Abstract
Age-related changes in reproductive hormone levels are a well-known risk factor for the development of cognitive dysfunction and dementia in women. We and others have shown an important contribution of gonadotropins in this process. Lowering serum gonadotropin levels is able to rescue cognitive function in Alzheimer’s disease and menopause models, but whether this is time-dependent and the exact mechanism through which gonadotropins regulate cognitive function is unknown. We show that pharmacologically lowering serum levels of luteinizing hormone lead to cognitive improvement immediately after ovariectomy and with a 4 month interval after ovariectomy, when the benefits of 17β-estradiol are known to disappear in rodents. Importantly, we show that these improvements are associated with spine density changes at both time points. These findings suggest a role of luteinizing hormone in learning and memory and neuroplasticity processes as well as provide an alternative therapeutic strategy of menopause associated cognitive loss.
Keywords: luteinizing hormone, spine density, ovariectomy, cognition, estrogen, aging, timing, menopause
1. Introduction
Hormonal changes due to aging, such as the loss of estrogens and the corresponding increase in serum luteinizing hormone (LH), are associated with cognitive decline. Historically, emphasis has been placed on understanding the mechanism underlying CNS dysfunction after estrogen loss at menopause. This is based on the recognized effects of 17β-estradiol (E2) on neuronal structure and function (Woolley and McEwen, 1992) and dysfunction after ovariectomy (OVX) at a neuronal (Woolley and McEwen, 1992; Waters et al. 2009; Tanapat et al., 1999) and functional level (reviewed McEwen et al. 2012). E2 treatment after OVX rescues cognitive function and associated mechanisms in mice and rats (Heikkenin et al., 2004, McLaughlin et al., 2008, Daniel et al., 2006). Additionally, E2 has benefits in humans, such as improving cognition in younger women who have undergone oophorectomy (Sherwin, 1988). However, introducing a long delay between OVX and treatment onset renders E2 ineffective in rodents at restoring cognition (Gibbs 2000, Heikkenin et al., 2004, Daniel et al., 2006) and rescuing spine density (McLaughlin et al. 2008). The differential effects of E2 have led to the critical period hypothesis (Daniel and Bohacek 2010) that E2 replacement has an ideal time frame of effectiveness in rodents. Associated with this theory is the healthy cell bias hypothesis which states that E2 is beneficial during health, but is detrimental in the diseased or stressed state, such as that of aging (Brinton 2008). Unfortunately, the underlying mechanisms remain unknown.
Independent of the ability of estrogens to regulate cognitive and neuronal function, increasing data support a role for LH on cognition. In this regard, serum LH levels are higher in Alzheimer’s disease (AD) patients (Short et al. 2001; Butchart et al., 2012), are associated with increased AD pathology in men (Verdile et al. 2008; Verdile et al., 2014) and cognitive dysfunction in older women (Rodrigues et al. 2008). In rodents, pharmacologically lowering LH levels with leuprolide acetate, a GnRHR super-agonist, is as effective as E2 replacement at improving cognitive function in ovariectomized mice (Bryan et al., 2010) as well as in AD models (Casadesus et al., 2006, Palm et al. 2014). Benefits of reducing serum LH levels on cognition have also been shown using the classic GnRHR antagonists such as antide in male and female rats (Ziegler et al. 2010, McConnell et al. 2012) and cetrorelix in rats and mice (Telegdy et al., 2009; Telegdy et al., 2010). Given the different mechanism of action of these drugs, but common end result (i.e. lowering LH levels), this suggests that peripheral LH rather than GnRHR signaling is associated with cognitive benefits. Importantly, a clinical trial in female AD patients revealed that in a subgroup taking acetylcholinesterase inhibitors, leuprolide acetate slows the progression of AD (Bowen et al., 2015).
In order to address whether pharmacologically lowering serum LH holds similar time dependent effects to that of E2 replacement on cognition and dendritic spine density as shown in previous studies in rodents (Gibbs 2000, Heikkenin et al., 2004, Daniel et al., 2006, McLaughlin et al. 2008) we determined: 1) whether leuprolide acetate rescues cognition after OVX both where E2 is effective (immediately after OVX) and ineffective (4 months after OVX) in middle-aged post-partus female mice and 2) whether changes in cognition observed in leuprolide acetate treated animals are associated with changes in spine density morphology as related to those observed for E2 replacement at both time points.
2. Methods
2.1 Animals and experimental timeline
C57Bl/6J retired breeders, 9 months of age, were purchased from Jackson Laboratory (Bar Harbor, Maine). Animals were group-housed in a humidity and temperature controlled room with a 12-hour light:dark cycle starting at 6AM. Throughout the duration of the study all animals were supplied with food and water ad libitum. Animals were allowed to acclimate to the animal facility for one week before any experimental manipulations. All experimental procedures and animal care was done in accordance with the Institutional Animal Care and Use Committee of Case Western Reserve University.
2.2 Experimental design
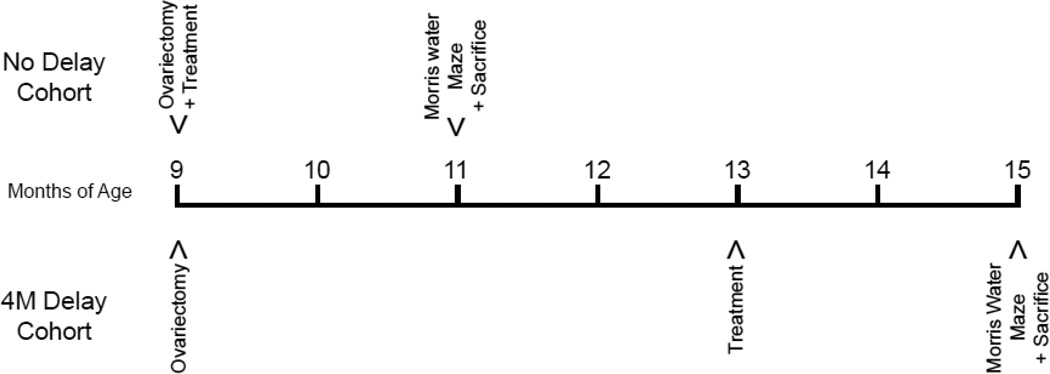
Animals were ovariectomized at 9 months of age and were either treated right after OVX or treated 4 months after OVX. All animals were treated for 8 consecutive weeks. During the last week of treatment, spatial learning and memory was assessed with the Morris water maze task, which occurred at 11 months of age for the no delay cohort and 15 months of age for the 4 month delay cohort (Figure 1).
