention to Prevent Catheter-Related Bloodstream Infection in the ICU: The Spanish Experience. Crit Care Med. 2013;41(10):2364–72. doi: 10.1097/CCM.0b013e3182923622. [DOI] [PubMed] [Google Scholar]
- 15.Dharmarajan K, Hsieh AF, Kulkarni VT, et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. Bmj. 2015;350:h411. doi: 10.1136/bmj.h411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.The Leapfrog Group. [accessed 10 August 2015];The Leapfrog Hospital Survey. 2012 http://leapfroghospitalsurvey.org/web/wp-content/uploads/2012/03/2012Survey.pdf.
- 18.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–60. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 19.Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309(8):792–9. doi: 10.1001/jama.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood) 2012;31(3):585–92. doi: 10.1377/hlthaff.2011.0719. [DOI] [PubMed] [Google Scholar]
- 21.Sutton M, Nikolova S, Boaden R, Lester H, McDonald R, Roland M. Reduced mortality with hospital pay for performance in England. N Engl J Med. 2012;367(19):1821–8. doi: 10.1056/NEJMsa1114951. [DOI] [PubMed] [Google Scholar]
- 22.Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among patients in VA hospitals in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):984–92. doi: 10.1001/jama.298.9.984. [DOI] [PubMed] [Google Scholar]
- 23.Donald SG, Lang K. Inference with difference-in-differences and other panel data. Rev Econ Stat. 2007;89:221–33. [Google Scholar]
- 24.Ryan AM. Effects of the Premier Hospital Quality Incentive Demonstration on Medicare patient mortality and cost. Health Serv Res. 2009;44(3):821–42. doi: 10.1111/j.1475-6773.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. Journal of clinical epidemiology. 2010;63(1):2–6. doi: 10.1016/j.jclinepi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health. 2008;53(3):165–7. doi: 10.1007/s00038-008-7068-3. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox ME, Chong CA, Niven DJ, et al. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med. 2013;41(10):2253–74. doi: 10.1097/CCM.0b013e318292313a. [DOI] [PubMed] [Google Scholar]
- 28.Iwashyna TJ, Angus DC. Declining Case Fatality Rates for Severe Sepsis: Good Data Bring Good News With Ambiguous Implications. JAMA. 2014 doi: 10.1001/jama.2014.2639. [DOI] [PubMed]
- 29.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality Related to Severe Sepsis and Septic Shock Among Critically Ill Patients in Australia and New Zealand, 2000–2012. JAMA. 2014 doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 30.Kahn JM. Volume, outcome, and the organization of intensive care. Crit Care. 2007;11(3):129. doi: 10.1186/cc5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahn JM. What’s new in ICU volume-outcome relationships? Intensive Care Med. 2013;39(9):1635–7. doi: 10.1007/s00134-013-2992-y. [DOI] [PubMed] [Google Scholar]
- 32.Kim MM, Barnato AE, Angus DC, Fleisher LA, Kahn JM. The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170(4):369–76. doi: 10.1001/archinternmed.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175–83. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
- 34.Pronovost PJ, Holzmueller CG, Clattenburg L, et al. Team care: beyond open and closed intensive care units. Curr Opin Crit Care. 2006;12(6):604–8. doi: 10.1097/MCC.0b013e32800ff3da. [DOI] [PubMed] [Google Scholar]
- 35.Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370(26):2506–14. doi: 10.1056/NEJMra1208795. [DOI] [PubMed] [Google Scholar]
- 36.Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012;307(13):1433–5. doi: 10.1001/jama.2012.404. [DOI] [PubMed] [Google Scholar]
- 37.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–43. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 38.Figueroa MI, Sepanski R, Goldberg SP, Shah S. Improving teamwork, confidence, and collaboration among members of a pediatric cardiovascular intensive care unit multidisciplinary team using simulation-based team training. Pediatr Cardiol. 2013;34(3):612–9. doi: 10.1007/s00246-012-0506-2. [DOI] [PubMed] [Google Scholar]
- 39.Garrouste-Orgeas M, Soufir L, Tabah A, et al. A multifaceted program for improving quality of care in intensive care units: IATROREF study. Crit Care Med. 2012;40(2):468–76. doi: 10.1097/CCM.0b013e318232d94d. [DOI] [PubMed] [Google Scholar]
- 40.Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235–9. doi: 10.1136/qshc.2005.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 42.Agency for Healthcare Research and Quality. [accessed 10 August 2015];2013 http://www.ahrq.gov/professionals/quality-patient-safety/cusp/index.html.
- 43. [accessed 10 August 2015];On the CUSP: Stop HAI. 2013 http://www.onthecuspstophai.org/about-us/background/
- 44.Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309. doi: 10.1136/bmj.c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.