Abstract
Background
Preoperative breast magnetic resonance imaging (MRI) use among Medicare beneficiaries with breast cancer has substantially increased from 2005 to 2009. We sought to identify factors associated with preoperative breast MRI use among women diagnosed with ductal carcinoma in situ (DCIS) or stage I-III invasive breast cancer (IBC).
Methods
Using Surveillance, Epidemiology, and End Results and Medicare data from 2005 to 2009 we identified women ages 66 and older with DCIS or stage I-III IBC who underwent breast conserving surgery or mastectomy. We compared preoperative breast MRI use by patient, tumor and hospital characteristics stratified by DCIS and IBC using multivariable logistic regression.
Results
From 2005 to 2009, preoperative breast MRI use increased from 5.9% to 22.4% of women diagnosed with DCIS and 7.0% to 24.3% of women diagnosed with IBC. Preoperative breast MRI use was more common among women who were younger, married, lived in higher median income zip codes and had no comorbidities. Among women with IBC, those with lobular disease, smaller tumors (< 1 cm) and those with estrogen receptor negative tumors were more likely to receive preoperative breast MRI. Women with DCIS were more likely to receive preoperative MRI if tumors were larger (> 2cm).
Conclusion
The likelihood of receiving preoperative breast MRI is similar for women diagnosed with DCIS and IBC. Use of MRI is more common in women with IBC for tumors that are lobular and smaller while for DCIS MRI is used for evaluation of larger lesions.
Keywords: breast magnetic resonance imaging, ductal carcinoma in situ, invasive breast cancer, preoperative
Introduction
For planning of surgical treatment of breast cancer, preoperative imaging with mammography, ultrasound or breast magnetic resonance imaging (MRI) allows for assessment of disease extent with the goal of improving patient outcomes such as re-excision rates, morbidity and breast cancer mortality. Breast MRI is increasingly used for this purpose, although there is no consensus that MRI improves short- or long-term outcomes (i.e. 5 year survival) over imaging with mammography or ultrasound, or preferentially benefits particular groups of women based on patient or tumor factors.(1)
Multiple studies have reported that preoperative breast MRI use among women with a breast cancer diagnosis increased dramatically from approximately 1% in 2001 to 25-53% in 2008/2009.(2-5) The current National Comprehensive Cancer Network (NCCN) guidelines state that breast MRI may be considered for women newly diagnosed with invasive breast cancer (IBC) in order to determine the extent of disease and to screen the contralateral breast, particularly for women at increased risk for mammographically occult disease.(1) In multiple studies of MRI in patients with newly diagnosed breast cancer, MRI has been shown to identify additional otherwise-occult disease, detecting additional breast cancer in approximately 16% of women.(6) However, studies to-date of breast MRI have not been associated with decreased re-excision rates, recurrence rates or improved survival.(7-9) Preoperative breast MRI has been shown to increase anxiety, as well as to be associated with more biopsies and higher mastectomy rates. Such additional procedures are for findings of otherwise occult disease and additional breast cancer.(10, 11)
We analyzed trends in the utilization of preoperative breast MRI from 2005-2009 as well as patient factors, tumor features and hospital characteristics associated with its use, separately for ductal carcinoma in-situ (DCIS) and stage I-III IBC. We sought to determine whether tumor features, such as estrogen receptor status, associated with use of breast MRI differed for DCIS compared to IBC. Although several prior studies have utilized Surveillance, Epidemiology, and End Results (SEER)-Medicare data to explore preoperative breast MRI diffusion and to identify factors associated with its use (3, 5), our study extends these previous studies by 1) examining DCIS and IBC separately; 2) more precisely defining the preoperative window; and 3) examining the impact of hospital characteristics on MRI use.
Material and Methods
Data Sources
We used data from the National Cancer Institute's linked SEER-Medicare data, which includes 17 population based cancer registries linked to Medicare administrative and health care claims data.(12) The SEER-Medicare data have been used to study health disparities, quality of care and cost of care across the cancer control continuum.(13) All patient data from the SEER-Medicare data used in this study were de-identified.
Study Population
The study cohort included women with DCIS or stage I-III IBC diagnosed between 2005 and 2009 who received BCS or mastectomy within 6 months of breast cancer diagnosis. Receipt of BCS and mastectomy was based on Current Procedural Terminology (CPT)/Healthcare Common Procedure Coding System (HCPCS) and International Classification of Disease (ICD-9) codes in the Medicare claims. We included women age 66 or older at breast cancer diagnosis with a pathologically confirmed diagnosis and for whom the reporting source of diagnosis was not a nursing home. To ensure complete capture of claims, we required women to be Medicare Parts A and B enrolled and non-HMO enrolled for one-year prior to and six-months post breast cancer diagnosis. We excluded women with a personal history of breast cancer because we were interested in incident disease. We further excluded women with unknown race, unknown residence, unknown census tract median income and ER borderline status because of small cell sizes.
Outcomes
Receipt of preoperative MRI was identified from Medicare outpatient or physician claims based on CPT/HCPCS and ICD-9 codes. We defined the preoperative window as the time between breast cancer diagnosis date (defined as the biopsy date closest to the SEER diagnosis date or the first of the month of the SEER diagnosis date if there was no biopsy date (approximately 3% of women)) and breast surgery claim date (either BCS or mastectomy) and limited the preoperative window to a maximum of 6 months (median preoperative window is 24 days with interquartile range of 14 to 39 days). Using SEER-Medicare data to define a preoperative window is not straightforward because SEER assigns the cancer diagnosis date as the 15th day of the month in which the cancer was diagnosed. To determine a more specific cancer diagnosis date and refine the preoperative window we identified the breast biopsy date closest to the SEER diagnosis date to anchor the beginning of the preoperative window. For this analysis we compared the use of preoperative MRI versus no preoperative MRI.