Figure 1. Schematic diagram for OVX, treatments and Morris water maze.
The no delay cohort was sham operated or ovariectomized at 9 months of age and immediately treated with saline, E2 or leuprolide acetate. After 8 weeks of treatment the no delay cohort was trained on the Morris water maze behavior paradigm. Animals were sacrificed on the last day of the Morris water maze. The delay cohort was sham operated or ovariectomized at 9 months of age, but no treatments were given on the day of surgery. Four months after surgery, the delay cohort began treatment with saline, E2 or leuprolide acetate. After 8 weeks of treatment the delay cohort underwent Morris water maze testing and on the final day the animals were sacrificed.
2.3 Ovariectomy and pump implant
Mice were deeply anesthetized and ovaries were either removed or exposed and reinserted (SHAM) bilaterally though a single incision. The no delay cohort was subcutaneously implanted with either physiological saline, E2 (1.1ng 17β-estradiol/day) or leuprolide acetate (3.6µg leuprolide acetate/day) in an osmotic pump (Alzet 1004, Durect Corperation, Cupertino, California) on the day of OVX (no delay) or after a 4 month delay between OVX and treatment onset (4 month treatment delay). It is known that serum E2 levels peak at 66pg/ml during estrous (Wood et al., 2007; Fata et al., 2001), and we have previously shown that this treatment regimen produces physiological levels of E2 in the bloodstream (12–85pg/ml, Palm et al., 2014). Both no delay and 4 month treatment delay cohorts underwent pump replacements monthly for the duration of the study. All procedures were performed in an aseptic environment.
2.4 Morris water maze
Mice were habituated to handling daily for two weeks prior to training on the Morris water maze (MWM) task. A 110 cm diameter pool in the center of a room with visual cues on each wall was filled with white opaque water maintained at 23°C and a submerged 10.5cm diameter platform was covered by 0.5cm of water. On the first day each animal was placed in the water and guided to the platform in order to acclimate the animal to swimming and the apparatus. During training, animals were placed gently into the water facing the wall of the pool from each quadrant and allowed to swim either until they found the platform or until 60 seconds elapsed, in which case they were gently guided to the platform and given 15 seconds on it prior to the start of the next trial. After 4 consecutive trials animals were dried and placed in the home cage on a heating pad until the start of the next session. Each animal was trained in 2 sessions of 4 trials each per day for 4 days. On the last trial of day 4 the platform was removed for a probe trial. During this trial, time spent in the target quadrant was recorded for one 60 second trial to determine spatial strategy use and hippocampal memory consolidation. All trials were tracked and analyzed using Ethovision 7.0 (Noldus, Leesburg, Virginia). Animals unable to swim for the entirety of the behavioral testing were excluded from analyses.
2.5 Serum Collection and Tissue Processing
On conclusion of behavioral testing, all animals were deeply anesthetized, weighed, and blood was collected by either cardiac puncture or via the orbital sinus. Blood was allowed to coagulate and after centrifugation the serum was decanted and stored at −20°C until it was shipped for analysis. Serum LH assays were performed by the Ligand Assay and Analysis Core at the University of Virginia. After blood collection, half of the animals were transcardially perfused with saline, then 4% paraformaldehyde before the brains were removed and bisected. One hemisphere was post-fixed in 4% paraformaldehyde for an additional hour, incubated in 30% sucrose overnight and then stored at −20°C in 30%glycerol 30%sucrose 0.2%NaN3. The other hemisphere was post-fixed in 4% paraformaldehyde for 4 hours, incubated in 30% sucrose overnight, and then frozen in tissue freezing medium for storage at −80°C. The uterine weight was taken for all animals at the end of the study in order to verify OVX status.
2.6 Spine Density
Hemispheres stored in 30%glycerol 30%sucrose 0.2%NaN3 were thawed, cut coronally at 250µm on a vibratome, and placed in PBS in a 12-well plate. A Helios gene gun was used with 1.6 µm gold particles to deliver lipophilic dialkylcarbocyanine (Staffend and Meisel, 2011) at 200 psi. The apical dendrite of pyramidal neurons residing in retrosplenial cortex and cingulate cortex layer II/III was imaged with a Zeiss LSM 510 Meta equipped with a motorized stage. Three dimensional reconstructions were made from z-stacks in Neuron studio (CNIC, Mount Sinai School of Medicine) and were used to trace apical dendrites, then perform Scholl analysis to classify and quantify spines 5 −15 um from the soma (Rodriguez et al., 2008). 3–5 mice were used for each treatment group and multiple neurons were quantified for each animal.
2.7 Statistic Analysis
All statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, Illinois). Statistical outliers were detected by using the Grubb’s outlier test. For the MWM task a repeated-measures ANOVA was used with the latency to the platform for training days as the within group and the treatment as between groups. The area under the curve (AUC) for latency during training was used to illustrate treatment effects (Heneka et al. 2013; D’Agostino et al. 2012; Walter et al. 2011). Duration in the target quadrant and spine density data were analyzed with a one-way ANOVA using post-hoc analysis where appropriate. Effect size is described by Cohen’s d for pairwise comparisons and eta squared for all ANOVA statistics where η2=SSeffect/SStotal.
3. Results
As expected a high serum LH level confirmed OVX status and a reduced serum LH level confirmed E2 and leuprolide acetate efficacy (Table 1). A two-way ANOVA was used to analyze serum LH levels and showed that there was no interaction between timing and treatment (F(3,73)=0.562; p>0.05; η2=0.004), but a significant difference was found with treatment (F(3,73)=101.89; p<0.001; η2=0.81). Serum LH levels were increased by OVX (no delay: p<0.01; d=2.98, 4M delay: p<0.01; d=3.42) and rescued to basal levels with leuprolide acetate (no delay: p>0.05; d=−0.81, 4M delay: p>0.05; d=−1.18) and E2 treatments (no delay: p>0.05; d=−0.95, 4M delay: p>0.05; d=−1.25) compared to SHAM in both no delay (F(3,35)=42.91; p<0.001; η2=0.80) and delay (F(3, 37)=60.21; p<0.001; η2=0.84) cohorts.
