Abstract
Background
There is growing concern about potential overuse of, and toxicity from, opioid analgesics. No nationally representative study has examined inter-state variations in opioid use and impact of policy on opioid use among older adults.
Methods
We used national Medicare data from 2007-2012 to assess temporal and geographic trends in rates of opioid prescription and relationship to opioid toxicity and different state regulations in Part D Medicare recipients. We excluded those with a cancer diagnosis. Multilevel, multivariable regression analyses evaluated rates of prolonged prescriptions for schedule II, schedule III, and combination II/III opioid for each state, adjusting for patient characteristics.
Results
The percent of Part D recipients receiving prescriptions for combined schedule II/III opioid more than 90 days in a year increased from 4.62% in 2007 to 7.35% in 2012. Large variations existed among states in rates of opioid prescriptions: from 2.84% in New York to 10.93% in Utah, in 2012 data. The state variation was larger for schedule III than schedule II. Individual characteristics independently associated with prolonged use included older age, female gender, white race, low-income, living in a lower education area, and comorbidity of drug abuse, rheumatoid arthritis, depression. Only state law regulating pain clinic was associated with reduction of schedule II opioid prescriptions. Prolonged opioid prescription use increased the odds of opioid overdose-related emergency room visits or hospitalization by 60%.
Conclusions
Analyses of Medicare Part D data demonstrated a substantial growth in opioid prescriptions from 2007 to 2011 and large variation in opioid prescriptions across states.
Keywords: Opioid, Regulations, Medicare, Overdose
Introduction
Opioid prescribing in the US has increased more than threefold over the last decade.1-3 Opioids offer important pain control for patients with cancer and other serious medical conditions. However, recent reports have raised concern about the safety and effectiveness of opioids—particularly use lasting for 90 days or more to treat chronic and acute non-cancer pain.4-7 More than 200 million opioid prescriptions are issued in the US each year.1,2,7 Approximately 16,000 people die annually from opioid overdose and many more experience opioid addiction.1,3-5,7-8 In response to the growing epidemic of opioid related deaths, the Drug Enforcement Administration (DEA) in 2014 changed hydrocodone combination products from a schedule III to a schedule II classification.
Understanding opioid use and the impact of federal and state policy holds particular clinical and public health relevance for the growing elderly population. Older adults are particularly prone to opioid toxicity—resulting in complications such as falls and fractures—because of the age-related decline in drug metabolism, their increased exposure to multiple medications, and the high prevalence of multiple co-morbidities.9-14 Adults ≥ 65 years are the largest consumers of prescription medications, including opioids and other psychoactive drugs.11-13
Current opioid prescribing practices are regulated by policies which vary substantially across states.8,14-16 To date, no nationally representative studies have examined variations in the use of opioids in older adults and how the use was associated with state regulations.5,17-18 To address this gap in knowledge, we use 2007-2012 national data of Medicare beneficiaries to investigate this escalating public health issue. Understanding multilevel factors associated with opioid use and outcomes in older adults and the impact of policy will offer important insights into developing guidelines for safe and effective use of opioids in this population.
Methods
Source of Data
Claims from 2006 to 2012 of a 5% national sample of Medicare beneficiaries were used, including Medicare beneficiary summary files, Medicare Provider Analysis and Review (MedPAR) files, Outpatient Standard Analytic Files (OUTSAF), Medicare Carrier files, and Prescription Drug Event (PDE) files.
Establishment of the Study Cohort
We selected Medicare beneficiaries aged 66 or above with Parts A, B and D coverage and not in a health maintenance organization (HMO) for the year prior to and the year of study or until death for each year from 2007 to 2012. Patients with a cancer diagnosis in the year prior to or the year of study were excluded. We identified cancer diagnosis using the listed International Classification of Diseases, 9th version (ICD-9) diagnosis codes for metastatic cancer, lymphoma, or solid tumor without metastasis under Elixhauser comorbidity measures19. We also excluded patients residing in long-term nursing homes identified through Evaluation and Management codes billed for long-term nursing home services. Also excluded were patients receiving palliative care identified through claims with the place of service listed as hospice.
Measures
Medicare enrollment files provided information on patient age, gender and race/ethnicity. We used a Medicaid indicator in the enrollment file or a low-income subsidy Part D program enrollment as a proxy for low income. Education for zip code areas was obtained from the 2010 Census data and categorized by quartiles. Elixhauser comorbidity measures for each enrollee were generated from all claims in the 12 months before each study year.19 Total number of hospitalizations in the 12 months before each study year was counted from MedPAR. We categorized type of residential area into metropolitan, non-metropolitan urban and ruralusing Rural-Urban Continuum Codes.20 State laws directed at reducing opioid misuse, abuse and toxicity published by the Centers for Disease Control and Prevention (CDC) in 2010 were used16. These laws include 7 domains: requiring a physical examination before prescribing, requiring tamper-resistant prescription forms, regulating pain clinics, setting prescription drug limits, prohibiting “Doctor Shopping”, requiring patient identification before dispensing, and providing immunity from prosecution at sentencing for persons seeking help during an overdose (Appendix).
Prolonged Opioid Use
The study outcomes were having at least 90 days’ prescriptions of schedule II opioid, schedule III opioid, or combination of schedule II and III filled at each year. We counted whether patients have an opioid prescription by days; therefore, the range of opioid use is from 0 to 365 days. This was determined by examining the PDE records for the study cohort. The RED BOOK Select Extracts database was used to identify the drug class and opioid schedules.21 Opioid given by injections were not included in the study.
Outcomes of Opioid Use
Emergency room (ER) visits or acute hospitalization related to potential overdose were identified from all claims. The following were included: any physician diagnoses for 1) opioid-related poisoning: ICD-9 965, E850.1, E950.0, E980.0, 2) opioid-specific adverse event: ICD-9 E935.0, E935.1, E935.2, or 3) overdose diagnosis: ICD-9 276.4, 292.1, 292.8, 486, 496, 518.81, 518.82, E950-E959 22.
