Abstract
Inability to engage with positive stimuli is a widespread problem associated with negative mood states across many conditions, from low self-esteem to anhedonic depression. Though attention retraining procedures have shown promise as interventions in some clinical populations, novel procedures may be necessary to reliably attenuate chronic negative mood in refractory clinical populations (e.g., clinical depression) through, e.g., more active, adaptive learning processes. In addition, a focus on individual difference variables predicting intervention outcome may improve the ability to provide such targeted interventions efficiently. To provide preliminary proof-of-principle, we tested a novel paradigm using operant conditioning to train eye gaze patterns towards happy faces. Thirty-two healthy undergraduates were randomized to receive operant conditioning of eye gaze towards happy faces (Train-Happy) or neutral faces (Train-Neutral). At the group level, the Train-Happy condition attenuated sad mood increases following a stressful task, in comparison to Train-Neutral. In individual differences analysis, greater physiological reactivity (pupil dilation) in response to happy faces (during an emotional face-search task at baseline) predicted decreased mood reactivity following stress. Preliminary results suggest that operant conditioning of eye gaze towards happy faces buffers against stress-induced effects on mood, particularly in individuals who show sufficient baseline neural engagement with happy faces. Eye gaze patterns to emotional face arrays may have a causal relationship with mood reactivity. Personalized medicine research in depression may benefit from novel cognitive training paradigms that shape eye gaze patterns through feedback. Baseline neural function (pupil dilation) may be a key mechanism, aiding in iterative refinement of this approach.
Keywords: attentional bias, attention training, eyetracking, mood, pupil
Introduction
Cognitive biases away from positive information, and towards negative information, are posited to play a critical role in the etiology and maintenance of negative affective conditions (e.g., Gotlib & Joormann, 2010) by promoting hyper-awareness of negative information, limiting awareness of positive information, and reinforcing maladaptive beliefs (e.g., Clark, Beck, & Alford, 1999; de Raedt & Koster, 2010). A growing literature suggests attention retraining techniques designed to modify attentional bias can effectively reduce clinical symptoms (MacLeod & Clarke, 2015). However, while these techniques appear to have relatively robust effects on some conditions (e.g., clinical anxiety), results of extant attention retraining studies in the context of other disorders (e.g., acute depression) have been mixed (reviewed by Mogoase, David, & Koster, 2014). Novel strategies to ameliorate attentional bias may help to address this gap in the clinical applicability of attention retraining techniques and broaden the range of individuals who might receive benefit.
Here, we sought to 1) provide preliminary proof-of-principal for one such novel strategy—operant (feedback-based) conditioning of eye gaze towards happy faces—by testing its effects on mood reactivity in a healthy sample, and 2) identify individual differences in neural engagement (indexed by pupil dilation) at baseline that can predict favorable response to the strategy, as an integral step towards further intervention refinement. Several specific features of the training paradigm were selected in the hopes of maximizing translational relevance to depressive patients. Foremost was an active, adaptive, operant learning process utilizing positive feedback—an effective means of enhancing learning (Masters, Maxwell, & Eves, 2009). We also used a training goal of increased focus on positive stimuli, designed to remediate a key attentional pattern in depression (e.g., Gotlib, McLachlan, & Katz, 1988); relatively ecologically valid stimuli (arrays of twelve emotional faces)(Dandeneau & Baldwin, 2004); and on-line, continuous measurement of eye gaze patterns to provide information proximal to the modification of visual gaze itself, rather than more ambiguous and complex measures such as manual response latencies.
Even if mood improvement is observed at the group level, individual differences in neural engagement may moderate training effects (Collier & Siegle, 2015; Siegle et al., 2014), providing clues for what psychophysiological processes must be engaged to maximize benefit. Thus, the second goal of the study was to evaluate baseline neural engagement as a predictor of post-training mood reactivity, using pupil dilation as a marker of neural engagement (Beatty, 1986; Koikegami & Yoshida, 1953) during a relevant face-search task. Consistent with previous pupil findings in depression (Collier & Siegle, 2015; Siegle et al., 2014), we specifically hypothesized that greater neural engagement with training-relevant stimuli (here, happy faces; i.e., greater pupil dilation when happy faces were presented) would predict more favorable post-training mood outcomes. Incorporating neurophysiological moderator analysis from the earliest stages of intervention development strongly promotes the ability to identify key intervention targets and make iterative refinements to expand the intervention’s reach, if and when dissemination becomes warranted.
