Abstract
Background
The use of statins increased among US adults with high coronary heart disease (CHD) risk following publication of the 2001 cholesterol treatment guidelines.
Methods and Results
We analyzed the association between lipids and CHD among 9,578 REasons for Geographic And Racial Differences in Stroke (REGARDS) study participants and 346,595 Kaiser Permanente Southern California (KPSC) members with baseline lipid measurements in 2003–2007. We performed the same analyses among 14,590 Atherosclerosis Risk In Communities (ARIC) study participants with lipid measurements in 1987–1989. Analyses were restricted to blacks and whites 45–64 years of age, without CHD, who were not taking statins at baseline. Total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides were measured at baseline. Low-density lipoprotein cholesterol (LDL-C), non-HDL-C, total-to-HDL-C and triglycerides-to-HDL-C ratios were calculated. The prevalence of diabetes, history of stroke and antihypertensive medication use increased at higher LDL-C in ARIC but not in REGARDS or KPSC. Over 8.9 years of follow-up, 225 CHD events occurred in REGARDS, 6,547 events in KPSC and 583 events in ARIC. After multivariable adjustment, less favorable lipid levels were associated with higher hazard ratios (HR) for CHD in ARIC. These associations were attenuated in REGARDS and KPSC. For example, the HR (95%CI) associated with the highest versus lowest quartile of LDL-C (≥146 mg/dL versus ≤102 mg/dL) was 1.89 (1.42–2.51) in ARIC, and 1.25 (0.81–1.92) in REGARDS and 1.49 (1.38–1.61) in KPSC.
Conclusions
The association between lipids and CHD in contemporary studies may be attenuated due to preferential use of statins by high risk individuals.
Keywords: lipids, coronary disease, epidemiology, follow-up studies
In 2001, the Third Report of the National Cholesterol Education Program, Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) provided guidelines for the diagnosis and treatment of dyslipidemia among US adults.1 These guidelines recommended healthcare providers use an individual’s low density lipoprotein cholesterol (LDL-C) level and coronary heart disease (CHD) risk in the decision to initiate statins for primary prevention. In ATP III, statin initiation was recommended for adults with high LDL-C or with low to moderate LDL-C levels but with a high estimated 10-year CHD risk or CHD risk equivalents.1 The percentage of US adults recommended lipid-lowering therapy by ATP III who were taking statins increased from 20% in 1999–2000 to 38% in 2003–2004 and 43% in 2009–2010.2, 3
Given the recommendations for the selective use of statins for primary prevention in ATP III, the group of individuals with high LDL-C who remain untreated may be enriched for adults who have low CHD risk. Therefore, it is possible that the association between LDL-C and incident CHD among adults not taking statins has changed since the publication of ATP III. This might have important implications for the interpretation of epidemiological studies conducted in the era of widespread statin use as well as for the accuracy of CHD and cardiovascular disease (CVD) risk prediction equations that were derived using data on lipids collected before the publication of ATP III.4–6
The main objective of the present analysis was to determine the association of LDL-C and other serum lipids including non-high density lipoprotein cholesterol (non-HDL-C), HDL-C, triglycerides (TG), total-to-HDL-C and TG-to-HDL-C ratios with incident CHD after the publication of the ATP III guidelines and the subsequent increase in statin use among US adults. For comparison, we conducted an identical analysis on the association between serum lipids and CHD incidence using data from an era before statin use became common in primary prevention. We hypothesized that the association of LDL-C and other serum lipids with CHD incidence in observational studies would be weaker among untreated adults in the era following publication of the ATP III guidelines due to the preferential use of statins by individuals with high LDL-C or low to moderate LDL-C but high CHD risk.
Methods
Study populations
We used data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study and Kaiser Permanente Southern California (KPSC) healthcare system to analyze the association between serum lipids and CHD risk for the time period following publication of the ATP III guidelines. For comparison, we used data from the public-use Atherosclerosis Risk In Communities (ARIC) study data set to analyze the same associations during the period prior to publication of the ATP III guidelines and the widespread use of statins.
The REGARDS study was designed to investigate reasons underlying the higher rate of stroke mortality among blacks compared to whites, and among residents of the Southeastern United States compared to other US regions.7 CHD events have been identified and adjudicated in an ancillary study.8 A total of 30,239 black and white men and women aged 45 years or older were recruited from all 48 contiguous US states and the District of Columbia between January 1, 2003 and October 31, 2007. KPSC is a healthcare delivery system which provides care for about 3.9 million members in the Southern California region.9 The ARIC study was designed to investigate the etiology of atherosclerosis in a cohort of black and white men and women aged 45 to 64 years from four US communities.10 A total of 15,792 participants were enrolled between 1987 and 1989. The REGARDS and ARIC studies were approved by the Institutional Review Boards at the participating centers and all participants provided written informed consent. The use of KPSC administrative data and electronic health records for the present analysis was approved by the KPSC IRB with a waiver for written informed consent. All analyses in KPSC were conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA) regulations.
