Abstract
Our knowledge of mechanisms responsible for both the development and maintenance of hypertension remains incomplete in the Goldblatt model (two kidney one clip, 2K1C). We tested the hypothesis that elevated sympathetic nerve activity (SNA) occurs before the onset of hypertension in 2K1C rats considering the time course of increase in SNA in relation to the onset of the hypertension is determined. We used a decorticated in situ working heart-brainstem preparation of male Wistar rats comprising: SHAM (n=7), three weeks post 2K1C, of which some were hypertensive (2K1C-H, n=6) and others normotensive (2K1C-N, n=9), as determined in vivo a priori. Perfusion pressure was higher in both 2K1C groups (2K1C-H: 76 ± 1; 2K1C-N: 74 ± 3 vs SHAM: 60 ± 2 mmHg, p< 0.05). The SNA was elevated significantly in both 2K-1C groups (2K1C-H: 47.7 ± 6.1; 2K1C-N: 32.8 ± 2.8 vs SHAM: 20.5 ± 2.5 μV, p< 0.05) due to its increased respiratory modulation; the chemoreflex was augmented and baroreflex depressed. Pre-collicular transection reduced SNA in all groups (2K1C-H: −32.5 ± 7.5, 2K1C-NH: −48 ± 6.9 vs SHAM: −13.2 ± 1%, p< 0.05). Subsequent medullary-spinal cord transection abolished SNA in both SHAM and 2K1C-N but only decreased it by 57± 5.5% in 2K1C-H. Thus, SNA is raised prior to hypertension onset by the 3rd week after renal artery clipping and this originates, in part, from its enhanced respiratory modulation. Spinal circuits contribute to the elevation of SNA in the 2K1C model but only when after hypertension has developed.
Keywords: Goldblatt model, hypertension, sympathetic nerve activity, respiratory modulation
Introduction
Renovascular hypertension is considered one of the most frequent causes of secondary hypertension. Its prevalence is estimated between 1-5% of the general population of hypertensives (Elliott, 2007). One of the most widely used models for the study of experimental hypertension has been proposed by Goldblatt (Goldblatt et al. 1934), which is renovascular hypertension induced by partial stenosis of a renal artery by implantation of a silver clip and called 2 Kidneys – 1 Clip (2R1C) (Goldblatt et al. 1934; Edmunds et al. 1991; Melaragno & Fink, 1996). Studies have shown that several factors are involved in the pathogenesis of hypertension including an elevation of renin-angiotensin-aldosterone system (RAAS) activity in which the cascade of events begins with increased renin secretion from the clipped kidney leading to increases in circulating angiotensin II (Ang II), sodium retention, blood volume expansion, increased peripheral arterial resistance and elevated activation of sympathetic nerve activity (SNA), all of which increase blood pressure (BP) (Plotw, 1983; Michell & Navar, 1995; Navar et al. 1998; Lerman et al. 2005).
Several studies have suggested a role of elevated SNA in the development of hypertension in 2K1C rats. One possible explanation for sympathetic activation is the central action of Ang II circulating in the periventricular tissue of the anterior ventral region of the third ventricle (AV3V). Brody and Johnson (1980) demonstrated that the electrolytic lesion AV3V region prevents or abolishes hypertension 2K1C. In 1982, Katholi demonstrated that renal denervation of the ischemic kidney improves hypertension in animals 2K1C independent of the levels of circulating angiotensin II and excretion of water and sodium, indicating participation of renal nerves (afferent and efferent) in this model. Later, Faber & Brody (1983), reported increased sympathetic tone in this model of hypertension. McElroy & Zimmerman (1989) demonstrated an increase of α1-adrenergic receptors affinity in the arterial vasculature of clipped kidney (2K1C) 2 weeks after clipping. At six weeks, the affinity of these receptors are found increased in both kidneys when compared to control animals, however, at 12 weeks there was no difference. Similar data were also found by Nakada et al. (1996), which showed increased SNA 4 weeks post-clipping animals. Finally, Johansson et al. (1999), also reported an increase in SNA in hypertensive humans with renovascular hypertension by using 2 independent techniques for examination of sympathetic outflow. Overall sympathetic outflow, as assessed from measurements of total body NE spillover, was increased 2-fold compared with healthy controls and correlated closely with muscle sympathetic nerve activity (MSNA), supporting the contention of an elevated state of efferent sympathetic traffic in renovascular hypertension. We reported that 6 weeks after renal artery clipping in rats, the 2K1C group showed