Method
Participants
Thirty-two healthy undergraduates (mean=20.56y, SD=3.94; 8 male), were randomized to experimental vs. control training (n=16/group) and completed the protocol (Figure-1). The local ethics committee approved the study. Informed consent was obtained from all participants.
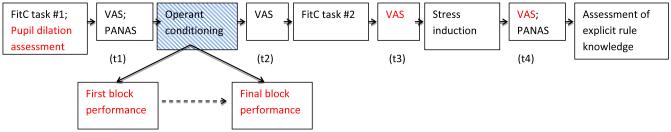
Figure 1.
Schematic of experimental procedures. Variables included in primary analyses are highlighted with red text. Primary mood outcomes were the VAS Happy and Sad scales administered at t3 & t4, immediately before and after the stress induction (Critical Feedback Test). Secondary mood measures (discussed in see Supplement) were the PANAS measures (administered at t1 and t4 only). Primary behavioral outcomes (red text) were based on performance during the first block and last block of the operant conditioning intervention. Secondary behavioral measures (discussed in see Supplement) were derived from the FitC task. Pupil dilation for moderation analyses was acquired during FitC task #1. FitC=face-in-the-crowd task; VAS=Visual Analogue Scales (happy and sad); PANAS=Positive and Negative Affect Scale.
Operant conditioning of eye movements
We trained participants to direct their attention towards a specific emotion (experimental condition: happy; control condition: neutral). Following a fixation cross (presented until 250ms gaze fixation was detected), pictures of 12 different women from the NimStim set were presented pseudo-randomly in a diamond pattern. Six different emotional expressions were displayed (happy, angry, sad, fearful, disgusted, neutral—two faces per emotion; details in Supplement).
Participants were instructed to attentively observe the pictures and were told they would hear a short, positive sound after responding “correctly.” No further instructions were given. When participants fixated on the target stimulus for 250ms within the time limit, a short sound designated correct completion. Otherwise, the trial ended and the experiment continued without feedback.
Participants were presented with at least 4 blocks of 36 trials. The first block began with a trial time limit of 3000ms. Continuation to faster blocks was adaptively based on performance. If 80% or more of trials were completed correctly, the time limit was decreased by 500ms (until the last block, which had a 1000ms limit). Otherwise, that speed was repeated. The experiment automatically stopped after the same speed was repeated 3 times (4 times for the initial block).
Behavioral outcomes of training
Training success was assessed using data from each participant’s first and last block. The primary outcome was latency to correct fixation. We also examined the time limit of final block (3000ms: no progress; 1000ms: maximal progress); % of trials with a correct fixation within the allowable window; and ‘Learning Rate’ calculated based on progression rates throughout training (see Supplement).
After final mood ratings, participants were asked in an open-ended format to state what was required to receive positive feedback during training. Responses were coded for presence/absence of explicit rule knowledge.
The Face in the crowd (FitC) task was used to assess generalization of training to a distinct visual search task (see Supplement) and for baseline pupillary assessment, as described below.
Stress induction
The Critical Feedback Task (Rossi & Pourtois, 2012)(see Supplement) is a mental counting task with bogus negative feedback. Participants were told results reflect intelligence and asked to tally instances of rare lines with a different in-plane orientation from distractors. Differences in orientation are extremely difficult to notice, creating high uncertainty. Participants were told the difficulty of each block would depend on performance. Unknown to them, participants always started with the most difficult block (minimal difference between target and distractor angles). End-of-block feedback was always negative and the following block was always easier.
Questionnaires
Two visual analogue scales (VAS) measured how happy and sad participants felt “at this moment” on a 0-10 scale (“not at all” to “very much”). The VAS scores used in primary analyses were collected just before and after the stress induction task (t3 & t4; Figure-1); additional VAS scores and secondary mood measures were not used in primary analyses, as the timing of assessments likely reduced sensitivity to stressor-related effects (see Supplement). Bonferonni correction was applied across the two primary measures; both corrected and uncorrected p-values are reported.
