Abstract
Background
Hand, foot and mouth disease mostly affects children and carries a substantial disease burden in the Western Pacific region. Enterovirus 71 (EV71) is the most virulent causative agent, and a monovalent vaccine against EV71 will soon become commercially available in China. An improved understanding of EV71 epidemiology could aid policy decisions regarding childhood immunization in China.
Objective
We aimed to assess and summarize information to date from individual seroepidemiologic studies of EV71 in mainland China in order to determine patterns of the age-specific risk of infection.
Methods
A systematic review and meta-analysis of studies of children aged 0–15 years, published in English or Chinese, was conducted. Estimates of seroprevalence were summarized by age group. A mixed-effects regression model was used to explore factors co-varying with EV71 seroprevalence.
Results
We identified 42 published studies, including 15 in English. We found that an average of 78% of neonates were seropositive to EV71 infection but such maternally conferred immunity almost completely waned by 5 months. The seroprevalence of EV71 antibody increased directly with age among pre-school children, from 26% (95% CI, 18–33%) at 1 year to 70% (95% CI, 62–78%) at 5 years. Age of subjects, sample size, sampling year, sampling method, geographic latitude and publication language were associated with variations of individual seroprevalence estimates.
Conclusions
Seroprevalence of EV71 antibody gradually declined during the first five months in infants. Infection with EV71 was most likely to occur between 2 and 4 years. Our findings may be useful in informing population-based EV71 vaccination strategies.
Keywords: enterovirus, seroprevalence, children, meta-analysis
INTRODUCTION
Hand, foot and mouth disease (HFMD) is a childhood disease causing substantial disease burden, particularly in Western Pacific Region [1–4]. Although HFMD is mainly a self-limited disease, severe complications may occur including brain-stem encephalitis, acute flaccid paralysis and aseptic meningitis [5, 6]. From 2008 through 2012, 7.2 million cases of HFMD were reported in mainland China, 2,457 were fatal [7]. Several serotypes of human enterovirus, including enterovirus 71 (EV71), Coxsackievirus A6 and A16 (CV-A6 and CV-A16), are considered to be common causative pathogens of HFMD [6]. In severe and fatal HFMD cases, EV71 is more commonly identified as the likely causative enterovirus type compared to the mild HFMD cases where CV-A16 was more commonly identified [6, 8, 9]. EV71 is therefore thought to be the most virulent serotype.
HFMD has been the most frequently reported notifiable infectious diseases and led to the largest number of deaths in children among pediatric notifiable diseases in mainland China since 2009 [10]. Severe EV71 cases develop to complications within 2 to 3 days after initially showed mild symptoms and the median time from diagnosis to death was 0.5 days for severe HFMD cases which were mostly caused by EV71 [10–14]. It is therefore necessary to develop a cost-effective vaccine particularly to prevent severe infections since currently no specific treatment is available for HFMD [6, 11]. Five manufactures from mainland China, Taiwan and Singapore have developed inactivated EV71 vaccines [11–15]. All three vaccines from mainland China were developed by the use of EV71 subgenotype C4a strains, while the Taiwanese and Singaporean vaccine used B4 and B2 strain, respectively [11, 12]. Phase III clinical trials have been finished on the three vaccines developed in mainland China. They all showed high efficacy (90.9–97.4%) in protecting against EV71-associated HFMD, and will likely be commercially available to the public soon [13–15]. Questions of the target population and immunization schedule are major considerations for Chinese policy makers when determining the potential impact and cost-effectiveness of population-based EV71 vaccination strategies [11].
The age-specific seroprevalence of EV71 antibody, estimated in the absence of vaccine, can provide a population profile of the risk of infection with the virus in different ages, contributing to decision making on vaccination implementation strategies. A number of serologic studies have been conducted in mainland China in different settings with various study designs. The objective of our study was to evaluate and summarize the information from these studies to guide vaccination policy decisions for mainland China. As part of our analysis, we explored potential factors that might affect the estimates of seroprevalence.
METHODS
Search Strategy
We searched for published studies on the prevalence of antibody against EV71 virus in children in mainland China. We searched PubMed for publications in any language, and the China National Knowledge Infrastructure (CNKI) and the Wanfang (WF) databases for publications in Chinese. The following keywords were used in the English literature search: (“hand foot and mouth disease” OR “HFMD” OR “enterovirus 71” OR “EV71” OR “HEV71”) AND (“seroprevalence” OR “seroprevalent” OR “seronegative” OR “seropositive” OR “seroepidemiology” OR “seroepidemiological” OR “serologic” OR “serological” OR “antibody”) AND (“China” OR “Chinese”). We used Chinese translations of the key words (Appendix Table 1) to search the Chinese databases.
Study Selection
We screened in the title, abstract and full text of all articles obtained from the database search. We only included original studies investigating the seroprevalence of EV71 antibody in recovery phase in children ≤15 years of age in mainland China. We excluded studies recruiting clinical HFMD patients only and EV71 vaccine trials that did not provide pre-vaccined seroprevalence data [16, 17].
Data extraction
We extracted relevant information from each study onto a standardized form including the sampling period, sampling method, geographic location, age of subjects, assay used for antibody detection, study endpoint, threshold to define seropositivity, and the estimates of seroprevalence in different age groups. Seroprevalence is defined as the proportion of children testing positive for EV71 serum antibody among all children providing blood samples. Seropositivity was pre-defined in studies, and the endpoint and cut-off for determination of seroposivity was summarized for each study. Potential heterogeneity associated with varied definitions for seropositivity was further explored in later analyses. We estimated the 95% confidence interval (95% CI) of seroprevalence estimates using the Wilson method [18, 19].
Risk of bias
The study was conducted following the Preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (Appendix Table 2) [20]. Sampling methods, assay methods and the publication language of each study were used as proxies of risk of bias for individual studies [21]. Effects of the potential bias on seroprevalence estimates were examined in the meta-regression analysis.