Table 1. Leuprolide acetate decreases serum LH levels after OVX.
Serum LH levels were analyzed by the UVA Center for Research in Reproduction Ligand Assay and Analysis Core. In both the no delay cohort and the 4 month treatment delay (4M Delay) cohort an increase in serum LH levels was observed with OVX. Treatment with E2 or leuprolide acetate (LA) lowered serum LH levels. Results are expressed as mean ± sem.
| Serum LH (ng/mL) |
No Delay | 4M Delay |
|---|---|---|
| SHAM+SAL | 0.31±0.12 | 0.52±0.15 |
| OVX+SAL | 3.29±0.44 | 4.01±0.47 |
| OVX+E2 | 0.06±0.02 | 0.04±0.00 |
| OVX+LA | 0.10±0.03 | 0.07±0.01 |
3.1 Leuprolide acetate rescues spatial memory after OVX regardless of onset of treatment
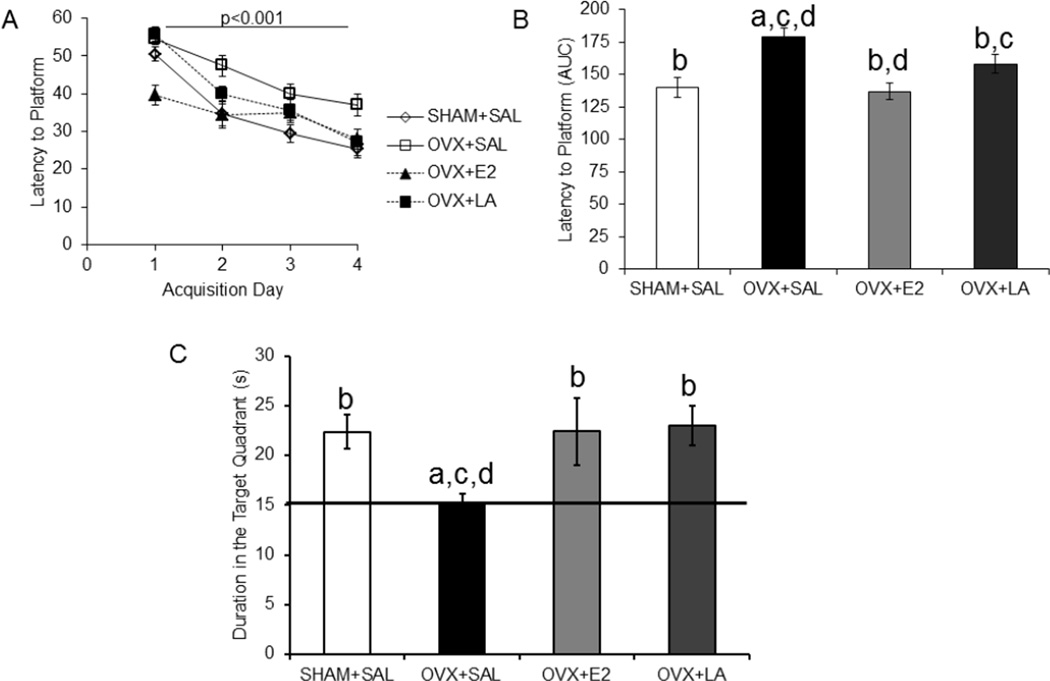
In the no delay cohort a repeated-measures ANOVA for escape latency, the time to find the hidden platform, showed a significant difference for the within subject factor (day of training) that illustrates the animals were successfully trained (F(3,108)=60.46; p<0.001; η2 = 0.58; Figure 2A). There was also a significant interaction between day and treatment (F(9,108)=2.72; p<0.01; η2 = 0.08). Post-hoc analysis indicated that ovariectomized animals took longer to locate the platform than SHAM (p<0.001; d=−1.71) and that this deficit was rescued by both E2 (p<0.01; d=−1.97) and leuprolide acetate (p<0.05; d=−0.95) treatments. Area under the curve (AUC) for latencies, which represents difference between treatment groups, is also shown (Figure 2B). In the probe trial there was a difference between treatment groups as shown by a one-way ANOVA (F(3,39)=3.46; p<0.05; η2=0.22). Post-hoc analysis showed that ovariectomized animals spent less time in the target quadrant than SHAM (p<0.05; d=1.62) and that this aspect was rescued by E2 (p<0.05; d=1.01) and leuprolide acetate (p<0.05; d=1.59) treatments (Figure 2C).
Figure 2. Leuprolide acetate rescues deficits on the Morris water maze task with no delay of treatment after OVX.