Statistical Analyses
Proportions of patients receiving prolonged opioid prescriptions (schedule II, schedule III, or combination) were calculated, plotted by year, and stratified by patient characteristics. Multilevel multivariable analyses using a hierarchical generalized linear model (HGLM) with a binary distribution and logit link, adjusted for clustering of patients within states, were conducted to evaluate the association of patient characteristics and state regulations with the likelihood of receiving schedule II (also III or combined) opioid prescriptions. HGLM was also used to examine the association between prolonged opioid use and ER visits or hospitalizations for potential opioid overdose. Spearman rank correlation was used to test the association between adjusted rates of opioid prescription for schedule II and schedule III across states, and the association between prolonged us and outcomes across states adjusted for state-level rates of Medicare HMO enrollment and part D enrollment. The adjusted rates of opioid prescription for each state were estimated by HGLM, controlling for patient characteristics. All tests of statistical significance were 2-sided. Analyses were performed with SAS version 9.3 (SAS Inc., Cary, NC).
Results
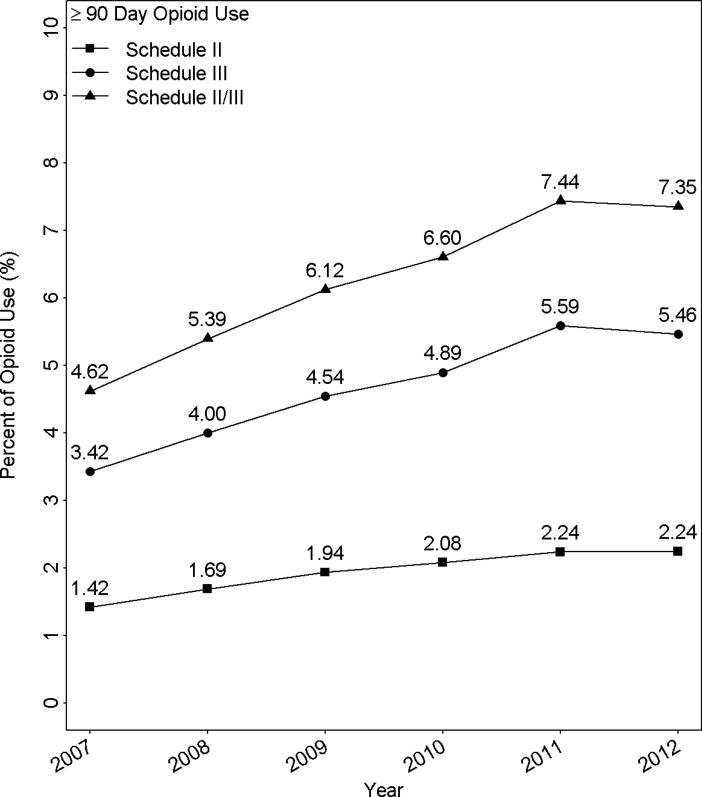
Our final sample consisted of 800,664 individuals aged 66 and older with 2,720,343 person-years of observation between 2007 and 2012. None of the sample subjects had a cancer diagnosis in the year prior to and the year of study. As shown in Figure 1, from 2007 to 2012, the percentage of Medicare patients receiving prolonged opioid prescription increased from 4.62% to 7.35%. The percentage given prescriptions for schedule II opioids went from 1.42% to 2.24%; for schedule III, it went from 3.42% to 5.46%. The increases were fairly steady over the 2007-2011 period, with a flat or a slight fall in 2012.
Figure 1.
Percent of Medicare patients in the U.S. who received prolonged opioid prescriptions for schedule II or schedule III or combination schedule II/III, from 2007 through 2012.
Table 1 shows the rate of opioid use in 2012 stratified by patient characteristics. Both unadjusted percentages and the adjusted odds ratios from a multilevel multivariable analysis are shown. The analyses for schedule II, III, and combination are presented separately. In the unadjusted results, there was a slight decline with age in prolonged use of combination opioid prescription. Female gender, black race, low socioeconomic status, comorbid conditions (especially drug abuse, rheumatoid arthritis, and depression), prior hospitalizations, and residence in a non-metropolitan or lower education area were all associated with higher percentages of receiving combination prescriptions for 90 days or more. The adjusted result showed the impact of age, race/ethnicity, and certain comorbidities was stronger for the odds of schedule II prescriptions than for schedule III prescriptions.
Table 1.