All REGARDS study participants, black and white KPSC members with a complete lipid panel between January 1, 2003 and October 31, 2007, and ARIC participants in the limited access public use data set were assessed for eligibility. A lipid panel including total cholesterol (TC), HDL-C and TG in 2003–2007 was required for KPSC members to match the enrollment period in REGARDS. Additionally, KPSC members were required to have 365 days of continuous healthcare coverage prior to their first lipid panel. This period was used to assess baseline characteristics. We excluded REGARDS study participants and KPSC members <45 or ≥65 years of age to match the age range of ARIC participants. Further exclusion criteria in REGARDS, KPSC and ARIC were having a history of CHD or use of lipid-lowering medications at baseline (defined below), and lack of post-baseline participant follow-up to identify the occurrence of CHD events. After these exclusions were applied, 9,578 REGARDS study participants, 346,595 KPSC members and 14,590 ARIC study participants were included in the present analysis (Figure 1).
Figure 1.
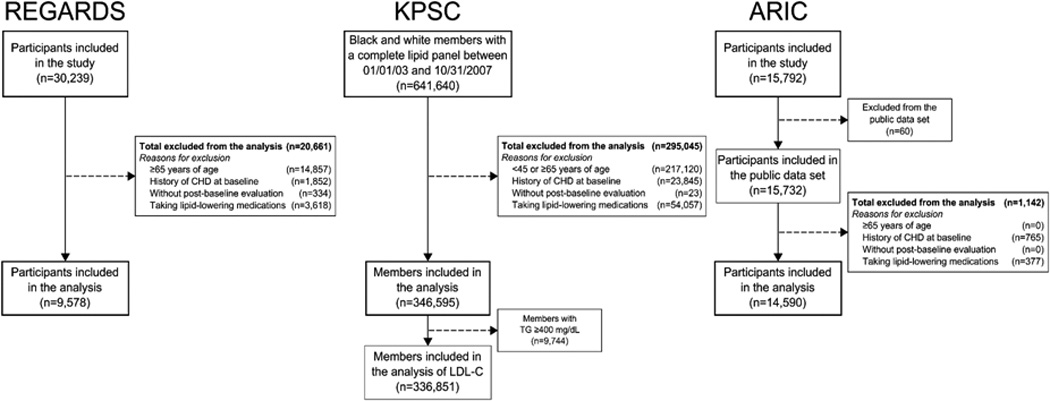
Flowchart of individuals from REGARDS, KPSC and ARIC included in the analysis. ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; KPSC: Kaiser Permanente Southern California; LDL-C: low-density lipoprotein cholesterol; REGARDS: REasons for Geographic And Racial Differences in Stroke; TG: triglycerides.
Baseline assessment
The REGARDS and ARIC studies
As part of the baseline evaluation in the REGARDS and ARIC studies, an interview was conducted, and a blood sample and an electrocardiogram were obtained for each participant. Self-reported information collected at baseline in the REGARDS and ARIC studies included age, race, sex, education, annual household income, alcohol consumption, current cigarette smoking, history of stroke and use of antihypertensive medication. History of CHD at baseline was defined by self-report of a prior diagnosis of myocardial infarction (MI), coronary bypass, coronary angioplasty (or stenting in REGARDS). Participants with evidence of a previous MI on the baseline electrocardiogram were also considered to have a history of CHD. Use of lipid-lowering medication was assessed by self-report in the REGARDS study and through the review of pill bottles for all medications participants reported taken during the two weeks prior to their baseline study visit in ARIC. For REGARDS study participants, use of statins was also assessed through the review of pill bottles for all medications taken during the two weeks preceding their baseline study visit. Pill bottle data to identify statin use at baseline is not included in the public use data set for the ARIC study.
Using the blood collected at baseline in REGARDS and ARIC, serum glucose, creatinine and lipids were measured. Diabetes was defined as self-reported treatment with oral anti-diabetes medications or insulin, fasting (8 hours or more) serum glucose ≥126 mg/dL or non-fasting serum glucose ≥200 mg/dL.11 For each participant, estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.12 Baseline serum creatinine levels from ARIC participants were calibrated as previously described.13 Reduced eGFR was defined as <60 ml/min/1.73 m2.14
KPSC
For KPSC members, baseline was defined by the date of their first lipid panel that included TC, HDL-C and TG between January 1, 2003 and October 31, 2007. Baseline characteristics of KPSC members prior to the lipid panel were identified using administrative databases and electronic health records. These characteristics included age, sex, race, education, income, alcohol consumption, smoking, diabetes, reduced eGFR, history of stroke and CHD, use of antihypertensive medications and lipid-lowering medications (Supplemental Table 1).
Serum lipids measurements at baseline
Six serum lipids were analyzed in the current study: LDL-C, non-HDL-C, HDL-C, TG, total-to-HDL-C and TG-to-HDL-C ratios. TC, HDL-C and fasting TG were measured by colorimetric reflectance spectrophotometry in REGARDS. In ARIC, automated enzymatic methods (Monotest Cholesterol and GPO Triglyceride procedures, Boehringer Mannheim) were implemented using a Cobas-Bio Analyzer© (Roche) to measure TC, HDL-C and fasting TG. TC, HDL-C and TG were measured by the Kaiser Permanente Regional Laboratory using enzymatic methods. The Friedewald equation was used to calculate LDL-C for individuals with TG <400 mg/dL in each population.15 We also calculated values for non-HDL-C (TC minus HDL-C), total-to-HDL-C ratio (TC divided by HDL-C) and TG-to-HDL-C ratio (TG divided by HDL-C).