In the no delay cohort, where treatment began on the day of OVX, OVX-associated cognitive dysfunction in the Morris water maze was rescued by 8 weeks of treatment with leuprolide acetate or E2. A: Animals were successfully trained over a four day period. B: Bar graphs represent area under the curve (AUC) for accumulative latency to the platform during training. Leuprolide acetate and E2 had a lower latency to the platform (repeated measure ANOVA). C: The duration the animal spent in the target quadrant was used to analyze the sixty second probe trial, where the platform was removed. E2 and leuprolide acetate treated animals spent a higher percentage of time in the target quadrant as compared to the ovariectomized group (one-way ANOVA). Results are expressed as mean ± sem. Significant difference (p<0.05) from SHAM+SAL (a), from OVX+SAL (b), from OVX+LA (c) and from OVX+E2 (d)
SHAM+SAL, sham ovariectomy treated with saline; OVX+SAL, ovariectomy treated with saline; OVX+E2, ovariectomy treated with 17β-estradiol; OVX+LA, ovariectomy treated with leuprolide acetate
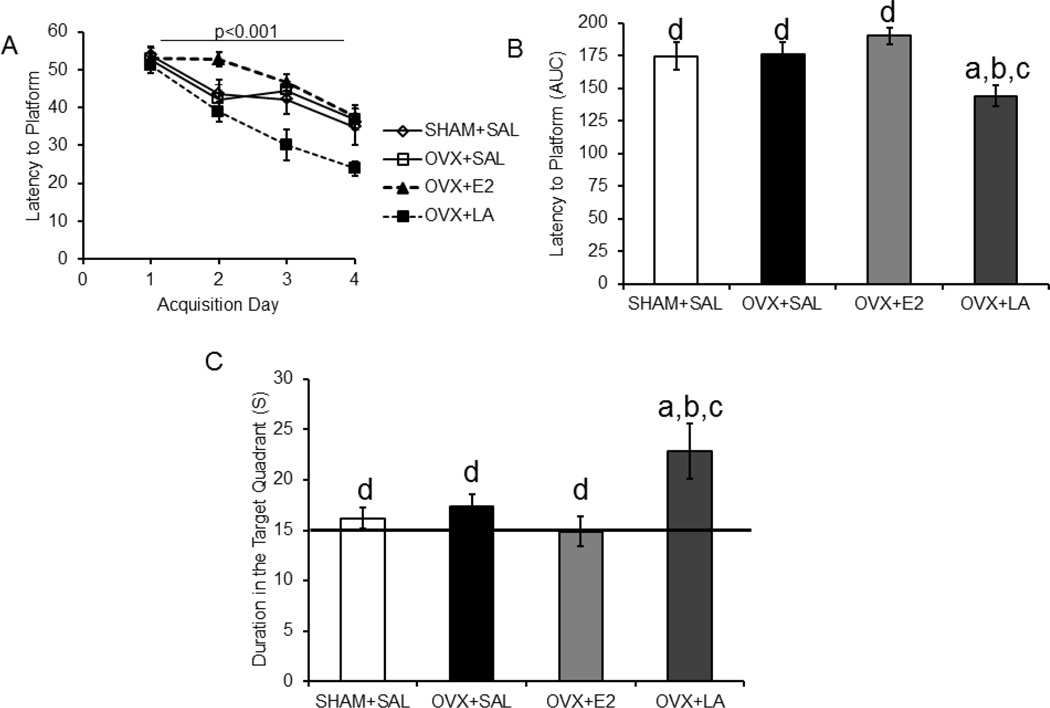
Morris water maze performance was also analyzed in the 4 month treatment delay cohort. Repeated-measures ANOVA analysis revealed a significant difference between treatment groups for latency to platform during training (F(3,30)=4.46; p<0.05; η2=0.30. Successful training was indicated by a significant difference in the within subjects factor (day of training) (F(3,90)=39.38, p<0.001; η2=0.52; Figure 3A). AUC for latency to the platform is shown to illustrate between group differences (Figure 3B). Post-hoc analysis revealed that the leuprolide acetate group took less time to locate the platform than any other group (SHAM: p<0.05; d=1.04, OVX: p<0.05; d=1.37, E2: p<0.05; d=2.27), but there was no difference between OVX and SHAM (p>0.05; d=−0.06). For the probe trial, one-way ANOVA analysis revealed a difference between treatment groups (F(3,34)=4.17; p<0.05; η2=0.29; Figure 3C). Post-hoc analysis demonstrated that the leuprolide acetate-treated mice spent more time in the target quadrant than any other group (SHAM: p<0.05; d=−1.16, OVX: p<0.05; d=−0.91, E2: p<0.05; d=−1.27, Figure 3C).
Figure 3. Leuprolide acetate rescues spatial learning and memory with a delay of 4 months after ovariectomy.
The 4 month delay cohort, who had a 4 month delay between OVX and treatment, showed improvements in the Morris water maze task only in the group treated for 8 weeks with leuprolide acetate. A: Similarly to the no delay cohort, animals were trained for 4 days. B: The leuprolide acetate group had a lower latency to the platform during training (repeated measures ANOVA). The bar graph represents the AUC for the latency to the platform for the training trials. C: The duration in the target quadrant during the probe trial showed a significant difference between treatment groups (one-way ANOVA), with the leuprolide acetate treatment spending more time in the target quadrant than any other group. Results are expressed as mean ± sem. Significant difference (p<0.05) from SHAM+SAL (a), from OVX+SAL (b), from OVX+LA (c) and from OVX+E2 (d) SHAM+SAL, sham ovariectomy treated with saline; OVX+SAL, ovariectomy treated with saline; OVX+E2, ovariectomy treated with 17β-estradiol; OVX+LA, ovariectomy treated with leuprolide acetate
3.2 LA treatment rescues spine density after OVX
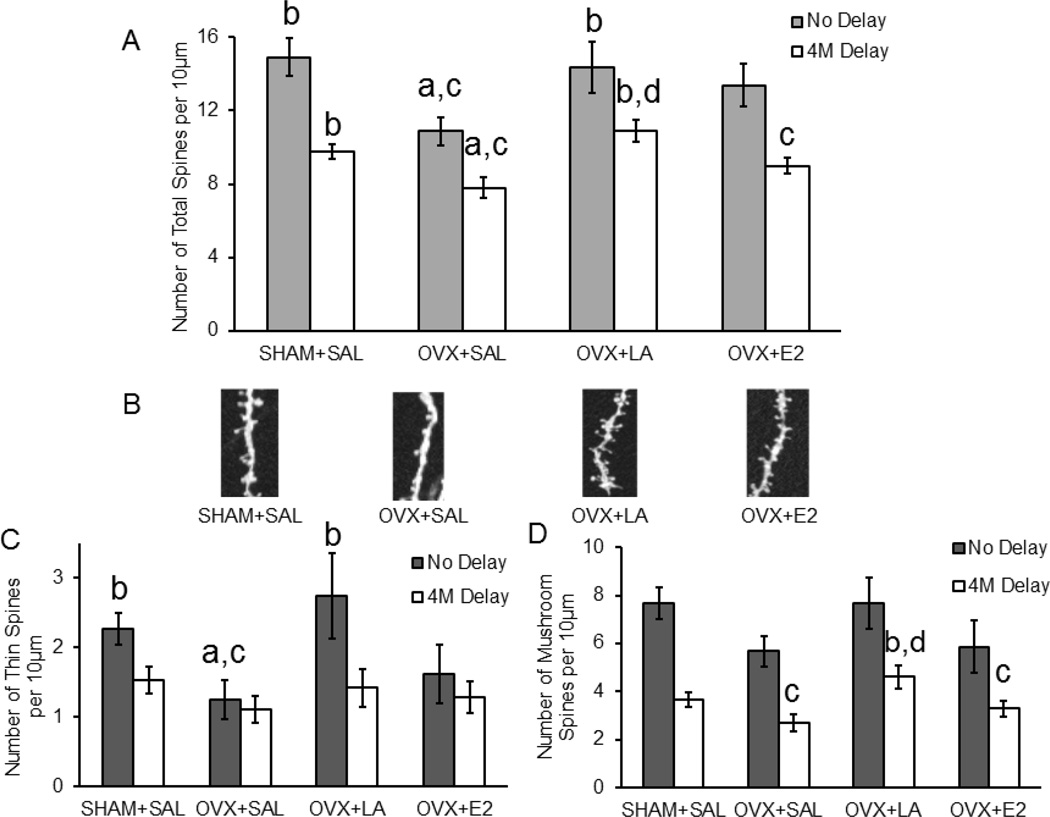
After Morris water maze testing animals were sacrificed and tissue was processed and diolistically labeled with a gene gun to evaluate changes in spine morphology of the apical dendrite in cortical layer II/III pyramidal neurons. A two-way ANOVA was used to analyze the effect of treatment group and treatment onset on spine density. There was no significant interaction between treatment group and treatment onset for spine density (F(3,96)=0.518; p>0.05; η2=0.01). However, there was a significant main effect of treatment onset between no delay and 4-month delay treatment (F(1,96)=18.79; p<0.001; η2=0.13) and a significant main effect of treatment group on spine density (F(3,96)=6.56; p<0.001; η2=0.14). For both the no delay (SHAM: n=17 cells/5 animals; OVX+SAL: n=15 cells/4 animals; OVX+LA: n=15 cells/4 animals; OVX+E2: n=12 cells/3 animals; F(3,58)=2.99; p<0.05; η2=0.14) and 4-month delay treatment (SHAM: n=45 cells/5 animals; OVX+SAL: n=27 cells/3 animals; OVX+LA: n=34 cells/4 animals; OVX+E2: n=35 cells/4 animals; F(3,140)=6.57; p<0.001; η2=0.12) cohorts the OVX group displayed fewer total spines compared to the SHAM group (no delay: p<0.05; d=0.94, 4M delay: p<0.05; d=0.73, Figure 4). E2 