Unadjusted percentages and adjusted odds ratios from a multilevel, multivariable analysis estimating the odds of having prolonged opioid prescription on Schedule II, Schedule III, or Combination for Medicare patients in 20121
| Schedule II | Schedule III | Combination of Schedule II or III | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | N | Use (%) | OR | 95% CI | Use (%) | OR | 95% CI | Use (%) | OR | 95% CI |
| Age (years) | ||||||||||
| 66 to 70 | 124,936 | 2.86 | Ref. | Ref. | 6.21 | Ref. | Ref. | 8.58 | Ref. | Ref. |
| 70+ to 75 | 126,637 | 2.10 | 0.75 | (0.71 to 0.79) | 5.29 | 0.85 | (0.82 to 0.88) | 7.06 | 0.81 | (0.78 to 0.84) |
| 75+ to 80 | 100,787 | 2.00 | 0.65 | (0.62 to 0.69) | 5.32 | 0.79 | (0.76 to 0.82) | 7.02 | 0.74 | (0.72 to 0.77) |
| 80+ to 85 | 80,464 | 1.99 | 0.62 | (0.58 to 0.66) | 5.24 | 0.77 | (0.73 to 0.80) | 6.94 | 0.71 | (0.69 to 0.74) |
| 85+ | 82,372 | 2.09 | 0.61 | (0.57 to 0.65) | 4.96 | 0.71 | (0.68 to 0.74) | 6.74 | 0.67 | (0.64 to 0.69) |
| Gender | ||||||||||
| Female | 346,446 | 2.47 | Ref. | Ref. | 6.06 | Ref. | Ref. | 8.12 | Ref. | Ref. |
| Male | 168,750 | 1.79 | 0.82 | (0.78 to 0.85) | 4.22 | 0.75 | (0.73 to 0.78) | 5.77 | 0.77 | (0.75 to 0.79) |
| Race | ||||||||||
| Whites | 421,327 | 2.39 | Ref. | Ref. | 5.46 | Ref. | Ref. | 7.47 | Ref. | Ref. |
| Blacks | 37,834 | 2.12 | 0.57 | (0.53 to 0.62) | 7.47 | 0.75 | (0.72 to 0.79) | 9.30 | 0.70 | (0.67 to 0.73) |
| Hispanics | 32,561 | 1.38 | 0.42 | (0.38 to 0.47) | 4.94 | 0.48 | (0.45 to 0.51) | 6.07 | 0.45 | (0.43 to 0.48) |
| Other | 23,474 | 0.98 | 0.34 | (0.29 to 0.39) | 2.95 | 0.36 | (0.33 to 0.39) | 3.81 | 0.35 | (0.32 to 0.37) |
| Medicaid Eligibility | ||||||||||
| No | 370,505 | 1.75 | Ref. | Ref. | 3.99 | Ref. | Ref. | 5.50 | Ref. | Ref. |
| Yes | 144,691 | 3.50 | 2.03 | (1.94 to 2.12) | 9.23 | 2.22 | (2.16 to 2.29) | 12.09 | 2.24 | (2.18 to 2.30) |
| Prior Hospitalization | ||||||||||
| 0 | 434,585 | 1.79 | Ref. | Ref. | 4.71 | Ref. | Ref. | 6.22 | Ref. | Ref. |
| 1 | 56,348 | 3.99 | 1.23 | (1.16 to 1.30) | 8.67 | 1.13 | (1.09 to 1.18) | 12.03 | 1.16 | (1.12 to 1.21) |
| 2 | 15,631 | 5.48 | 1.13 | (1.03 to 1.24) | 10.74 | 1.05 | (0.98 to 1.12) | 15.37 | 1.09 | (1.03 to 1.16) |
| 3+ | 8,632 | 8.02 | 1.02 | (0.91 to 1.15) | 12.66 | 0.86 | (0.79 to 0.94) | 19.25 | 0.92 | (0.86 to 1.00) |
| Proportion of Education > 12 years | ||||||||||
| Q1 (lowest) | 127,201 | 2.36 | Ref. | Ref. | 7.71 | Ref. | Ref. | 9.62 | Ref. | Ref. |
| Q2 | 125,733 | 2.54 | 1.05 | (0.99 to 1.11) | 6.13 | 0.93 | (0.89 to 0.96) | 8.23 | 0.95 | (0.92 to 0.98) |
| Q3 | 127,877 | 2.26 | 0.97 | (0.92 to 1.03) | 4.66 | 0.84 | (0.80 to 0.87) | 6.61 | 0.87 | (0.84 to 0.90) |
| Q4 (highest) | 125,975 | 1.82 | 0.88 | (0.83 to 0.94) | 3.25 | 0.67 | (0.64 to 0.70) | 4.87 | 0.72 | (0.69 to 0.75) |
| Rural/urban | ||||||||||
| Metro | 388,232 | 2.17 | Ref. | Ref. | 4.93 | Ref. | Ref. | 6.78 | Ref. | Ref. |
| Non-metro urban | 110,783 | 2.47 | 0.98 | (0.93 to 1.03) | 7.10 | 1.09 | (1.06 to 1.12) | 9.10 | 1.07 | (1.04 to 1.10) |
| Rural | 16,181 | 2.53 | 1.03 | (0.92 to 1.15) | 6.80 | 1.01 | (0.94 to 1.08) | 8.96 | 1.03 | (0.97 to 1.10) |
| Comorbidity | ||||||||||
| Congestive heart failure | 35,003 | 4.73 | 1.15 | (1.07 to 1.23) | 10.44 | 1.14 | (1.09 to 1.20) | 14.31 | 1.15 | (1.10 to 1.20) |
| Valvular disease | 28,707 | 2.96 | 0.84 | (0.77 to 0.91) | 6.19 | 0.85 | (0.80 to 0.90) | 8.73 | 0.84 | (0.80 to 0.88) |
| Pulmonary circulation disease | 9,377 | 5.48 | 1.11 | (1.00 to 1.24) | 10.20 | 1.07 | (0.99 to 1.16) | 14.87 | 1.10 | (1.03 to 1.18) |
| Hypertension | 303,057 | 2.75 | 1.32 | (1.26 to 1.39) | 6.89 | 1.59 | (1.54 to 1.64) | 9.20 | 1.53 | (1.49 to 1.58) |
| Peripheral vascular disease | 42,240 | 4.01 | 1.29 | (1.21 to 1.37) | 8.18 | 1.20 | (1.15 to 1.25) | 11.55 | 1.23 | (1.19 to 1.28) |
| Paralysis | 4,746 | 5.29 | 1.19 | (1.03 to 1.37) | 9.67 | 1.03 | (0.92 to 1.15) | 14.29 | 1.10 | (1.00 to 1.21) |
