Abstract
Background
Continuous-flow (CF) left ventricular assist devices (LVADs) are standard of care for bridging patients to cardiac transplantation. However, existing data about preoperative factors influencing early post-transplant survival in this population are limited. We sought to determine risk factors for mortality using a large international database.
Methods
All patients in the International Society for Heart and Lung Transplantation Transplant Registry who were bridged to transplantation with CF LVADs between June 2008 and June 2012 were included. Risk factors for mortality within 30 days of transplant were identified. Statistical approach included multivariable analysis and Kaplan-Meier survival analysis.
Results
During the study period, 2,152 CF LVAD patients underwent heart transplantation. Post-transplant survival was 95.5% at 30 days. Risk factors for mortality during this window included ventilator support at transplant (HR 5.00, 95% CI 1.51–16.58), female recipient/male donor (compared to male recipient/male donor, HR 3.29, 95% CI 1.90–5.72), history of hemodialysis (HR 2.51, 95% CI 1.14–5.51), and history of coronary bypass grafting (HR 1.89, 95% CI 1.19–3.00). Increasing recipient age (p=0.002), body mass index (p=0.002), creatinine (p=0.004), and total bilirubin (p<0.001) were also associated with an increase in mortality.
Conclusions
In patients supported with CF LVADs, risk factors for early mortality can be identified pre-transplant, including ventilator support, female recipient/male donor, and increasing recipient age and body mass index. Despite the inherent complexities of reoperative surgery, patients bridged to transplant with CF LVADs have excellent perioperative survival.
Background and Significance
Continuous-flow Left Ventricular Assist Devices (CF LVADs) have emerged as the mainstay of therapy for patients who require bridging to heart transplantation.1 Patients bridged with CF LVADs have been shown to have similar post-transplant survival as those patients who are bridged with traditional pulsatile-flow LVADs.2 Continuous-flow devices have largely replaced pulsatile-flow devices due to decreased complication rates, improved mechanical performance, and smaller size.3,4
While there has been considerable research regarding pulsatile flow devices and the corresponding outcomes after heart transplant, less information is available regarding continuous flow devices.5–12 In particular, published data correlating CF LVAD use at it relates to transplant outcomes are largely derived from single-center analyses, stratify outcomes by a single variable (gender or age, for example), or have multiple indications in the study cohort (destination therapy combined with bridge-to-transplant). Currently, information derived from a large, multi-institutional analysis of pre-transplant risk factors influencing post-transplant mortality in patients bridged to heart transplantation with CF LVADs is sparse.12,13 Some studies have described increased risk of early post-transplant mortality in patients bridged with CF LVADs.12,14,15 A comprehensive evaluation of risk factors which predispose patients to early, 30-day mortality after transplantation has, however, not been done.
Large multi-institutional databases such as the International Society for Heart and Lung Transplantation (ISHLT) Transplant Registry provide an opportunity for large-scale investigation into post-transplant survival outcomes. Due to the fact that multiple pre-transplant data points are collected on patients bridged with CF LVADs and annual post-transplant follow-up information is collected on each of these patients, the registry serves as a valuable tool for assessing the effects of pre-transplant variables on post-transplant outcomes.
We therefore sought to determine which pre-transplant risk factors influence early (30 day) post-transplant survival in patients bridged to transplantation with CF LVADs, using data from the ISHLT transplant registry.
Methods
The ISHLT Transplant Registry was queried for all patients transplanted between July 1, 2008 and June 30, 2012 who were age 18 years and above at the time of transplant and were bridged to transplant with a durable CF LVAD (Table 1). Patients with a prior transplant of any organ, biventricular support, or simultaneous transplant of any other organ were excluded. Patients were analyzed for survival at 30 days post-transplant.
Table 1.
Types of Continuous Flow LVADs in Patients Bridged to Transplant Between July 1, 2008 and June 30, 2012.
| VAD Type | (n = 2,152) |
|---|---|
| Thoratec HeartMate II | 1,883 |
| Jarvik 2000 | 33 |
| MicroMed DeBakey | 1 |
| Heartware HVAD | 139 |
| Terumo DuraHeart | 26 |
| Ventracor VentrAssist | 63 |
| WorldHeart Levacor | 7 |
LVAD, left ventricular assist device, VAD, ventricular assist device.