CHD incidence
Trained personnel in both the REGARDS and ARIC studies used similar approaches to adjudicate incident CHD events.16 REGARDS participants were contacted by telephone every 6 months and medical records were retrieved when a heart-related hospitalization was reported by the participant or a proxy respondent. When possible CHD-related deaths were reported, interviews with next-of-kin or proxies, medical records in the last year of life, death certificates and autopsy reports were used to determine if a CHD event was the underlying cause of death. Follow-up of ARIC participants included annual telephone interviews and in-person clinic visits at 3 year intervals. Additional information on CHD incidence was retrieved from hospital records and death certificates through a community surveillance system. The definition of CHD events from the REGARDS and ARIC studies included definite or probable MI or CHD death (death preceded by cardiac symptoms or signs or a history of CHD and with no evidence of non-coronary causes).
Incident CHD events following the baseline lipid panel identified among KPSC members included non-fatal MIs, defined by an inpatient stay with a discharge diagnosis code of 410.× according the International Classification of Diseases, 9th version (ICD-9) in any position, or CHD deaths, defined by an underlying cause of death ICD, 10th version (ICD-10) code I20–I25. KPSC members’ deaths were identified from health plan databases, State of California death certificate files and Social Security Administration Death files.
Adjudicated CHD outcomes were available through December 31st, 2011 in REGARDS (maximum follow-up: 8.9 years). To match the follow-up time in REGARDS, ARIC participants and KPSC members who remained free of CHD events and alive were censored after 8.9 years of follow-up.
Statistical analysis
Baseline characteristics of individuals in REGARDS, KPSC and ARIC included in the current analysis were calculated. For the main analysis, each lipid was divided into four levels defined by quartile cut-points in the REGARDS study. We chose to use quartiles because few individuals were available in some categories (e.g., LDL-C ≥ 160 mg/dL) when clinical cut-points were applied. To examine preferential treatment of high risk individuals with statins following the publication of ATP III, baseline characteristics of individuals in REGARDS, KPSC and ARIC not taking lipid-lowering medications were calculated by category of LDL-C.
Age, race and sex adjusted CHD incidence rates were calculated for each lipid category using Poisson regression. Cox proportional hazards models were used to calculate hazard ratios (HR) for CHD associated with categories of each serum lipid, using the most favorable category as the reference (e.g., lowest LDL-C, highest HDL-C). Three models with progressive adjustment were conducted. The first model included adjustment for age, race and sex. The second model included the covariates from the first model plus education, household income, alcohol consumption and current smoking. The third model included variables in the second model plus diabetes, reduced eGFR, history of stroke and use of antihypertensive medication. All regression models from REGARDS also included adjustment for region of residence (stroke belt, stroke buckle and non-belt regions).7 Trends in incidence rates and HRs across categories of each lipid were calculated by modeling the lipid categories as an ordinal variable. A lower HR for CHD associated with the highest category of LDL-C in contemporary studies may be driven by fewer individuals with very high LDL-C. Therefore, we repeated the analysis for LDL-C after creating a fifth category for individuals with LDL-C ≥190 mg/dL. In sensitivity analyses, we calculated HRs for CHD associated with serum lipids as continuous variables and, separately, with categories of lipids defined by quartile cut-points derived using ARIC study data.
All analyses in REGARDS and ARIC were conducted using multiple imputation to replace missing data including covariates and non-fasting LDL-C and TG values. For each lipid analyzed, we imputed 25 data sets using chained equations. Multiple imputation was based on observed values from all the covariates included in the fully adjusted Cox regression models and the outcome.17, 18 The results were similar in analyses conducted without multiple imputation (data not shown). Analyses in KPSC were conducted using missing indicator variables rather than multiple imputation due to the large number of observations. All analyses were conducted using Stata/I.C. 12.1 (Stata Corporation, College Station, TX).
RESULTS
Participant characteristics
The mean age of individuals included in the current study was 56.7, 53.4 and 54.0 years in REGARDS, KPSC and ARIC, respectively (Table 1). By design, a high percentage of REGARDS study participants was black. REGARDS participants were more likely to have at least a high school education compared with KPSC members and ARIC study participants. An annual household income ≥$25,000 was more common among REGARDS participants and KPSC members as compared with ARIC participants. A smaller percentage of REGARDS participants and KPSC members was currently smoking cigarettes as compared with ARIC participants. Antihypertensive treatment was more common in the REGARDS study and among KPSC members. The mean HDL-C and TG-to-HDL-C ratio were similar in REGARDS, KPSC and ARIC. The mean TC, LDL-C, and non-HDL-C were lower for REGARDS participants as compared with KPSC members and ARIC participants. Mean total-to-HDL-C ratios were lower in REGARDS participants and KPSC members as compared with ARIC participants.
Table 1.
