Abstract
Parental experience and hormones play a large role in the common marmoset (Callitrhix jacchus) father’s care of their offspring. We tested the effect of exogenous estradiol or testosterone on the responsiveness of common marmosets to respond to infant distress vocalizations and whether males who haven’t become fathers yet (paired males) would have increased responsiveness to infant distress calls with either steroid or whether parental experience is the most important component for the onset of paternal care. Sixteen male marmosets (8 fathers, 8 paired males) received a vehicle, low dose or high dose of estradiol and additional 16 males were tested with testosterone at three doses for their response either to a vocal control or a recording of an infant distress call for 10 minutes. Without steroid stimulation fathers were significantly more likely to respond to the infant distress stimulus than paired males. Low dose estradiol stimulation resulted in a significant increase in father’s behavioral response toward the infant distress stimulus but not in paired males. Fathers also showed a significant increase in infant responsiveness from the vehicle dose to the estradiol low dose treatment, but not to the estradiol high dose treatment. Testosterone treatment did not show significant differences between infant responsiveness at either dose and between fathers and paired males. We suggest that neither steroid is involved in the onset of paternal care behaviors in the marmoset but that estradiol may be involved in facilitating paternal motivation in experienced fathers.
Keywords: paternal care, estradiol, testosterone, infant distress cry, marmoset
Introduction
Becoming a parent involves a change in the perception of infants and in the motivation to respond to infant sensory cues. Our basis for understanding the organizational effects involved in the onset of maternal care behaviors has come from years of studying the female rat, Rattus norvegicus (Rosenblatt, 1969). Female virgin rats will either avoid unknown pups or attack them (Dulac et al., 2014; Stern, 1989). Female pregnant rats begin to show an increase in responsiveness to pup sensory cues just prior to parturition and continuing from birth through to weaning (Mayer and Rosenblatt, 1984; Bridges, 1975). The cascade of hormonal changes occurring prior to parturition have been shown in the female rat to be involved in the induction of maternal behavior (Moltz et al., 1970; Zarrow et al., 1971).
In female mammals multiple endocrine changes occur during pregnancy and at parturition that are associated with an increase in spontaneous expression of maternal care (Bridges 2015). Estrogens play an essential role in the induction of maternal care in mammals. This has been demonstrated in many species, including the rat, mice, and ewes (Siegel and Rosenblatt 1975; Ribeiro et al. 2012; Poindron et al. 1988). Estradiol is also involved in enhancing the acquisition and retention of behavioral preferences for pup isolation calls (Caras 2013). Additionally, the actions of other hormones such as progesterone, prolactin and oxytocin are dependent upon exposure to estrogens (Bridges 2015). Little is known about the effect of estradiol on the onset of paternal behavior in males. If estrogen works in males as it does in females, estrogen should increase parenting motivation in fathers and in non-fathers to respond to infant auditory calls. In a bi-parental rodent, the effect of estrogens has been shown to be via testosterone aromatization into estradiol in the brain (Kirkpatrick et al. 1994; Lee and Brown 2002).
Testosterone, however, has primarily been shown to have a negative relationship with paternal care and is low in bi-parental rodents following the birth of their offspring (Bales and Saltzman 2015). Infant crying is a primary modality of infant communication and a few studies have investigated testosterone reactivity to audiotaped or simulated infant distress. Human males with higher testosterone are less responsive to novel infant cries (Fleming et al. 2002). However, testosterone also has been shown to have a role in the onset of paternal care through its aromatization to estradiol in the bi-parental California mouse (Peromyscus californicus) (Trainer and Marler 2001). In many species, testosterone has an inverse relationship with prolactin where prolactin is associated with paternal care (See Ziegler et al. 2009).
Common marmoset fathers have physical and hormonal changes while their mates are pregnant (Ziegler et al. 2009). Males gain weight during the gestation period of their mate and lose weight while performing infant care behaviors and this appears to be under the control of prolactin (Ziegler et al 2006; Ziegler et al. 2009). Prolactin, estradiol, testosterone and cortisol increase during pregnancy. Testosterone declines significantly following birth of infants and has an inverse relationship with prolactin. Testosterone also significantly declines in fathers after smelling the scent of their dependent infants while estrogens increase (Prudom et al. 2008; Ziegler et al. 2011). In marmosets, and the closely related cotton-top tamarin, Sauginus oedipus, males have very high levels of estrogens circulating and excretion into the urine (Ziegler et al. 2000; Ziegler et al. 2009). In the male cotton-top tamarin, estradiol is mainly of gonadal origin and therefore aromatized by testosterone (Ziegler et al. 2000). However, there is no evidence of an induction of paternal behavior in this species without becoming a father.
We have developed a motivation test for our marmosets where males can respond to infant distress calls (Zahed et al. 2008). Fathers who have experienced at least one birth are significantly more responsive to the infant cries than males who have never had their own infants even though they have participated in carrying younger siblings (Zahed et al. 2008; Ziegler et al. 2009). Therefore, either experience or hormonal induction is causing the increase in response to infant distress cries. We gave long acting estradiol or testosterone to fathers or males who had not become fathers yet (paired males) to determine if estradiol or testosterone (through aromatizing to estradiol) would increase male’s response to infant distress cries. We hypothesize that if the paired males increase their responsiveness behavior towards infant distress calls when given estradiol or testosterone treatment, then we would expect the steroid to be involved in the onset of paternal behaviors. If not, then it would add support for experience as a father being the most important condition for the onset of paternal care.