Risk factors for early mortality, including recipient factors, donor factors, and transplant factors were analyzed (Table 2). Some variables of interest were excluded from the analysis. Exercise oxygen consumption was excluded due to poor data quality, and donor and recipient Human Immunodeficiency Virus and Human T-Lymphotropic Virus status were excluded due to insufficient sample size.
Table 2.
Risk Factors Analyzed for 30-Day Mortality
| Characteristic | BTT with CF LVAD (n=2,152)
|
|
|---|---|---|
| N | % or median (5th–95th percentile) | |
| Demographics | ||
| Male Recipient | 1,756 | 81.6% |
| Male Donor | 1,660 | 77.1% |
| Female Recipient/Female Donor | 206 | 9.6% |
| Female Recipient/Male Donor | 190 | 8.8% |
| Male Recipient/Female Donor | 286 | 13.3% |
| Male Recipient/Male Donor | 1,470 | 68.3% |
| ABO Identical | 1,899 | 88.2% |
| Recipient Age (years) | 2,152 | 56.0 (27.0–68.0) |
| Recipient Body Mass Index (kg/m^2) | 2,152 | 27.7 (20.4–36.3) |
| Donor Age (years) | 2,152 | 29.0 (17.0–51.0) |
| Donor Height (cm) | 2,146 | 177.8 (160.0–190.0) |
| Donor Weight (kg) | 2,146 | 83.0 (59.0–118.0) |
| Diagnosis | ||
| Idiopathic Cardiomyopathy | 938 | 43.6% |
| Ischemic Cardiomyopathy | 911 | 42.3% |
| Other Cardiomyopathy | 259 | 12% |
| Valvular Heart Disease | 24 | 1.1% |
| Congenital Heart Disease | 12 | 0.6% |
| Other Heart Disease | 8 | 0.4% |
| Condition at Transplant | ||
| ICU | 185 | 8.6% |
| Hospitalized, not ICU | 271 | 12.6% |
| ECMO | 5 | 0.2% |
| IABP | 15 | 0.7% |
| Ventilator | 15 | 0.7% |
| Inotropes | 160 | 7.5% |
| Medical History | ||
| Diabetes | 646 | 30% |
| History of Dialysis | 52 | 2.5% |
| Hypertension | 948 | 49.4% |
| COPD | 91 | 5.3% |
| Antiarrhythmic Medications | 633 | 38% |
| Implantable Defibrillator | 1,776 | 87.6% |
| Prior Cardiac Surgery | 1,520 | 70.9% |
| Serum Creatinine (mg/dL) | 2,135 | 1.1 (0.7–2.0) |
| Total Bilirubin (mg/dL) | 2,084 | 0.7 (0.3–2.4) |
| Serum Albumin (g/dL) | 1,727 | 3.7 (2.5–4.6) |
| Ischemic Time (hours) | 2,115 | 3.3 (1.6–5.0) |
| Serology | ||
| Recipient CMV IgG | 1,249 | 59% |
| Recipient CMV IgM | 74 | 3.6% |
| Recipient Hepatitis B Core Antibody | 99 | 4.7% |
| Recipient Hepatitis B Surface Antigen | 34 | 1.6% |
| Recipient Hepatitis C Serostatus | 55 | 2.6% |
| Recipient Epstein Barr Virus Serostatus | 1,630 | 79.6% |
| Donor Anti-CMV Antibody | 1,301 | 60.7% |
| Donor HBV Core Antibody | 44 | 2.1% |
| Donor HBV Surface Antigen | 2 | 0.1% |
| Donor Anti-HCV Antibody | 0 | 0% |
| Donor EBV IgG | 1,883 | 90.1% |
| Donor EBV IgM | 58 | 2.8% |
| Donor RPR-VDRL | 4 | 0.2% |
| Donor/Recipient CMV Mismatch | 1,009 | 46.9% |
| Donor/Recipient EBV Mismatch | 303 | 14.1% |
BTT, Bridge to Transplant; CF, Continuous Flow; CMV, Cytomegalovirus; COPD, Chronic Obstructive Pulmonary Disease; EBV, Epstein Barr Virus; ECMO, Extracorporeal Membrane Oxygenation; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; IABP, Intraaortic Balloon Pump; ICU, Intensive Care Unit; IgG, Immunoglobulin G; IgM, Immunoglobulin M; LVAD, Left Ventricular Assist Device; RPR-VDRL, Rapid Plasma Reagin – Venereal Disease Research Laboratory.