replacement was not able to significantly increase total spine number in neither the no delay nor the 4-month delay interventions (no delay: p>0.05; d=0.80, 4M delay: p>0.05; d=0.46, Figure 4A). However, lowering luteinizing hormone levels with leuprolide acetate was able to rescue total spine densities to SHAM levels as compared to OVX (no delay: p<0.05; d=0.89, 4M delay: p<0.001; d=0.96, Figure 4A). Importantly, we also identified differences in spine type across groups. For example, in the no delay cohort the SHAM and leuprolide acetate groups showed a significantly increased number of thin spines compared OVX (F(3,58)=6.15; p<0.05; η2=0.15, SHAM: p<0.05; d=0.91, LA: p<0.01; d=0.92, Figure 4C). In the 4-month delay cohort an increase in the number of mushroom spines was observed (F(3,140)=3.95; p<0.05; η2=0.08) with leuprolide acetate treatment compared to both the OVX and E2 groups (OVX: p<0.05; d=−0.75, E2: p<0.05; d=−0.53, Figure 4D).
Figure 4. Leuprolide acetate rescues the total number of spines on dendritic outgrowths after ovariectomy.
OVX-associated decreases in spine density are rescued by leuprolide acetate treatment in the no delay cohort, and leuprolide acetate increases spine density when a 4 month delay is present between treatment and OVX (4M Delay). A: Leuprolide acetate exhibited an ability to rescue the total number of spines regardless of treatment onset after OVX (one-way ANOVA). B: Representative images of dendritic segments. C: The number of thin spines was rescued when treatment with leuprolide acetate began on the OVX day. D: The number of mushroom spines was increased by leuprolide acetate treatment with a 4 month delay between OVX and treatment onset. Results are expressed as mean ± sem. Significant difference (p<0.05) from SHAM+SAL (a), from OVX+SAL (b), from OVX+LA (c) and from OVX+E2 (d) SHAM+SAL, sham ovariectomy treated with saline; OVX+SAL, ovariectomy treated with saline; OVX+E2, ovariectomy treated with 17β-estradiol; OVX+LA, ovariectomy treated with leuprolide acetate
4. Discussion
We compared the effects of pharmacologically lowering serum LH and E2 replacement on cognition and spine density in middle-aged ovariectomized female mice when treatments were administered immediately after OVX (no delay) or after an interval of 4 months between OVX and treatment onset (4 month delay). Our data demonstrate that peripheral LH is a key player in driving ovariectomy-related cognitive dysfunction and spine density loss. Importantly, we have also determined that unlike E2 replacement, lowering peripheral LH is effective at improving cognition and spine density beyond the “critical” period of E2 replacement effectiveness in rodents.
Importantly, we show that ovariectomized animals treated with leuprolide acetate to lower peripheral levels of LH show improvements in spatial memory both when treatment onset is immediately after or 4 months after OVX. In contrast, E2 replacement was only effective at improving cognition when administered immediately after OVX. While the mechanisms underlying these differences is unknown, one explanation for the differences between treatments may lie in the fact that E2 treatment is beneficial while neuronal health remains stable, but E2 treatment becomes detrimental when neurons are in poor health (Brinton, 2008).
Interestingly, in our 4 month delay cohort we did not identify significant differences in cognitive function between SHAM and OVX or E2 treated animals, but did observe that leuprolide acetate treated animals showed significant cognitive improvements. This suggests an independent mechanism of action of LH on cognition. The fact that SHAM control animals were not different from OVX or E2 in the 4 month delay cohort is not surprising since this cohort was tested at 15 months of age, an age when deficits in the MWM task are prevalent in normal animals (de Fiebre et al., 2006). However, our data highlights the fact that lowering serum LH in aged animals, despite of their E2 status is beneficial and will need to be explored in depth in future studies.
In addition to cognitive benefits, leuprolide acetate treatment maintained the total number of spines, a marker of synaptic plasticity, at SHAM levels in both no delay and 4 month treatment delay cohorts. Dendritic spines have a low rate of turnover in adults, but morphology changes rapidly and spine plasticity is strongly associated with learning and memory (Gutierrez and Davies, 2011). Therefore, our data suggest that a potential mechanism underlying improvements in cognition by lowering peripheral LH levels involves its ability to regulate spine density.