Predictor Variables
Demographic data included age, race (black, white, and other (Asian, Hispanic, Native American Indian, and other)), ethnicity, marital status, geographic region based on SEER registry, rural/urban location based on county of residence (urban includes big metropolitan, metropolitan, and urban; rural includes less urban and rural; see http://appliedresearch.cancer.gov/seermedicare/medicare/Sumden.requests.pdf for additional information), median income of the 2000 census tract in which the woman resided, diagnosis year and a measure of comorbidity based on a validated claims-based algorithm.(14) Using the SEER behavior codes and stage variable, we classified women with DCIS or invasive disease (including stage I-III). Tumor characteristic data was obtained from SEER sources and includes histology (invasive ductal cancer (IDC), invasive lobular cancer (ILC), invasive lobular and ductal, and other (papillary, tubular, mucinous, medullary, inflammatory, metaplastic, sarcoma)), grade, AJCC stage, size, and estrogen receptor (ER) status. From the SEER-Medicare provider and hospital files we ascertained characteristics of the hospitals where the surgery was performed including teaching hospital and if the hospital participated in at least one National Cancer Institute (NCI)-sponsored cooperative group trial at the time of the breast surgery.
Statistical Analysis
We compared the unadjusted distribution of preoperative MRI use by woman and tumor characteristics stratified by cancer type (DCIS and invasive) using the chi-square test. A multivariable logistic regression model identified patient, tumor and hospital characteristics associated with receipt of preoperative breast MRI, for women with DCIS and those with invasive disease. SAS 9.3 (15) was used for all analyses and a two-sided p-value of 0.05 defined statistical significance.
We conducted a sensitivity analysis to determine the impact that adjustment of our preoperative window to capture MRI conducted in the 30 days prior to the diagnosis date would have on the results.
Results
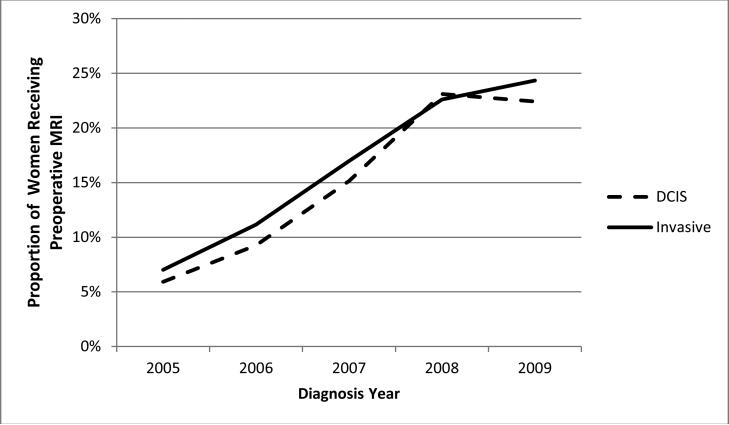
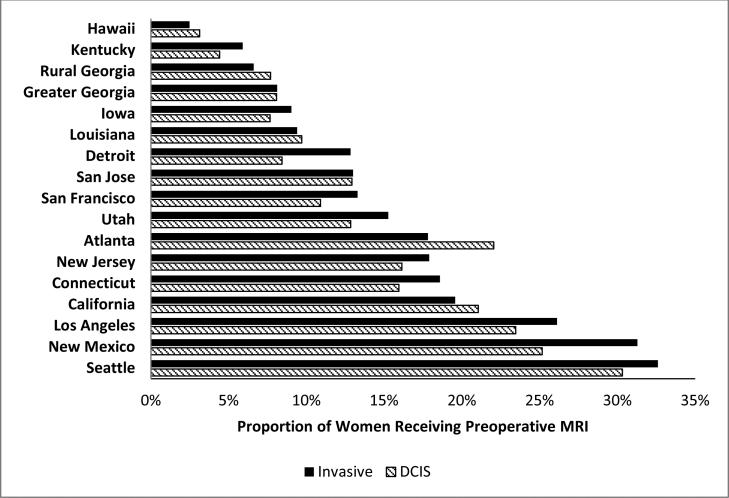
Of the 55,334 women with non-metastatic breast cancer who underwent BCS or mastectomy, 8,979 (16.2%) received preoperative MRI. From 2005 to 2009 the use of preoperative breast MRI increased significantly (p-value for trend <0.0001) at approximately the same rate for DCIS and IBC (Figure 1). Preoperative breast MRI utilization rose from 5.9% in 2005 to 22.4% in 2009 for women with DCIS and from 7.0% to 24.3% during the same time period for women with IBC. Geographic variation in use was evident across SEER sites with lower rates in the southeast and higher rates in the west (Figure 2).
Figure 1.
Proportion of women with non-metastatic breast cancer receiving preoperative breast MRI by year and cancer type, SEER-Medicare 2005-2009
Figure 2.
Proportion of women with non-metastatic breast cancer receiving preoperative breast MRI by SEER location and cancer type, SEER-Medicare 2005-2009
Women who were younger, white, lived in urban areas, were married, lived in a zip code with a higher median income and had a comorbidity score of zero were more likely to receive MRI (Table 1). Among women with DCIS, those with low grade, larger tumors and those whose surgery was performed at a hospital that participated in an NCI cooperative group trial were more likely to receive preoperative breast MRI. For women with invasive cancer, those with ILC, high to intermediate grade, smaller size, who had their surgery at a teaching hospital or a hospital that participated in an NCI sponsored cooperative group trial were more likely to receive preoperative breast MRI.