Baseline characteristics of individuals from REGARDS, KPSC and ARIC included in the analysis.
| REGARDS (n = 9,578) |
KPSC (n = 346,595) |
ARIC (n = 14,590) |
|
|---|---|---|---|
| Age in years, mean (SE) | 56.7 (0.05) | 53.4 (0.01) | 54.0 (0.05) |
| Men, % | 39.3 | 42.5 | 43.3 |
| Blacks, % | 43.7 | 20.5 | 27.6 |
| Region of residence,* % | |||
| Stroke belt (buckle states) | 21.4 | -- | -- |
| Stroke belt (non-buckle states) | 36.1 | -- | -- |
| Other contiguous US states | 42.5 | -- | -- |
| High school or higher education level, % | 92.5 | 81.1 | 76.6 |
| Annual household income, % | |||
| <US$25,000 | 24.1 | 2.8 | 38.5 |
| ≥US$25,000 | 75.9 | 91.2 | 61.5 |
| Unknown | -- | 6.0 | -- |
| Alcohol consumption, % | |||
| None | 57.5 | 45.4 | 61.4 |
| Moderate | 37.8 | 38.9 | 30.0 |
| Heavy | 4.7 | 8.3 | |
| Unknown | -- | 15.8 | -- |
| Current smoking, % | |||
| Yes | 18.7 | 14.7 | 26.3 |
| No | 81.3 | 78.8 | 73.7 |
| Unknown | -- | 6.5 | -- |
| Diabetes, % | 12.7 | 7.9 | 11.1 |
| Reduced eGFR, % | 2.8 | 4.8 | 2.6 |
| History of stroke, % | 2.6 | 1.2 | 1.4 |
| Taking antihypertensive medication, % | 36.7 | 29.3 | 23.9 |
| Total cholesterol (mg/dL), mean (SE) | 202.2 (0.40) | 214.1 (0.07) | 214.1 (0.35) |
| LDL-C (mg/dL), mean (SE) | 123.5 (0.36) | 132.9 (0.06) | 136.2 (0.34) |
| Non-HDL-C (mg/dL), mean (SE) | 148.9 (0.40) | 159.9 (0.07) | 161.9 (0.37) |
| HDL-C (mg/dL), mean (SE) | 53.3 (0.17) | 54.2 (0.03) | 52.3 (0.14) |
| TG (mg/dL), mean (SE) | 126.6 (0.95) | 134.8 (0.12) | 128.4 (0.72) |
| Total-to-HDL-C ratio, mean (SE) | 4.1 (0.01) | 4.2 (0.003) | 4.5 (0.01) |
| TG-to-HDL-C ratio, mean (SE) | 2.9 (0.04) | 2.9 (0.004) | 3.0 (0.03) |
ARIC: Atherosclerosis Risk In Communities; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; KPSC: Kaiser Permanente Southern California; LDL-C: low-density lipoprotein cholesterol; REGARDS: REasons for Geographic And Racial Differences in Stroke; SE: standard error; TG: triglycerides; US$: United States dollars.
Stroke belt (buckle states) includes coastal North Carolina, South Carolina and Georgia. Stroke belt (non-buckle states) includes the remaining parts of the stroke buckle states and Tennessee, Mississippi, Alabama, Louisiana and Arkansas.
Association between baseline characteristics and LDL-C
At higher LDL-C levels, ARIC participants had a greater burden of cardiovascular risk factors (Table 2). ARIC participants with higher LDL-C were older, more likely to be men, have diabetes, reduced eGFR, history of stroke, and to be taking antihypertensive medication. In contrast, many of these associations were not present in REGARDS and KPSC. For example, the prevalence of diabetes and use of antihypertensive medications were lower at higher LDL-C levels among REGARDS participants and KPSC members.
Table 2.
Baseline characteristics of individuals from REGARDS, KPSC and ARIC by category of LDL-C.
| Category of LDL-C* | Trend | ||||
|---|---|---|---|---|---|
| LDL-C (mg/dL), range | 1 ≤102 |
2 103 to 123 |
3 124 to 145 |
4 ≥146 |
p-value |
| REGARDS (n=9,578) | |||||
| Individuals, n | 2,470 | 2,411 | 2,346 | 2,351 | - |
| Age in years, mean (SE) | 56.2 (0.11) | 56.6 (0.11) | 56.8 (0.11) | 57.0 (0.11) | <0.001 |
| Blacks, % | 45.7 | 41.8 | 42.8 | 44.6 | 0.64 |
| Men, % | 39.8 | 39.1 | 39.1 | 39.2 | 0.71 |
| Current smoking, % | 19.5 | 18.1 | 18.3 | 18.8 | 0.62 |
| Diabetes, % | 15.6 | 12.9 | 10.4 | 11.5 | <0.001 |
| Reduced eGFR, % | 3.0 | 2.4 | 2.6 | 3.1 | 0.79 |
| History of stroke, % | 2.6 | 2.7 | 2.6 | 2.6 | 0.99 |
| Antihypertensive medication, % | 39.3 | 36.9 | 36.3 | 34.4 | 0.002 |
| KPSC (n=336,851) | |||||
| Individuals, n | 66,101 | 73,786 | 82,927 | 114,037 | - |
| Age in years, mean (SE) | 52.8 (0.02) | 53.2 (0.02) | 53.5 (0.02) | 53.9 (0.02) | <0.001 |
| Blacks, % | 22.9 | 20.2 | 19.3 | 20.3 | <0.001 |
| Men, % | 40.0 | 41.1 | 43.2 | 44.2 | <0.001 |
| Current smoking, % | 15.0 | 13.7 | 14.3 | 15.7 | 0.002 |
| Diabetes, % | 12.2 | 8.7 | 6.7 | 5.9 | <0.001 |
| Reduced eGFR, % | 4.9 | 4.3 | 4.3 | 4.7 | 0.28 |
| History of stroke, % | 1.5 | 1.1 | 1.1 | 1.1 | <0.001 |
| Antihypertensive medication, % | 30.6 | 29.5 | 29.0 | 29.0 | <0.001 |
| ARIC (n=14,590) | |||||
| Individuals, n | 2,686 | 2,858 | 3,345 | 5,701 | - |
| Age in years, mean (SE) | 52.7 (0.11) | 53.4 (0.11) | 54.0 (0.10) | 54.8 (0.08) | <0.001 |
| Blacks, % | 30.4 | 27.3 | 24.8 | 28.2 | 0.09 |
| Men, % | 36.7 | 41.2 | 46.0 | 45.9 | <0.001 |
| Current smoking, % | 26.1 | 25.9 | 25.8 | 26.8 | 0.45 |
| Diabetes, % | 9.2 | 9.4 | 11.1 | 12.8 | <0.001 |
| Reduced eGFR, % | 1.7 | 2.3 | 2.5 | 3.3 | <0.001 |
| History of stroke, % | 0.8 | 1.0 | 1.6 | 1.8 | <0.001 |
| Antihypertensive medication, % | 21.8 | 22.9 | 22.3 | 26.4 | <0.001 |
ARIC: Atherosclerosis Risk In Communities; eGFR: estimated glomerular filtration rate; KPSC: Kaiser Permanente Southern California; LDL-C: low-density lipoprotein cholesterol; REGARDS: REasons for Geographic And Racial Differences in Stroke; SE: standard error.