Statistical analysis
Random-effects model was used to summarize the pooled mean of seroprevalence in each subgroup [22]. Cochran’s Q test and the I2 statistic were used to identify and quantify heterogeneity among included studies [20, 21]. An I2 value more than 75% indicates high heterogeneity [24]. A linear mixed-effects meta-regression model was used to investigate the effect of potential factors on the estimate of seroprevalence by weighting the estimates with the inverse of their variances. An omnibus test was used for the moderator test in the mixed-effects model [25]. The potential factors that were examined in the model included: language of publication, age of subjects, year of the study, season of sampling, sampling method, sample size, laboratory assay used for antibody detection and latitude of the study location. We divided China into two main epidemic regions – North and South – by the latitude of around 35°N according to previous epidemiological studies [7]. We defined the HFMD season according to the location of a study, i.e. the season of HFMD for studies conducted in northern China was from April to July while it was from April to October for studies in southern China, since HFMD occurs in the autumn in the south in addition to the main peak in the spring/summer observed both in the north and south of the country. We classified the sampling method used in each study as either (1) “random sampling”, referring to studies using a random or stratified random sampling method for selection of subjects; (2) “physical examination”, referring to studies using residual sera that were originally collected for the purpose of medical assessments; or (3) “trial cohort”, referring to studies using baseline sera collected from children participating in randomized controlled trials of EV71 vaccines. All analyses were conducted in R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
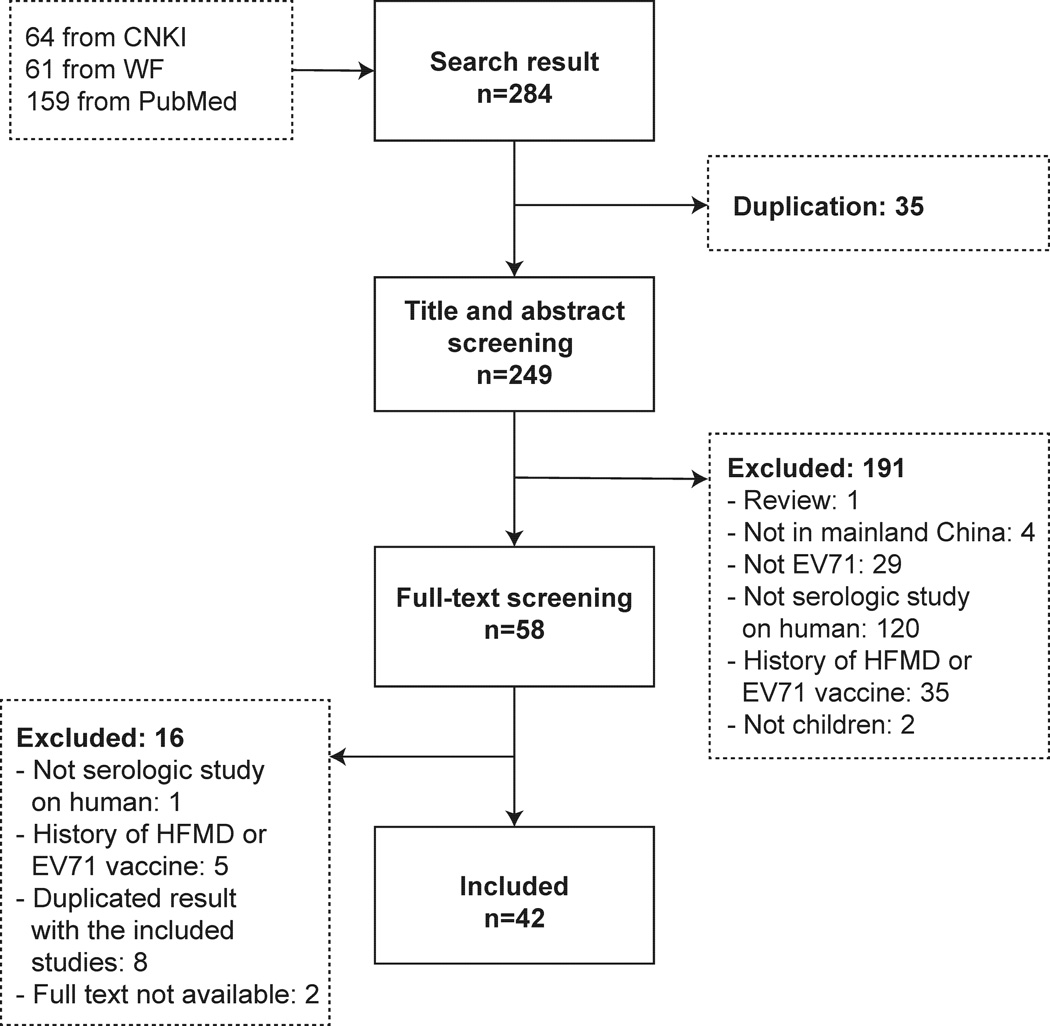
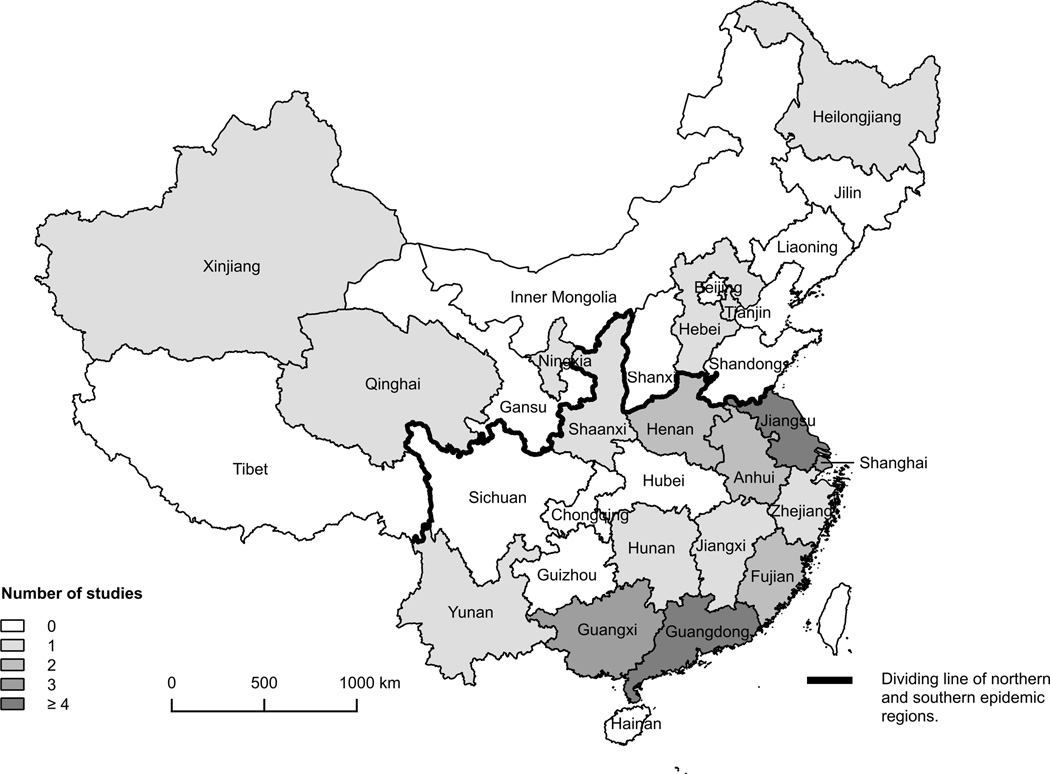
We identified 42 articles that met the eligibility criteria [12, 13, 16, 17, 23–60], 15 studies in English and 27 in Chinese (Figure 1). Studies were conducted in 18 of the 31 provinces in mainland China, mostly (34/42) in the east and south (Figure 2). One study was conducted across 6 provinces [34], and we extracted separate data for each of the 6 provinces. Guangdong and Jiangsu were the most frequently studied provinces with 12 and 11 reports respectively. Table 1 summarizes the characteristics and methods used in each study.