Demographic and clinical characteristics were analyzed using univariate analysis. Kaplan-Meier survival with log rank analysis was used to evaluate post-transplant survival. Multivariable analysis in the form of Cox proportional hazards regression was used to evaluate the relationship between risk factors and mortality. Continuous risk factors were included in the models using a restricted cubic spline. This method assigns a hazard ratio of 1.0 to the median value of a particular risk factor, with the hazard ratios of other values for that risk factor compared relative to the median value. Continuous risk factors with missing values were imputed using multiple imputation. Variables that were found to be significant (p < 0.05) were designated as having an association with the given outcome.
The University of Utah Investigational Review Board waived the need for formal approval and individual consent for this study.
Results
A total of 2,152 patients were transplanted while being supported by a CF LVAD during the study period. The frequency of risk factors, including donor and recipient characteristics and transplant process variables, are listed in Table 2. Patients were predominantly male (81.6%), had a median age of 56 years, and received hearts from donors with a median age of 29 years. Idiopathic cardiomyopathy was the most common cause of heart failure (43.6%), followed closely by ischemic cardiomyopathy (42.3%). Hypertension and diabetes were present in 49.4% and 30.1% of patients, respectively. Previous cardiac surgery had been performed in 70.9% of the patients.
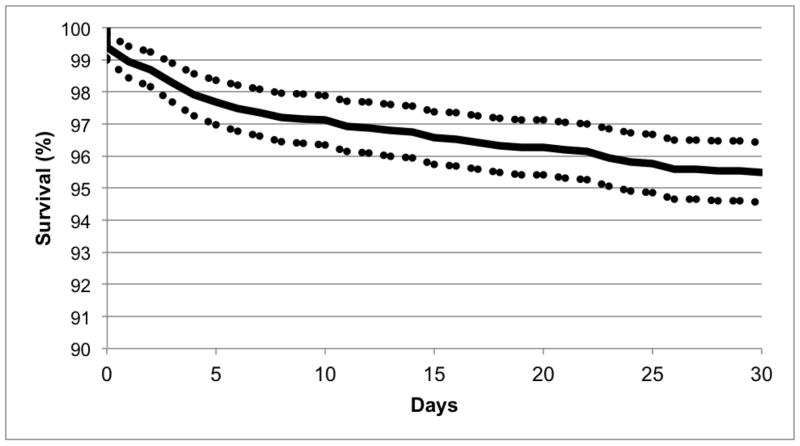
Overall survival to 30 days post-transplant in this population was 95.5% (Figure 1), with a 95% confidence interval (CI) of 94.6–96.4%.
Figure 1.
Thirty-Day Post-Transplant Survival in Patients Bridged to Transplantation with a Continuous Flow Left Ventricular Assist Device.
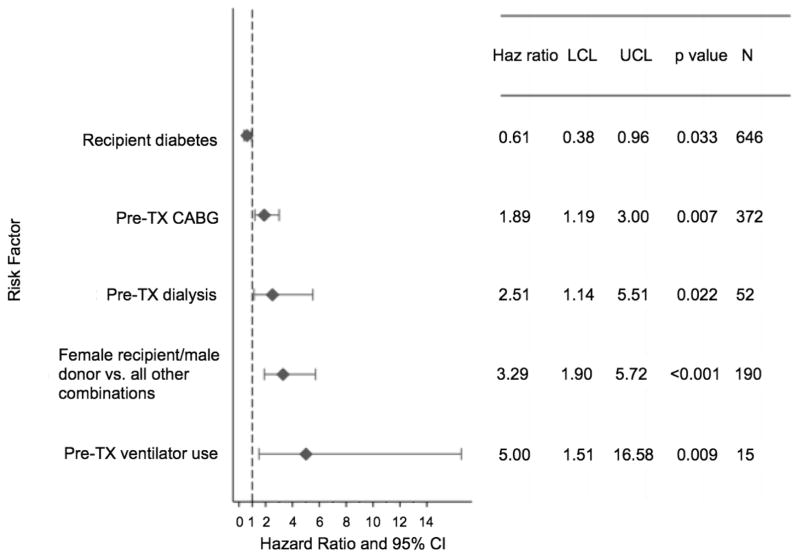
Among categorical risk factors tested, the need for ventilator use in a heart transplant candidate at the time of transplant had the highest hazard ratio (HR) for mortality within the first 30 days post-transplant, with a 5-fold increase in mortality risk compared to those without use of a ventilator (HR 5.00, 95% CI 1.51–16.58) (Figure 2). Other categorical risk factors for increased mortality within the first 30 days post-transplant after being bridged to transplant with a CF LVAD included being a female recipient of a male donor allograft versus all other combinations (HR 3.29, 95% CI 1.90–5.72), a history of pre-transplant dialysis (HR 2.51, 95% CI 1.14–5.51), and pre-transplant coronary artery bypass grafting (HR 1.89, 95% CI 1.19–3.00). The presence of diabetes in this study population appeared to be associated with a lower risk of mortality in the first 30 days post-transplant (HR 0.61, 95% CI 0.38–0.96).