Contrary to previous studies showing increases in spine density with E2 treatment (Woolley and McEwen, 1992; Gould et al., 1990; Bailey et al., 2011), we did not observe such benefits. While E2 did show a trend toward increasing OVX-associated loss of spine density when administered immediately after OVX surgery, these differences were not statistically significant. These findings may be explained by differences in age, parturition status of the females, treatment length, and/or different methods of delivery of E2 across studies. It is also possible that because the animals were 11 months of age when scarified, based on the healthy-bias hypothesis of E2 (Brinton et al., 2008) that the effects of estrogen were mitigated by the aging, more stressful, neuronal environment. However, given that lowering of peripheral LH to SHAM levels increased spine density like E2 has been shown to do, our data suggest that some of the E2 effects may be secondary to lowering OVX-induced rises in peripheral LH.
Alternatively, it is also possible that OVX-related loss of E2 and rises in peripheral LH regulate neuronal plasticity in parallel but independently both via the regulation of spine density and other signals. This latter aspect is highlighted by the fact that while SHAM operated animals showed significantly higher levels of spines compared to OVX and E2 animals, SHAM operated animals did not show differences in cognitive function when compared to OVX or E2 animals in the 4 month delay treatment cohort. Previous published data from our group demonstrates that lowering LH with leuprolide acetate alters signaling molecules associated with development and stabilization of spines (Palm et al., 2014) and leads to activation of signaling cascades associated with long term potentiation (Bryan et al., 2010). Therefore it is plausible that additional mechanisms are involved in the ability of leuprolide acetate to improve cognition above and beyond the performance observed in SHAM treated animals in the 4-month delay cohort.
In addition to changes in total spine density, our data also show that restoring OVX-related increases in serum LH levels also alters spine type. Mature mushroom spines have large postsynaptic densities and are generally viewed as stable, while the plastic, thin spines are less stable due to their dynamic nature (Bailey et al., 2011). It is thought that the conversion from thin spines to mushroom spines can occur with the induction of long-term potentiation and long-term depression. Our data revealed that depending on treatment onset, different types of spines were more prone to being altered. For example, in the no delay cohort, when animals were younger, lowering peripheral LH rescued OVX-related loss in thin spines to SHAM levels, indicative of changes in plasticity, but not mushroom spines. These findings are similar to those in an aging study where thin and stubby spines were decreased due to aging, and were affected by stress (Bloss et al., 2011). Though mushroom spines are associated with stability of synapses, an increase in spine head diameter has been observed with aging, despite the loss of thin and stubby spines (Bloss et al., 2011). In our data leuprolide acetate treatment increased the number of mushroom spines in the older animals even though no decrease was observed in the OVX group when compared to control.
Previously published data support the role of LH in cognition. For example, we and others have demonstrated that pharmacologically lowering OVX-related increases in serum LH improves cognitive function in rodents (Bowen et al., 2004; Bryan et al., 2010; Casadesus et al., 2006; Kovacs et al., 2001; Palm et al., 2014; Telegdy et al., 2009; Ziegler and Thornton, 2010). While a non-specific pharmacological effect of leuprolide acetate cannot be ruled out, positive effects on cognition achieved by lowering peripheral LH levels have been attained using different types of drugs. Both competitive antagonists like antide or cetrorelix (Kovacs et al., 2001; Telegdy et al., 2009; Ziegler and Thornton, 2010) as well as leuprolide acetate (Bryan et al., 2010; Casadesus et al., 2006; Palm et al., 2014), a super agonist that drives its effects by downregulating rather than blocking the GnRHR, improve OVX-associated cognitive deficits. As such, while these drugs work differently at the GnRHR, all have a common end result, to reduce peripheral LH levels. Together these data suggest that it is the reduction of peripheral LH rather than non-specific effect of leuprolide that drives benefits on cognition and plasticity. Additionally, we provide supplemental data (figure 1 supplemental materials) showing that levels of hypothalamic GnRH mRNA are similar in OVX and leuprolide-treated animal suggesting that changes in GnRH processing is also an unlikely factor to drive the observed effects of leuprolide. Lastly, studies show that peripheral injections of an LH analogue (hCG) attenuate E2 associated rescues in spatial memory (Barron et al., 2010; Berry et al., 2008). Given that hCG, like LH, signals through the LH receptor these data suggest that peripheral activation of the LH receptor impact cognition negatively despite of E2 status; an aspect that has been shown for conditions associated with high LH levels such as polycystic ovarian syndrome (Barnard et al., 2007; Yen et al., 1970).
The mechanism through which lowering peripheral levels of LH restores cognition and spine density is unknown, but evidence from our lab supports a role for LH signaling in the brain. We have shown that AD patients have lower levels of LH mRNA in both the hippocampus and cortex (Palm et al., 2014) despite having higher plasma levels compared to controls (Short et al., 2001; Butchart et al., 2012). In the rodent we have shown that ovariectomy-associated increases in serum LH are associated with reductions in LH immunoreactivity in cognition-associated areas, and that these LH levels are restored with leuprolide acetate treatment (Palm et al., 2014). Similarly, by observing LH levels in rats throughout the estrous cycle it has been shown that hypothalamic LH decreases during proestrus, when pituitary and serum LH levels are high (Emanuele et al., 1981). Due to the presence of LH mRNA (Palm et al., 2014), the presence of functional LH protein in the brain (Blair et al., 2015; Palm et al., 2014; Emanuel et al., 1983; Hostetter et al., 1981) and the inverted relationship between pituitary/serum LH levels and brain LH levels (Palm et al., 2014; Emanuele et al., 1981), we believe that LH is made in the brain, It is also known that LH receptor is localized to regions of the brain important for spatial reasoning, such as the hippocampus (Apaja et al., 2004). Additionally, activating LH receptor increases differentiation in PC12 cells, a neuron-like cell (Meng et al., 2007), and in vivo LH receptor activation affects courtship songs in Xenopus laevis (Yang et al., 2007) and activity and neurogenesis in rodents (Lukacs et al., 1995; Mak et al., 2007). Therefore, one potential mechanism is that serum LH levels regulate, through some yet to be discovered feedback mechanism, the production of brain-derived LH. This, in turn, drives LH receptor signaling in the brain, which regulates cognition and plasticity (reviewed Blair et al., 2015). One such mechanism could be the short-loop feedback mechanism between the pituitary and hypothalamus (Emanuele et al., 1981; Conway and McCann, 1990). In fact, projections from the hypothalamus may be supplying LH as a neurotransmitter in higher quantities when the peripheral levels of LH are decreased, but future studies are necessary to test this hypothesis.