Figure 1. Flowchart of study selection.
Figure 2. Number of studies included in mainland China.
The Study that collected sera from more than one province counted separately for each involved province.
Table 1.
Detailed information on the 42 included studies.
| Reference | Province | Location | Sampling year |
Sampling period |
Sampling method | Age range | Sample size |
Assay method |
Endpoint | Positive threshold |
Language |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bai Y. 2012 | Ningxia | Yinchuan | 2011 | Unknown | Physical examination | 0–65 yrs | 204 | ELISA | OD450, OD680 | 0.1+ ODc | CHN |
| Cai M. 2013 | Guangdong | Shenzhen | 2012 | Unknown | Unknown | 0–62 yrs | 240 | ELISA | OD450 | 0.16 | CHN |
| Chen R. 2013 | Guangdong | Futian | 2011–12 | Unknown | Random sampling | all ages | 471 | NTA | 50% CPE | 1:256 | CHN |
| Chen X. 2012 | Guangxi | Mengshan | 2010 | Aug | Random sampling | 0–15 yrs | 519 | NTA | Unknown | 1:8 | CHN |
| Chen X. 2013 | Jiangsu | Donghai | 2010 | Aug | Random sampling | 0–15 yrs | 420 | NTA | Unknown | 1:8 | CHN |
| Deng H. 2012 | Shaanxi | Xi’an | 2010 | Sep | Unknown | 1–4 yrs | 312 | ELISA | OD450 | Unknown | CHN |
| Ding Q. 2011 | Jiangsu | Ganyu | 2010 | Aug | Random sampling | 0–15 yrs | 400 | NTA | 50% CPE | 1:8 | CHN |
| Guo R. 2011 | Hebei | Handan | 2009 | Unknown | Random sampling | 0–15 yrs | 856 | ELISA | OD450 | Unknown | CHN |
| Guo X. 2009 | National | Unkown | 2005 | Sep | Random sampling | 1–6 yrs | 371 | NTA | 50% CPE | 1:8 | CHN |
| Hou H. 2012 | Guangdong | Futian | 2010 | Jan–Jun | Physical examination | 0–28 yrs | 436 | ELISA | OD450 | Unknown | CHN |
| Hu Y. 2013 | Jiangsu | Jurong | 2012–13 | Jan12–Mar13 | Random sampling | 0.5–5 yrs | 1400 | NTA | 50% CPE | 1:8 | ENG |
| Huang X. 2010 | Henan | Zhengzhou, Kaifeng, Nanyang, Anyang, Sanmenxia |
2010 | Jan–Feb | Unknown | 0–12 yrs | 103 | NTA | No CPE | 1:8 | CHN |
| Ji H. 2012 | Jiangsu | Ganyu, Donghai | 2010 | Aug | Random sampling | 0–15 yrs | 840 | NTA | 50% CPE | 1:8 | ENG |
| Kuang L. 2011 | Guangdong | Guangzhou | 2010 | Jan–Mar | Physical examination | 0–14 yrs | 819 | ELISA | OD450, OD630 | Amax(YOUDEN index) | CHN |
| Li J. 2011 | Tianjin | Tianjin | 2009–10 | Jan09–Nov10 | Random sampling | 0–50 yrs | 1611 | NTA | 50% CPE | 1:4 | CHN |
| Li J. 2012 | Guangdong | Longgang | 2012 | Jun | Unknown | 0–5 yrs | 528 | ELISA | OD450 | 0.1+ ODc | CHN |
| Li W. 2013a* | Guangdong | Guangdong | 2007–09 | Unknown | Random sampling | 0–9 yrs | 715 | NTA | 50% CPE | 1:8 | ENG |
| Li W. 2013b* | Guangdong | Guangdong | 2010 | Jan, Aug | Random sampling | all ages | 707 | NTA | 50% CPE | 1:8 | ENG |
| Li Y. 2012 | Guangxi | Mengshan | 2010 | Dec10–Jun11 | Trial cohort | 0.5–11 yrs 18–49 yrs |
168 | NTA | 50% CPE | 1:8 | ENG |
| Li Z. 2013 | Guangxi | Lipu | 2010 | Aug | Random sampling | 0–15 yrs | 445 | NTA | 50% CPE | 1:8 | CHN |
| Liao Y. 2012 | Fujian | Longyan | 2006, 2009 | Unknown | Random sampling | all ages | 250 | NTA | No CPE | 1:8 | CHN |
| Lin X. 2007 | Guangdong | Shantou | 2001 | Unknown | Physical examination | 0–90 yrs | 380 | NTA | No CPE | 1:10 | CHN |
| Liu F. 2013 | Guangdong | Longgang | 2011–12 | Jan–Jun | Physical examination | 0–6 yrs | 464 | ELISA | OD450 | Unknown | CHN |
| Mao Q. 2009 | Henan | Kaifeng | 2004 | Unknown | Unknown | 0.5–2.5 yrs | 349 | NTA | Unknown | 1:8 | CHN |
| Mao Q. 2010 | Jiangsu | Jiangsu | 2007–09 | Sep07–Jul09 | Physical examination | 2, 7 mons | 133 | NTA | 50% CPE | 1:8 | ENG |
| Ni H. 2012 | Zhejiang | Cixi | 2011 | Apr | Unknown | all ages | 258 | NTA | 50% CPE | 1:8 | ENG |
| Xiong Y. 2013 | Jiangxi | Nanchang | 2010 | Jan–Feb | Random sampling | all ages | 1144 | NTA | 50% CPE | 1:8 | CHN |
| Xu M. 2012 | Shanghai | Shanghai | 2010–11 | Jul10–Jan11 | Physical examination | children | 164 | NTA | 50% CPE | 1:8 | CHN |
| Xu W. 2013 | Jiangsu | Changzhou | 2006 | Unknown | Unknown | 1–5 yrs | 252 | NTA | 50% CPE | 1:8 | CHN |
| Yang X. 2010 | Fujian | Fujian | 2006, 2009 | Unknown | Random sampling | 0–68 yrs | 2374 | NTA | 50% CPE /No CPE |
1:8/1:4 | CHN |
| Yu H. 2011 | Anhui | Luan | 2005–08, 2010 |
Dec05–Mar08; Mar–Apr10 |
Unknown | 0–15 yrs | 555 | NTA | 50% CPE | 1:8 | ENG |
