Abstract
Background and Aims
Multiple European studies report increased prevalence of selective Immunoglobulin A deficiency (SIgAD) and partial Immunoglobulin A deficiency (PIgAD) in celiac disease (CD) patients. However; prospective data representing North American adults are lacking. While SIgAD precludes the use of IgA-tissue-transglutaminase antibody (IgA-tTG), the effect of PIgAD on IgA-tTG sensitivity is not well documented. We aim to determine the prevalence and impact of IgA deficiency on CD presentation and diagnosis in North American adult patients.
Methods
We reviewed 1000 consecutive patients undergoing IgA-tTG testing and 243 healthy controls. Eligible sera were tested for IgA-tTG, serum immunoglobulins and IgA/IgG-Deamidated Gliadin Peptide (IgA/IgG-DGP).
Results
Prevalence of SIgAD was marginally higher in CD patients (1.9%) compared with healthy controls (0.4%, p=0.24) and non-CD patients (0.7% p=0.173). Prevalence of PIGAD was similar in CD patients (4.8%) compared with healthy controls (5.9% p=0.57) and non-CD patients (7.2% p=0.22). 1 (16.7%) of 6 CD patients with SIgAD and all 15 (100%) with PIGAD tested IgA-tTG positive prior to GFD initiation. CD patients with SIGAD showed lower frequency of GI symptoms (33% vs 82% p=0.01) and more co-morbid autoimmune disease (67% vs 23% p=0.03) when compared to CD patients with normal IgA.
Conclusions
The prevalence of SIgAD in North American CD patients is comparable with European data but not significantly different than control populations. CD patients with SIgAD exhibit decreased IgA-tTG sensitivity and lack of gastrointestinal symptoms. PIgAD is common in patients with GI disorders but does not alter CD presentation or IgA-tTG sensitivity.
Keywords: Celiac disease, IgA deficiency, transglutaminases, United states
Introduction
Selective immunoglobulin A deficiency (SIgAD) is considered to be the most common primary immunodeficiency and defined as undetectable serum Immunoglobulin A (IgA) in the presence of normal serum levels of Immunoglobulin G (IgG) and Immunoglobulin M (IgM), in patients older than 4 years of age, in whom other causes of hypogammaglobulinemia have been excluded.(1–4) With constantly improving sensitivity of diagnostic assays, working definition of SIGAD has changed over years depending upon the lowest detectable level of serum IgA. SIgAD is estimated to affect 2%–3% patients with celiac disease (CD), a frequency approximately 10–15 times higher than the general population.(5–7) Prevalence of SIgAD is also known to vary significantly amongst different ethnicities and age groups. (4, 8–10) As the majority of prospective data regarding SIGAD in CD patients come from European pediatric populations, data may not be representative of the adult North American patient population.
Partial IgA deficiency (PIgAD) is defined as detectable level of serum IgA that is two standard deviations below normal for age (Serum IgA level 3 – 70 mg/dl) in the presence of normal IgG and IgM levels.(4) This is commonly encountered in clinical practice and creates uncertainty in interpretation of negative IgA tissue-transglutaminase (IgA-tTG) testing during evaluation for CD. Although rare exceptions exist, SIgAD precludes the use of IgA-tTG based screening for CD. Only retrospective data evaluating the prevalence of SIgAD, PIgAD and the effect of PIgAD on diagnostic performance of IgA-tTg are currently available for adult CD populations in the U.S.(6)
We combined prospective collection of sera with retrospective chart review on an adult North American cohort consisting of patients with CD, non-CD gastrointestinal disorders and healthy controls to determine the prevalence of IgA deficiency states. Our secondary aims were to determine the impact of SIgAD and PIgAD on the clinical presentation of CD and sensitivities of IgA-tTG and Deamidated Gliadin Peptide (IgA/IgG-DGP) testing.
Methods
We collected sera from 1000 consecutive patients who underwent IgA-tTG testing at Beth Israel Deaconess Medical Center between August 2010 and March 2011. These patients were seen in the outpatient clinics. After sera collection was completed, we waited 6 months to allow completion of workup for new patients and performed a retrospective review of clinical and demographic information. We excluded patients with known immune deficiency, iatrogenic immune suppression, and malignancy. We also excluded patients in whom CD could not be diagnosed or excluded confidently by the time of data analysis due to either incomplete workup or unequivocal results. The remaining patients were classified into those with CD (Group 1) and those with a non-CD gastrointestinal illness (Group 2). CD was diagnosed on the basis of characteristic small bowel histology findings with villous atrophy together with either positive celiac-specific serology (IgA-tTG or IgA/IgG-DGP) or, improvement of histology on a gluten free diet (GFD). Conversely, a normal biopsy or negative IgA-tTG (with normal total IgA and IgA/IgG-DGP) result while on a normal diet were used to exclude CD.