Table 1.
Characteristics of the cohort by preoperative breast magnetic resonance imaging (MRI) use and cancer type (Ductal Carcinoma In-Situ (DCIS) versus Invasive Breast Cancer (IBC)), SEER-Medicare, 2005-2009
| DCIS | IBC | |||||||
|---|---|---|---|---|---|---|---|---|
| No MRI N=7,595 (84.7%) | MRI N=1,372 (15.3%) | No MRI N=38,770 (83.7%) | MRI N=7,607 (16.3%) | |||||
| Characteristic | N | %a | N | %a | N | %a | N | %a |
| Age Group, years | ||||||||
| 66-69 | 1679 | 22.1 | 436 | 31.8 | 7072 | 18.2 | 2232 | 29.3 |
| 70-74 | 2083 | 27.4 | 451 | 32.9 | 9266 | 23.9 | 2381 | 31.3 |
| 75-79 | 1939 | 25.5 | 309 | 22.5 | 9114 | 23.5 | 1670 | 22.0 |
| 80-84 | 1245 | 16.4 | 138 | 10.1 | 7624 | 19.7 | 939 | 12.3 |
| 85+ | 649 | 8.6 | 38 | 2.8 | 5694 | 14.7 | 385 | 5.1 |
| Race | ||||||||
| White | 6433 | 84.7 | 1209 | 88.1 | 33858 | 87.3 | 6936 | 91.2 |
| Black | 689 | 9.1 | 79 | 5.8 | 2938 | 7.6 | 321 | 4.2 |
| Other | 473 | 6.2 | 84 | 6.1 | 1974 | 5.1 | 350 | 4.6 |
| Residenceb | ||||||||
| Urban | 6900 | 90.9 | 1313 | 95.7 | 34486 | 89.0 | 7189 | 94.5 |
| Rural | 695 | 9.2 | 59 | 4.3 | 4284 | 11.1 | 418 | 5.5 |
| Marital status | ||||||||
| Not married | 3725 | 49.1 | 510 | 37.2 | 20937 | 54.0 | 3248 | 42.7 |
| Married | 3498 | 46.1 | 806 | 58.8 | 16401 | 42.3 | 4056 | 53.3 |
| Unknown | 372 | 4.9 | 56 | 4.1 | 1432 | 3.7 | 303 | 4.0 |
| Median Income of Zip Code | ||||||||
| < $35,801 | 1923 | 25.3 | 180 | 13.1 | 10591 | 27.3 | 1130 | 14.9 |
| $35,801 - 47,300 | 1858 | 24.5 | 292 | 21.3 | 10126 | 26.1 | 1675 | 22.0 |
| $47,301 - 64,200 | 1900 | 25.0 | 381 | 27.8 | 9608 | 24.8 | 2074 | 27.3 |
| > $64,200 | 1914 | 25.2 | 519 | 37.8 | 8445 | 21.8 | 2728 | 35.9 |
| Comorbidity indexc | ||||||||
| 0 | 4671 | 61.5 | 979 | 71.4 | 23514 | 60.7 | 5458 | 71.8 |
| 1 | 1941 | 25.6 | 283 | 20.6 | 9569 | 24.7 | 1526 | 20.1 |
| 2+ | 983 | 12.9 | 110 | 8.0 | 5687 | 14.7 | 623 | 8.2 |
| Histology | ||||||||
| DCIS | 7595 | 100.0 | 1372 | 100.0 | ||||
| Invasive Ductal | 28935 | 74.6 | 5206 | 68.4 | ||||
| Invasive Lobular | 3881 | 10.0 | 1221 | 16.1 | ||||
| Invasive Ductal and Lobular | 2490 | 6.4 | 726 | 9.5 | ||||
| Invasive, Other | 3464 | 8.9 | 454 | 6.0 | ||||
| Grade | ||||||||
| High | 935 | 12.3 | 140 | 10.2 | 9331 | 24.1 | 1943 | 25.5 |
| Intermediate | 2451 | 32.3 | 455 | 33.2 | 16851 | 43.5 | 3563 | 46.8 |
| Low | 2876 | 37.9 | 580 | 42.3 | 10597 | 27.3 | 1781 | 23.4 |
| Unknown | 1333 | 17.6 | 197 | 14.4 | 1991 | 5.1 | 320 | 4.2 |
| Stage | ||||||||
| 0 | 7595 | 100.0 | 1372 | 100.0 | ||||
| I | 21650 | 55.8 | 4421 | 58.1 | ||||
| II | 13097 | 33.8 | 2541 | 33.4 | ||||
| III | 4023 | 10.4 | 645 | 8.5 | ||||
| Tumor Size | ||||||||
| <1cm | 2101 | 27.7 | 393 | 28.6 | 8426 | 21.7 | 1782 | 23.4 |
| 1 to <2 cm | 1486 | 19.6 | 294 | 21.4 | 14721 | 38.0 | 3154 | 41.5 |
| 2 to <5cm | 1090 | 14.4 | 265 | 19.3 | 12722 | 32.8 | 2214 | 29.1 |
| 5+ cm | 266 | 3.5 | 78 | 5.7 | 2329 | 6.0 | 361 | 4.8 |
| Unknown | 2652 | 34.9 | 342 | 24.9 | 572 | 1.5 | 96 | 1.3 |
| Estrogen Receptor Status | ||||||||
| Positive | 4480 | 59.0 | 883 | 64.4 | 30703 | 79.2 | 6305 | 82.9 |
| Negative | 1154 | 15.2 | 242 | 17.6 | 5869 | 15.1 | 1053 | 13.8 |
| Unknown | 1961 | 25.8 | 247 | 18.0 | 2198 | 5.7 | 249 | 3.3 |
| Teaching Hospitald | ||||||||
| No | 2520 | 33.2 | 442 | 32.2 | 15401 | 39.7 | 2573 | 33.8 |
| Yes | 2829 | 37.3 | 549 | 40.0 | 15220 | 39.3 | 3425 | 45.0 |
| Unknown | 2246 | 29.6 | 381 | 27.8 | 8149 | 21.0 | 1609 | 21.2 |
| Membership in an NCI Cooperative Group Triale | ||||||||
| No | 2477 | 32.6 | 292 | 21.3 | 15312 | 39.5 | 1912 | 25.1 |
| Yes | 2902 | 38.2 | 705 | 51.4 | 15464 | 39.9 | 4129 | 54.3 |
| Unknown | 2216 | 29.2 | 375 | 27.3 | 7994 | 20.6 | 1566 | 20.6 |
The percent shown is the column percent.