Categories of LDL-C were defined based on quartiles from REGARDS participants.
Lipids and CHD incidence
Over 8.9 years of follow-up, 225 CHD events occurred in REGARDS, 6,547 in KPSC and 583 in ARIC (Supplemental Table 2). With the exception of LDL-C in REGARDS, less favorable lipid levels were associated with a higher age-race-sex adjusted incidence of CHD in each population (Table 3). LDL-C was not associated with CHD incidence after age, race and sex adjustment in REGARDS. For each lipid, the association with CHD incidence was stronger in ARIC as compared with REGARDS and KPSC.
Table 3.
Age, race and sex adjusted incidence rates for coronary heart disease associated with serum lipids among individuals from REGARDS, KPSC and ARIC (per 1,000 person-years).
| Category of serum lipids* | Trend | ||||
|---|---|---|---|---|---|
| 1 IR (95% CI) |
2 IR (95% CI) |
3 IR (95% CI) |
4 IR (95% CI) |
p-trend | |
| LDL-C, range (mg/dL) | ≤102 | 103 to 123 | 124 to 145 | ≥146 | -- |
| REGARDS | 3.65 (2.55–4.75) | 3.26 (2.22–4.31) | 4.09 (2.93–5.25) | 4.11 (2.89–5.33) | 0.41 |
| KPSC | 2.44 (2.29–2.60) | 2.31 (2.17–2.45) | 2.45 (2.32–2.59) | 3.12 (2.99–3.26) | <0.001 |
| ARIC | 3.21 (2.39–4.03) | 3.53 (2.69–4.36) | 4.57 (3.70–5.45) | 6.39 (5.61–7.18) | <0.001 |
| Non-HDL-C, range (mg/dL) | ≤122 | 123 to 146 | 147 to 172 | ≥173 | -- |
| REGARDS | 2.90 (1.97–3.83) | 3.50 (2.49–4.52) | 4.21 (3.07–5.34) | 4.55 (3.36–5.75) | 0.02 |
| KPSC | 2.12 (1.98–2.28) | 2.11 (1.98–2.25) | 2.46 (2.33–2.60) | 3.53 (3.39–3.67) | <0.001 |
| ARIC | 2.67 (1.92–3.43) | 3.57 (2.77–4.36) | 4.35 (3.56–5.15) | 6.90 (6.08–7.73) | <0.001 |
| HDL-C, range (mg/dL) | ≤41 | 42 to 51 | 52 to 63 | ≥64 | -- |
| REGARDS | 4.73 (3.54–5.92) | 3.85 (2.82–4.88) | 3.41 (2.34–4.49) | 2.83 (1.82–3.84) | 0.02 |
| KPSC | 4.31 (4.11–4.51) | 2.82 (2.69–2.97) | 2.16 (2.04–2.29) | 1.74 (1.62–1.87) | <0.001 |
| ARIC | 8.22 (7.13–9.32) | 5.14 (4.27–6.01) | 3.53 (2.79–4.26) | 2.11 (1.50–2.71) | <0.001 |
| TG, range (mg/dL) | ≤77 | 78 to 105 | 106 to 151 | ≥152 | -- |
| REGARDS | 2.18 (1.37–2.99) | 3.40 (2.35–4.46) | 4.02 (2.90–5.13) | 5.67 (4.24–7.11) | <0.001 |
| KPSC | 1.71 (1.59–1.83) | 2.18 (2.05–2.33) | 2.76 (2.62–2.91) | 3.82 (3.66–3.98) | <0.001 |
| ARIC | 2.77 (2.14–3.41) | 3.66 (2.90–4.42) | 5.49 (4.61–6.37) | 7.79 (6.68–8.91) | <0.001 |
| Total-to-HDL-C ratio, range | <3.12 | 3.12 to <3.90 | 3.90 to <4.90 | ≥4.90 | -- |
| REGARDS | 2.97 (1.97–3.97) | 3.12 (2.12–4.11) | 3.61 (2.55–4.67) | 5.28 (4.00–6.56) | 0.004 |
| KPSC | 1.72 (1.59–1.86) | 1.96 (1.83–2.09) | 2.58 (2.44–2.71) | 4.23 (4.05–4.41) | <0.001 |
| ARIC | 1.94 (1.32–2.56) | 2.62 (1.94–3.31) | 5.05 (4.20–5.91) | 7.94 (6.98–8.89) | <0.001 |
| TG-to-HDL-C ratio, range | <1.31 | 1.31 to <2.06 | 2.06 to <3.37 | ≥3.37 | -- |
| REGARDS | 2.07 (1.20–2.94) | 3.52 (2.44–4.60) | 3.78 (2.68–4.88) | 5.68 (4.26–7.10) | <0.001 |
| KPSC | 1.58 (1.47–1.70) | 2.15 (2.02–2.29) | 2.74 (2.60–2.89) | 4.10 (3.93–4.28) | <0.001 |
| ARIC | 2.11 (1.52–2.71) | 3.65 (2.88–4.42) | 5.27 (4.40–6.13) | 8.42 (7.28–9.55) | <0.001 |
ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; CI: confidence interval; HDL-C: high-density lipoprotein cholesterol; ICD-9: International Classification of Diseases, 9th version; ICD-10: International Classification of Diseases, 10th version; IR: incidence rate; KPSC: Kaiser Permanente Southern California; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; REGARDS: REasons for Geographic And Racial Differences in Stroke; TG: triglycerides.
Categories of serum lipids were defined based on quartiles from REGARDS participants.
CHD definition from REGARDS and ARIC includes definite or probable MI or CHD death. CHD definition for KPSC includes non-fatal MI (defined by an inpatient stay with a discharge ICD-9 diagnosis code of 410.× in any position) or CHD death (defined by an underlying cause of death ICD-10 code I20–I25).
Analysis from REGARDS includes 225 events from 9,578 participants. Analysis from KPSC is limited to 8.9 years of follow-up and includes 6,547 events from 346,595 members (6,163 events from 336,851 members for the analysis of LDL-C). Analysis from ARIC is limited to 8.9 years of follow-up and includes 583 events from 14,590 participants.
All analyses include adjustment for age (mean age=55 years old), race (50% blacks) and sex (50% males).
With the exception of LDL-C, HDL-C and total-to-HDL-C in REGARDS, less favorable lipid levels were associated with a higher multivariable adjusted HR for CHD in each study (Figure 2 and Supplemental Table 3). The association between each lipid category and incident CHD was stronger in ARIC as compared with REGARDS and KPSC. LDL-C ≥190 mg/dL was associated with an increased HR for incident CHD as compared with a LDL-C of ≤102 mg/dL in each study (Supplemental Table 4). The HR for incident CHD associated with LDL-C of 146 to 189 mg/dL was lower in REGARDS (HR 1.18; 95% CI 0.78–1.80) and KPSC (HR 1.38; 95% CI 1.27–1.49) as compared with ARIC (HR 1.76; 95% CI 1.30–2.38). A stronger association between each serum lipid and incident CHD was also present among ARIC participants as compared with REGARDS participants and KPSC members when serum lipids were analyzed as continuous variables (Supplemental Table 5) and using categories defined by ARIC quartile cut-points (Supplemental Table 6).
Figure 2.
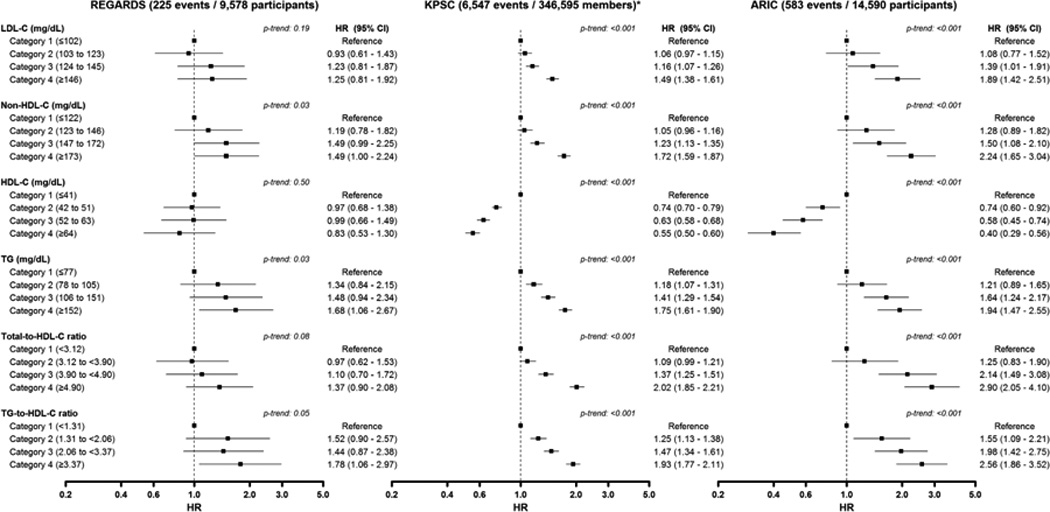
Forest-plot of hazard ratios for coronary heart disease by lipid categories in REGARDS, KPSC and ARIC. * 6,163 events from 336,851 members for the analysis of LDL-C. All analyses include adjustment by age, race, sex, education and income levels, alcohol consumption, current smoking, diabetes, reduced eGFR, stroke and use of antihypertensive medications. Analyses from REGARDS also include adjustments for region of residence. CHD definition from REGARDS and ARIC includes definite or probable MI or CHD death. CHD definition for KPSC includes non-fatal MI (defined by an inpatient stay with a discharge ICD-9 diagnosis code of 410.× in any position) or CHD death (defined by an underlying cause of death ICD-10 code I20–I25). Analyses in KPSC and ARIC are limited to 8.9 years of follow-up. Categories of serum lipids were defined based on quartiles from REGARDS study participants. ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; KPSC: Kaiser Permanente Southern California; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; REGARDS: REasons for Geographic And Racial Differences in Stroke; TG: triglycerides.