| Neurological disorders | 28,540 | 4.41 | 1.16 | (1.08 to 1.24) | 7.81 | 0.96 | (0.92 to 1.02) | 11.42 | 1.02 | (0.97 to 1.06) |
| Chronic pulmonary disease | 60,899 | 5.05 | 1.60 | (1.53 to 1.68) | 11.14 | 1.64 | (1.59 to 1.70) | 15.24 | 1.67 | (1.62 to 1.72) |
| Diabetes | 117,106 | 2.96 | 1.04 | (0.99 to 1.10) | 7.63 | 1.16 | (1.12 to 1.19) | 10.15 | 1.14 | (1.11 to 1.17) |
| Diabetes Complications | 31,553 | 4.09 | 1.26 | (1.17 to 1.35) | 9.08 | 1.14 | (1.08 to 1.19) | 12.54 | 1.18 | (1.13 to 1.23) |
| Hypothyroidism | 67,034 | 3.26 | 1.10 | (1.04 to 1.16) | 6.98 | 1.01 | (0.98 to 1.05) | 9.73 | 1.04 | (1.01 to 1.08) |
| Renal failure | 34,412 | 3.96 | 0.96 | (0.90 to 1.03) | 8.99 | 1.00 | (0.95 to 1.05) | 12.36 | 0.99 | (0.95 to 1.03) |
| Liver disease | 3,805 | 5.55 | 1.41 | (1.21 to 1.66) | 8.36 | 1.01 | (0.89 to 1.15) | 13.30 | 1.19 | (1.07 to 1.32) |
| Peptic ulcer | 189 | 4.76 | 1.27 | (0.60 to 2.69) | 5.29 | 0.58 | (0.29 to 1.16) | 9.52 | 0.80 | (0.47 to 1.37) |
| Acquired immune deficiency syndrome | 326 | 6.14 | 1.95 | (1.17 to 3.24) | 5.83 | 0.84 | (0.50 to 1.40) | 11.35 | 1.19 | (0.81 to 1.77) |
| Rheumatoid arthritis | 17,988 | 7.72 | 3.17 | (2.97 to 3.38) | 14.12 | 2.61 | (2.49 to 2.74) | 20.26 | 2.97 | (2.85 to 3.10) |
| Coagulopathy | 8,668 | 4.36 | 1.02 | (0.90 to 1.14) | 8.48 | 1.02 | (0.94 to 1.11) | 12.25 | 1.04 | (0.96 to 1.12) |
| Obesity | 17,072 | 5.82 | 1.27 | (1.17 to 1.37) | 12.35 | 1.31 | (1.24 to 1.39) | 17.21 | 1.34 | (1.28 to 1.41) |
| Weight loss | 8,306 | 6.31 | 1.30 | (1.17 to 1.45) | 9.94 | 1.02 | (0.94 to 1.11) | 15.15 | 1.11 | (1.04 to 1.19) |
| Fluid and electrolyte disorders | 35,192 | 5.36 | 1.07 | (1.00 to 1.15) | 10.16 | 0.99 | (0.94 to 1.04) | 14.58 | 1.01 | (0.97 to 1.06) |
| Chronic blood loss anemia | 2,757 | 5.19 | 0.95 | (0.79 to 1.15) | 10.23 | 1.03 | (0.90 to 1.18) | 14.73 | 1.03 | (0.92 to 1.16) |
| Deficiency Anemias | 56,515 | 4.55 | 1.35 | (1.27 to 1.43) | 9.42 | 1.26 | (1.21 to 1.31) | 13.21 | 1.30 | (1.26 to 1.35) |
| Alcohol abuse | 2,338 | 5.90 | 0.68 | (0.55 to 0.83) | 9.58 | 0.89 | (0.76 to 1.04) | 14.71 | 0.81 | (0.70 to 0.92) |
| Drug abuse | 1,248 | 33.17 | 8.96 | (7.77 to 10.33) | 29.25 | 3.29 | (2.85 to 3.79) | 55.29 | 7.80 | (6.84 to 8.89) |
| Psychoses | 15,667 | 5.86 | 1.34 | (1.24 to 1.45) | 9.18 | 1.08 | (1.01 to 1.15) | 14.11 | 1.19 | (1.13 to 1.25) |
| Depression | 27,448 | 7.75 | 2.12 | (2.00 to 2.25) | 13.00 | 1.73 | (1.65 to 1.81) | 19.30 | 1.94 | (1.87 to 2.02) |
OR: Odds ratio; CI: Confidence Interval
The amount of variation (residual intraclass correlation coefficient or residual ICC) in receiving prolonged opioid prescriptions attributed to states was 2.4%, 7.2% and 3.5% for schedule II, schedule III and schedule II or III, respectively.
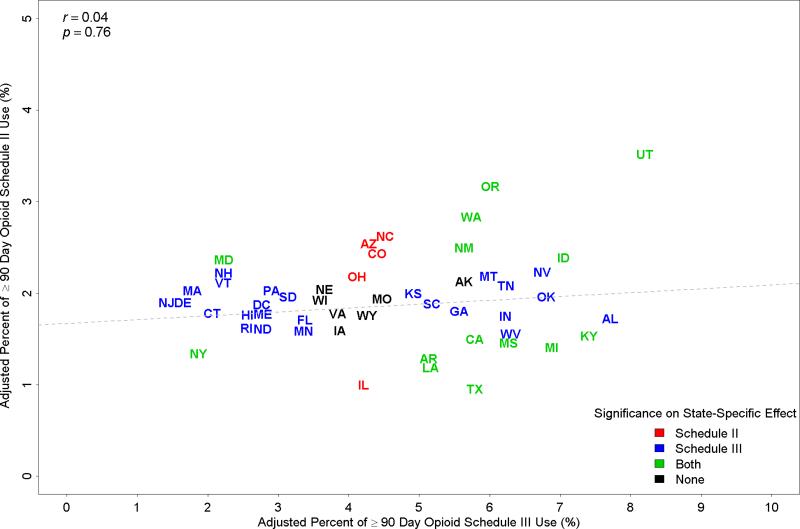
We next examined variation among states in rates of opioid prescriptions. After adjusting for patient characteristics shown in Table 1, the rate of prolonged use of combination prescription opioids varied from 2.84% in New York to 10.93% in Utah estimated from the multi-level, multivariable model. Figure 2 plots the adjusted percentages of patients who received prolonged schedule III prescription (X-axis) versus prolonged schedule II prescription (Y-axis) for each state and the District of Columbia, in 2012. For schedule II, the rates of prescription ranged from 0.87% in Texas to 3.44% in Utah. For schedule III, the rates varied from 1.23% in New Jersey to 8.0% in Utah. Many of the states in the northeast of the US had lower than average rates of schedule III opioid prescription, with rates for schedule II opioid prescription closed to the average. In contrast, Texas had high schedule III use with the lowest schedule II use among states. Interestingly, there was no correlation at the state level between the schedule III and II rates (r = 0.04, p = 0.76).
Figure 2.