Residence is based on the woman's county of residence at time of breast cancer diagnosis and is categorized into urban which includes big metropolitan, metropolitan, and urban versus rural which includes less urban and rural counties.
Comorbidity Index is based on the method of Klabunde et al.(14)
Teaching hospital is based on the hospital where the breast surgery was performed.
Membership in an NCI Cooperative group trial indicates if the hospital where the surgery was performed participated in at least one NCI trial.
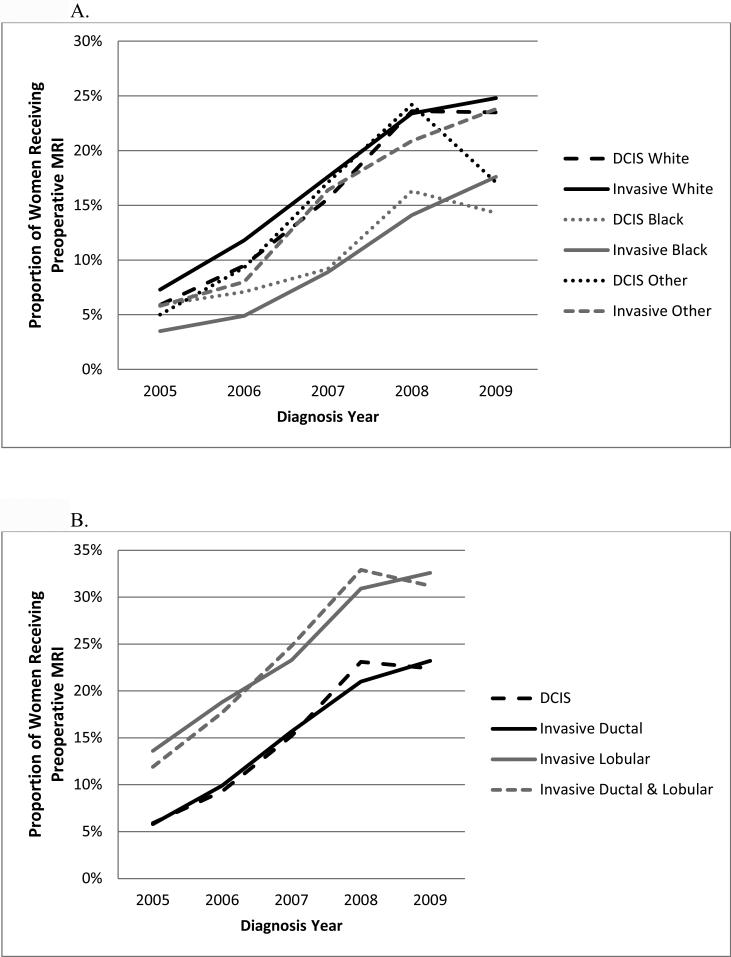
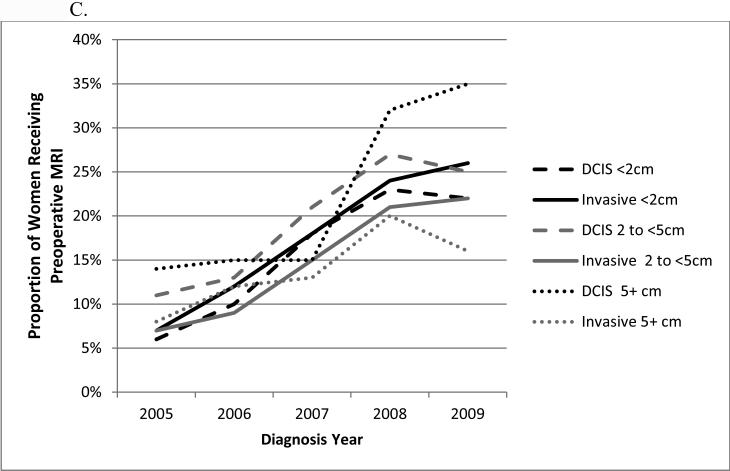
The use of preoperative breast MRI increased for all race groups from 2005 to 2008 but then decreased in 2009 for black and other non-white/non-black women with DCIS (Figure 3A). Among women with DCIS, there was no significant difference in MRI use between blacks and whites in 2005 and 2006 but from 2007 to 2009, MRI use was consistently higher among white than black women. For women with invasive cancer, preoperative MRI use was consistently higher for white compared with black women. The proportion of women receiving preoperative breast MRI was higher for those with ILC from 2005 to 2009 (Figure 3B). With regard to tumor size, there was a sharp increase in use of preoperative MRI from 2007 to 2008 for DCIS tumors 5 cm or larger (Figure 3C). There was little variation in preoperative breast MRI use by ER status and cancer type within each year (data not shown). Use of preoperative breast MRI at hospitals with an NCI sponsored cooperative group trial were consistently higher than at hospitals with no such capacity with utilization growing at a similar rate (data not shown).