| Zeng M. 2012 | Shanghai | Shanghai | 2010–11 | Nov10–Apr11 | Physical examination | 0–5 yrs | 614 | NTA | 50% CPE | 1:8 | ENG |
| Zhang D. 2011 | Guangdong | Futian | 2011 | Unknown | Random sampling | 0–59 yrs | 382 | ELISA | OD450 | 0.16 | CHN |
| Zhao S. 2011 | Qinghai | Xining | 2009 | May-Aug | Unknown | 1–6 yrs | 181 | NTA | 50% CPE | 1:8 | CHN |
| Zhou S. 2007 | Guangdong | Shenzhen | 1999–2003 | Unknown | Unknown | all ages | 584 | ELISA | OD450 | Unknown | CHN |
| Zhu F. 2012a* | Jiangsu | Jiangsu | 2007–09 | Nov07–Aug09 | Random sampling | 2, 7, 12 mons | 975 | NTA | 50% CPE | 1:8 | ENG |
| Zhu F. 2012b* | Jiangsu | Donghai | 2011 | Mar–Jun | Trial cohort | 0.5–5 yrs | 332 | NTA | 50% CPE | 1:8 | ENG |
| Zhu F. 2013c* | Jiangsu | Donghai | 2011 | Aug–Sep | Trial cohort | 0.5–3 yrs | 1106 | NTA | 50% CPE | 1:8 | ENG |
| Zhu F. 2013d* | Jiangsu | Donghai, Pizhou, Baoying |
2012–13 | Jan12–Mar13 | Trial cohort | 0.5–3 yrs | 1219 | NTA | 50% CPE | 1:8 | ENG |
| Zhu F. 2014e* | Jiangsu | Ganyu, Taixing, Sheyang |
2012 | Jan | Trial cohort | 0.5–3 yrs | 1150 | NTA | 50% CPE | 1:8 | ENG |
| Zhu W. 2013 | Shanghai | Shanghai | 2011 | Jul–Aug | Physical examination | 0–8 yrs | 93 | NTA | 50% CPE | 1:8 | CHN |
| Zhu Z. 2010 | Anhui, Guangdong, Heilongjian, Hunan, Xinjiang and Yunnan |
Anhui, Guangdong, Heilongjiang, Hunan, Xinjiang and Yunnan |
2005 | Aug | Random sampling | 0–5 yrs | 900 | NTA | 50% CPE | 1:8 | ENG |
Although serum samples were collected between 1999 and 2013, most were from 2010 (15 studies) or 2011 (10 studies). Ten studies, eight in Chinese, failed to describe their sampling methods explicitly. Neutralization assays (NTA) were used by most of the studies (32/42). Among studies using NTA, twenty-four studies set the endpoint value as the titer inhibiting 50% of cytopathogenic effect (CPE) and defined the positive cut-off as antibody titer ≥1:8. All ten studies applying the Enzyme-linked Immunosorbent Assay (ELISA) to detect EV71 antibody used the optical value density at 450nm (OD450), while the cut-off for seropositivity was not reported in most (8/10) studies.
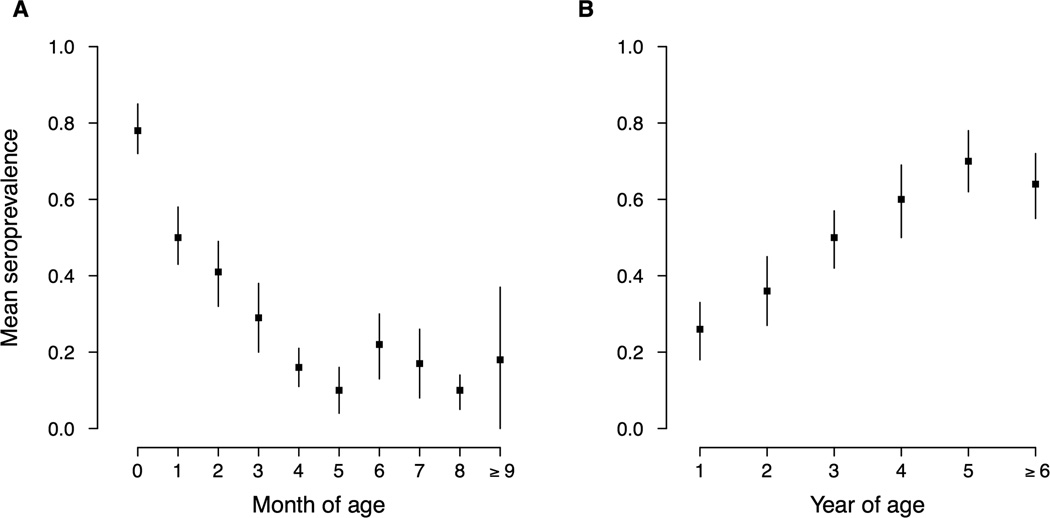
Seroprevalence among infants 0–12 months of age was reported in 23 studies. 9 reported seroprevalence at individual months of age, which mainly focused on infants aged 8 months and younger (Appendix Figure 1). In general, seroprevalence declined with age in younger infants, from 78% (95% CI, 72–85%) at birth to 10% (95% CI, 4–16%) at five months of age, and fluctuated at a generally low level (10–22%) from 6 months through 1 year of age (Figure 3, Appendix Figure 1). Limited estimates of individual month groups were available among infants ≥ 9 months, estimates based on studies on a broader age group however revealed a similarly low level of seroprevalence (Appendix Figure 1). Weighting seroprevalence estimates by population sizes of provinces involved into these studies did not substantially change the pooled estimates of seroprevalence (data not shown). Seroprevalence estimates among infants were largely consistent across different studies within each one-month group (I2 statistics range: 0–63.6%, p>0.05) except for the 2 and 7 months groups (I2 statistics: 76.1% and 89.3% respectively, p<0.01).
Figure 3. Mean seroprevalence of EV71 antibody among infants and children.
Panel A: Mean seroprevalence of EV71 antibody among infants. Panel B: Mean seroprevalence of EV71 antibody among children. Estimates are the pooled mean seroprevalence derived from random-effects models for each age group.
We identified 108 estimates of seroprevalence for children of 1–15 years from 21 studies reporting seroprevalence for individual ages (Figure 3, Appendix Figure 2). The seroprevalence of EV71 antibody directly increased with age among pre-school children, from 26% (95% CI, 18–33%) at 1 year to 70% (95% CI, 62–78%) at 5 years. Among children ≥6 years of age, declining seroprevalence with age was reported in one study [57]. There was substantial heterogeneity in estimates of seroprevalence within each age group (I2 statistics range: 70.1–94.6%, p<0.001). The results of a sensitivity analysis, weighting the estimates of seroprevalence with the population size of the underlying province, were similar with unweighted estimates (results not shown).