Additional sera from 243 healthy individuals, (age matched within 5 years of CD patients) attending annual health screening visits were collected (Group 3). To exclude the possibility of silent CD in apparently healthy patients, their sera were tested for IgA-tTG level using ELISA (INOVA Diagnostics San Diego, California; 0–20 normal, 20–39 weakly positive, 40 and above (2XULN) strongly positive). Healthy Individuals found to have positive IgA-tTG were excluded from further analysis.
After careful review, sera from patients not meeting any of the exclusion criteria, were tested for total serum Immunoglobulin A, M and G using ELISA (MILLIPLEX MAG Human Immunoglobulin Magnetic Bead Panel). Sera with total IgA level less than 77mg/dl (within 10% of lower limit of normal i.e. 70mg/dl) were rechecked for immunoglobulin levels in duplicate. All patients with deficiency of multiple immunoglobulin classes were excluded from further analysis.
All IgA deficient sera were tested for IgA/IgG-DGP ELISA screen (INOVA Diagnostics San Diego, California) to confirm the presence or absence of CD.
For all patients in group 1, determined to have CD and SIgAD, we performed IgA/IgG-DGP testing. For patients determined to have CD and SIgAD, who were already on GFD at the time of sera collection, we retrieved sera from prior to adoption of the GFD from an existing serum repository to evaluate the performance of IgA-tTG. When there were no pre-GFD sera available, patient charts were reviewed for pre-GFD IgA-tTG and IgA/IgG-DGP levels.
The frequencies of partial and selective IgA deficiency were calculated and compared in all three groups. For CD patients with PIgAD, IgA-tTG levels were correlated with total serum IgA.
Fisher’s exact test and student’s t-test (for normally distributed data) and Mann–Whitney U test (for non-normally distributed data) were used to assess differences between the study cohort and the overall celiac population, as appropriate. The institutional review board at BIDMC approved this study.
Results
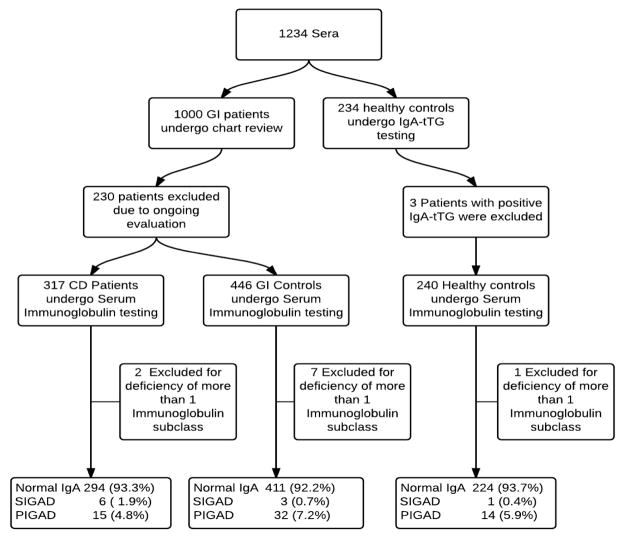
Of the 1000 patient sera initially collected (groups 1 and 2), 230 met one or more of the exclusion criteria (duplicates, indeterminate diagnosis, known malignancy or other known immune disorders). Three out of 243 healthy controls (group 3) (1.2%) were excluded after they tested positive for IgA-tTG (Range 25 – 94).
Finally a total of 1010 sera were tested for total serum immunoglobulin of which groups 1,2 and 3 contained 317, 453 and 240 patients respectively. Based on total serum Immunoglobulin testing, 10 patients (Two (0.6%) CD, seven (1.5%) GI controls and one (0.4%) healthy control) were excluded due to deficiency of more than one immunoglobulin leaving behind a total of 1000 patients for final analysis of which 315, 446 and 239 patients belonged to groups 1,2 and 3 respectively. 30 of 317 CD patients were newly diagnosed during the study and 287 had a previous diagnosis of CD. The frequency of SIgAD was 1.9% in group 1, 0.7% in group 2 and 0.4% in group 3. Frequency of PIgAD was 4.4% in group1 7.2% in group 2 and 5.9% in group 3. (Table 1) These differences were not statistically significant.