Figure 3.
Proportion of women with non-metastatic breast cancer receiving preoperative breast MRI by year, cancer type, and patient/tumor characteristics: A, race; B, histology; C, tumor size.
In multivariable analysis for women with DCIS, factors associated with increased odds of receiving preoperative breast MRI included younger age, being married, living in a zip code with a higher median income, having no comorbidities and being diagnosed in more recent years (Table 2). Women with DCIS were more likely to receive preoperative MRI if their tumor size was ≥ 2cm (Odds Ratio (OR)=1.30, 95% Confidence Interval (CI): 1.07-1.58 for 2-4.9cm versus <1cm; OR=1.68, 95%CI: 1.24-2.28 for 5+cm versus <1cm). Additionally, having surgery at a hospital that participated in at least one NCI sponsored cooperative group trial was associated with increased odds of receiving MRI (OR=1.97, 95%CI: 1.66-2.33).
Table 2.
Multivariable logistic regression models for association between receipt of MRI and patient, tumor, and hospital characteristics, SEER-Medicare 2005-2009
| Ductal Carcinoma In-Situ | Invasive Breast Cancer | ||||
|---|---|---|---|---|---|
| Characteristic | Category | OR (95% CI)a | p-value | OR (95% CI)a | p-value |
| Age Group, years | 66-69 | Referent | <0.0001 | Referent | <0.0001 |
| 70-74 | 0.88 (0.75, 1.03) | 0.80 (0.74, 0.86) | |||
| 75-79 | 0.67 (0.56, 0.80) | 0.60 (0.55, 0.65) | |||
| 80-84 | 0.44 (0.36, 0.56) | 0.39 (0.36, 0.43) | |||
| 85+ | 0.23 (0.16, 0.33) | 0.20 (0.18, 0.23) | |||
| Race | White | Referent | 0.84 | Referent | <0.0001 |
| Black | 0.92 (0.69, 1.21) | 0.77 (0.67, 0.88) | |||
| Other | 0.89 (0.68, 1.18) | 0.79 (0.69, 0.90) | |||
| SEER Registry | California | Referent | <0.0001 | Referent | <0.0001 |
| Utah | 0.52 (0.31, 0.87) | 0.65 (0.54, 0.79) | |||
| Seattle | 1.37 (1.06, 1.77) | 1.72 (1.54, 1.91) | |||
| San Jose | 0.45 (0.29, 0.71) | 0.51 (0.42, 0.62) | |||
| San Francisco | 0.32 (0.21, 0.47) | 0.44 (0.37, 0.53) | |||
| Rural Georgia | 0.41 (0.05, 3.43) | 0.43 (0.19, 0.95) | |||
| New Mexico | 1.58 (0.98, 2.55) | 2.09 (1.74, 2.51) | |||
| New Jersey | 0.70 (0.57, 0.87) | 0.69 (0.63, 0.76) | |||
| Louisiana | 0.53 (0.38, 0.74) | 0.53 (0.45, 0.61) | |||
| Los Angeles | 1.20 (0.93, 1.55) | 1.54 (1.38, 1.71) | |||
| Kentucky | 0.18 (0.12, 0.29) | 0.28 (0.23, 0.33) | |||
| Iowa | 0.35 (0.23, 0.52) | 0.42 (0.35, 0.49) | |||
| Hawaii | 0.10 (0.04, 0.29) | 0.08 (0.04, 0.15) | |||
| Greater Georgia | 0.38 (0.28, 0.51) | 0.40 (0.35, 0.46) | |||
| Detroit | 0.31 (0.22, 0.45) | 0.53 (0.46, 0.62) | |||
| Connecticut | 0.68 (0.52, 0.89) | 0.75 (0.66, 0.84) | |||
| Atlanta | 0.84 (0.60, 1.17) | 0.64 (0.54, 0.76) | |||
| Residenceb | Urban | Referent | 0.49 | Referent | 0.87 |
| Rural | 1.12 (0.80, 1.57) | 1.01 (0.89, 1.15) | |||
| Married | Married | Referent | <0.0001 | Referent | <0.0001 |
| Not married | 0.75 (0.65, 0.86) | 0.87 (0.82, 0.92) | |||
| Median Income | <= $35,800 | Referent | <0.0001 | Referent | <0.0001 |
| $35,801-$47,300 | 1.42 (1.14, 1.76) | 1.32 (1.21, 1.45) | |||
| $47,301-$64,200 | 1.62 (1.3, 2.02) | 1.58 (1.44, 1.73) | |||
| >$64,200 | 2.02 (1.62, 2.52) | 2.21 (2.01, 2.43) | |||
| Comorbidity Scorec | None | Referent | <0.0001 | Referent | <0.0001 |
| 1 | 0.77 (0.66, 0.90) | 0.78 (0.73, 0.84) | |||
| 2+ | 0.60 (0.47, 0.75) | 0.57 (0.51, 0.62) | |||
| Diagnosis Year | 2005 | Referent | <0.0001 | Referent | <0.0001 |
| 2006 | 1.57 (1.20, 2.06) | 1.67 (1.50, 1.86) | |||
| 2007 | 2.98 (2.31, 3.85) | 3.05 (2.75, 3.39) | |||
| 2008 | 5.41 (4.25, 6.90) | 4.42 (4.00, 4.89) | |||
| 2009 | 4.94 (3.87, 6.31) | 4.94 (4.47, 5.46) | |||
| Histology | Invasive Ductal | Referent | <0.0001 | ||
| Invasive Lobular | 1.95 (1.80, 2.12) | ||||
| Invasive Lobular & Ductal | 1.57 (1.42, 1.74) | ||||
| Invasive Other | n/a | 0.87 (0.78, 0.98) | |||
| Grade | Low | Referent | 0.56 | Referent | 0.01 |
| Intermediate | 0.91 (0.78, 1.07) | 1.10 (1.02, 1.19) | |||
| High | 0.96 (0.77, 1.20) | 1.09 (1.00, 1.10) | |||