Proportions of Part D Medicare patients who received prolonged prescriptions for schedule II vs. schedule III opioid in 2012, controlling for patient characteristics, for each state. Patients with a cancer diagnosis were excluded. States colored as red had adjusted rates of schedule II opioid prescription significantly higher or lower than the average. States colored as blue had adjusted rates of schedule III opioid prescription significantly higher or lower than the average. States colored as green had both adjusted rates of schedule II and adjusted rates of schedule III opioid prescriptions significantly higher or lower than the average. States colored as black had adjusted rates of schedule II and adjusted rates of schedule III opioid prescriptions similar to the average. These adjusted rates were estimated from hierarchical generalized linear models that included all variables listed in Table 1.
Also indicated in Figure 2 are the states with rates that were significantly different from the mean of all states. There was considerable variation in both schedule II and III use. For schedule II, 10 states had adjusted rates significantly higher than the state average, with a mean rate of 2.58%; nine states has significantly lower rates, with a mean rate of 1.22%. For schedule III, 22 states had significantly higher rates than the state average, with a mean rate of 6.02%; 17 states had significantly lower rates, with a mean rate of 2.24%. The amount of variation in receiving prolonged opioid prescription attributed to states was 2.4% for schedule II and 7.2% for schedule III, measured by residual intra-class correlation coefficient.
Table 2 shows the rate of opioid use stratified by the state laws regulating opioid prescription. Both unadjusted percentages and the adjusted odds ratios are presented. Except for the law regulating pain clinics, which was associated with prolonged schedule II opioid prescription (OR: 0.64, 95% CI = 0.47-0.89), none of the other laws had a significant impact on opioid use. We also found that state laws mediated 29.2% and 11.1% of the inter-state variation for schedule II and III prescriptions, respectively.
Table 2.
Unadjusted percentages and adjusted odds ratios from a multilevel, multivariable analysis estimating odds of having prolonged opioid prescription on Schedule II, Schedule III, or combination associated with state laws regulating opioid prescriptions for Medicare patients in 20121
| Schedule II | Schedule III | Combination of Schedule II or III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Use (%) | OR | 95% CI | Use (%) | OR | 95% CI | Use (%) | OR | 95% CI | |
| Drug Law2 | |||||||||||
| Law 1 | No | 52,685 | 2.82 | Ref. | Ref. | 5.31 | Ref. | Ref. | 7.76 | Ref. | Ref. |
| Yes | 462,511 | 2.18 | 0.89 | (0.70 to 1.13) | 5.48 | 0.96 | (0.62 to 1.49) | 7.30 | 0.96 | (0.71 to 1.29) | |
| Law 2 | No | 209,307 | 2.44 | Ref. | Ref. | 4.88 | Ref. | Ref. | 7.02 | Ref. | Ref. |
| Yes | 305,889 | 2.11 | 0.96 | (0.83 to 1.11) | 5.85 | 1.15 | (0.87 to 1.52) | 7.58 | 1.08 | (0.90 to 1.31) | |
| Law 3 | No | 441,226 | 2.34 | Ref. | Ref. | 5.39 | Ref. | Ref. | 7.37 | Ref. | Ref. |
| Yes | 73,970 | 1.67 | 0.64 | (0.47 to 0.89) | 5.86 | 1.33 | (0.72 to 2.47) | 7.20 | 1.05 | (0.69 to 1.60) | |
| Law 4 | No | 107,231 | 2.54 | Ref. | Ref. | 6.63 | Ref. | Ref. | 8.71 | Ref. | Ref. |
| Yes | 407,965 | 2.17 | 0.95 | (0.80 to 1.14) | 5.15 | 0.73 | (0.53 to 1.02) | 6.99 | 0.83 | (0.66 to 1.04) | |
| Law 5 | No | 382,632 | 2.22 | Ref. | Ref. | 5.59 | Ref. | Ref. | 7.44 | Ref. | Ref. |
| Yes | 132,564 | 2.32 | 1.06 | (0.88 to 1.26) | 5.07 | 0.86 | (0.61 to 1.20) | 7.09 | 0.92 | (0.73 to 1.16) | |
| Law 6 | No | 274,943 | 2.27 | Ref. | Ref. | 5.25 | Ref. | Ref. | 7.16 | Ref. | Ref. |
| Yes | 240,253 | 2.22 | 1.01 | (0.86 to 1.20) | 5.69 | 1.02 | (0.75 to 1.41) | 7.57 | 1.03 | (0.83 to 1.28) | |
| Law 7 | No | 491,093 | 2.20 | Ref. | Ref. | 5.51 | Ref. | Ref. | 7.35 | Ref. | Ref. |
| Yes | 24,103 | 3.06 | 1.34 | (0.98 to 1.83) | 4.44 | 0.92 | (0.52 to 1.61) | 7.30 | 1.11 | (0.75 to 1.64) | |
OR: Odds ratio; CI: Confidence Interval
These models included all patient characteristics listed in Table 1. The amount of variation (residual intraclass correlation coefficient or residual ICC) in receiving prolonged opioid prescriptions attributed to states was 1.7%, 6.4% and 3.1% for schedule II, schedule III and schedule II or III, respectively.
Law 1 means laws requiring a physical examination before prescribing; Law 2 means laws requiring tamper-resistant prescription forms; Law 3 means laws regulating pain clinics; Law 4 means laws setting prescription drug limits; Law 5 means laws prohibiting doctor shopping or fraud; Law 6 means laws requiring patient identification before dispensing; and Law 7 means laws providing immunity from prosecution or mitigation at sentencing for individuals seeking assistance during an overdose.
Table 3 shows the rate of ER visits and hospitalizations related to potential overdose, stratified by prolonged opioid prescription of schedule II drugs, schedule III drugs, and combination. Prolonged combined opioid prescription was associated with higher rates of overdose-related acute care events. Rates of ER visits were 8.7% vs. 3.0% for patients with and without opioid prescriptions for schedule II/III combinations, respectively; for hospitalizations, these rates were 10.0% vs. 3.7%. After adjusting for patient characteristics, the odds of having an ER visit related to potential overdose was larger for schedule II than for schedule III prescriptions (OR: 1.74, 95% CI = 1.62 – 1.82 vs. OR: 1.46, 95% CI = 1.38 – 1.54). Results for hospitalization were similar. A high correlation was found between the rate of prolonged opioid prescription use and the rates of ER visits and hospitalizations across states (ER: r = 0.39, p = 0.0044 and hospitalization: r = 0.45, p=0.0009).