We conducted a meta-regression analysis on the seroprevalence of EV71 antibody reported for children 1–5 years. 76% of the heterogeneity can be explained by our model (Table 2). Except for the type of laboratory assays and the season of study period, factors included in the meta-regression model substantially affected estimates of seroprevalence (Ommibus test: p <0.001). After accounting for potential factors and biases, the estimates of seroprevalence still positively correlate with age (Table 2). Studies conducted in larger epidemic years or in the north of China were more likely to report higher estimates of seroprevalence. Sampling methods may introduce bias in estimation of seroprevalence as studies using non-random sampling methods tended to report lower estimates of seroprevalence compared with studies using randomly sampled sera (Table 2). “Trial cohort” was not included in the final meta-regression model since estimates from the vaccine clinical trials were all by grouped ages. Published language is another potential source of bias, noting studies published in English-language journals were likely to report lower estimates compared with those published in Chinese journals (coefficient: −0.133, 95%CI (−0.213, −0.053), p < 0.05).
Table 2.
Meta-regression of factors correlated with seroprevalence among children 1–5 years of age.
| Characteristic | Number of estimates |
β coefficient | 95% CI |
|---|---|---|---|
| Total | 98 | 0.002 | (0.001, 0.003)** |
| Age | |||
| 1 year | 25 | ref | |
| 2 years | 21 | 0.083 | (0.002, 0.164)* |
| 3 years | 20 | 0.226 | (0.142, 0.310)** |
| 4 years | 20 | 0.312 | (0.230, 0.395)** |
| 5 years | 12 | 0.386 | (0.285, 0.487)** |
| Sampling year | |||
| 2008 or before | 23 | ref | |
| 2009 | 15 | −0.176 | (−0.315, −0.037)* |
| 2010 | 42 | 0.186 | (0.012, 0.360)* |
| 2011 or after | 18 | 0.261 | (0.092, 0.431)** |
| Sampling method | |||
| Random sampling | 62 | ref | |
| Physical examination | 9 | −0.437 | (−0.573, −0.302)** |
| Unknown | 27 | −0.227 | (−0.310, −0.145)** |
| Assay method | |||
| ELISA | 18 | ref | |
| NTA | 80 | 0.067 | (−0.030, 0.164) |
| Seasonality | |||
| Season | 46 | ref | |
| Non-season | 17 | −0.045 | (−0.152, 0.062) |
| Unknown | 35 | 0.082 | (−0.061, 0.225) |
| Epidemic region | |||
| North | 10 | ref | |
| South | 84 | −0.203 | (−0.376, −0.029)* |
| Published language | |||
| Chinese | 60 | ref | |
| English | 38 | −0.133 | (−0.213, −0.053)** |
p < 0.05;
p < 0.01.
I2 = 79.9% (95%CI, 72.0–85.9%; p < 0.001). QM (df=15) = 240.80; p < 0.001.
DISCUSSION
We were able to identify important patterns in the risk of infection by age and geography. Our meta-analysis showed that, on average, 78% of neonates in China investigated were seropositive (Figure 3, Appendix Figure 1), somewhat higher than reported in studies conducted in Viet Nam, Thailand and Singapore (ranging from 44–67%) [61–63], suggesting a high level of maternal antibody against EV71 infection in China, perhaps due to higher historical incidence of infections among adults [17, 29, 41, 64]. In general, the titer and duration of maternal immunity of infants are associated with the level of mother’s immunity [32]. Maternal antibody waned at about 5 months, which is comparable with the waning time of maternal immunity to EV71 in other countries and regions and to maternal immunity to CV-A16 [65, 66]. The age pattern of seroprevalence to EV71 in infants observed in our study was consistent with the age distribution of confirmed HFMD cases infected with EV71 reported through national surveillance. During 2008–2012, cases under 6 months only accounted for around 2% of all reported EV71 cases, while 15% of reported EV71 cases were 6–11 months of age [7].
The mean seroprevalence of EV71 antibody increased from 24% at 1 year of age to 70% at 5 years of age, indicating a substantial incidence of infection (Figure 3, Appendix Figure 1). Studies in other regions also revealed similar trends suggesting that preschool children are at a high risk of infection with EV71 [61, 62, 67, 68]. Notifiable disease surveillance data in China showed that about 50% of probable and confirmed EV71-associated HFMD cases occur in children 2 to 4 years of age [7]. One factor possibly driving this high risk of EV71 infection in young children is admission to kindergarten at around 3 years of age in China. In children 6 to 15 years of age, EV71 seroprevalence decreased with age, although this observation is based on estimates from just one study [57]. Previous findings as well as the estimates reported in our included studies of broader age groups (Appendix Figure 3) show that seroprevalence tends to fluctuate after 6 years of age at a generally high level [61, 67], suggesting the possibility of the presence of a long-lasting immunity. However, the geometric mean titer (GMT) of EV71 antibody wanes after peaking at around 5 years of age [32, 44, 46, 50, 56]. The relation between the titer level and protection against re-infection is unknown [69], so immunity to EV71 infection in older children is not clear at present [11].
Substantial heterogeneity was not detected in estimates of seroprevalence among infants except for those at age of 2 and 7 months (Appendix Figure 1). We noticed that one study with a relatively large sample size conducted by Zhu et al. [26] reported very high estimates of seroprevalence for children at age of 2 and 7 months. It might lead to an overestimated mean seroprevalence in the 7-month group, which however is not likely to substantially affect our main findings. Relatively high heterogeneity was observed in estimates for 1–5 years while most variations can be explained by variables included in the meta-regression model. The unaccounted variability may be due to some unmeasured factors in studies, e.g. possible variations in timing of specimen collection, storage of sera or choice of virus strains in laboratory testing.
In our study, EV71 seroprevalence in China and in larger epidemic years was higher than estimates from other Asian areas (ranging from 27.8% to 60.9%) [61, 63, 67, 70]. Our analysis revealed an increasing trend in seroprevalence with a more recent year of sampling except for the year of 2009, which is consistent with the causative pathogens in China HFMD epidemics. Similar findings were also reported in Japan. It has not been determined whether the seroprevalence of EV71 antibody is a reliable predictor for the HFMD incidence in the future [71, 72]. Studies conducted in northern China reported a higher EV71 seroprevalence although the southern areas tend to have longer periods of EV71 activity with two epidemics in some years [7]. However several large outbreaks of HFMD have been reported in northern China recently [73, 74]. Russia, a high-latitude country, also reported a relatively higher seroprevalence (83% in children 5 years) but with lower HFMD incidence in a recent study [73]. Although higher environmental temperatures and humidity are associated with the higher HFMD incidence in China [75–77], HFMD occurrence is likely to be driven by a range of factors not limited to environmental conditions, such as population structure, birth rates, urbanization and the prevalent circulating strains [63, 75, 78]. Due to limited data, we were not able to further compare the age-specific risk of EV71 infection between northern and southern China.