| Unknown | 0.89 (0.73, 1.09) | 0.93 (0.80, 1.08) | |||
| Stage | I | Referent | 0.01 | ||
| II | n/a | 1.18 (1.09, 1.28) | |||
| III | 1.07 (0.94, 1.21) | ||||
| Tumor Size | <1cm | Referent | <0.0001 | Referent | 0.0003 |
| 1 to <2cm | 1.09 (0.91, 1.31) | 1.04 (0.97, 1.12) | |||
| 2cm to <5cm | 1.30 (1.07, 1.58) | 0.83 (0.75, 0.92) | |||
| 5+ cm | 1.68 (1.24, 2.28) | 0.76 (0.65, 0.90) | |||
| Unknown | 0.82 (0.68, 0.97) | 0.95 (0.74, 1.22) | |||
| Estrogen Receptor | Positive | Referent | 0.05 | Referent | 0.001 |
| Negative | 1.13 (0.94, 1.35) | 1.14 (1.04, 1.24) | |||
| Unknown | 0.84 (0.70, 0.99) | 0.81 (0.69, 0.94) | |||
| Teaching Hospitald | No | Referent | 0.58 | Referent | 0.01 |
| Yes | 0.95 (0.81, 1.12) | 1.09 (1.02, 1.17) | |||
| Unknown | 0.63 (0.23, 1.72) | 1.44 (0.98, 2.11) | |||
| Member of NCI Cooperative Group Triale | No | Referent | <0.0001 | Referent | <0.0001 |
| Yes | 1.97 (1.66, 2.33) | 1.94 (1.81, 2.07) | |||
| Unknown | 2.08 (0.75, 5.78) | 0.97 (0.66, 1.43) | |||
OR = odds ratio; 95%CI = 95% confidence interval.
Residence is based on the woman's county of residence at time of breast cancer diagnosis and is categorized into urban which includes big metropolitan, metropolitan, and urban versus rural which includes less urban and rural counties.
Comorbidity Index is based on the method of Klabunde et al.(14)
Teaching hospital is based on the hospital where the breast surgery was performed.
Membership in an NCI Cooperative group trial indicates if the hospital where the surgery was performed participated in at least one NCI trial.
Among women with IBC, results of multivariable analysis indicate that preoperative MRI use was associated with younger age, white race, being married, living in a zip code with a higher median income, having a comorbidity score of zero and having a more recent diagnosis. Compared to women with IDC, women with ILC were more likely to receive MRI (OR=1.95, 95%CI: 1.90-2.12) as were women with invasive combined lobular/ductal disease (OR=1.57, 95%CI: 1.42-1.74). Women with IBC with ER-negative tumors were more likely to receive MRI compared to women with ER-positive tumors (OR=1.14, 95%CI: 1.04-1.24). Similar to the finding observed for women with DCIS, women with IBC who received breast surgery at a hospital that participated in at least one NCI sponsored cooperative group trial were more likely to receive breast MRI. The main difference between the multivariable results for women with DCIS versus those with invasive disease was in regard to tumor size; larger DCIS tumor size was associated with receipt of preoperative MRI, whereas women with smaller invasive tumors (defined as <1 cm) were more likely to receive preoperative MRI.
Our sensitivity analysis adjusting the preoperative window to capture MRIs conducted in the 30 days prior to the diagnosis date had no notable impact on results (data not shown).
Discussion
Recent studies across community settings indicate that approximately 15-20% of breast MRIs are performed to determine the extent of disease and inform treatment decisions.(16, 17) Multiple studies have found MRI to be more sensitive than conventional imaging in assessing the extent of both DCIS and invasive disease.(18-32) However, the value of MRI use in guiding practice is uncertain, in part as studies have not yet demonstrated improved outcomes such as fewer re-operations or recurrences when MRI is employed.(8, 33) The randomized controlled COMICE Trial that included women scheduled for breast-conserving therapy found no significant difference in re-operations rates for the MRI compared to the no-MRI group (33), but the trial had methodology limitations including mastectomies without pre-operative tissue verification for MRI findings and some low volume facilities which may impact generalizability. Further, it has not been definitively shown that MRI provides added advantage in particular subgroups of women based on factors such as age, breast density or cancer type.