Table 3.
Unadjusted percentages and adjusted odds of emergency room (ER) visits or hospitalizations associated with potential overdose from a multilevel, multivariable analysis estimating odds of ER visit or hospitalization for Medicare patients in 20121
| ER Visit | Hospitalization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 90 Day Opioid Use | N | % | OR | 95% CI | N | % | OR | 95% CI | ||
| Schedule II | No | 503,631 | 3.26 | Ref. | Ref. | No | 503,631 | 4.02 | Ref. | Ref. |
| Yes | 11,565 | 10.44 | 1.74 | (1.62 to 1.86) | Yes | 11,565 | 11.98 | 1.78 | (1.66 to 1.90) | |
| Schedule III | No | 487,073 | 3.14 | Ref. | Ref. | No | 487,073 | 3.89 | Ref. | Ref. |
| Yes | 28,123 | 8.25 | 1.46 | (1.38 to 1.54) | Yes | 28,123 | 9.50 | 1.47 | (1.40 to 1.54) | |
| Combination of Schedule II or III | No | 477,330 | 3.00 | Ref. | Ref. | No | 477,330 | 3.74 | Ref. | Ref. |
| Yes | 37,866 | 8.70 | 1.60 | (1.53 to 1.67) | Yes | 37,866 | 10.04 | 1.61 | (1.54 to 1.68) | |
OR: Odds ratio; CI: Confidence Interval
These models included all patient characteristics listed in Table 1.
Discussion
This national study of opioid prescriptions in Medicare patients enrolled in Part D showed substantial growth from 2007 to 2011 in the percentage of older adults receiving prolonged (≥ 90 day) schedule III or schedule II opioids, with a slight fall in 2012. There were many similarities in individual characteristics associated with prolonged use of schedule III, schedule II, or a combination of schedule II/III medication. However, there was no association at the state level between rates of prolonged schedule II and III prescriptions. Adjusting for individual patient characteristics had little effect on the marked variation among states.
The variation among states was greater for schedule III than for schedule II opioids, as shown in Figure 2. This is consistent with our hypothesis that state law enforcement has a larger impact on schedule III variation than schedule II variation. This result may reflect the tighter federal controls on schedule II opioids, with a special prescription form, a maximum 30 day supply for each prescription and the prohibition of refills.23 In contrast, control of schedule III medication is mostly at the state level. One might expect a reduction in the state-to-state variation in prescribing for oxycodone/acetaminophen combination products after the federal-government mandated change from schedule III to schedule II in late 2014.24
Increasing attention has been paid to toxicity from opioids and also to diversion of prescription opioids into illegal use.25-26 This in turn has led to attempts within states to more strictly monitor these drugs,14 such that, by mid-2012, laws have been enacted in all states to establish prescription drug monitoring programs (PDMPs).15 Large differences exist among states in the level of regulation and control of opioid prescriptions.16 For example, as of 2012, the year used to generate the data in Figure 2, only physicians can prescribe schedule II opioids in Texas and Arkansas, whereas Utah and Washington had no such restrictions, both states in which nurse practitioners and physician assistants are allowed to prescribe schedule II and above opioids.27-28 On examining the individual impact of the 7 categories of state laws, we found that only the state law regulating pain clinic was significantly associated with lower odds of schedule II prescription. Together, all 7 categories of state laws mediated 29.2% of the inter-state variation in schedule II prescriptions and 11.1% of the inter-state variation in schedule III opioid prescriptions.
The overall use of prescription opioids in the elderly is high. By 2012, 7.35% of Part D Medicare recipients were on opioid prescription for more than 90 days. Our results is higher than the 3% reported among patients aged 66 years or older who were receiving prescriptions for opioids for more than 90 days after major surgery29 but comparable to the 10% prolonged opioid use rate after minor ambulatory surgery30. Overall, our finding is much lower than the rates reported in younger populations31. Among 892 patients with back pain, 25% had prolonged opioid prescriptions31. Most of other studies in younger populations are about the non-medical use of opioids, with use rate data derived in the setting of illicit drugs, diverted drugs, and other illegal activities. However, most seniors receive their opioids legally from licensed prescribers, and their patterns and outcomes of use and misuse are likely different from those of younger populations.
The availability of Medicare Part D data allows investigators to generate national estimates of prescription drug use in the elderly. A major limitation is that only approximately 70% of Medicare recipients are accessible in Part D data. By 2014, about 26.6% enrolled in managed care plans and 43.4% in traditional fee-for-service Medicare had not enrolled in Part D plans.32-33 Also, the percent of Medicare patients enrolled in Part D varies somewhat by state, a fact which may contribute to the differences among states in the estimate of percent receiving opioid prescriptions. However, there were no significant correlations between state level opioid prescription and state level HMO or Part D enrollment (HMO: r = 0.16, p = 0.2595; Part D: r = −0.12, p = 0.4004).
Several factors may contribute to the decline in rates of both schedules II and III prescriptions in 2012, after a steady rise between 2007 and 2011. The largest increase in enactment of new opioid-regulating laws in the US occurred in 2010-2011, especially in Texas and Florida, two states with the highest number of clinics engaged in high-volume prescribing of opioids for non-medical use.14-15,34-37 Also, a study using state-level databases of laws governing the operation of prescription monitoring programs (PMPs) found that states with PMPs laws have increased from 25 in 2005 to 46 in 2011.14 These new laws were enacted partly in response to the growing epidemic of opioid-related deaths and overdoses reported by the CDC and other public health agencies in 2010 and 2011.1-3,37 Also, in 2010, extended-release oxycodone was reformulated by its maker to be tamper- and abuse-resistant.38 Thus, it is possible that the 2012 decline in opioid prescriptions seen in our study reflects the flurry of new laws and regulations enacted one to two years earlier.39 In Florida, for example, deaths from prescribed opioid analgesics declined by 26% from 2,560 deaths in 2010 to 1,892 in 2012, after the implementation of laws regulating pain clinics.37
Both the unadjusted rates for schedule II and III opioid use and the rates adjusted for patient characteristics were higher in women, those with lower income, those with lower education, and those with a higher burden of illness. These findings are consistent with past reports that older women have the highest prevalence of long term opioid use.2,39 However, our findings are different from an analysis of opioid prescription for older adults in 1999-2010 from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey ,40 which found no differences in opioid prescription rates by age, race/ethnicity, or region.