Materials and methods
Subjects
The study used a total of 32 adult male common marmosets: 16 parentally experienced (Mean ± SD: 5.04 ± 1.68 years) and 16 parentally inexperienced (Mean ± SD: 4.3 ± 1.59 years), Table 1. There was no significant difference by age between the two groups (t=1.3, df=30, P=0.21). All subjects were housed in social groups comprised either of a pair mate or a family (pair mate and their offspring of various ages) in the Marmoset Colony of the Wisconsin National Primate Research Center, University of Wisconsin-Madison. Subjects and their social groups were housed with 12-hour light:dark cycles (6:30–18:30 light), a steady temperature of 27°C, and humidity approximately 40%. The marmosets were fed twice daily at approximately 0800h and 1300h in standardized meals consisting of marmoset chow (Mazuri 5MI6, LandO’Lakes) and supplemental fruit, mealworms, and vegetables. Water was provided ad libitum. Cage size varied between 1.22 × 0.61 × 1.83 m (family groups) and 0.6 × 0.91 × 1.83 m (pairs). This study was reviewed and approved by the Graduate School Animal Care and Use Committee at the University of Wisconsin-Madison and the experiments were conducted in accordance with international standards on animal welfare as well as being compliant with national regulations. Adequate measures were taken to minimize pain or discomfort.
Table 1.
Marmoset males as fathers (experienced) and paired males (inexperienced) in paternal care with their age, the number of sibling sets they have raised and number of offspring sets raised.
| Male ID# | Experience level | Male age (years) | Sibling sets raised | Offspring sets raised |
|---|---|---|---|---|
| cj0863 | experienced | 8.71 | 4 | 7 |
| cj0955 | experienced | 7.79 | 2 | 3 |
| cj1301 | experienced | 4.53 | 2 | 2 |
| cj1271 | experienced | 4.73 | 2 | 4 |
| cj1305 | experienced | 4.3 | 0 | 3 |
| cj1157 | experienced | 6.04 | 1 | 9 |
| cj1237 | experienced | 5.1 | 3 | 3 |
| cj1263 | experienced | 4.77 | 1 | 4 |
| cj1229 | experienced | 4.77 | 0 | 3 |
| cj1049 | experienced | 6.53 | 2 | 5 |
| cj1115 | experienced | 6.48 | 1 | 8 |
| cj1483 | experienced | 3.18 | 0 | 2 |
| cj1403 | experienced | 3.97 | 2 | 2 |
| cj1560 | experienced | 2.57 | 1 | 2 |
| cj1427 | experienced | 3.96 | 1 | 2 |
| cj1533 | experienced | 3.27 | 0 | 2 |
| cj1450 | inexperienced | 2.41 | 1 | N/A |
| cj1489 | inexperienced | 2.15 | 0 | N/A |
| cj1479 | inexperienced | 2.21 | 0 | N/A |
| cj1381 | inexperienced | 2.97 | 2 | N/A |
| cj1065 | inexperienced | 6.4 | 9 | N/A |
| cj1285 | inexperienced | 4.44 | 3 | N/A |
| cj1197 | inexperienced | 5.24 | 2 | N/A |
| cj1099 | inexperienced | 6.57 | 2 | N/A |
| cj1163 | inexperienced | 5.26 | 0 | N/A |
| cj1165 | inexperienced | 5.26 | 1 | N/A |
| cj1213 | inexperienced | 4.8 | 3 | N/A |
| cj1345 | inexperienced | 4.2 | 3 | N/A |
| cj1430 | inexperienced | 2.83 | 1 | N/A |
| cj1490 | inexperienced | 2.38 | 0 | N/A |
| cj1053 | inexperienced | 6.36 | 3 | N/A |
| cj1239 | inexperienced | 5.29 | 3 | N/A |
Study Design
All study subjects were tested for their behavioral response to a pre-recorded infant distress vocalization and to a pre-recorded artificial control acoustic stimulus (vocal control). The 32 subjects were divided into two categories based on whether or not they had yet experienced the birth of their own offspring. Sixteen of the subject males had previously sired and provided paternal care for their own offspring (“fathers”) and were housed with their pair mate and offspring for the duration of this study, and the remaining 16 males had never sired their own offspring (“paired males”) and were housed with only their pair mate for the duration of this study. Subjects in both the “fathers” and “paired males” categories had previous alloparenting experience in their natal groups prior to being paired with their own mate before the start of this study, Table 1. (Mean ± SD [Range]: N=16 fathers 1.38 ± 1.15 [0–4] sibling litters, N=16 paired males 2.06 ± 2.21 [0–9] sibling litters). Fathers had raised 2 to 9 litters of their own offspring prior to the start of this study (Mean ± SD: N=16 fathers 3.81 ± 2.29 [2–9] offspring litters).