Table 1.
Comparison between CD patients, GI controls and Healthy controls
| Total | GI controlsa (Group 2) | P value CDb vs GI controls | CD (Group 1) | P value CD vs Healthy controls | Healthy Controls (Group 3) | |
|---|---|---|---|---|---|---|
| Total | 1000 | 446 | - | 315 | - | 239 |
| Mean Age (years) | 43.7 (18 – 93) | 44.1 (18 – 89) | .6515 | 44.6 (18 – 92) | .0249* | 41.7 (18–93) |
| Females | 662 (66.20%) | 298 (66.82%) | .078 | 230 (73%) | <.0001* | 134 (56.07%) |
| SIgADc n (%) | 10 (1%) | 3 (0.67%) | .173 | 6 (1.90%) | .248 | 1 (0.42%) |
| PIgADd n (%) | 61 (6.10%) | 32 (7.17%) | .22 | 15 (4.76%) | .57 | 14 (5.86%) |
| Mean IgA (mg/Dl) | 202 (0 – 800) | 201 (0 – 800) | .12 | 215 (0 – 800) | .0030* | 185 (0 – 800) |
Non Celiac Disease patients with gastrointestinal symptoms,
Celiac Disease,
Selective immunoglobulin A deficiency,
Partial Immunoglobulin A deficiency,
Significant P value
Demographics
The mean age at testing for group 1 was higher compared to group 3 (44.6 vs 41.7 years p = .024) and similar to group 2 (44.6 vs 44.1 years p = .65). Female predominance was highest in the group 1 (73%) followed by group 2 (66.8% vs 73% p=.078) and group 3 (56.1%vs 73% p<.0001). (Table 1) Mean IgA levels were significantly higher in group 1 when compared with group 3 (215 mg/dl vs 185 mg/dl p=0.003).
Performances of IgA-tTG and IgA/IgG-DGP in IgA deficient subjects
Within group 1, all patients with PIgAD tested IgA-tTG positive when tested prior to initiating GFD (range 20 to 273 IU/ml). Of the six patients in group 1 diagnosed with SIgAD, just one tested positive for IgA-tTG (>120 IU/ml). Pre-GFD sera were available in four out of six above mentioned patients. All 4 sera tested positive for IgA/IgG-DGP (range 22 – 99 IU/ml, normal level less than 20 IU/ml). Pre-GFD sera or documentation of pre-GFD IgA/IgG-DGP testing results were not available for the remaining 2 patients. Both these patients tested IgA/IgG-DGP negative while on GFD.
Characteristics of CD patients with and without SIGAD
Age at testing and gender distribution was not significantly different between CD patients with and without IgA deficiency. Although average age at diagnosis was higher for those with CD and SIGAD as compared to CD patients with normal IGA levels, the difference was not significant (48.7 vs 42.7 years p=.273). (Table 2) Compared to CD patients with normal IgA levels, CD patients with SIgAD were more likely to have a co-existing autoimmune disease (67% vs 23.5%; OR 6.37 95% CI 1.14 – 35.56 P = 0.034) and lack classic gastrointestinal symptoms of CD (66.7% vs 17.7% p = .012). (Table 2) Autoimmune diseases seen in the CD+SIgAD cohort included autoimmune hypothyroidism in two patients, rheumatoid arthritis and Primary Biliary cirrhosis/autoimmune hepatitis overlap picture in one each. Mean age at diagnosis (41.29 vs 44.70 p = .0596) as well as mean ages at data collection (47.0 vs 50.67 p = .0494) were higher in the CD patients with additional autoimmune disorder.
Table 2.