Taken together our data suggest that LH associated mechanisms are key in the regulation of learning and memory and underlying mechanisms of this function such as dendritic spine density. Importantly, lowering OVX-associated rises in serum LH, unlike E2 replacement, is effective regardless of timing of treatment onset in rodents. Whether the benefits of lowering LH are dependent or independent of estrogens remains unclear and a goal for future studies. However, at the very least these data clearly demonstrate that lowering peripheral LH levels induced by loss of estrogens is more effective than E2 replacement in the aged rodent brain. Furthermore, our data also suggests that additional mechanisms, beyond spine density, may be influencing the benefits on cognition that are observed after lowering levels of serum LH. Though the root mechanisms are yet to be discovered, these data and the recently published findings by Bowen et al., 2015, which show leuprolide acetate treatment benefits female patients with AD, highlight the potential for leuprolide acetate as a therapeutic agent and the need for further studies characterizing the role of LH in cognition.
Supplementary Material
Highlights.
Leuprolide acetate and E2 rescue deficits on the Morris water maze task when treated immediately after OVX
At a time point after OVX where E2 is not beneficial, leuprolide acetate improves spatial learning and memory
OVX-associated loss of spines density on dendritic outgrowths is rescued by leuprolide acetate
Acknowledgements
This work was supported by grants from the National Institute on Aging (R01 AG032325). The authors would like to thank the Neuroscience Imaging Center of Case Western Reserve University. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core which is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Apaja PM, Harju KT, Aatsinki JT, Petäjä-Repo UE, Rajaniemi HJ. Identification and structural characterization of the neuronal luteinizing hormone receptor associated with sensory systems. Journal of Biological Chemistry. 2004;279(3):1899–1906. doi: 10.1074/jbc.M311395200. [DOI] [PubMed] [Google Scholar]
- Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience. 2011;191:148–158. doi: 10.1016/j.neuroscience.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard L, Balen AH, Ferriday D, Tiplady B, Dye L. Cognitive functioning in polycystic ovary syndrome. Psychoneuroendocrinology. 2007;32(8):906–914. doi: 10.1016/j.psyneuen.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Barron AM, Verdile G, Taddei K, Bates KA, Martins RN. Effect of Chronic hCG Administration on Alzheimer’s-Related Cognition and Aβ Accumulation in PS1KI Mice. Endocrinology. 2010;151(11):5380–5388. doi: 10.1210/en.2009-1168. [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-β levels in female rats. Hormones and behavior. 2008;54(1):143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JA, Bhatta S, McGee H, Casadesus Smith G. Luteinizing Hormone: Evidence for direct action in the CNS. Hormones and Behavior. 2015 doi: 10.1016/j.yhbeh.2015.06.020. (Pre-press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. The Journal of Neuroscience. 2011;31(21):7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A Clinical Study of Lupron Depot in the Treatment of Women with Alzheimer's Disease: Preservation of Cognitive Function in Patients Taking an Acetylcholinesterase Inhibitor and Treated with High Dose Lupron Over 48 Weeks. Journal of Alzheimer's Disease. 2015;44(2):549–560. doi: 10.3233/JAD-141626. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in neurosciences. 2008;31:529. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee HG, Zhu X, Smith MA, Casadesus G. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. Journal of neurochemistry. 2010;112(4):870–881. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart J, Birch B, Bassily R, Wolfe L, Holmes C. Male sex hormones and systemic inflammation in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2013;27(2):153–156. doi: 10.1097/WAD.0b013e318258cd63. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-β deposition in Alzheimer APP transgenic mice. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2006;1762(4):447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Molecular and cellular endocrinology. 2007;269(1):107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139(6):1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway S, McCann SM. The Role of Lh in the Autofeedback Inhibition of Lh and Fsh Secretion in Ovariectomized Rats. Endocrine research. 1990;16(3):403–413. doi: 10.1080/07435809009033015. [DOI] [PubMed] [Google Scholar]
- D'Agostino G, Russo R, Avagliano C, Cristiano C, Meli R, Calignano A. Palmitoylethanolamide protects against the amyloid-β25–35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology. 2012;37(7):1784–1792. doi: 10.1038/npp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochimica et Biophysica Acta. 2010;1800(10):1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147(1):607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- De Fiebre NE, Sumien N, Forster MJ, De Fiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. Age. 2006;28(3):235–253. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele NV, Anderson J, Andersen E, Connick E, Baker G, Kirsteins L, Lawrence AM. Extrahypothalamic brain luteinizing hormone: characterization by radioimmunoassay, chromatography, radioligand assay and bioassay. Neuroendocrinology. 1983;36(4):254–260. doi: 10.1159/000123464. [DOI] [PubMed] [Google Scholar]
- Emanuele N, Oslapas R, Connick E, Kirsteins L, Lawrence AM. Hypothalamic LH may play a role in control of pituitary LH release. Neuroendocrinology. 1981;33(1):12–17. doi: 10.1159/000123194. [DOI] [PubMed] [Google Scholar]
- Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17β-estradiol during the estrous cycle. Biology of reproduction. 2001;65(3):680–688. doi: 10.1095/biolreprod65.3.680. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of aging. 2000;21(1):107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. The Journal of Neuroscience. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Davies AM. Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends in neurosciences. 2011;34(6):316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen T, Poutanen M, Huhtaniemi I. Age-and sex-specific promoter function of a 2-kilobase 5′-flanking sequence of the murine luteinizing hormone receptor gene in transgenic mice. Endocrinology. 1999;140(11):5322–5329. doi: 10.1210/endo.140.11.7115. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puoliväli J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Experimental gerontology. 2004;39(9):1277–1283. doi: 10.1016/j.exger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng T, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer/'s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter G, Gallo RV, Brownfield MS. Presence of immunoreactive luteinizing hormone in the rat forebrain. Neuroendocrinology. 1981;33(4):241–245. doi: 10.1159/000123238. [DOI] [PubMed] [Google Scholar]