Discussion
In the current study, we contrasted the association of serum lipids with incident CHD using data collected before and after the publication of ATP III and the subsequent increase in the use of statins among US adults. Among participants not taking lipid-lowering medications in the ARIC study, which was conducted before the publication of the ATP III guidelines, less favorable lipid levels were associated with a large increase in risk for CHD. In contrast, the association between less favorable lipid levels and increased CHD risk was weaker among REGARDS study participants and KPSC members not taking lipid-lowering medications, two populations studied after publication of the ATP III guidelines. Also, ARIC participants with higher LDL-C were more likely to have concomitant CHD risk factors, but these associations were not present for REGARDS participants and KPSC members. These findings suggest the observed association between serum lipids and CHD in contemporary studies may be attenuated because of preferential use of lipid-lowering medications by high-risk individuals, representing a challenge for observational research on lipids and CHD.
Many previous observational studies have documented strong, graded associations between serum lipids and CHD risk.19–23 For example, the Emerging Risk Factors Collaboration, which used participant-level data from 8 observational studies and 44,243 participants, reported that a 33 mg/dL higher LDL-C was associated with 38% increased risk of CHD (HR 1.38; 95%CI 1.09–1.73).24 However, most of the data used in these studies were collected prior to the widespread use of statins. A series of randomized clinical trials showing a significant reduction of both LDL-C and CHD risk associated with the use of statins were published in the 1990s and early 2000s.25–31 As a consequence, LDL-C became a target for CHD risk reduction therapy and the use of statins rose substantially.2, 3
In 2001, the ATP III introduced risk stratification to help guide the initiation of statin therapy, emphasizing a patient’s global CHD risk in addition to LDL-C.1, 32 This recommendation resulted in the preferential use of statin therapy by individuals with high LDL-C or with low to moderate LDL-C but with other CHD risk factors. In a study by Aronow et al., 90.8% of statin users in Medicare and 70.8% of statin users in US commercial health plans had hypertension, diabetes, chronic kidney disease, CHD, angina, or a history of stroke or transient ischemic attack.33 In our analysis, there was a marked shift in the distribution of CHD risk factors across categories of LDL-C among individuals not taking lipid-lowering medications from before to after publication of the ATP III guidelines. This shift is consistent with the contemporary use of lipid-lowering medications by individuals with high CHD risk. ARIC study participants with higher levels of LDL-C were more likely to be male, have diabetes, reduced eGFR, and a history of stroke and use antihypertensive medications. In contrast, among REGARDS study participants and KPSC members, those with higher LDL-C levels were less likely to have diabetes or take antihypertensive medication.
Results from the present study should not be interpreted as evidence of a lack of a causal effect of serum lipids on incident CHD. Instead, our results suggest that the ATP III guidelines could have contributed to the attenuation of the association between serum lipids and CHD in contemporary populations. In ATP III, the recommendation to initiate lipid-lowering medications is based on both LDL-C levels and the presence of other CHD risk factors.1 Preferential initiation of lipid-lowering therapy in individuals with high LDL-C and/or high burden of CHD risk factors could lead to collider stratification bias in populations after the publication of ATP III when analyses are conducted conditioning on lipid-lowering medication use.34 Adults who are not taking lipid-lowering medication in contemporary populations have lower LDL-C compared with their counterparts before the publication of ATP III. Also, among adults with high LDL-C in contemporary populations, the average CHD risk will be lower than among individuals with equivalent LDL-C levels in earlier eras. Taken together, these changes can weaken the association between lipids and CHD in contemporary populations. An example of how ATP III may result in collider stratification bias was provided when studying the prevalence of diabetes by LDL-C levels in REGARDS and KPSC versus ARIC (i.e., diabetes was more common at higher LDL-C in ARIC but was more common at lower LDL-C in KPSC and REGARDS). While we adjusted for known CHD risk factors, unmeasured factors that are associated with lipid-lowering medication use may be present. A collider stratification bias was likely not present in populations before the publication of ATP III because the recommendation to initiate lipid-lowering medication in prior guidelines was primarily based on serum lipid levels.32 Although including individuals taking lipid-lowering medication in the analysis of contemporary populations could avoid collider stratification bias, the results using this approach would also be biased because lipid-lowering therapy modifies both serum lipid levels and CHD incidence. Another possible explanation for the attenuation of the association between serum lipids and incident CHD in contemporary populations is that individuals with high LDL-C who are untreated may be more likely to initiate statins during follow-up as compared with individuals before the publication of ATP III. An attenuation in the association between serum lipids and CHD may have important implications for modern studies. For instance, the association of new lipid markers with CHD risk may be obscured. This might also change the role of lipids as potential confounders in the association of other risk factors with CHD risk. Most importantly, there is a need to evaluate the validity of CHD and CVD risk prediction models that were developed using lipid data collected before ATP III was published.6
The current analysis has a number of strengths. ARIC and REGARDS were population-based studies, they included large sample sizes, with the collection of blood samples and a long active follow-up period for CHD events that were rigorously adjudicated following similar approaches in each study. Results in the contemporary era were replicated using data from KPSC, a large healthcare system with a high degree of generalizability.9 The current analysis also has potential and known limitations. We only analyzed a single study from the era prior to the publication of ATP III. Also, REGARDS, KPSC and ARIC had differences in their design and methods for data collection including the assay used to measure lipids which may explain differences in results across these studies. We were not able to investigate some relevant cardiovascular risk factors, including C-reactive protein and albuminuria, which could have changed among individuals not taking lipid-lowering medication from before to after publication of the ATP III guidelines as this information was not available for all three populations analyzed. In KPSC, information on fasting status for lipid panels at baseline was not available and incident CHD events were not adjudicated. Additionally, we did not analyze information on the initiation of statins following baseline.