We found that use of MRI for preoperative evaluation of both DCIS and IBC increased substantially from 2005 to 2008 and leveled off in 2009. We also observed a 6-fold geographic variation during the five-year study period. Correlates of preoperative MRI use were similar for women with DCIS and IBC, with the exception of tumor size, which had a positive relationship with MRI use in DCIS (larger tumors) but an inverse relationship with MRI use in invasive cancers (small tumors). Women with IBC receiving preoperative MRI, consistent with previous studies, tended to have tumors that have been shown to be poorly visualized on mammography and associated with positive surgical margins, such as lobular disease and ER-negative disease. Similarly, we found preoperative breast MRI use was associated with large DCIS tumors that are typically associated with greater frequency of positive margins.
Our findings are consistent with prior SEER-Medicare studies (3, 5, 34) and add to the existing knowledge, specifically for women with ILC, and ER-negative disease. We found higher preoperative breast MRI use among women with ILC compared to IDC. ILC is challenging to identify with conventional detection methods, likely attributable to its diffuse invasive growth pattern. Mammographic false negative rates for ILC have been reported as high as 19% (35, 36), and compared to IDC, malignancies that are ILC are more frequently larger, bilateral and result in positive surgical margins.(37) In a review of the literature, pooled data demonstrated that MRI detected additional otherwise-occult ipsilateral lesions in 32% of ILC cases and contralateral lesions in 7%.(38) However, a differential benefit of MRI in women with ILC compared to those with IDC has not been definitively demonstrated. Our results demonstrating more frequent MRI use in ILC likely reflect perceived advantages given the limitations of conventional modes of detection.
Several studies have shown that mammography is less sensitive at detecting ER-negative compared to ER-positive tumors (39, 40), likely due to the fact that ER-negative cancers grow quickly and may be difficult to identify on mammography or lack the typical suspicious characteristics of ER positive tumors.(40, 41) In contrast, MRI has been shown to better identify ER-negative and triple negative breast cancer.(41) Given that ER-negative tumors have worse prognosis with a higher risk of metastasis and higher risk of local recurrence, additional imaging of these tumors with MRI prior to surgical treatment may be warranted.(42, 43)
We also found for women with DCIS or IBC, hospital participation in NCI cooperative group trials is associated with receipt of preoperative breast MRI. The fact that women seen at a hospital that participated in an NCI-sponsored cooperative group trial were approximately two times more likely to receive preoperative breast MRI suggests that despite a lack of clear evidence of improved outcomes from using preoperative breast MRI, this emerging technology is being utilized differentially and more frequently among academic or research-focused practices, perhaps due to the NCCN guidelines.
There are several limitations of using SEER-Medicare data. Due to the nature of SEER data, several tumor variables (size, stage) may be those from final surgery, and not reflect the preoperative tumor characteristics. Since the SEER program collects tumor characteristics based on all available evidence within the initial treatment window, some information such as pathologic findings may differ substantially from preoperative estimates. In addition, we are unable to assess the use of neoadjuvant chemotherapy because of the challenges of doing so with claims data.
Overall, we found that the frequency with which preoperative breast MRI is performed is similar for women diagnosed with DCIS and IBC. Among women with IBC, those with ILC and those with ER-negative tumors were more likely to receive preoperative breast MRI suggesting that MRI is being performed more commonly among women diagnosed with invasive tumors that may not be well visualized on mammography. For women with DCIS, MRI was associated with larger lesions, which may be associated with greater frequency of positive margins. To better understand the use of this imaging tool, future studies should examine additional patient factors such as family history of breast cancer and breast density, tumor subtypes, and physician and hospital characteristics of women receiving preoperative MRI and also include a population of younger women.
Acknowledgments
This work was supported by funding from the National Institute of Health, National Cancer Institute under grant R01 CA149365-01.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflict of Interest - Dr. Lehman has been a paid consultant to GE Healthcare, Bayer Healthcare, and Phillips Healthcare. All other authors have no conflicts of interest to disclose.
References
- 1.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7(2):193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- 2.Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191(2):332–9. doi: 10.2214/AJR.07.3207. [DOI] [PubMed] [Google Scholar]
- 3.Killelea BK, Long JB, Chagpar AB, et al. Trends and clinical implications of preoperative breast MRI in Medicare beneficiaries with breast cancer. Breast Cancer Res Treat. 2013;141(1):155–63. doi: 10.1007/s10549-013-2656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer CA, Stitzenberg KB, Tolleson-Rinehart S, Carpenter WR, Carey TS. Breast MRI utilization in older patients with newly diagnosed breast cancer. J Surg Res. 2011;170(1):77–83. doi: 10.1016/j.jss.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttle TM, Jarosek S, Durham S, et al. Use of preoperative MRI among older women with ductal carcinoma in situ (DCIS) and early invasive breast cancer: use of preoperative breast MRI. 2012. Data Points #13 (prepared by the University of Minnesota DEcIDE Center, under Contract No. HHSA29020100013I). AHRQ Publication No. 12-EHC086-EF. [PubMed]
- 6.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2008;26(19):3248–58. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 7.Hwang N, Schiller DE, Crystal P, Maki E, McCready DR. Magnetic resonance imaging in the planning of initial lumpectomy for invasive breast carcinoma: its effect on ipsilateral breast tumor recurrence after breast-conservation therapy. Ann Surg Oncol. 2009;16(11):3000–9. doi: 10.1245/s10434-009-0607-1. [DOI] [PubMed] [Google Scholar]
- 8.Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26(3):386–91. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull L. Magnetic resonance imaging in breast cancer: results of the COMICE trial. Breast Cancer Res. 2008;10(Suppl 3):P10. [Google Scholar]
- 10.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209(2):180–7. doi: 10.1016/j.jamcollsurg.2009.04.010. quiz 294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virnig B. Diagnosis and management of DCIS. In: AHRQ, editor. Evidence Report/Technology Assessment No. 185. Rockville, MD: [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results Program (SEER) Medicare web site available at http://healthservices.cancer.gov/seermedicare. Accessed July 9, 2014.