Moderator analysis: Pupil dilation
Pupil diameter was recorded by the eyetracker throughout the pre-training FitC task and preprocessed (see Supplement). Analysis focused on the trial type containing 5 angry distractors and 1 happy target face (5Angry1Happy), which we expected to be most relevant to mood outcomes given high emotional content and the need for simultaneous disengagement from negative faces and engagement with positive faces. To assess moderation effects on mood, pre-post-stress change scores for each mood outcome (VAS Happy/Sad) were calculated. Hierarchical regression analyses, performed separately at every timepoint in the waveform, tested whether pupil × training-group interaction terms explained additional variance in VAS change, over and above main effects of group and pupil. Type I error was held at p<.05 across all timepoints using temporal contiguity threshholding (Guthrie & Buchwald, 1991), as used regularly in pupillary waveform analysis, e.g., (Price et al., 2013)(see Supplement). Whenever a contiguous sequence of timepoints surpassing the p<.05 waveform-wise threshold was obtained, average pupil dilation throughout this “empirically derived window” was extracted for follow-up analysis.
Results
Group-level effects: self-reported mood
A manipulation check confirmed that the stress induction task was effective in reducing happy mood and increasing sad mood (Table). Effects on sad mood were moderated by training group in a preliminary ANOVA (Group × Time interaction: F1,30=4.6, puncorrected=.04, pcorrected=.08, partial η2=.13). Explicit knowledge of the training rule was tested post hoc and found to be an appropriate covariate, given that it was significantly related to the dependent variable (mood: F1,29=5.5, p=.03, partial η2=.16); but not to the independent variable (group: χ2=1.4, p=.24) and did not interact with the independent variable (p>.30; see further exploration in Supplement). ANCOVA (controlling for rule-learning) confirmed a Group × Time interaction (F1,29=5.7, puncorrected=.024, pcorrected=.048, partial η2=.16) explained by a significant increase in sad ratings in Train-Neutral (p<.001) but not Train-Happy (p=.25) participants. No significant group × time interactions were observed for VAS Happy ratings, with or without the covariate, nor were significant effects detected on secondary mood outcomes (see Supplement).
Table.
Mood scale scores and behavioral effects of training as a function of timepoint and group
| Full Sample (n=32) |
Train-Happy (n=16) |
Train-Neutral (n=16) | |
|---|---|---|---|
| VAS Sad | |||
| Pre-Stressor (t3) | .93 (.28)*** | .33 (.41) | 1.52 (2.1)*** |
| Post-Stressor (t4) | 1.47 (.76)*** | .59 (.76) | 2.35 (2.1)*** |
| VAS Happy | |||
| Pre-Stressor (t3) | 4.56 (3.3)** | 4.48 (3.7) | 4.63 (3.0)* |
| Post-Stressor (t4) | 3.97 (3.1)** | 4.03 (3.5) | 3.91 (2.9)* |
|
| |||
|
Latency to correct fixation
(ms) |
|||
| First block | 1462.5 (138.8)*** | 1455.6 (124.6)*** | 1469.5 (155.5)** |
| Final block | 1219.5 (223.0)*** | 1129.4 (240.3)*** | 1309.6 (180.1)** |
|
| |||
| Final block time limit | |||
| 3000ms | n=10 | n=3a | n=7a |
| 2500ms | n=5 | n=1a | n=4a |
| 2000ms | n=12 | n=7a | n=5a |
| 1500ms | n=4 | n=4a | n=0a |
| 1000ms | n=1 | n=1a | n=0a |
|
| |||
| % trials with correct fixation | |||
| First block | 73.9% (14.8)b | 81.6% (13.3)b | 66.2% (12.0)b |
| Last block | 66.3% (13.2)b | 69.3% (10.9)b | 63.3% (14.9)b |
|
| |||
| ‘Learning Rate’ points | 1.14 (.97) | 1.64 (.96)c | .65 (.71)c |
|
| |||
| Explicit rule knowledge | n=23 (72%) | n=13 (81%) | n=10 (63%) |
Note: Values reported as mean (SD).
Within-groups values differ between timepoints according to paired t-tests: (p<.05);
Within-groups values differ between timepoints according to paired t-tests: (p<.01);
Within-groups values differ between timepoints according to paired t-tests: (p<.001).
Distribution of final block speeds suggested learning in both groups, but differed across groups at the trend level (χ2=8.7, p=.07).