Figure 2.
Categorical Risk Factors for Death within 30 Days Among Patients Bridged to Transplantation with a Continuous Flow Left Ventricular Assist Device. Results of a multivariable logistic regression analysis.
CABG, Coronary Artery Bypass Grafting; CI, Confidence Interval; Haz, Hazard; LCL, Lower Confidence Limit; TX, Transplant; UCL, Upper Confidence Limit
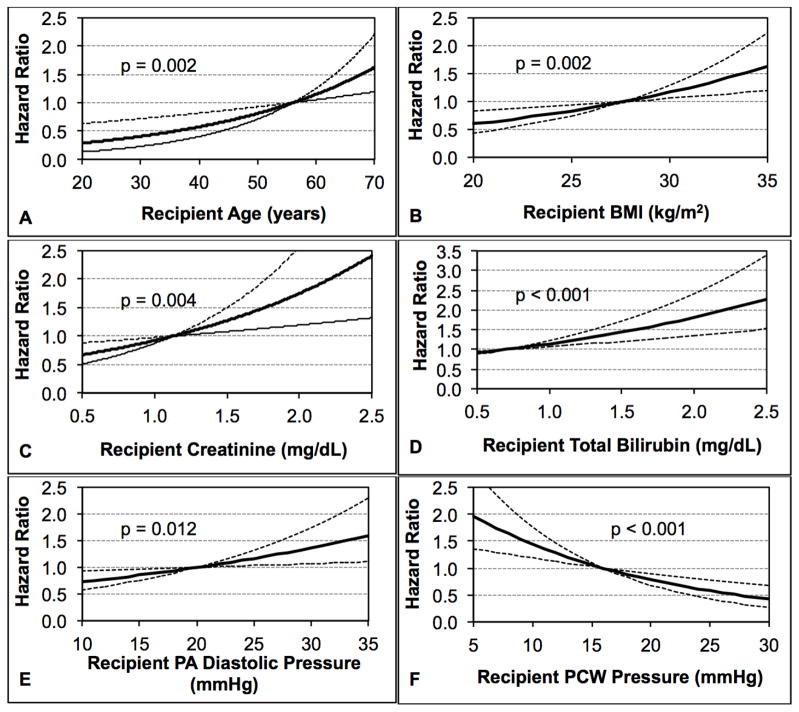
Continuous risk factors from both the recipient and donor were found to be significant. Increasing recipient age, with a median value of 56 years, was found to be a risk factor for mortality in this group (p = 0.002, Figure 3A). Increasing recipient body mass index (BMI), with a median value of 28 kg/m2 (p = 0.002) was also found to be a risk factor for early post-transplant mortality (Figure 3B). Four other continuous risk factors in the recipient were associated with an increased risk of mortality in the first 30 days after heart transplantation. The first was increasing recipient creatinine, which had a median value of 1.1 mg/dL (p = 0.004, Figure 3C). The second was increasing serum total bilirubin, which had a median value of 0.7 mg/dL (p < 0.001, Figure 3D). The third was increasing PA diastolic pressure, with a median value of 20 mmHg (p = 0.012, Figure 3E). The fourth, with a median value of 16 mmHg (p < 0.001, Figure 3F), was pulmonary wedge pressure.
Figure 3.