- King JC, Anthony ELP, Damassa DA, Elkind-Hirsch KE. Morphological evidence that luteinizing hormone-releasing hormone neurons participate in the suppression by estradiol of pituitary luteinizing hormone secretion in ovariectomized rats. Neuroendocrinology. 1987;45(1):1–13. doi: 10.1159/000124698. [DOI] [PubMed] [Google Scholar]
- Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr Expression in Granulosa Cells: Roles for PKA-Phosphorylated β-Catenin, TCF3, and FOXO1. Molecular Endocrinology. 2013;27(8):1295–1310. doi: 10.1210/me.2013-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132(5):2262–2270. doi: 10.1210/endo.132.5.8477671. (1993). [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Hormones and behavior. 1995;29(1):42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone–stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nature neuroscience. 2007;10(8):1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women's Health Initiative 10 years on. Climacteric. 2012;15(3):256–262. doi: 10.3109/13697137.2012.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Hormones and behavior. 2012;61(4):479–486. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behavioral neuroscience. 2012;126(1):4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Hormones and behavior. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XL, Rennert OM, Chan WY. Human chorionic gonadotropin induces neuronal differentiation of PC12 cells through activation of stably expressed lutropin/choriogonadotropin receptor. Endocrinology. 2007;148(12):5865–5873. doi: 10.1210/en.2007-0941. [DOI] [PubMed] [Google Scholar]
- Okada H, Doken Y, Ogawa Y. Persistent suppression of the pituitary-gonadal system in female rats by three-month depot injectable microspheres of leuprorelin acetate. Journal of pharmaceutical sciences. 1996;85(10):1044–1048. doi: 10.1021/js960123a. [DOI] [PubMed] [Google Scholar]
- Oliver C, Mical RS, Porter JC. Hypothalamic-pituitary vasculature: evidence for retrograde blood flow in the pituitary stalk. Endocrinology. 1977;101(2):598–604. doi: 10.1210/endo-101-2-598. [DOI] [PubMed] [Google Scholar]
- Palm R, Chang J, Blair J, Garcia-Mesa Y, Lee HG, Castellani RJ, Casadesus G. Down-regulation of serum gonadotropins but not estrogen replacement improves cognition in aged-ovariectomized 3xTg AD female mice. Journal of neurochemistry. 2014;130:115–125. doi: 10.1111/jnc.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288(17):2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated Three-Dimensional Detection and Shape Classification of Dendritic Spines from Fluorescence Microscopy Images. PLoS ONE. 2008;3(4):e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Verdile G, Foster JK, Hogervorst E, Joesbury K, Dhaliwal S, Martins RN. Gonadotropins and cognition in older women. Journal of Alzheimer's Disease. 2008;13(3):267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Reichelt C, Scherbaum WA. Neuroendocrinology of aging in humans: attenuated sensitivity to sex steroid feedback in elderly postmenopausal women. Neuroendocrinology. 1994;59(4):355–362. doi: 10.1159/000126678. [DOI] [PubMed] [Google Scholar]
- Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. Journal of neurochemistry. 2006;96(4):1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Halmo GA. Hypothalamic hormones and cancer. Frontiers in neuroendocrinology. 2001;22(4):248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- Shaw ND, Histed SN, Srouji SS, Yang J, Lee H, Hall JE. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. The Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1955–1961. doi: 10.1210/jc.2009-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The Critical Period Hypothesis: Can It Explain Discrepancies in the Oestrogen-Cognition Literature? Journal of neuroendocrinology. 2006;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Short RA, O’Brien PC, Graff-Radford NR, Bowen RL. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clinic Proceedings. 2001;76(9):906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic labeling of neurons in tissue slices: a qualitative and quantitative analysis of methodological variations. Frontiers in neuroanatomy. 2011;5:14. doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. The Journal of neuroscience. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Tanaka M, Schally AV. Effects of the LHRH antagonist Cetrorelix on the brain function in mice. Neuropeptides. 2009;43(3):229–234. doi: 10.1016/j.npep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Telegdy G, Adamik A, Tanaka M, Schally AV. Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regulatory peptides. 2010;159(1):142–147. doi: 10.1016/j.regpep.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Verdile G, Yeap BB, Clarnette RM, Dhaliwal S, Burkhardt MS, Chubb SP, Martins RN. Luteinizing hormone levels are positively correlated with plasma amyloid-β protein levels in elderly men. Journal of Alzheimer's Disease. 2008;14(2):201–208. doi: 10.3233/jad-2008-14208. [DOI] [PubMed] [Google Scholar]
- Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Martins RN. Associations between gonadotropins, testosterone and β amyloid in men at risk of Alzheimer’s disease. Molecular Psychiatry. 2014;19:69–75. doi: 10.1038/mp.2012.147. [DOI] [PubMed] [Google Scholar]
- Walter J, Keiner S, Witte OW, Redecker C. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiology of aging. 2011;32(10):1906–1914. doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C, Bader M. Sustained Long Term Potentiation and Anxiety in Mice Lacking the Mas Protooncogene. Journal of Biological Chemistry. 1998;273(19):11867–11873. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain research. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 292(24):2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion Neuroendocrine modulation and repercussions of female reproductive aging. Recent progress in hormone research. 2002;57(1):235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KL, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133(5):1035–1044. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. The Journal of Neuroscience. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Nasipak BT, Kelley DB. Direct action of gonadotropin in brain integrates behavioral and reproductive functions. Proceedings of the National Academy of Sciences. 2007;104(7):2477–2482. doi: 10.1073/pnas.0608391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SS, Vela P, Rankin J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. The Journal of Clinical Endocrinology & Metabolism. 1970;30(4):435–442. doi: 10.1210/jcem-30-4-435. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Young WS., III Changes in cellular levels of messenger ribonucleic acid encoding gonadotropin releasing hormone in the anterior hypothalamus of female rats during the estrous cycle. Endocrinology. 1988;123(3):1688–1689. doi: 10.1210/endo-123-3-1688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.