Comparison between Celiac Disease patients with and without IgA deficiency
| CDa patients with SIgADb | P value CD patients with SIgAD vs CD patients with Normal IgA | CD patients with Normal serum IgA | P value CD patients with Normal IgA vs CD patients with PIgADc | CD patients with PIgAD | |
|---|---|---|---|---|---|
| N | 6 | 294 | 15 | ||
| Mean Age (yrs) | 50.2 | .42 | 44.6 | .60 | 42.4 |
| Mean age at diagnosis (yrs) | 48.7 | .27 | 42.7 | .16 | 37.3 |
| Females n (%) | 6 (100%) | .19 | 213 (72.45%) | 1.0 | 4 (26.67%) |
| Mean IgA (mg/dl) | 0 | <.0001* | 229 (79 – 800) | <.0001* | 52 (21 – 68) |
| IgA-tTG Positive n (%) | 1 (16.67%) | <.0001* | 290 (98.64%) | 1.0 | 15 (100%) |
| Autoimmune Disease | 4 (66.67%) | .033* | 69 (23.47%) | .33 | 5 (33.33%) |
| GI symptoms n (%) | 2 (33.33%) | .012* | 242 (82.31%) | .15 | 11 (73.33%) |
| Diarrhea n (%) | 1 (16.67%) | .11 | 151 (51.36%) | .07 | 4 (26.67%) |
| Abdominal Pain n (%) | 2 (33.33%) | .68 | 138 (46.94%) | .42 | 5 (33.33%) |
| Weight Loss n (%) | 2 (33.33%) | .64 | 73 (24.83%) | 1.0 | 4 (26.7%) |
| Bloating n (%) | 1 (16.67%) | 1.0 | 69 (23.47%) | 1.0 | 3 (20.0%) |
Celiac Disease
Selective Immunoglobulin A deficiency,
Partial Immunoglobulin A deficiency,
Significant P value
Discussion
We found the prevalence of Selective Immunoglobulin A deficiency in North American adults with CD to be comparable with the European data (1.9%). While numerically the incidence of SIgAD was higher in the CD group, it was not statistically significant and may represent under powering. SIgAD was responsible for false negative IgA-tTg in 1.6% of CD patients in our study. Because of such false negatives, CD patients with SIgAD may elude diagnosis thereby lowering the apparent frequency of IgA deficiency in the celiac disease population. Our finding that IgG based testing (DGP-IgA/IgG screen in this case) is useful for detection of CD in patients with SIgAD, is in keeping with prior studies.(11–15) DGP-IgA/IgG screen also performed well in ruling out CD in controls with either SIgAD or PIgAD.
While both SIGAD (3, 16, 17) and CD(18) are known to be associated with autoimmunity, the prevalence of autoimmune diseases (67%) observed in our cohort of CD patients with SIgAD was much higher than reported in either cohort separately. Higher than expected rates of autoimmunity have been reported in CD patients with coexisting SIGAD and Type 1 Diabetes Mellitus (18–20).
Two main hypotheses have been proposed to explain this relationship; 1) effects of ongoing inflammation and antigen spreading as a result of prolonged gluten exposure and 2) a common predisposition to auto-immunity, either genetically determined or acquired.(21)
Ventura et al (18) studied 909 CD patients and found a close correlation between increasing age at diagnosis and increased prevalence of other autoimmune disease in patients with CD thereby suggesting that duration of gluten exposure may be directly proportional to the degree of immune dysregulation.
In our study, baseline prevalence of a second autoimmune disease (23.5% in patients with CD and normal IgA) was very similar to that observed by Ventura et al (23.6% for CD patients with age of diagnosis > 10yrs). In order to test their hypothesis, we compared the mean ages at diagnosis for patients with CD only vs. patients with CD and at least one more autoimmune disease. In a cross-sectional manner we showed that mean ages at diagnosis and data collection were higher in the CD patients with additional autoimmune disorder. However it is unclear whether the higher prevalence of autoimmune disorders is attributable to prolonged gluten exposure or advancing age or both.
One patient with SIgAD had a highly positive IgA-tTG at diagnosis (>120), a finding that has rarely been reported.(13, 22, 23) On further review we discovered that at initial testing this patient also had a serum total IgA level of 12 mg/dl. At the time of serum collection for this study, the patient’s IgA-tTG had decreased to 7 (normal 0 – 19) in response to a GFD, at which time the total serum IgA was undetectable. We have observed a similar phenomenon in a another research subject who participated in a gluten challenge study.(24) This finding can be explained by the fact that SIgAD does not exclude a residual capacity to mount an IgA antibody response.(22) We hypothesize that in some patients with SIGAD and celiac disease; gluten related immune activation with stimulation of celiac antibody production could lead to detectable serum IgA levels.. Our theory is supported by findings from a recent study that observed that tissue-transglutaminase 2 (tTG2) specific plasma cells are markedly expanded within the duodenal mucosa in individuals with active CD. Authors attributed this phenomenon to a germ line repertoire with high affinity for tTG2 that can favor massive generation of auto reactive B cells.(25) This preferential activation of tTG2 reactive B cells may also explain detectable IgA-tTG antibodies in CD patients with PIgAD as discussed in the next paragraph.