The analysis results (Table 2) suggest that biases in seroprevalence estimates could be introduced by sampling methods and language of publication. Studies using residual sera originally collected for physical examination were likely to report lower estimates compared with those studies using random sampling because some “physical examination” studies reported that sera from subjects who reported previous history of HFMD or fever were excluded from the analysis and therefore less likely to be infected by EV71. Higher estimates of seroprevalence of EV71 antibody were reported in studies published in Chinese-language journals suggesting the possibility of selective reporting by local literature. Similar local literature bias was suggested in a previous study for 13 different diseases [80]. Laboratory testing assays were not suggested to be a factor partially explaining the heterogeneity across the studies, which is consistent with a previous study on IgM antibody against EV71 [80].
Our study shows that infants become susceptible to EV71 infection after 5 months of age, and a substantial fraction of infections occur between 2 and 4 years of age. Therefore, it was proposed that the forthcoming 2-dose EV71 vaccination should be given to children at 6 and 7 month of age respectively with a 4-week interval between two dosages [12]. A booster dose might be given at ages of 18–24 months [12] given the undetermined duration of vaccine derived immunity and protective antibody [12–14]. Vaccine-derived EV71 antibody titer experienced a slightly decrease in the first 6–7 months after injection of the second dose (two-dose vaccines) and stayed relatively stable in the following 6 months [13, 15]. Currently we lack information on the long-term duration of vaccine derived EV71 antibody which however tends to be shorter than the natural immunity for other vaccine-preventable disease [83]. Further evidence is needed to determine the optimal time period for a booster dose to avoid a potential window of EV71 susceptibility. Population at high risk of EV71 infection may shift to older age groups after implementation of childhood EV71 mass vaccination because of waning of the vaccine-derived immunity and declined risk of infection in whole population. Low incidence of EV71 infection in post-vaccination period would also decrease the natural immunity in women of childbearing age, which as a result would lead to a lower level and possibly shorter duration of maternal immunity in neonates and infants [32, 84, 85].
There are some limitations of our study. First, estimates included in our analysis were mainly from the east and south of China which are more densely populated and economically developed and may not represent the population in other areas of China. Second, we found relatively high heterogeneity among the seroprevalence estimates which could not be entirely explained in the meta-regression (Table 2). There are likely to be other factors affecting seroprevalence that were not considered in this review.
CONCLUSION
Data from 42 published studies of the seroprevalence of EV71 antibody in Chinese children suggested that younger infants are likely to be protected by maternal antibody against EV71 infection and become susceptible to the infection since 5 months of age, and that EV71 infection is more likely to occur in young children at 2–4 years of age. Incidence of infection was relatively higher in the north of China compared to the south. EV71 vaccines should be administered in the window between 5 months and 1 year of age.
Supplementary Material
Estimates are ordered by the year of sampling and the estimate of seroprevalence.
Estimates are ordered by the year of sampling and the estimate of seroprevalence.
Estimates are ordered by age and the estimate of seroprevalence.
ACKNOWLEDGMENTS
The authors thank Vicky Fang for technical support.
FUNDING
This project was supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), and a commissioned grant from the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
GML has received speaker honoraria from Hongkong and Shanghai Banking Corporation (HSBC) and Credit Lyonnais Securities Asia (CLSA). BJC has received research funding from MedImmune Inc. and Sanofi Pasteur, and consults for Crucell NV.
Footnotes
CONFLICTS OF INTEREST
The authors report no other potential conflicts of interest.
REFERENCES
- 1.Lee MS, Lin TY, Chiang PS, et al. An investigation of epidemic enterovirus 71 infection in Taiwan, 2008: clinical, virologic, and serologic features. Pediatr Infect Dis J. 2010;29:1030–1034. doi: 10.1097/INF.0b013e3181e52945. [DOI] [PubMed] [Google Scholar]
- 2.Chan LG, Parashar UD, Lye MS, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis. 2000;31:678–683. doi: 10.1086/314032. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu H, Utama A, Yoshii K, et al. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997–1998. Jpn J Infect Dis. 1999;52:12–15. [PubMed] [Google Scholar]
- 4.Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–e1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch Dis Child. 1980;55:583–588. doi: 10.1136/adc.55.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 7.Xing W, Liao Q, Viboud C, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. 2014;14:308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardosa MJ, Krishnan S, Tio PH, Perera D, Wong SC. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet. 1999;354:987–991. doi: 10.1016/S0140-6736(98)11032-2. [DOI] [PubMed] [Google Scholar]
- 9.Hsueh C, Jung SM, Shih SR, et al. Acute encephalomyelitis during an outbreak of enterovirus type 71 infection in Taiwan: report of an autopsy case with pathologic, immunofluorescence, and molecular studies. Mod Pathol. 2000;13:1200–1205. doi: 10.1038/modpathol.3880222. [DOI] [PubMed] [Google Scholar]
- 10.National Health and Family Planning Commission of the PRC. National notifiable disease situation in 2014. [Accessed May 9 2015]; Available at: http://www.nhfpc.gov.cn.