- 13.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.SAS 9.3 System Options: Reference. Second Edition SAS Institute Inc.; Cary, NC: 2011. [Google Scholar]
- 16.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Internal Medicine. 2014;174(1):114–21. doi: 10.1001/jamainternmed.2013.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wernli KJ, Demartini WB, Ichikawa L, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Internal Medicine. 2014;174(1):125–32. doi: 10.1001/jamainternmed.2013.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98(3):468–73. doi: 10.1002/cncr.11490. [DOI] [PubMed] [Google Scholar]
- 19.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 20.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213(3):881–8. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 21.Lee SG, Orel SG, Woo IJ, et al. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: preliminary results. Radiology. 2003;226(3):773–8. doi: 10.1148/radiol.2263020041. [DOI] [PubMed] [Google Scholar]
- 22.Lehman CD, Blume JD, Thickman D, et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: results from the International Breast Magnetic Resonance Consortium (IBMC) trial. J Surg Oncol. 2005;92(1):9–15. doi: 10.1002/jso.20350. discussion -6. [DOI] [PubMed] [Google Scholar]
- 23.Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2003;180(4):901–10. doi: 10.2214/ajr.180.4.1800901. [DOI] [PubMed] [Google Scholar]
- 24.Liberman L, Morris EA, Kim CM, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003;180(2):333–41. doi: 10.2214/ajr.180.2.1800333. [DOI] [PubMed] [Google Scholar]
- 25.Mumtaz H, Hall-Craggs MA, Davidson T, et al. Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol. 1997;169(2):417–24. doi: 10.2214/ajr.169.2.9242745. [DOI] [PubMed] [Google Scholar]
- 26.Orel SG, Schnall MD, Powell CM, et al. Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology. 1995;196(1):115–22. doi: 10.1148/radiology.196.1.7784554. [DOI] [PubMed] [Google Scholar]
- 27.Pediconi F, Venditti F, Padula S, et al. CE-Magnetic Resonance Mammography for the evaluation of the contralateral breast in patients with diagnosed breast cancer. Radiol Med. 2005;110(1-2):61–8. [PubMed] [Google Scholar]
- 28.Rieber A, Merkle E, Bohm W, Brambs HJ, Tomczak R. MRI of histologically confirmed mammary carcinoma: clinical relevance of diagnostic procedures for detection of multifocal or contralateral secondary carcinoma. J Comput Assist Tomogr. 1997;21(5):773–9. doi: 10.1097/00004728-199709000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Schelfout K, Van Goethem M, Kersschot E, et al. Contrast-enhanced MR imaging of breast lesions and effect on treatment. Eur J Surg Oncol. 2004;30(5):501–7. doi: 10.1016/j.ejso.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Schnall MD, Blume J, Bluemke DA, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol. 2005;92(1):32–8. doi: 10.1002/jso.20381. [DOI] [PubMed] [Google Scholar]
- 31.Slanetz PJ, Edmister WB, Yeh ED, Talele AC, Kopans DB. Occult contralateral breast carcinoma incidentally detected by breast magnetic resonance imaging. Breast J. 2002;8(3):145–8. doi: 10.1046/j.1524-4741.2002.08304.x. [DOI] [PubMed] [Google Scholar]
- 32.Viehweg P, Rotter K, Laniado M, et al. MR imaging of the contralateral breast in patients after breast-conserving therapy. Eur Radiol. 2004;14(3):402–8. doi: 10.1007/s00330-003-2086-2. [DOI] [PubMed] [Google Scholar]
- 33.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375(9714):563–71. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang SY, Virnig BA, Tuttle TM, Jacobs DR, Jr., Kuntz KM, Kane RL. Variability of preoperative breast MRI utilization among older women with newly diagnosed early-stage breast cancer. Breast J. 2013;19(6):627–36. doi: 10.1111/tbj.12177. [DOI] [PubMed] [Google Scholar]
- 35.Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol. 1993;161(5):957–60. doi: 10.2214/ajr.161.5.8273634. [DOI] [PubMed] [Google Scholar]
- 36.Le Gal M, Ollivier L, Asselain B, et al. Mammographic features of 455 invasive lobular carcinomas. Radiology. 1992;185(3):705–8. doi: 10.1148/radiology.185.3.1438749. [DOI] [PubMed] [Google Scholar]
- 37.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008;107(1):1–14. doi: 10.1007/s10549-007-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Annals of Internal Medicine. 2011;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR. American Journal of Roentgenology. 2010;194(4):1160–6. doi: 10.2214/AJR.09.2355. [DOI] [PubMed] [Google Scholar]
- 41.Dogan BE, Turnbull LW. Imaging of triple-negative breast cancer. Annals of Oncology: official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 6):vi23–9. doi: 10.1093/annonc/mds191. [DOI] [PubMed] [Google Scholar]
- 42.Dominici LS, Mittendorf EA, Wang X, et al. Implications of constructed biologic subtype and its relationship to locoregional recurrence following mastectomy. Breast Cancer Research: BCR. 2012;14(3):R82. doi: 10.1186/bcr3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2008;26(14):2373–8. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]