The study has some limitations. First, prescription data do not contain information on whether patients actually take opioids. Second, the indication of opioid use is not available in the Part D data. However, it is unlikely there are substantial differences in indications for opioid use across states, or large changes in indications for opioid use over time. Third, we excluded patients with HMO enrollment and those without Part D coverage. The results of opioid use may not be generalized to these populations. In addition, without Minimum Data Set and hospice claims, our exclusion of patients residing in long-term facilities or receiving hospice care might be incomplete. However, we do not expect the amount of this incomplete information to vary across states. Last, our study likely underestimated the total use of opioids due to the exclusion of opioid injection and lack of information on opioids obtained from the internet, friends, the street, and mail order.
The rise in use of opioids over the years and the recent slight decline is suggestive of greater awareness of this epidemic by both the federal and state governments. This awareness likely accelerated the introduction of stricter opioid regulation laws across the US. The recent DEA reclassification of hydrocodone products will likely bring forth a further decline in schedule III narcotic use. The nil correlation seen between schedule II and III opioid prescription at the state level and the insignificant association between opioid use and six out of seven state laws challenges the utility of state-wide opioid regulating policy. The limited utility of these policies needs to be further investigated. Future longitudinal studies are needed to evaluate the effectiveness of the growing number of opioid regulation laws enacted by state and federal governments. The findings from such studies can provide age-appropriate information to guide public policy and clinical practice for safe and effective use of opioids in older adults.
Clinical Significance.
Use of prescription opioids for 90 days or more grew substantially from 2007 to 2011 among Medicare beneficiaries, declining slightly in 2012.
Except for laws regulating pain management clinics, no state opioid-related laws had significant impact on prescription opioid use.
State laws mediated 11.1% and 29.2% of state-level variation in schedule III and II opioid prescriptions, respectively.
Prolonged opioid prescription use increased the odds of opioid overdose-related emergencies by 60%.
Acknowledgments
This work was supported by grants R24-HS022134 from the Agency for Healthcare Research and Quality, R01-AG033134, P30-AG024832, and UL1TR001439 from the National Institutes of Health.
Appendix
Appendix.
| State | Law 1 | Law 2 | Law 3 | Law 4 | Law 5 | Law 6 | Law 7 |
|---|---|---|---|---|---|---|---|
| AK | V | V | |||||
| AL | V | V | V | ||||
| AR | V | V | |||||
| AZ | V | V | |||||
| CA | V | V | V | ||||
| CO | V | V | V | ||||
| CT | V | V | V | V | |||
| DC | V | V | V | ||||
| DE | V | V | V | V | |||
| FL | V | V | V | V | V | V | |
| GA | V | ||||||
| HI | V | V | V | ||||
| IA | V | V | V | ||||
| ID | V | V | V | ||||
| IL | V | V | V | ||||
| IN | V | V | V | ||||
| KS | V | V | |||||
| KY | V | V | V | V | |||
| LA | V | V | V | V | V | ||
| MA | V | V | V | ||||
| MD | V | V | V | ||||
| ME | V | V | V | V | |||
| MI | V | V | |||||
| MN | V | V | V | ||||
| MO | V | V | |||||
| MS | V | V | V | ||||
| MT | |||||||
| NC | V | V | V | ||||
| ND | V | ||||||
| NE | V | ||||||
| NH | V | V | V | ||||
| NJ | V | V | |||||
| NM | V | V | V | ||||
| NV | V | V | V | V | V | ||
| NY | V | V | V | V | V | ||
| OH | V | V | V | ||||
| OK | V | V | V | V | |||
| OR | V | V | V | ||||
| PA | V | V | |||||
| RI | V | V | |||||
| SC | V | V | V | V | |||
| SD | V | V | |||||
| TN | V | V | V | ||||
| TX | V | V | V | V | V | ||
| UT | V | V | |||||
| VA | V | V | |||||
| VT | V | V | V | V | |||
| WA | V | V | |||||
| WI | V | V | |||||
| WV | V | V | V | V | V | ||
| WY | V | V |
Abbreviation on 50 states and District of Columbia.
The law 1 means laws requiring a physical examination before prescribing; the law 2 means laws requiring tamper-resistant prescription forms; the law 3 means laws regulating pain clinics; the law 4 means laws setting prescription drug limits; the law 5 means laws prohibiting doctor shopping or fraud; the law 6 means laws requiring patient identification before dispensing; the law 7 means laws providing immunity from prosecution or mitigation at sentencing for individuals seeking assistance during an overdose.
The checkmark ‘V’ indicates ‘Yes’. The law is required by the state.
Centers for Disease Control and Prevention. Injury Prevention and Control: Prescription drug overdose. (Accessed April 20, 2015 at http://www.cdc.gov/homeandrecreationalsafety/Poisoning/laws/state/index.html.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial, personal or potential conflicts of interest to disclose.
YFK contributed to conception, design, analysis, and interpretation of data, drafting the article, and final approval of the draft. MAR contributed to conception, design, and interpretation of data, drafting the article, and final approval of the draft. NWC contributed to analysis, interpretation of data, and final approval of draft. HH contributed to interpretation of data and final approval of draft. JSG contributed to conception, design, interpretation of data, drafting the article, and final approval of draft.