- 11.Li L, Yin H, An Z, Feng Z. Considerations for developing an immunization strategy with enterovirus 71 vaccine. Vaccine. 2015;33:1107–1112. doi: 10.1016/j.vaccine.2014.10.081. [DOI] [PubMed] [Google Scholar]
- 12.Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis. 2015;60:797–803. doi: 10.1093/cid/ciu852. [DOI] [PubMed] [Google Scholar]
- 13.Zhu FC, Meng FY, Li JX, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–2032. doi: 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–837. doi: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 15.Zhu F, Xu W, Xia J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–828. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 16.Huang XY, Liu GH, Chen HM, et al. Seroepidemiological study of enterovirus 71 in Henan province. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi. 2010;5:617–639. [Google Scholar]
- 17.Xu MH. Fudan University. Shanghai: Fudan University; 2012. Pathogen composition causing hand foot and mouth disease (HFMD) and seroepidemiology of enterovirus 71 infection in Shanghai, China. Vol. Master. [Google Scholar]
- 18.Wilson EB. Probable inference, the law of succession, and statistical inference. Journal of the American Statistical Association. 1927;22:209–212. [Google Scholar]
- 19.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychological methods. 1998;3:486–504. [Google Scholar]
- 23.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 26.Zhu FC, Liang ZL, Meng FY, et al. Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PLoS One. 2012;7:e37206. doi: 10.1371/journal.pone.0037206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo XB, Zhu SL, Wang DY. Seroepidemiology investigation of HEV71 in healthy children of 1–6 years old in three counties of China in 2005. Zhongguo Yi Miao He Mian Yi. 2009;15:141–144. [PubMed] [Google Scholar]
- 28.Xu WG, Wang YL. Seroepidemiological study of Enterovirus 71 infection in children aged 1 through 6 years in Changzhou in 2006. Shi Yong Yu Fang Yi Xue. 2013;20:179–180. [Google Scholar]
- 29.Mao QY, Yang ZW, Yu X, et al. Epidemic tendency of neutralizing antibody against Enterovirus 71 and Coxsackievirus A 16 in infants in rural area of Kaifeng City, Henan Province, China. Zhongguo Sheng Wu Zhi Pin Xue Za Zhi. 2009;22:911–922. [Google Scholar]
- 30.Zhou SL, Li LL, He YQ. Serological epidemiology investigation of Enterovirus type 71 infection in Shenzhen. Re Dai Yi Xue Za Zhi. 2007;7:66–67. [Google Scholar]
- 31.Deng HL, Ma SW, Mi B, et al. Survey on Enterovirus subclinical infection of healthy children in Xi'an. Yi Xue Lin Chuang Yan Jiu. 2012;29:204–208. [Google Scholar]
- 32.Mao QY, Liao XY, Yu X, et al. Dynamic change of mother-source neutralizing antibodies against enterovirus 71 and coxsackievirus A16 in infants. Chin Med J (Engl) 2010;123:1679–1684. [PubMed] [Google Scholar]
- 33.Ni H, Yi B, Yin J, et al. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Ningbo, China, 2008–2011. J Clin Virol. 2012;54:342–348. doi: 10.1016/j.jcv.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Zhu FC, Liang ZL, Li XL, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2013;381:1037–1045. doi: 10.1016/S0140-6736(12)61764-4. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Wang M, Chang H, et al. Prevalence of antibodies against enterovirus 71 in children from Lu'an City in Central China. Jpn J Infect Dis. 2011;64:528–532. [PubMed] [Google Scholar]
- 36.Zhu FC, Wang JZ, Li XL, et al. Reactogenicity and immunogenicity of an enterovirus 71 vaccine in Chinese healthy children and infants. Pediatr Infect Dis J. 2012;31:1158–1165. doi: 10.1097/INF.0b013e31826eba74. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Zhu S, Guo X, et al. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J. 2010;7:300. doi: 10.1186/1743-422X-7-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YP, Liang ZL, Gao Q, et al. Safety and immunogenicity of a novel human Enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine. 2012;30:3295–3303. doi: 10.1016/j.vaccine.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Zeng M, El Khatib NF, Tu S, et al. Seroepidemiology of Enterovirus 71 infection prior to the 2011 season in children in Shanghai. J Clin Virol. 2012;53:285–289. doi: 10.1016/j.jcv.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Yi L, Su J, et al. Seroepidemiology of human enterovirus71 and coxsackievirusA16 among children in Guangdong province, China. BMC Infect Dis. 2013;13:322. doi: 10.1186/1471-2334-13-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji H, Li L, Liu Y, et al. Seroepidemiology of human enterovirus71 and coxsackievirusA16 in Jiangsu province, China. Virol J. 2012;9:248. doi: 10.1186/1743-422X-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Yi L, Su J, et al. Seroprevalence of human enterovirus 71 and coxsackievirus A16 in Guangdong, China, in pre- and post-2010 HFMD epidemic period. PLoS One. 2013;8:e80515. doi: 10.1371/journal.pone.0080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai YS, Yang AN, Zhang J, Sun WW, Wang J, Guo AL. Survey on prevalence of EV71 and ECHO infection among population received physical examination in Yinchuan City. Ningxia Yi Ke Da Xue Xue Bao. 2012;34:453–455. [Google Scholar]
- 44.Ding Q, Zhu FC, Li L, Li DH, Liu YM. Serum epidemiology survey of HEV 71 among children in Ganyu County. Jiangsu Yu Fang Yi Xue. 2011;22:4–6. [Google Scholar]
- 45.Hou HB, Zhou HT, Zeng HS, Chen RL. IgG antibody levels in healthy people of Futian District of Shenzhen City. Zhi Ye Yu Jian Kang. 2012;28:3107–3108. [Google Scholar]
- 46.Liu FR, Liu Q, Li G, Li JM, Ye WX, Ye BL. Seroepidemiology study on EV71 infection among healthy children in Longgang District, Shenzhen City. Hua Nan Yu Fang Yi Xue. 2013;39:40–46. [Google Scholar]
- 47.Liao YH, Chen QJ, Liao LH, Cao CY, Yang XH, Luo ZF. Seroepidemiology study on Enterovirus 71 in Longyan City. Hai Xia Yu Fang Yi Xue Za Zhi. 2012;18:28–30. [Google Scholar]
- 48.Zhang DX, Yang F, Wang B, et al. Subclinical infection of hand-foot-mouth disease in Shenzhen City. Zhongguo Re Dai Yi Xue. 2011;11:1332–1333. [Google Scholar]
- 49.Zhu W, Ju LW, Jiang LF, Shen HG, Wang QL, Jiang QW. Serum levels of antibody against enterovirus 71 in healthy children at Shanghai in 2011. Zhonghua Chuan Ran Bing Za Zhi. 2013;31:650–653. [Google Scholar]
- 50.Li JM, Xu YJ, Wang QY. Investigation of enterovirus 71 antibody levels among children in the Longgang District, City of Shenzhen. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi. 2012;7:924–926. [Google Scholar]
- 51.Li JM, Zhang Y, Gao L, et al. Seroepidemiology investigation of neutralizing antibody against enterovirus 71 among healthy people in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:568–570. [PubMed] [Google Scholar]
- 52.Yang XH, Yan YS. Cause of HFMD epidemic caused by HEV71 in Fujian Province: seroepidemiology and molecular epidemiology of HEV71. 10th Conference on Epidemiology in Eastern China; Hefei, China. [Google Scholar]