Repeated-measures ANOVA comparing % trials with correct fixations on the first vs. last block showed a decrease in accuracy from first to last block (F1,30=5.5, p=.03, partial η2=.16), suggesting the adaptive design successfully created increasing task difficulty across blocks. Train-Happy participants also showed higher overall accuracy (75%) than Train-Neutral participants (65%) (F1,30=11.4, p=.002, partial η2=.28); the group × time interaction was not significant (F1,30=2.1, p=.16, partial η2=.07).
‘Learning Rate’ differed significantly by group (t30=3.3, p=.003). Explicit rule knowledge did not differ significantly across the two training groups (χ2=1.4, p=.24).VAS=Visual Analogue Scale
Group-level effects: behavior
Participants in both training conditions exhibited successful operant learning (see Table for all behavioral outcomes). ANOVA comparing mean latency to correct fixation on the first vs. last block reflected pre-post-training performance improvements (F1,30=34.9, p<.001, partial η2=.54). An effect of group (F1,30=4.3, p=.048, partial η2=.12) and a trend-level group × time interaction (F1,30=4.1, p=.052, partial η2=.12) reflected slightly faster responses and larger improvements in the Train-Happy group. Behavioral measures were uncorrelated with change on VAS scales (within groups or across the entire sample; p’s>.4) and the group × time interaction on VAS Sad remained significant after covarying each index (all p’s<.05), suggesting group differences in learning success did not explain differential mood reactivity.
Robust generalization effects to a distinct visual search task (FitC) were not observed, although several near-significant, moderate effect sizes in the expected direction were apparent (see Supplement).
Pupillary moderation of mood effects
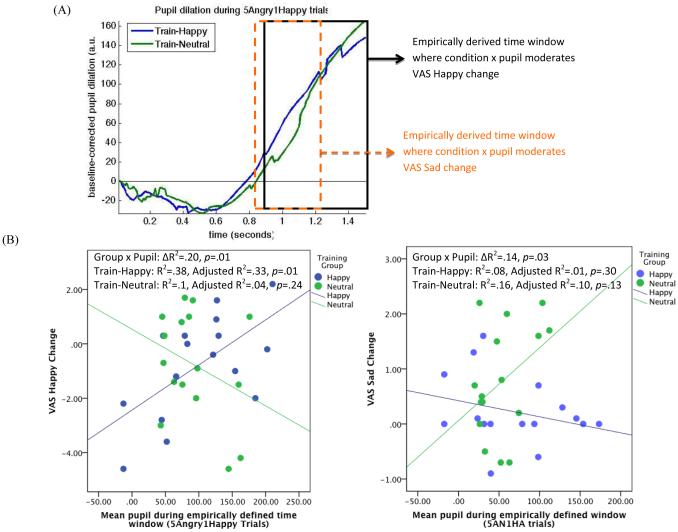
Pupil waveform analyses revealed pupil dilation from 0.9-1.5sec after face array onset interacted with training condition to predict VAS Happy change (ΔR2=.20, puncorrected=.01, pcorrected=.02; Figure-2), over and above main effects of pupil dilation and group. The interaction was driven by a significant effect in the Train-Happy group: Train-Happy participants with greater baseline pupil dilation exhibited a more positive response to the stressor, showing increases (or attenuated decreases) in happy mood following stress. Similarly, pupil dilation from 0.85-1.25sec after face onset moderated VAS Sad change (ΔR2=.14, puncorrected=.03, pcorrected=.06). The moderation pattern was similar, but non-significant in both groups: Train-Happy participants with larger pupil dilation exhibited smaller increases in negative mood—i.e., a less negative response to the stressor; whereas an opposing pattern was observed in Train-Neutral participants. Together these findings are consistent with a mood buffering effect in Train-Happy participants with highest pupillary engagement. In spite of small samples, moderation findings were robust in Monte-Carlo simulations (see Supplement). The Supplement presents additional sensitivity analyses (e.g., pupil from other FitC trial types).
Figure 2.