References
- 1.Centers for Disease Control and Prevention Vital signs: overdoses of prescription opioid pain relievers — United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–92. [PubMed] [Google Scholar]
- 2.Epidemic: Responding to America's prescription drug abuse crisis. Office of National Drug Control Policy; Washington, DC: 2011. [April 15, 2015]. (at http://www.whitehouse.gov/sites/default/files/ondcp/issues-content/prescription-drugs/rx_abuse_plan.pdf.) [Google Scholar]
- 3.Betses M, Brennan T. Abusive prescribing of controlled substances–a pharmacy view. N Engl J Med. 2013;369:989–91. doi: 10.1056/NEJMp1308222. [DOI] [PubMed] [Google Scholar]
- 4.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–59. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention CDC Grand Rounds: Prescription drug overdoses – a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10–3. [PubMed] [Google Scholar]
- 8.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55:325–31. doi: 10.1002/ajim.21998. [DOI] [PubMed] [Google Scholar]
- 9.Koechl B, Unger A, Fischer G. Age-related aspects of addiction. Gerontology. 2012;58:540–4. doi: 10.1159/000339095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller M, Stürmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–8. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culberson JW, Ziska M. Prescription drug misuse/abuse in the elderly. Geriatrics. 2008;63:22–31. [PubMed] [Google Scholar]
- 12.Simoni-Wastila L, Yang HK. Psychoactive drug abuse in older adults. Am J Geriatr Pharmacother. 2006;4:380–94. doi: 10.1016/j.amjopharm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000-2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–9. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis CS, Pierce M. Dasgupta, N. Evolution and convergence of state laws governing controlled substance prescription monitoring programs,1998-2011. Am J Public Health. 2014;104:1389–95. doi: 10.2105/AJPH.2014.301923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public Health Reports. 2014;129:139–47. doi: 10.1177/003335491412900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Public Health Law Program. [September 20, 2015];Prescription Drugs. (at http://www.cdc.gov/phlp/publications/topic/prescription.html)
- 17.Rosen D, Engel RJ, Hunsaker AE, Engel Y, Detlefsen EG, Reynolds CF., 3rd Just say know: an examination of substance use disorders among older adults in gerontological and substance abuse journals. Soc Work Public Health. 2013;28:377–87. doi: 10.1080/19371918.2013.774668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12:1336–57. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris RD, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture [April 15, 2015];Measuring Rurality: Rural-Urban Continuum Codes. (at http://www.ers.usda.gov/Data/RuralUrbanContinuumCodes/.)
- 21.RED BOOK Select Extracts. Truven Health Analytics; Ann Arbor, MI: 2011. [December 16, 2013]. (at http://sites.truvenhealth.com/redbook/.) [Google Scholar]
- 22.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opiod prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Title 21 Code of Federal Regulations: Part 1306.12: Prescriptions: Controlled Substances Listed in II. US Department of Justice. Drug Enforcement Administration. Office of Diversion Control; [April 20, 2015]. (at http://www.deadiversion.usdoj.gov/21cfr/cfr/1306/1306_12.htm.) [Google Scholar]
- 24.Rescheduling of Hydrocodone Combination Products from Schedule III to Schedule II. US Department of Justice. Drug Enforcement Administration. Office of Diversion Control; [April 20, 2015]. FR Doc. 2014-19922 Filed 8-21-14; 8:45 am. (at http://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0822.htm.) [Google Scholar]
- 25.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 26.Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802–3. doi: 10.1001/jamainternmed.2013.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [April 13, 2015];Federal Opioid Treatment Guidelines. 2013 (at http://www.dpt.samhsa.gov/pdf/FederalGuidelinesforOpioidTreatment5-6-2013revisiondraft_508.pdf.)
- 28.State-by-state opioid prescribing policies. [April 13, 2015];Medscape. (at http://www.medscape.com/resource/pain/opioid-policies.)
- 29.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014 Feb 11;:348. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. Mar 12. 2012;172(5):425–30. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 31.Krebs EE, Lurie JD, Fanciullo G, et al. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain. Jan. 2010;11(1):44–52. doi: 10.1016/j.jpain.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoadley J, Summer L, Hargrave E, Cubanski J, Neuman T. Medicare Part D in its ninth year: the 2014 marketplace and key trends, 2006-2014. The Henry J. Kaiser Family Foundation; [April 11, 2015]. (at http://kff.org/report-section/medicare-part-d-in-its-ninth-year-section-1-part-d-enrollment-and-plan-availability/.) [Google Scholar]
- 33.Medicare Advantage Fact Sheet. The Henry J. Kaiser Family Foundation; [April 11, 2015]. (at http://kff.org/medicare/fact-sheet/medicare-advantage-fact-sheet/.) [Google Scholar]
- 34.SpecialEmphasis on Pain Management Clinics. National Alliance for Model State Drug Laws Report; 2011. [April 9, 2015]. State Statutes and Regulations Relative to Chronic Pain and Pain Management. (at http://www.namsdl.org/library/1704DF70-1372-636CDD7CFA1EDE075078/.) [Google Scholar]
- 35.Kuehn BM. CDC: Major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312:684–6. doi: 10.1001/jama.2014.9253. [DOI] [PubMed] [Google Scholar]
- 36.Hanson K. Prescription drug abuse is the fastest growing form of substance abuse. National Conference of State Legislatures Report; Mar, 2010. [April 15, 2015]. (at http://www.ncsl.org/research/health/a-pill-problem-rx-abuse-is-fastest-growing.aspx.) [PubMed] [Google Scholar]
- 37.Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes — Florida, 2010–2012. MMWR Morb Mortal Wkly Rep. 2014;63:569–74. [PMC free article] [PubMed] [Google Scholar]
- 38.Severtson S, Bartelson B, Davis J, et al. Difference in rates of abuse following reformulation of extended release oxycodone using data from the RADARS System Poison Center Program. Ann Emerg Med. 2012;60:S34. [Google Scholar]
- 39.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–7. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinman MA, Komaiko KD, Fung KZ, Ritchie CS. Use of opioids and other analgesics by older adults in the United States, 1999-2010. Pain Med. 2015;16:319–27. doi: 10.1111/pme.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]