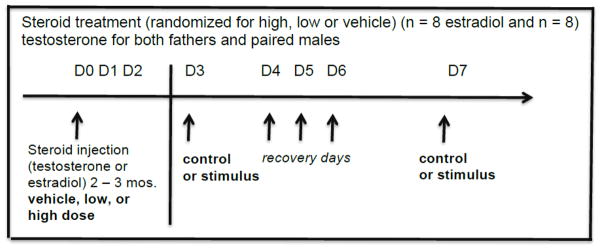
Within the two conditions, fathers (N=16) and paired males (N=16) were each further sub-divided according to hormone treatment (estrogen or testosterone) at three different levels for each subject (vehicle, low dose, high dose), resulting in two groups of eight animals for each hormone treatment, Figure 1. Each animal was given two days of habituation to the testing cage prior to the start of treatments and each animal received one of the three levels of hormone treatment in randomized order. Three days following hormone treatment, each male was tested for his behavioral response to one of the two auditory stimuli (vocal control, infant distress call) selected at random. He was returned to his social group immediately following behavioral testing, and two-to-four days later, he was tested with the other auditory stimulus. Thus, each animal in each experience condition was tested for behavioral response under five different treatment levels, once for each possible combination of auditory stimulus (vocal control, infant distress call) and hormone level (vehicle, low dose, high dose). Each male was tested at three different times 2 – 3 months apart, randomized for which steroid dose they would receive (i.e., vehicle, low or high dose).
Figure 1.
Steroid treatment protocol for the father and paired male conditions. A total of 16 males received estradiol treatment (i.e., vehicle, low dose, high dose) on 3 different months, 2–3 months apart, and an additional 16 males (father and paired males) received the testosterone treatment (vehicle, low, high dose) on 3 different months, 2–3 months apart. Males were tested under each treatment with the vocal control and the infant distress stimulus three days apart.
Both fathers and paired males were tested using the infant stimuli response test as reported in Zahed et al. (Zahed et al. 2008). The test cage was situated in a room removed from the smells and sounds of the marmoset colony. The cage consisted of two pair cages (0.6 × 0.91 × 1.83 m) separated by a distance of 0.6 m but connected by a mesh bridge attached 1.17 m above the floor. The bridge connected the two cages where one cage was where the male was let into (home cage) and the other cage was where the auditory stimuli were located (stimulus cage). The auditory stimulus was turned on to either emit infant distress or vocal control vocalizations. Males were transferred from their home environment in a metal nest box that was attached to the inside of the home cage. Once the stimulus was started, the male would be allowed to leave the nest box and the 10-minute test would begin. During this time males could chose to remain in the home component of the test cage or cross the bridge to interact with the auditory stimulus in the stimulus cage.
Behavioral tests
For each different treatment combination, pre-defined behaviors were sampled for each male in the study (Table 2). The response variables represent typical behavioral responses to infant distress vocalizations in this species and were chosen to encompass a broad range of individual variation in the expression of motivation to provide infant care. These behaviors: enter bridge, enter stimulus cage, look at stimulus, investigate stimulus source, look at stimulus nestbox, and look at stimulus cage were selected as a subset of behaviors described in Zahed et al. that were significantly related to motivation to respond to a distressed infant (Zahed et al. 2008). Additionally, we collected other behaviors that were labeled “response behaviors unrelated to infant stimulus” and these were: long call vocals, chirp vocals, investigate the home cage nest box, and look out of the cage and away from stimulus. These were thought to indicate a lack of interest in the vocal stimulus.
Table 2.
Behaviors tested in response to the Infant stimulus; a distressed infant vocalization played on a MP3 player. Males had to cross a bridge to enter the infant cage where stimulus was playing.
|
Infant response behaviors
| |
| Enter bridge | Male leaves his cage and enters into the bridge connecting to the infant stimulus cage |
| Enter stimulus cage | Male crosses the bridge and enters the infant stimulus cage |
| Look at stimulus | Male looks at the MP3 player |
| Investigate stimulus source | Male investigates for the source of the vocalizations Including looking into the nestbox where MP3 sits |
| Look at stimulus nestbox | Male is in his cage and looks at the stimulus cage |
|
| |
| Response behaviors unrelated to stimulus | |
|
| |
| Long call vocals | Male is calling-trying to contact his group |
| Chirp vocals | Male is producing short chirping directed towards observer |
| Investigate home nestbox | Male is looking and entering the home nestbox |
| Look out of cage and away from stimulus | Male’s attention is directed outside of his or the stimulu’s cage |
Auditory Stimuli
With presentation of our control stimulus, created by S. K. Zahed (Ph.D. Thesis), there was no difference in behavioral response to the non-vocal control (silence) versus the vocal control stimulus, indicating a lack of species-relevant information contained in the control stimulus. However, rather than testing animals with no control, a vocal control stimulus was necessary to prevent animals from attending and responding to ambient noises in the environment, which may vary unpredictably between subjects (C. T. Snowdon, pers. comm.). In other words, a vocal control stimulus provided a noise to which each subject would attend, without eliciting fear or flight, but to which subjects would not behaviorally react in a confounding way. Since we wanted to ensure that the animals were responding to an infant call rather than to any pulsatile sound, the auditory components of both the vocal control and infant distress cries were similarly complex in amplitude, volume, tempo, and frequency. The vocal control stimulus was digitally mixed to achieve the appropriate acoustic properties, but the infant cry was not remixed. Marmoset calls of the general category Infant Distress Call can include various discrete vocal components, typically including “chirp” a brief single note and “trill”, an interrupted composite call of equally spaced ascending or descending notes. The Infant Distress Call used for this study included an average 69 vocalizations/min (28 “chirps” and 41 “trills”). The vocal control stimulus was digitally constructed as a synthetic chirp based on a sampled human “e” sound, and mixed to 45 sounds/min. A random sample of the vocal control stimulus (Avisoft SASLab Pro) showed a frequency of 2,790 Hz and a foundation frequency of 340 Hz for each synthetic chirp (human “e”).