(A) Waveforms representing mean pupil response during analyzed timepoints of 5Angry1Happy trials in the Train-Happy and Train-Neutral groups. Pupil diameter represents change from baseline (trial onset). On average, pupil dilation increased in all participants as the trial progressed, particularly during the latter stages of the trial. Boxed areas represent the empirically derived time windows where pupil x training group moderated VAS Happy (.9-1.5s, outlined in black) and VAS Sad (.85 to 1.26, outlined in dashed orange), respectively. A.u.=arbitrary units, recorded by EyeLink II software on the basis of the number of pixels considered to be pupil within the camera image. (B) Scatterplots showing, for the empirically-derived time windows depicted in (A), the relationship between pupil dilation and change in mood measures (baseline to post-stress) as a function of training group (Train-Happy vs. Train-Neutral). Change scores calculated as t4 – t3 (positive values indicate increase in mood post-stress; negative values indicate decrease in mood post-stress). P-values derived from waveform analysis with timeseries-wise error correction
Discussion
The current study a) supports the ability to train intended gaze patterns in healthy volunteers using a novel, operant conditioning-based attention retraining paradigm and b) provides preliminary evidence for buffering effects on mood reactivity following a stressful task. In comparison to a control condition, training eye gaze patterns towards happy faces resulted in attenuated sad mood responses following a stressor, consistent with cognitive models suggesting information processing patterns have downstream effects on emotional vulnerability (Clark et al., 1999). Individual differences in neural engagement with happy faces at baseline (assessed peripherally via pupil dilation) moderated training effects on mood reactivity. Given that this intervention was designed in the hopes of maximizing translational relevance to depressed patients, initial results provide promising proof-of-concept for an operant conditioning-based, eye gaze-dependent, adaptive, active, attention retraining approach. Attention to individual differences in neural engagement at baseline may promote the ability to further improve on this intervention via efforts to increase the salience of target stimuli.
Novel features of the current intervention included an active, adaptive learning process utilizing positive feedback, guided by on-line, continuous measurement of eye gaze patterns. This represents a substantial departure from previous attention retraining methods, which have relied exclusively on manual responses to visual stimuli and have rarely incorporated feedback. Additional features included a training goal of increasing focus on positive stimuli and the use of relatively ecologically valid stimuli (arrays of emotional faces). The current findings could help clarify previous findings from manual response attention training paradigms (e.g., the dot-probe)—which conflate a number of possible visual and non-visual mechanisms—by suggesting that conditioning overt visual attention itself via gaze patterns is sufficient to impact mood reactivity.
The current intervention exhibited a group-level buffering effect specifically on sad mood ratings, suggesting the intervention, despite its primary focus on positive stimuli, had its broadest impact on dysphoric responses to a stressor, an important vulnerability factor for depression (de Raedt & Koster, 2010). Notably, our control condition constituted an active training as well, in that participants were trained to gaze at neutral faces while inhibiting gaze towards an array of mostly negative (sad, angry, disgusted, fearful) faces. The control condition was therefore similar to the active training utilized routinely in attention retraining procedures, where attention is trained towards neutral targets and away from negative. Results therefore provide a conservative estimate of the intervention’s capacity to buffer against mood reactivity, which might partially explain why group-level effects were constrained to sad mood.
Pupil moderation effects on both happy and sad mood suggest that sufficient neural representation of trials containing happy faces is needed in order to fully capitalize on the training, consistent with findings in clinically depressed patients completing cognitive training (Siegle et al., 2014). An opposing pattern in Train-Neutral participants may suggest that, for individuals with high baseline engagement with happy stimuli, training attention away from happy faces is particularly ill-advised. Findings suggest multiple useful modifications of the current paradigm to increase its breadth, e.g., pilot-testing target stimuli to maximize pupillary engagement potential; using idiographic, self-relevant target stimuli; and/or enhancing neural engagement prior to—or during—eye gaze training via operant feedback of pupil dilation (“neurofeedback”).
Limitations
We cannot rule out the influence of expectancy effects on self-reported mood ratings, given that most participants developed explicit knowledge of the training rule; however, participants were likely unaware of study hypotheses related to the stress manipulation. Although samples were small, they were consistent with many existing attention retraining studies (Hallion & Ruscio, 2011), and Type I and II error control appeared adequate (see Supplement). In supplementary analyses, we failed to identify reliable generalization of changes to a similar, but distinct, visual search task (although several suggestive, near-significant findings were present). This might be due to inadequate test-retest reliability of attentional bias measures (Price et al., In press) adversely effecting power (see Supplement) and/or the distinct properties of the generalization task (FitC). For example, the FitC simply asks whether faces are all the same or different, which could be done through relatively low-level stimulus features, particularly given that only angry, happy, and neutral faces were used; while the training task required strategically identifying a categorization rule and fixating on the correct face type from within a much more complex emotional array. In moderation analyses, pupil dilation during fixation periods was averaged across multiple trials with variable timecourses, leaving uncertain which specific aspects of attentional responding were indexed (e.g., face processing, preparation for a subsequent saccade). Finally, explicit testing of this intervention in clinical (e.g., depressed) samples is needed to show that preliminary findings are clinically relevant.