Partial IgA deficiency (PIgAD) is defined as detectable level of serum IgA that is two standard deviations below normal for age (Serum IgA level 3 – 70 mg/dl) in the presence of normal IgG and IgM levels.(4) The incidence of PIgAD was not significantly different between CD, GI controls and healthy controls. Partial IgA deficiency can be viewed as a maturation delay within the IgA system, and the residual capacity of antigen-specific IgA responses in patients with CD has been previously described.(22, 26) Our results strongly suggest that PIGAD does not affect the diagnostic sensitivity of IgA-tTg. Confirmation of this finding in larger studies could help avoid unnecessary endoscopy in asymptomatic individuals undergoing testing due to family history of CD and individuals undergoing CD evaluation for non-GI symptoms. This can avoid unnecessary delay and expense in diagnosis of the actual underlying etiology of their symptoms.
Although we feel that this study provides relevant clinical information and is the largest studied cohort of CD patients with PIgAD, we do note some limitations. Subjects in this study were identified at a referral center for CD diagnosis and management. Seronegative CD patients with low suspicion index who did not undergo endoscopy could have been misdiagnosed as not having CD. A majority patients determined to have CD were follow up patients therefore there is a theoretical risk of overestimation of IgA sufficiency given the effect of SIgAD on IgA-tTG sensitivity. However this risk is attenuated by our practice of routinely examining serum IgA levels as well duodenal pathology in patients suspected of having CD who test negative for IgA-Ttg. Also, while this study was large in comparison to prior reports, it is possible that differences between the prevalence of SIgAD and PIgAD in CD and controls did not reach statistical significance due to under powering. Our finding of higher incidence of autoimmune disease and lesser GI symptoms in patients with both CD and SIgAD is based on just six patients and should be carefully interpreted.
In conclusion, prevalence of SIgAD in adults with CD in the US is similar to European literature but not significantly different than control populations. The prevalence of a co-morbid autoimmune disease in patients with both CD and SIgAD is much higher, at 67%), as compared to either disease separately. False negative serology and lack of classic gastrointestinal symptoms in the majority of CD patients with SIgAD may make CD more difficult to detect. We also observed that a small number of CD patients with SIgAD test positive for IgA-tTG possibly reflecting the ability of tTG-2 to active a robust B cell response. What factors decide whether CD patients with SIgAD may be seropositive or seronegative is unclear. PIgAD is a common occurrence both in those with GI disorders and in the healthy population and did not affect the diagnostic performance of IgA-tTG serology in screening for CD.
Figure 1.
Study flowchart. GI controls; non–celiac disease patients with gastrointestinal symptoms, CD; celiac disease, SIgAD; selective immunoglobulin A deficiency, PIgAD; partial Immunoglobulin A deficiency.
Acknowledgments
We would like to thank Zakera Shums and INOVA Diagnostics San Diego, California for their contribution.
This work was conducted with support from: NIH Award R03 DK095937.
Footnotes
None of the authors have any conflict of interest relevant to this article.
BIBLIOGRAPHY
- 1.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93(3):190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 2.Geha RS, Notarangelo LD, Casanova JL, Chapel H, Conley ME, Fischer A, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120(4):776–94. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. Journal of clinical immunology. 2001;21(5):303–9. doi: 10.1023/a:1012241117984. Epub 2001/11/27. [DOI] [PubMed] [Google Scholar]
- 4.Yel L. Selective IgA deficiency. Journal of clinical immunology. 2010;30(1):10–6. doi: 10.1007/s10875-009-9357-x. Epub 2010/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneghan MA, Stevens FM, Cryan EM, Warner RH, McCarthy CF. Celiac sprue and immunodeficiency states: a 25-year review. J Clin Gastroenterol. 1997;25(2):421–5. doi: 10.1097/00004836-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chow MA, Lebwohl B, Reilly NR, Green PH. Immunoglobulin A Deficiency in Celiac Disease. J Clin Gastroenterol. doi: 10.1097/MCG.0b013e31824b2277. [DOI] [PubMed] [Google Scholar]