Behavior Data Collection Procedure
Behavior data were collected by continuous-frequency measurement during 10-min focal-animal sampling sessions (Martin and Bateson 1987). Two different people collected behavioral data over the course of the study with inter-observer accuracy of 95%. All subjects and social groups were thoroughly habituated to the presence of each observer prior to the start of the study. Only a single observer was present in the testing room for release of the subject into the testing apparatus and data collection. Data were collected using a hand-held palm pilot.
Hormone Treatment Procedure
Following the second habituation day, hormone treatments were administered to each animal by intramuscular injection into the thigh three days before the observation period for the first of the two auditory stimuli. This allowed the males to be primed with the steroid prior to the motivation test. Each male received a high steroid treatment, low steroid treatment and vehicle in random order. Eight males received the long-acting sterilized testosterone-propionate at a low dose of 2.25 mg and a high does at 4.5 mg infused into sterile sesame oil. Sesame oil served as the vehicle control. The other eight males received long-acting sterilized 17-β-estradiol-benzoate at a low dose of 35 μg and a high dose at 70 μg infused into sterilized sesame oil. These doses were selected to be at or near the physiologic levels of the steroids and had been used for marmosets previously (Dixson 1993, Hodges and Hearn 1978).
Statistical Analysis
The data was not normally distributed, as some fathers never responded to the stimulus, therefore we used nonparametric analyses. Wilcoxon matched-pairs test was used to compare vocal control and infant distress vocalization for the individual behaviors. Comparisons by behavior between fathers and paired males in the vehicle control condition (no steroid enhancement) were analyzed by Mann Whitney test. Spearman Rank correlations were performed to compare father’s combined response score (sum of the stimulus directed behaviors) with the number of sibling births, age, number of offspring and paired males with the same, except with number of offspring. Wilcoxon signed rank test was used to compare the fathers to the paired males at different doses of either estradiol or testosterone. Friedman test was used as a nonparametric 1way ANOVA for comparisons between the different steroid concentrations for either fathers or paired males.
Results
To determine if the males responded differently to the infant distress vocalization than to the vocal control, we tested 16 fathers without any hormone manipulation. We found the behaviors: look at stimulus and search for the stimulus source were significantly different between the infant vocal and the vocal control for our most salient responses: Look at stimulus: U = 74.00, p = 0.03, mean ± s.e.m., vocal control = 0.38 ± 0.18, infant distress stimulus = 8.38 ± 4.36), Search for stimulus source: U = 2.0, p = 0.001, mean ± s.e.m. vocal control = 0.33 ± 0.21, infant distress stimulus = 3.0 ± 1.41. Therefore, we used only the infant distress vocalization for the remaining analyses.
Differences between fathers and paired males during the vehicle steroid treatment (control)
Without steroid stimulation, the 16 fathers and 16 paired males showed significantly different results in several behaviors during the vehicle treatment. This confirms what we have seen before. The frequency of the behaviors: enter stimulus cage (U = 68.50, ns = 14,14, P = 0.04) and investigate stimulus source (U = 85.50, n = 14,14, P=0.01) were significantly higher for the fathers than for the paired males (Figure 2). The behavior: enter bridge (U = 95.50, n = 14,14, P=0.07) showed a trend for significant differences between the fathers and the paired males. For all non-stimulus directed behaviors there were no differences between the two groups in the vehicle control condition.
Figure 2.
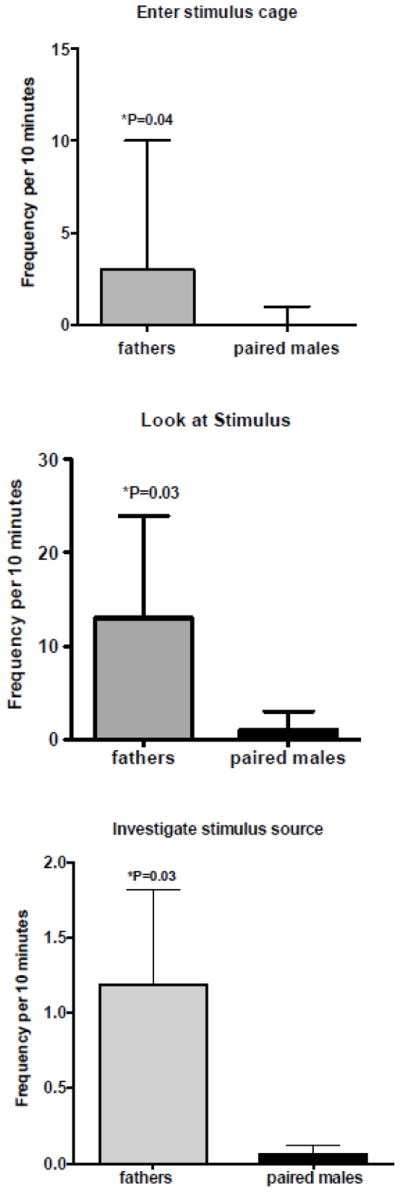
Differences between fathers and paired males in steroid non-stimulated, vehicle treatment for three infant-directed behaviors: enter stimulus cage, look at stimulus, investigate stimulus source.