In conclusion, there is a need for novel training paradigms to fulfill the promise of attention retraining as a clinical intervention strategy, particularly for patients not helped by existing paradigms in widespread use (e.g., acutely depressed patients). A novel attention retraining paradigm based on operant conditioning of eye gaze patterns was successful in training intended gaze patterns and reducing sad mood reactivity following stress. Behavioral training effects did not generalize to a similar visual search task, and training effects on happy mood were observed only in participants who strongly engaged with happy faces at baseline. Upon replication and extension of current findings in clinical samples, this paradigm could have clinical utility within the growing spectrum of cognitive training interventions, particularly if optimized based on these preliminary findings.
Supplementary Material
Acknowledgement
This research was supported by Grant BOF10/GOA/014 for a Concerted Research Action of Ghent University awarded to Rudi De Raedt and Ernst Koster. Dr. Price is supported by a Career Development Award from the National Institute of Mental Health (K23MH100259). Thanks to Dr. Valentina Rossi for providing and explaining the Critical Feedback Task.
References
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, et al. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J. The pupil system. In: Coles M, Donchin E, Porges S, editors. Psychophysiology: Systems, Processes, and Application. Guilford; New York: 1986. pp. 43–50. [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory and therapy of depression. Wiley; Hoboken, NJ: 1999. [Google Scholar]
- Collier A, Siegle G. Individual differences in response to prediction bias training. Clinical Psychological Science. 2015;3(1):79–90. [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Dandeneau SD, Baldwin MW. The Inhibition of Socially Rejecting Information Among People with High Versus Low Self-Esteem: The Role of Attentional Bias and the Effects of Bias Reduction Training. Journal of Social and Clinical Psychology. 2004;23:584–603. [Google Scholar]
- de Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, McLachlan AL, Katz AN. Biases in visual attention in depressed and nondepressed individuals. Cognition & Emotion. 1988;2:185–200. [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011 doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry. 2005;58(6):495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Koikegami H, Yoshida K. Pupillary dilation induced by stimulation of amygdaloid nuclei. Folia psychiatrica et neurologica japonica. 1953;7:109–125. doi: 10.1111/j.1440-1819.1953.tb00600.x. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Clarke P. The Attentional bias modification approach to anxiety intervention. Clinical Psychological Science. 2015;3(1):58–78. [Google Scholar]
- Masters RS, Maxwell JP, Eves FF. Marginally perceptible outcome feedback, motor learning and implicit processes. Consciousness and Cognition. 2009;18(3):639–645. doi: 10.1016/j.concog.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Mogoase C, David D, Koster EHW. Clinical Efficacy of Attentional Bias Modification Procedures: An Updated Meta-analysis. Journal of Clinical Psychology. Journal of Clinical Psychology. 2014;70:1133–1157. doi: 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Niemi P. Inducing affective states with success-failure manipulations: a meta-analysis. Emotion. 2004;4(2):207–214. doi: 10.1037/1528-3542.4.2.207. [DOI] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. doi: 10.1037/pas0000036. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle G, Silk JS, Ladouceur CD, McFarland A, Dahl RE, et al. Sustained neural alterations in anxious youth performing an attentional bias task: A pupilometry study. Depression and Anxiety. 2013;30:22–30. doi: 10.1002/da.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi V, Pourtois G. State-dependent attention modulation of human primary visual cortex: A high density ERP study. NeuroImage. 2012;60:2365–2378. doi: 10.1016/j.neuroimage.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Price RB, Jones N, Ghinassi F, Painter T, Thase ME. You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for depression. Clinical Psychological Science. 2014;2(4):455–471. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biological Psychiatry. 2011;69(8):726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, et al. Pupillary reactivity to emotional information in child and adolescent depression: links to clinical and ecological measures. American Journal of Psychiatry. 2007;164(12):1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87(2):245–251. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.