Over 50 years of research including both observational studies and randomized trials demonstrate serum lipids levels are independent risk factors for CHD and that lipid-lowering medications, mainly statins, reduce this risk.19–23, 25–31 As a consequence, the use of statins among US adults has increased substantially over the last 15 years. The current analysis suggests that the preferential use of statins for primary prevention in high risk populations may obscure the association between serum lipids and CHD. Results from the current analysis need to be confirmed in future studies. Until more data become available, caution should be taken when interpreting the association between lipids and CHD risk in contemporary observational studies.
Supplementary Material
Clinical Perspectives.
Prior studies have shown that statins are effective in reducing coronary heart disease (CHD) incidence. The proportion of US adults taking statins increased after the publication of the 2001 Adult Treatment Panel (ATP III) cholesterol treatment guidelines which recommended statin therapy based on low-density lipoprotein cholesterol (LDL-C) levels and predicted CHD risk. We compared the characteristics of adults not taking lipid-lowering medications by level of LDL-C and the association between serum lipids and CHD using data from populations before (Atherosclerosis Risk In Communities [ARIC]) and after (Kaiser Permanente Southern California [KPSC], and REasons for Geographic And Racial Differences in Stroke [REGARDS]) the publication of ATP III. The prevalence of diabetes, history of stroke and antihypertensive medication use increased at higher levels of LDL-C in ARIC but not in REGARDS or KPSC. These findings were consistent with a preferential use of statins by high risk individuals or individuals with high LDL-C in the contemporary era. Less favorable levels of each lipid analyzed (LDL-C, high-density lipoprotein cholesterol [HDL-C], non-HDL-C, triglycerides, total-to-HDL-C and triglycerides-to-HDL-C) were associated with an increased risk for CHD in ARIC but these associations were attenuated in REGARDS and KPSC. Results from our study suggest that the preferential use of statins by individuals with high CHD risk in the contemporary era may induce a bias when analyzing the association between serum lipids and CHD. This bias may have important implications for future studies of lipids and CHD risk as well as for CHD risk prediction.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Funding Sources: This study was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke and R01-HL80477 and K24- HL111154 from the National, Heart, Lung and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Disclosures: LDC was supported by Fulbright Scholarship. VB has received research support from Amgen Inc., Astra Zeneca, Bayer Healthcare, Janssen, Pfizer, and Sanofi-Aventis, and has served on advisory panels for Amgen Inc. and Eli Lilly. KR and SFD have received research support from Merck & Co. EBL, RSR, STK, MMS and PM have received grant support from Amgen Inc.
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Levitan EB, Brown TM, Sharma P, Zhao H, Bittner V, Glasser S, Kilgore M, Yun H, Woolley JM, Farkouh ME, Rosenson RS. Trends in the prevalence, awareness, treatment and control of high low density lipoprotein-cholesterol among United States adults from 1999–2000 through 2009–2010. Am J Cardiol. 2013;112:664–670. doi: 10.1016/j.amjcard.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 5.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 7.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 8.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 19.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 20.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 22.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 23.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 24.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 26.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 27.MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 28.Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 29.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 31.Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talwalkar PG, Sreenivas CG, Gulati A, Baxi H. Journey in guidelines for lipid management: From adult treatment panel (ATP)-I to ATP-III and what to expect in ATP-IV. Indian J Endocrinol Metab. 2013;17:628–635. doi: 10.4103/2230-8210.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronow HD, Dutro MP, Kuznik A, Cwengros J. Prevalence of cardiovascular comorbidities and utilization of evidence-based treatment strategies among statin users in a Medicare and commercial health plan. Curr Med Res Opin. 2009;25:205–213. doi: 10.1185/03007990802611828. [DOI] [PubMed] [Google Scholar]
- 34.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, Poole C. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