Continuous, Recipient-Related Risk Factors Independently Associated with Mortality within 30 Days of Transplant Among Patients Bridged to Transplantation with a Continuous Flow Left Ventricular Assist Device Showing the Impact of: (A) Recipient Age; (B) Recipient BMI; (C) Recipient Creatinine (D) Recipient Total Bilirubin; (E) Recipient PA Diastolic Pressure; and (F) Recipient PCW Pressure
BMI, Body Mass Index; PA, Pulmonary Artery; PCW, Pulmonary Capillary Wedge
One transplant-related factor was also found to be statistically significant. Increasing donor age, with a median value of 29 years, was also predictive of early recipient mortality (p = 0.011, Figure 4).
Figure 4.
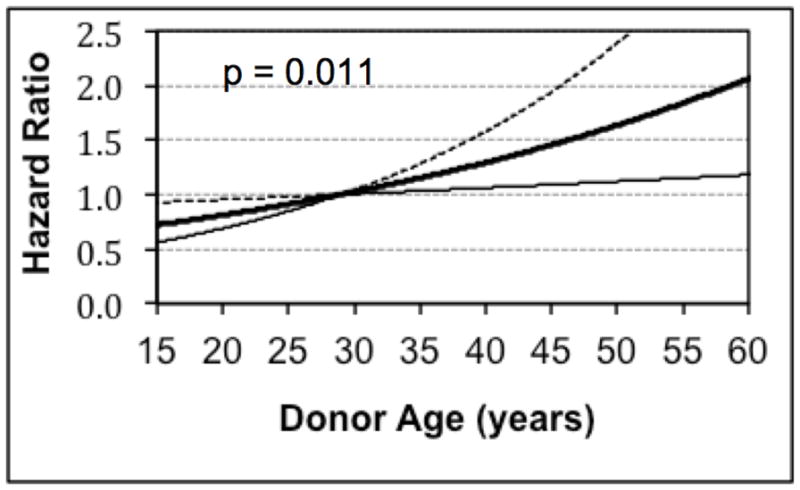
Risk of Donor Age for Mortality within 30 Days of Transplant Among Patients Bridged to Transplantation with a Continuous Flow Left Ventricular Assist Device
Discussion
This study used data from a large, international heart transplant database to evaluate 30-day post-transplant survival in patients bridged to transplant with durable CF LVADs. The ISHLT Transplant Registry is an ideal information source for this type of study because the data points are derived from multiple countries and include more device types than are available in other data sources, resulting in a broader sample of patients. This results in a data set that is reflective of the real-world practice of transplant medicine. Our study identified a number of risk factors associated with early post-transplant mortality in patients bridged to transplant with durable CF LVADs.
Despite previously published data that typically demonstrated a steep perioperative down-spike in survival after transplant,12,14,15 this contemporary series demonstrates an impressive 95.5% survival rate for the entire cohort at 30 days after transplant. As shown in Figure 1, deaths occurred at a relatively steady rate, rather than as a precipitous drop in the first 5–10 days after transplant. The possible reasons for the lack of an immediate post-operative survival drop-off include the current wide availability of advanced therapies for primary graft dysfunction at centers that also perform durable VAD bridging to transplant, such as extracorporeal membrane oxygenation (ECMO) and temporary VADs. These approaches provide an opportunity for recovery in transplant recipients with reversible graft dysfunction or, alternatively, eventual withdrawal of care later in the postoperative course if a patient’s condition does not improve.
We identified a number of risk factors for 30-day mortality. These included pre-transplant coronary artery bypass grafting, being a female recipient of a male donor heart, pre-transplant use of a ventilator, or pre-transplant renal failure requiring hemodialysis. Other significant risk factors included increasing recipient age, BMI, creatinine, bilirubin, pulmonary artery diastolic pressure and decreasing pulmonary wedge pressure. Increasing donor age was also associated with poor outcomes in the early post-transplant period.
Several of these risk factors have been previously reported to be associated with poor heart transplant outcomes in general, regardless of whether an LVAD was used for a bridge. For example, the use of a ventilator in patients undergoing heart transplantation has been associated with increased inhospital, 90-day, and 1-year mortality.13,16,17 Similarly, older recipient age and liver and kidney dysfunction have been identified as risk factors in the general transplant candidate population.
BMI at extremes has been shown to negatively influence survival following heart transplantation, though being overweight (BMI 25–30 kg/m2) may not have an effect.18,19 The median heart transplant recipient BMI in this cohort was 28 kg/m2, with an increased risk of post-transplant mortality associated with rising values for BMI. Interestingly, because the median BMI in this cohort is consistent with being overweight, patients with a normal BMI (20–25 kg/m2) are at a relatively lower risk of mortality after transplant.