Not all of the fathers showed the stimulus directed behaviors. We found only 6 fathers out of 16 looked at the stimulus and investigated the stimulus source while only 5 paired males looked at the stimulus. Therefore, not all of the fathers displayed the most salient behaviors in response to the infant distress calls. We then examined whether the number of previous offspring raised by fathers, father’s age or the number of sibling births the males had experienced correlated to their combined response factor (combined score for significant response behaviors: enter the stimulus cage, look at the stimulus, investigate the stimulus source). The number of previous offspring (rs = −0.21, P = 0.44, 2-tailed, n = 15) and the age of the male (rs = 0.24, P = 0.38, n = 15) were not correlated with their combined response factor but the number of siblings raised did correlate significantly (rs = 0.62, P = 0.01, n = 15). Therefore, experience with sibling infants may have influenced the father’s motivation although not all fathers showed a combined response and not all fathers had experienced raising siblings. Paired males were also examined for their combined response factor and number of siblings raised and age of the male. Unlike fathers, paired males did not show an effect of number of siblings raised (rs = 0.28, P = 0.32, n = 15) or for age of the male (rs = 0.22, P = 0.43, n = 15).
Effect of estradiol stimulation
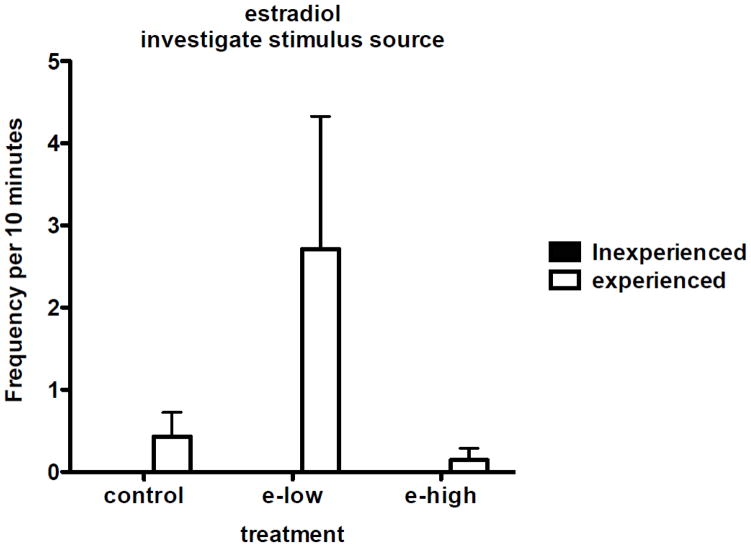
Both fathers and paired males were tested for their response to infant distress calls following a vehicle, low dose and high dose injection of estradiol in random order. At the estradiol low dose we found that fathers were significantly higher in their response, investigating the stimulus source, than the paired males (W = −21.00, n = 8,8, P = 0.03, Figure 3). None of the paired males exhibited this behavior in any treatment condition (vehicle, low, or high dose of estradiol). Under the vehicle treatment 3 out of 8 of the fathers investigated the stimulus source and under the low dose estradiol treatment 6 out of 8 of the fathers investigated the stimulus source while two fathers never showed a response. There were significant differences between the three estradiol concentrations in fathers for the behavior, investigate the stimulus source (F = 7.5, n = 8, P = 0.02, Figure 3), and the low estradiol treatment had a significantly higher response than the vehicle (W = 15.00, n = 8,8, P = 0.03) and the high estradiol treatment (W = 15.0, n = 8,8, P = 0.03).
Figure 3.
Investigate the stimulus source behavior response to estradiol stimulation of fathers and paired males under three treatments: vehicle, estradiol low (E2 low), estradiol high (E2 high). For fathers the E2 low response was significantly higher than to the vehicle or E2 high dose. Paired males did not show any response to the estradiol treatment.
Effect of testosterone stimulation
We tested a different set of males as paired males and fathers after testosterone treatment (vehicle, low, or high dose). No significant difference between the fathers and the paired males where found in their stimulus directed behaviors with the low testosterone dose: enter stimulus cage (W = −1.0, n = 7,7, P = 0.50), look at stimulus (W = −1.0, n = 7,7, P = 1.00) and investigate stimulus source (W = −1.0 n = 7,7, P = 0.50). No significant difference occurred with the high testosterone dose between paired males and fathers: enter the stimulus cage (W = −1.0, n = 8,8, P = 0.5), look at the stimulus (W = 4.0, n = 8,8, P = 0.39) or investigate the stimulus source (W = 0.50, n = 8,8, P = 1.0).
Behaviors that indicated the males were not attending to the infant distress stimulus were chirp vocals, long calls or looking out of the cage and away from the stimulus source. No significant difference was found between paired males and fathers for these behaviors either with low or high dose estradiol or low or high dose testosterone, Table 3.
Table 3.