- 7.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut. 1998;42(3):362–5. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.al-Attas RA, Rahi AH. Primary antibody deficiency in Arabs: first report from eastern Saudi Arabia. Journal of clinical immunology. 1998;18(5):368–71. doi: 10.1023/a:1023247117133. [DOI] [PubMed] [Google Scholar]
- 9.Feng L. Epidemiological study of selective IgA deficiency among 6 nationalities in China. Zhonghua Yi Xue Za Zhi. 1992;72(2):88–90. 128. [PubMed] [Google Scholar]
- 10.Kanoh T, Mizumoto T, Yasuda N, Koya M, Ohno Y, Uchino H, et al. Selective IgA deficiency in Japanese blood donors: frequency and statistical analysis. Vox Sang. 1986;50(2):81–6. doi: 10.1111/j.1423-0410.1986.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 11.Prince HE, Norman GL, Binder WL. Immunoglobulin A (IgA) deficiency and alternative celiac disease-associated antibodies in sera submitted to a reference laboratory for endomysial IgA testing. Clin Diagn Lab Immunol. 2000;7(2):192–6. doi: 10.1128/cdli.7.2.192-196.2000. Epub 2000/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin a deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol. 2002;9(6):1295–300. doi: 10.1128/CDLI.9.6.1295-1300.2002. Epub 2002/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korponay-Szabo IR, Dahlbom I, Laurila K, Koskinen S, Woolley N, Partanen J, et al. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut. 2003;52(11):1567–71. doi: 10.1136/gut.52.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villalta D, Tonutti E, Prause C, Koletzko S, Uhlig HH, Vermeersch P, et al. IgG antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem. 2010;56(3):464–8. doi: 10.1373/clinchem.2009.128132. Epub 2009/12/22. [DOI] [PubMed] [Google Scholar]
- 15.Vermeersch P, Geboes K, Marien G, Hoffman I, Hiele M, Bossuyt X. Diagnostic performance of IgG anti-deamidated gliadin peptide antibody assays is comparable to IgA anti-tTG in celiac disease. Clin Chim Acta. 2010;411(13–14):931–5. doi: 10.1016/j.cca.2010.02.060. Epub 2010/02/23. [DOI] [PubMed] [Google Scholar]
- 16.Jacob CM, Pastorino AC, Fahl K, Carneiro-Sampaio M, Monteiro RC. Autoimmunity in IgA deficiency: revisiting the role of IgA as a silent housekeeper. Journal of clinical immunology. 2008;28(Suppl 1):S56–61. doi: 10.1007/s10875-007-9163-2. [DOI] [PubMed] [Google Scholar]
- 17.Edwards E, Razvi S, Cunningham-Rundles C. IgA deficiency: clinical correlates and responses to pneumococcal vaccine. Clin Immunol. 2004;111(1):93–7. doi: 10.1016/j.clim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117(2):297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 19.Not T, Tommasini A, Tonini G, Buratti E, Pocecco M, Tortul C, et al. Undiagnosed coeliac disease and risk of autoimmune disorders in subjects with Type I diabetes mellitus. Diabetologia. 2001;44(2):151–5. doi: 10.1007/s001250051593. [DOI] [PubMed] [Google Scholar]
- 20.Collin P, Maki M, Keyrilainen O, Hallstrom O, Reunala T, Pasternack A. Selective IgA deficiency and coeliac disease. Scand J Gastroenterol. 1992;27(5):367–71. doi: 10.3109/00365529209000089. Epub 1992/05/01. [DOI] [PubMed] [Google Scholar]
- 21.Fasano A. Systemic autoimmune disorders in celiac disease. Current opinion in gastroenterology. 2006;22(6):674–9. doi: 10.1097/01.mog.0000245543.72537.9e. Epub 2006/10/21. [DOI] [PubMed] [Google Scholar]
- 22.Valletta E, Fornaro M, Pecori S, Zanoni G. Selective immunoglobulin A deficiency and celiac disease: let’s give serology a chance. J Investig Allergol Clin Immunol. 21(3):242–4. Epub 2011/05/10. [PubMed] [Google Scholar]
- 23.Donaldson MR, Firth SD, Wimpee H, Leiferman KM, Zone JJ, Horsley W, et al. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5(5):567–73. doi: 10.1016/j.cgh.2007.01.003. Epub 2007/04/13. [DOI] [PubMed] [Google Scholar]
- 24.Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2012 doi: 10.1136/gutjnl-2012-302196. Epub 2012/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Niro R, Mesin L, Zheng NY, Stamnaes J, Morrissey M, Lee JH, et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med. 2012;18(3):441–5. doi: 10.1038/nm.2656. Epub 2012/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeves GE, Squance ML, Duggan AE, Murugasu RR, Wilson RJ, Wong RC, et al. Diagnostic accuracy of coeliac serological tests: a prospective study. Eur J Gastroenterol Hepatol. 2006;18(5):493–501. doi: 10.1097/00042737-200605000-00006. [DOI] [PubMed] [Google Scholar]