The significance of increased mortality associated with increasing pulmonary artery diastolic pressures and decreasing pulmonary wedge pressures is not entirely obvious, as these entities are coexistent, at least at the cohort level. One possible explanation is that the patients at highest risk suffer from primary pulmonary hypertension, accounting for a high pulmonary artery diastolic pressure in the setting of a normal wedge pressure.
Gender mismatch in heart transplantation is the source of some controversy, as questions remain as to whether worse outcomes due to gender mismatch are due to gender per se, or whether gender mismatch is a surrogate for size mismatch. This investigation showed that female recipients of male heart donors were at increased risk for early post-operative mortality. Though this analysis did not control for size/weight of the heart, it did control for recipient and donor body weight. The results described here are consistent with other recent studies. Khush and colleagues, using ISHLT Transplant Registry data, have shown that female recipients of female allografts have lower adjusted mortality compared to female recipients of male allografts.20 Similarly, Maltais and colleagues used the Scientific Registry for Transplant Recipients to show that gender match was independently associated with increased graft survival in patients bridged to transplant using mechanical circulatory support compared with gender mismatched patients.21 To account for mass effect, Reed and colleagues used the United Network for Organ Sharing transplantation registry and controlled for differences in predicted total heart mass. They found worse adjusted post-transplant survival was associated with gender mismatch in female patients.22 While reasons for this are speculative, Reed and colleagues speculate that this may be related to an immunogenic phenomenon due to first-time exposure to a Y chromosome.
In comparing this study to previously performed studies examining 90-day post-transplant survival in similar cohorts of patients bridged to transplant with CF LVADs,12,13 age was the only risk factor common to all three studies. The lack of concordance with other identified risk factors may be due to differences in sample size, methodology, and the outcome period in question (30 day vs. 90 day).
There are certain limitations to this study. First, the study included multiple device types, which creates some heterogeneity with regard to the pre-transplant management of the patients. The majority (1,883/2,152 or 87.5%) of the devices were HeartMate II LVADs (Thoratec Corporation, Pleasanton, CA). The inclusion of multiple device types was intentional on the part of the authors, as the advantage of increased sample size was felt to offset the perceived disadvantage of device heterogeneity. Furthermore, since the end of the study period other continuous flow devices such as the Heartware HVAD (Heartware Incorporated, Framingham, MA) have become more common, rendering device homogeneity in studies such as this one less relevant because device homogeneity is not the norm in clinical practice.
Second, the data in this study was derived from the ISHLT Transplant Registry. While contributing centers make a genuine effort to ensure good quality data entry, some level of inaccuracy in data entry is likely present.
Lastly, there are additional variables in the ISHLT Transplant Registry that could be analyzed to determine the influence on early post-transplant mortality. Out of 443 possible variables in the ISHLT Transplant Registry, 50 were used in this analysis. The variables that were included were chosen based on the likelihood of a relationship with outcome based on previously published work in transplantation, clinical judgment, and data availability and quality. All the variables used in our analysis are presented in Table 2. However, these results should not be interpreted as a comprehensive list of all possible variables that may influence 30-day post transplant mortality in patients bridged to transplant with a CF LVAD.
In conclusion, in this retrospective analysis of more than 2,000 patients bridged to transplant with durable CF LVADs, we identified a number of risk factors associated with early post-transplant mortality. Our analysis focused exclusively on this rapidly growing group of patients. We present hazard ratios associated with the presence of particular risk factors, which will assist clinicians in quantification of the risk of performing transplant in their patients. Some, but not all, of these risk factors are modifiable. Now that LVAD use as a permanent therapy can be a very efficient treatment approach in patients with advanced heart failure, transplant teams need to be cognizant of the effect of the described risk factors on short-term post-transplant mortality when making decisions regarding listing of patients with CF LVADs for transplant.