Comparison of behaviors indicating the test male was not attending to the infant distress stimulus. No significant difference was found between paired males and fathers at low and high dose estradiol and testosterone treatment.
| Paired males | Fathers | ||||
|---|---|---|---|---|---|
|
| |||||
| Behavior | Dose | mean±sem | mean±sem | Mann-Whitney U | P |
| Chirp | Low E2 | 67.29 | 158.00 | 24.00 | 1.00 |
| High E2 | 108.10 | 119.60 | 23.50 | 0.95 | |
| Low T | 75.43 | 229.90 | 20.00 | 0.61 | |
| High T | 78.29 | 165.00 | 22.00 | 0.79 | |
|
| |||||
| Long Call | Low E2 | 27.71 | 11.86 | 16.00 | 0.30 |
| High E2 | 22.00 | 10.71 | 16.50 | 0.33 | |
| Low T | 24.83 | 4.86 | 10.00 | 0.12 | |
| High T | 28.50 | 10.29 | 10.50 | 0.14 | |
|
| |||||
| Look out cage | Low E2 | 40.29 | 19.14 | 21.00 | 0.70 |
| High E2 | 27.00 | 20.29 | 17.50 | 0.41 | |
| Low T | 30.14 | 22.71 | 18.50 | 0.48 | |
| High T | 22.29 | 20.29 | 22.50 | 0.85 | |
DISCUSSION
Based on our results, estradiol treatment significantly increased the response of all fathers that had responded to the infant distress cry, plus three males who had not in the vehicle treatment, but not for any of the paternally inexperienced paired males. Hormones are known to have an organizational effect on maternal care in rodents and increased estrogens along with progesterone promote the onset of maternal behavior (Bridges 1984). Additionally, estradiol has an activational effect on maternal care in primates. Infant directed behavior postpartum is correlated with elevated urinary estradiol during late pregnancy in the female red-bellied tamarin monkey, Saguinus labiatus (Pryce et al. 1988). Elevated estradiol in pigtail macaques, Macaca nemestrina, during late pregnancy is associated with increased maternal responsiveness after birth and estrogen treatment in ovariectomized rhesus females increases infant interaction significantly over no treatment (Maestripieri and Zehr 1998). In these studies where estradiol levels were assessed in pregnant females near term, progesterone levels were also elevated prior to parturition, and also likely responsible for maternal responsiveness. However, the estrogen treated-only rhesus ovariectomized females show higher infant handling rates without progesterone treatment and progesterone levels remained low throughout the estrogen treatment. Late pregnant common marmoset females where estradiol and progesterone ratio are maximal also increase infant responsiveness and non-pregnant females treated with progesterone and estradiol at levels of late pregnancy also have increased infant responsiveness (Pryce et al. 1993).
While bi-parental rodents lack evidence of serum estrogens changing with parental status, cotton-top tamarin fathers show significantly increased estrogens in the final month of their mate’s gestation (Ziegler et al. 2004) and estradiol remains elevated in marmoset males after their infants are born (Ziegler et al. 2009). In our study an organizational effect on paternal care by estradiol treatment was not seen in marmoset males since the paired males did not show an effect of the estradiol treatment. However, our results illustrate that estradiol does enhance paternal infant responsiveness in experienced fathers. Our study was a causal study to determine if estrogen treatment would enhance male’s responsiveness, or motivation, to interact with an infant stimulus. Only two of the eight fathers never showed a response to the infant stimulus in any estrogen treatment condition and three fathers showed an onset of responding with the low estradiol treatment. Other studies on estrogen and paternal care have monitored changes in estrogen levels prior to and following human infant-father interaction and shown no significant change in estrogen with specific behaviors (Gettler et al. 2013). A different species of marmoset, Callithrix geoffroyi, has also shown no significant change in estrogen metabolites with paternal responsiveness (Cavanaugh & French 2013). The lack of change in estrogens with paternal behaviors does not necessarily indicate estrogens are not involved. Estrogens may be elevated during the postpartum period in males and not changing in response to father-infant interactions. While estrogen is likely involved in onset of paternal care, they may not act alone or the actual learning experience provides the foundation for which the steroid changes act.
In the bi-parental species of rodent, Phodopus campbelli, females express higher levels of the estrogen receptor, ERα in the maternal regions of the brain such as the medial pre-optic area, bed nucleus of the stria terminalis and the media amygdala than the paternal male (Timonin et al. 2008). Conversely, in the California mouse, Peromyscus californicus, both the medial preoptic area and the basolateral amygdala have a role in male and female parental behaviors (Lee & Brown 2007) indicating receptor activation but not necessarily via estradiol. Little is known about the estrogen receptor distribution in bi-parental marmosets or whether paternal primates have increased activation in the paternal brain areas (Saltzman and Ziegler, 2014).
Experience appears to be the primary mediator of motivation for paternal care in common marmoset males. Males who have been fathers show higher motivation to respond to infants. Parentally experienced fathers have higher hormonal changes than first-time fathers (Ziegler et al. 2004) and show long-term changes in dopamine and oxytocin in their hypothalamus as well as elevated prolactin (Woller et al. 2012). Even past experience of caring for younger siblings has not been linked to an increased responsiveness to infants in marmosets until our current results (Zahed et al. 2008; Ziegler et al. 2009) and there is no evidence to date that paternal behavior in marmosets is regulated by developmental effects of estrogen on the brain during early life (Timonin et al, 2008). While it is unclear how the initial experience with becoming a father would induce the onset of physical and behavioral changes, there is some evidence that it comes from the pair bonding and close association with the mother (Ziegler et al. 2004; Berg and Wynne-Edwards 2001).