- 53.Xiong Y, Gong T, Shi Y, et al. Seroepidemiology investigation on HEV71 in the population in Nanchang in early 2010. Xian Dai Yu Fang Yi Xue. 2013;40:7–10. [Google Scholar]
- 54.Cai MS, Ren Y. Investigation on HFMD recessive infection in healthy population in some community of Shenzhen in 2012. Zhongguo Wei Sheng Jian Yan. 2013;23:2355–2357. [Google Scholar]
- 55.Zhao SC, Zhang SJ, Yue JN, Ma YC, Jiang SY. Seroepidemiologic investigation of HEV71 among children in Xining city. Zhongguo Gong Gong Wei Sheng. 2011;27:361–362. [Google Scholar]
- 56.Kuang L, Wang CB, Liang ZF, zhong JY, Xiao MS, Zhu B. Investigation on enterovirus 71 antibody levels among children in Guangzhou area. Zhongguo Xun Zheng Er Ke Za Zhi. 2011;6:211–214. [Google Scholar]
- 57.Guo RL, Deng J, Li Yx, Ma Yx. Serum epidemiological study of HFMD in Handan, 2009. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi. 2011;6:848–850. [Google Scholar]
- 58.Chen XL, Wen SQ, Wei D. Survey on level of antibody against EV71 and Cox A 16 among children aged 0 to 15 in Mengshan County. Yi Yao Qian Yan. 2012;2:115–116. [Google Scholar]
- 59.Chen XQ, Zhang ZY, Pang HY. Monitoring and analysis of the levels of neutralizing antibody against enterovirus 71 in individuals under the age of 15 in Donghai County. Xian Dai Yu Fang Yi Xue. 2013;40:3875–3877. [Google Scholar]
- 60.Lin XW, Xie RN, Zeng C, Zheng YY. Investigation of Human Enterovirus 71 infection among clinical cases and healthy people in Chaoshan District. Yi Xue Jian Yan Yu Lin Chuang. 2007;18:66–67. [Google Scholar]
- 61.Chen RL, Pan RY, Lai ZF, Mo HL, Xhang Y, Zhou HT. The microneutralization assay for the detection of neutralizing antibodies against EV71 and CV A 16 among healthy population. Zhongguo Yu Fang Yi Xue Za Zhi. 2013;14:362–365. [Google Scholar]
- 62.Li ZF, Liu TX, Yang GP, Xia xT. Seroepidemiological investigation on enterovirus 71 among children in Lipu County, Guangxi in 2010. Ying Yong Yu Fang Yi Xue. 2013;19:281–283. [Google Scholar]
- 63.Hu YM, Wang X, Wang JZ, et al. Immunogenicity, safety, and lot consistency of a novel inactivated enterovirus 71 vaccine in Chinese children aged 6 to 59 months. Clin Vaccine Immunol. 2013;20:1805–1811. doi: 10.1128/CVI.00491-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002;8:995–997. doi: 10.3201/eid0809.10.3201/eid0809.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tran CB, Nguyen HT, Phan HT, et al. The seroprevalence and seroincidence of enterovirus71 infection in infants and children in Ho Chi Minh City, Viet Nam. PLoS One. 2011;6:e21116. doi: 10.1371/journal.pone.0021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linsuwanon P, Puenpa J, Huang SW, et al. Epidemiology and seroepidemiology of human enterovirus 71 among Thai populations. J Biomed Sci. 2014;21:16. doi: 10.1186/1423-0127-21-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo ST, Chiang PS, Chao AS, et al. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis. 2009;15:581–584. doi: 10.3201/1504.081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ang LW, Phoon MC, Wu Y, Cutter J, James L, Chow VT. The changing seroepidemiology of enterovirus 71 infection among children and adolescents in Singapore. BMC Infect Dis. 2011;11:270. doi: 10.1186/1471-2334-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diedrich S, Weinbrecht A, Schreier E. Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch Virol. 2009;154:1139–1142. doi: 10.1007/s00705-009-0413-x. [DOI] [PubMed] [Google Scholar]
- 70.Rabenau HF, Richter M, Doerr HW. Hand, foot and mouth disease: seroprevalence of Coxsackie A16 and Enterovirus 71 in Germany. Med Microbiol Immunol. 2010;199:45–51. doi: 10.1007/s00430-009-0133-6. [DOI] [PubMed] [Google Scholar]
- 71.Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88. doi: 10.1542/peds.109.6.e88. [DOI] [PubMed] [Google Scholar]
- 72.Lu CY, Lee CY, Kao CL, et al. Incidence and case-fatality rates resulting from the 1998 enterovirus 71 outbreak in Taiwan. J Med Virol. 2002;67:217–223. doi: 10.1002/jmv.2210. [DOI] [PubMed] [Google Scholar]
- 73.Akhmadishina LV, Eremeeva TP, Trotsenko OE, Ivanova OE, Mikhailov MI, Lukashev AN. Seroepidemiology and molecular epidemiology of enterovirus 71 in Russia. PLoS One. 2014;9:e97404. doi: 10.1371/journal.pone.0097404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Tan XJ, Wang HY, et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol. 2009;44:262–267. doi: 10.1016/j.jcv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Zhu Z, Yang W, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu M, Li Z, Wang J, et al. Determinants of the incidence of hand, foot and mouth disease in China using geographically weighted regression models. PLoS One. 2012;7:e38978. doi: 10.1371/journal.pone.0038978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng H, Duan G, Zhang R, Zhang W. Time series analysis of hand-foot-mouth disease hospitalization in Zhengzhou: establishment of forecasting models using climate variables as predictors. PLoS One. 2014;9:e87916. doi: 10.1371/journal.pone.0087916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng T, Huang Y, Yu S, et al. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PLoS One. 2013;8:e56943. doi: 10.1371/journal.pone.0056943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao LX, Wu B, Bao WX, et al. Epidemiology of hand, foot, and mouth disease and genotype characterization of Enterovirus 71 in Jiangsu, China. J Clin Virol. 2010;49:100–104. doi: 10.1016/j.jcv.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Pan Z, Trikalinos TA, Kavvoura FK, Lau J, Ioannidis JP. Local literature bias in genetic epidemiology: an empirical evaluation of the Chinese literature. PLoS Med. 2005;2:e334. doi: 10.1371/journal.pmed.0020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu F, Yan Q, Wang H, et al. Performance of detecting IgM antibodies against enterovirus 71 for early diagnosis. PLoS One. 2010;5:e11388. doi: 10.1371/journal.pone.0011388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang Z, Mao Q, Gao Q, et al. Establishing China's national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine. 2011;29:9668–9674. doi: 10.1016/j.vaccine.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 83.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24:S58–S61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- 84.Waaijenborg S, Hahne SJ, Mollema L, et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Infect Dis. 2013;208:10–16. doi: 10.1093/infdis/jit143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimates are ordered by the year of sampling and the estimate of seroprevalence.
Estimates are ordered by the year of sampling and the estimate of seroprevalence.
Estimates are ordered by age and the estimate of seroprevalence.