Acknowledgments
Grant Support: Research funded by a 2013 ISHLT Transplant Registry Early Career Award (AHH)
Footnotes
This work was presented at the 34th Annual Meeting of the International Society of Heart and Lung Transplantation, April 12, 2014, in San Diego, CA
Conflict of Interest Statement:
Healy – a 2013 ISHLT Transplant Registry Early Career Award funded this project
Stehlik – none
Edwards – none
McKellar – none
Drakos - none
Selzman - none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Nativi JN, Drakos SG, Kucheryavaya AY, et al. Changing outcomes in patients bridged to heart transplantation with continuous- versus pulsatile-flow ventricular assist devices: an analysis of the registry of the international society for heart and lung transplantation. J Heart Lung Transplant. 2011;30:854–61. doi: 10.1016/j.healun.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Pagani FD. Continuous-flow rotary left ventricular assist devices with “3rd generation” design. Semin Thorac Cardiovasc Surg. 2008;20:155–63. doi: 10.1053/j.semtcvs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Donneyong M, Cheng A, Trivedi JR, et al. The association of pre-transplant HeartMate II left ventricular assist device placement and heart transplantation mortality. ASAIO J. 2014;60:294–9. doi: 10.1097/MAT.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5.Bogaev RC, Pamboukian SV, Moore SA, et al. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–22. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 6.John R, Pagani FD, Naka Y, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: Impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg. 2010;140:174–81. doi: 10.1016/j.jtcvs.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Sandner SE, Zimpfer D, Zrunek P, et al. Age and outcomes after continuous-flow left ventricular assist device implantation as bridge to transplantation. J Heart Lung Transplant. 2009;28:367–72. doi: 10.1016/j.healun.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8.John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227–34. doi: 10.1016/j.athoracsur.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Garatti A, Bruschi G, Colombo T, et al. Clinical outcome and bridge to transplant rate of left ventricular assist device recipient patients: comparison between continuous-flow and pulsatile-flow devices. Eur J Cardiothorac Surg. 2008;34:275–80. doi: 10.1016/j.ejcts.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Healy AH, Mason NO, Hammond ME, et al. Allograft rejection in patients supported with continuous-flow left ventricular assist devices. Ann Thorac Surg. 2011;92:1601–7. doi: 10.1016/j.athoracsur.2011.05.119. [DOI] [PubMed] [Google Scholar]
- 11.Cowger J, Sundareswaran K, Roger JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–21. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Sabashnikov A, Mohite PN, Zych B, et al. Outcomes and predictors of early mortality after continuous-flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J. 2014;60:162–69. doi: 10.1097/MAT.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnaoutakis GJ, George TJ, Kilic A, et al. Risk factors for early death in patients bridged to transplant with continuous-flow left ventricular assist devices. Ann Thorac Surg. 2012;93:1549–54. doi: 10.1016/j.athoracsur.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Healy AH, Baird BC, Drakos SG, Stehlik J, Selzman CH. Impact of ventricular assist device complications on posttransplant survival: an analysis of the united network for organ sharing database. Ann Thorac Surg. 2013;95:870–5. doi: 10.1016/j.athoracsur.2012.10.080. [DOI] [PubMed] [Google Scholar]
- 16.Singh TP, Almond CS, Semigran MJ, Piercey G, Gauvreau K. Risk prediction for early in-hospital mortality following heart transplantation in the United States. Circ Heart Fail. 2012;5:259–66. doi: 10.1161/CIRCHEARTFAILURE.111.965996. [DOI] [PubMed] [Google Scholar]
- 17.Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT) Ann Thorac Surg. 2011;92:914–21. doi: 10.1016/j.athoracsur.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Russo MJ, Hong KN, Davies RR, et al. The effect of body mass index on survival following heart transplantation: do outcomes support consensus guidelines? Ann Surg. 2010;251:144–52. doi: 10.1097/SLA.0b013e3181b5db3c. [DOI] [PubMed] [Google Scholar]
- 19.Lietz K, John R, Burke EA, et al. Pretransplant cachexia and morbid obesity are predictors of increased mortality after heart transplantation. Transplantation. 2001;72:277–83. doi: 10.1097/00007890-200107270-00020. [DOI] [PubMed] [Google Scholar]
- 20.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. 2012;31:459–66. doi: 10.1016/j.healun.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maltais S, Jaik NP, Feurer ID, et al. Mechanical circulatory support and heart transplantation: donor and recipient factors influencing graft survival. Ann Thorac Surg. 2013;96:1252–8. doi: 10.1016/j.athoracsur.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Reed RM, Netzer G, Hunsicker L, et al. Cardiac size and sex-matching in heart transplantation: size matters in matters of sex and the heart. JACC Heart Fail. 2014;2:73–83. doi: 10.1016/j.jchf.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]