We found that not all of the experienced fathers showed the salient behaviors directed towards the infant stimulus. Therefore, a wide variation occurs even within experienced fathers towards their response to infant cries. Fathers may provide other parenting behaviors but not show the motivation to respond the distress calls. The marmoset fathers who showed high levels of response to infant distress calls may provide benefits to their infant’s growth and social development. Fathers of bi-parental species, such as humans, some nonhuman primates and rodents, contribute significantly to offspring survival (Gubernick and Teferi 2000) and behavioral development through paternal investment (Braun and Champagne 2014). Extensive direct involvement of human fathers has been associated with positive cognitive and social behavioral development including improved weight gain, breastfeeding rates, higher receptive language skills and higher academic achievement (Garfield and Isacco 2006). Primate fathers likely have more plasticity in their learned or environmentally influenced paternal behaviors. Offspring may learn from their fathers parenting style or experience an epigenetic effect where highly responsive fathers have sons who will also be highly responsive to their infant’s needs.
We didn’t see any significant behavioral changes in either the paired males or the fathers due to the testosterone treatments. Increased testosterone didn’t increase behaviors that were related to their attention being directed away from the infant stimulus or infant directed behaviors. If testosterone had increased infant responsiveness in experienced males, then it would be likely due to testosterone aromatization to estrogen in the brain. We know that males reduce their testosterone and exhibit increased estrogens to infant olfactory stimulus (Ziegler et al. 2009). These differences between the marmoset and the California mouse may be due to the behavioral flexibility found in the primate or other factors or due to the dosing we used in this study.
We have validated the results we obtained in our previous work showing that males without paternal experience show significantly less response to the infant distress signal. The use of an infant distress call, or infant crying, is known to activate the anterior and posterior cingulate cortices in mothers during MRI and these brain structures are known to be involved in caregiving behavior (Lorberbaum et al. 2002). Additionally, infant cries are known to have a higher valence for parents than non-parents. Fathers are more responsive than non-fathers and fathers with lower testosterone are more responsive to infant cries. Those fathers who can physically respond to a crying infant have lower testosterone (Fleming et al. 2002; van Anders 2012). Fathers responding to infant cries activate the areas of the brain involved in empathy, anterior insula and inferior frontal gyrus (Mascaro et al 2014). Empathy reduces the aversive components of the cry stimulus and appears to be more effective in experienced fathers than nonfathers. Therefore, in humans as well as the marmosets, the response to infant distress calls is influenced by experience.
In conclusion, our treatment of male marmosets with either testosterone or estradiol did not induce the onset of paternal care behaviors. While mothers show induction of maternal behaviors with estradiol changes, this did not occur in male marmosets without parental experience. Only experienced fathers show increased responsiveness to infant distress calls with the estradiol treatment. We suggest that parental experience is important for the production of parenting behaviors in male marmosets.
Highlights.
Fathers show large variability in responding to infant distress calls.
Treatment with estradiol increased the number of responsive fathers
No effect of estradiol treatment occurred in the parentally naïve paired males.
Testosterone treatment had no effect on either fathers or parentally naïve males.
Neither steroid influenced the onset of paternal motivation in the common marmoset.
Acknowledgments
Anita Ginther, Ph.D contributed to the organization of the data and descriptions of the infant vocal control during her postdoctoral work in the lab. We are grateful to Charles T. Snowdon for further assistance and advise on the use and description of infant vocal and control stimulus recordings. We thank Allison Bernard for her assistance in testing the marmosets. The expertise of the animal care staff for their special care of the marmosets are acknowledged. This work was funded by the NIH: HD057684 to TEZ and in part by the Wisconsin National Primate Research Center, P51OD011106 and the Institute for Clinical and Translational Research grant UL1TR000427.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Hearn JP. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978;53:155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- Braun K, Champagne FA. Paternal influences on offspring development: behavioural and epigenetic pathways. J Neuroendocrinol. 2014;26:697–706. doi: 10.1111/jne.12174. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiology and Behavior. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Biochemical basis of parental behavior in the rat. In: Rosenblatt JS, Snowdon CT, editors. Prental Care: Evolution, Mechanisms, and Adaptive Significance. Academic Press; San Diego: 1996. p. 715. [Google Scholar]
- Bridges RS. Neuroendocrine regulation of maternal behavior. Frontiers in Neuroendocrinology. 2015;36:178–196. doi: 10.1016/j.yfrne.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Murdoch T, Murphy PR, Moger WH. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, French JA. Post-partum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Horm Behav. 2013;63:551–558. doi: 10.1016/j.yhbeh.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson AF. Effects of testosterone propionate upon the sexual and aggressive behavior of adult male marmosets (Callithrix jacchus) castrated as neonates. Horm Behav. 1993;27:216–230. doi: 10.1006/hbeh.1993.1016. [DOI] [PubMed] [Google Scholar]
- Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299:551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Garfield CF, Isacco A. Fathers and the well-child visit. Pediatrics. 2006;117:e637–645. doi: 10.1542/peds.2005-1612. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Agustin SS, Kuzawa CW. Progesterone and estrogen responsiveness to father-toddler interaction. Am J Hum Biol. 2013;25:491–498. doi: 10.1002/ajhb.22396. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Prolactin, fatherhood, and reproductive behavior in human males. Am J Phys Anthropol. 2012;148:362–370. doi: 10.1002/ajpa.22058. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Teferi T. Adaptive significance of male parental care in a monogamous mamal. Proc Biol Sci. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JK, Hearn JP. A positive feedback effect of oestradiol on LH release in the male marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978;52:83–86. doi: 10.1530/jrf.0.0520083. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Research. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring behaviour: An introductory guide Paul Martin and Patrick Bateson, Cambridge University, Cambridge. Vol. 1986. 1987. p. 200. Price: pound20.00 hardback 6.95 paperback, 1987/12/01 ed. [Google Scholar]
- Lee AW, Brown RE. Medial preoptic lesions disrupt parental behavior in both male and female California mice (Peromyscus californicus) Behavioral Neuroscience. 2002;116:968–975. doi: 10.1037//0735-7044.116.6.968. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Zehr JL. Maternal responsiveness increases during pregnancy and after estrogen treatment in macaques. Horm Behav. 1998;34:223–230. doi: 10.1006/hbeh.1998.1470. [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Gouzoules H, Lori A, Rilling JK. Behavioral and genetic correlates of the neural response to infant crying among human fathers. Soc Cogn Affect Neurosci. 2014;9:1704–1712. doi: 10.1093/scan/nst166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Prepartum changes in maternal responsiveness and nest defense in Tattus norvegicus. Journal of Vomp Psychol. 1984;98:177–188. [PubMed] [Google Scholar]
- Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav. 1970;5:1373–1377. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Poindron P, Levy F, Krehbiel D. Genital, olfactory, and endocrine interactions in the development of maternal behavior in the parturient ewe. Psychoneuroendo. 1988;13:99–125. doi: 10.1016/0306-4530(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Prudom SL, Broz CA, Schultz-Darken N, Ferris CT, Snowdon C, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus) Biol Lett. 2008;4:603–605. doi: 10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Abbott DH, Hodges JK, Martin RD. Maternal behavior is related to prepartum urinary estradiol levels in red-bellied tamarin monkeys. Physiol Behav. 1988;44:717–726. doi: 10.1016/0031-9384(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dobeli M, Martin RD. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J Comp Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, Pfaff DW. siRNA silencing of estrogen receptor-alpha expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc Natl Acad Sci. 2012;109:16324–16329. doi: 10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51:731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. The development of maternal responsiveness in the rat. Am J Orthopsychiatry. 1969;39:36–56. doi: 10.1111/j.1939-0025.1969.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Ziegler TE. Functional significance of hormonal changes in mammalian fathers. J Neuroendocrinol. 2014;26:685–696. doi: 10.1111/jne.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt Estrogen-induced maternal behavior in hysterectomized-overictomized virgin rats. Physiol Behav. 1975;14:465–471. doi: 10.1016/0031-9384(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Ziegler TE. Variation in prolactin is related to variation in sexual behavior and contact affiliation. PLoS One. 2015;10:e0120650. doi: 10.1371/journal.pone.0120650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM. Maternal behavior priming in virgin and caesarean-delivered Long-Evans rats: effects of brief contact or continuous exteroceptive pup stimulation. Physiol Behav. 1983;31:757–763. doi: 10.1016/0031-9384(83)90271-8. [DOI] [PubMed] [Google Scholar]
- Timonin ME, Cushing BS, Wynne-Edwards KE. In three brain regions central to maternal behavior, neither male nor female Phodopus dwarf hamsters show changes in oestrogen receptor alpha distribution with mating or parenthood. J Neuroendocrinol. 2008;20:1301–1309. doi: 10.1111/j.1365-2826.2008.01797.x. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behavior in a monogamous mammal via conversion to oestrogen. Royal Society Proceedings Biological Science. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anders SM, Tolman RM, Volling BL. Baby cries and nurturance affect testosterone in men. Horm Behav. 2012;61:31–36. doi: 10.1016/j.yhbeh.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Sosa ME, Chiang Y, Prudom SL, Keelty P, Moore JE, Ziegler TE. Differential hypothalamic secretion of neurocrines in male common marmosets: parental experience effects? J Neuroendocrinol. 2012;24:413–421. doi: 10.1111/j.1365-2826.2011.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus) Am J Primatol. 2008;70:84–92. doi: 10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Gandelman R, Denenber VH. Prolactin; Is it an essential hormone for maternal behavior in the mammal? Hormones and Behavior. 1971;2:343–354. [Google Scholar]
- Ziegler TE, Prudom SL, Schultz-Darken NJ, Kurian AV, Snowdon CT. Pregnancy weight gain: marmoset and tamarin dads show it too. Biol Lett. 2006;2:181–183. doi: 10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner F. Prolactin’s mediative role in male parenting in parentally experienced marmosets (Callithrix jacchus) Horm Behav. 2009;56:436–443. doi: 10.1016/j.yhbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate’s pregnancy. Horm Behav. 2004;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wegner FH, Snowdon CT. Hormonal responses to parental and nonparental conditions in male cotton-top tamarins, Saguinus oedipus, a New World primate. Horm Behav. 1996;30:287–297. doi: 10.1006/hbeh.1996.0035. [DOI] [PubMed] [Google Scholar]