a significant increase in renal SNA (and arterial pressure) when compared with the control group in urethane anaesthetized rats (Oliveira-Sales et al. 2008, Campos et al., 2015). Additionally, the increase in SNA in the 2K1C model seems to have both a direct and indirect mechanism both operating via the RAAS; indirectly sympathetic activation produces greater release of renin (Miyajima et al. 1991; Johansson et al. 1999; Head & Burke, 2004). Burke et al. (2010) found that chronic rilmenidine (an imidazoline and α2-adrenoceptor agonist) treatment not only normalized arterial pressure and reduced renal SNA, but that it also markedly reduced basal plasma renin activity. This suggests that rilmenidine is an effective antihypertensive therapy for renovascular hypertension. Furthermore, the sympathetic component may also be involved in other mechanisms, such as increased sodium resorption (Dibona & Kopp, 1997). However, whether renal or autonomic dysfunction is the predominant contributor to systemic hypertension is still debated (Esler et al. 2010; Navar, 2010) and whether elevated SNA is a cause of high blood pressure in renovascular hypertension remains unknown and controversial.
Previously, we described the temporal profile of underlying cardiovascular autonomic changes that occur in the Goldblatt rat model of hypertension followed out to 6 weeks post-clipping (Oliveira-Sales et al. 2014). The parameters analysed were power spectral density analysis of both arterial pressure and pulse interval, and spontaneous cardiac baroreceptor reflex gain in conscious freely-moving rats fitted with radio-telemetry devices. Our findings indicated that the 2K1C Goldblatt model is associated with an immediate loss of cardiac baroreflex sensitivity accompanied with an apparent withdrawal of cardiac vagal tone, and increase in neurohumoral control of arterial pressure. By around the fourth week post clipping there was a large increase in low frequency spectra of systolic blood pressure, indicative of an elevated sympathetic vasomotor drive, that accompanied the most steep development of the hypertension (Oliveira-Sales et al. 2014). This sympathetic hyperactivity was also observed when measured directly from the renal nerve in anesthetized animals 6 weeks after clipping (Oliveira-Sales et al. 2008), suggesting that the maintenance phase of the hypertension was also dependent on high sympathetic drive. However, exactly when the increase in SNA was initiated relative to the onset of the hypertension could not be determined in our previous studies (Oliveira-Sales et al. 2010, 2014), as we were limited to the relatively insensitive and indirect measurement of SNA as inferred from power spectral analysis of systolic blood pressure. Moreover, the relative contributions of brainstem and spinal cord mechanisms in the generation of the increased SNA remain unknown.
In the present study, our hypothesis was that SNA is elevated before the development of hypertension in the Goldblatt rat model and this is mediated, in part, by greater modulation from the central respiratory pattern generator; the latter mechanism has been seen in at least two other models of hypertension (Moraes et al. 2014; Simms et al. 2009; Zoccal et al. 2008). Therefore, we analyzed cardiovascular autonomic function in the third week after renal artery clipping to assess its relationship with the onset of hypertension in both in vivo and a non-anaesthetized decorticated in situ rat preparations. At this stage, blood pressure and sympathetic modulation of the cardiovascular system increases, however, it is not well established if the blood pressure increases before or after sympathetic overactivity (Oliveira-Sales et al. 2014).
Methods
The authors have read, and the experiments comply with, the policies and regulations of Experimental Physiology given by Grundy (2015).
Animals
All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were approved by the University of Bristol Ethical Review Committee (Personal Licence No. 30/8202). Weaned juvenile male Wistar rats (n=22, 30g; Charles River Laboratories, UK) were housed individually, allowed normal rat chow and drinking water ad libitum, and kept on a 12-hour light/12-hour dark cycle.
Experimental Protocol
In the first day of the protocol the left kidney was clipped in juvenile rats and three weeks later, the systolic arterial pressure (SBP) was measured before making the working heart-brainstem preparation (WHBP) by inserting an arterial catheter, in vivo under anesthesia (halothane). Once these data were acquired rats were decorticated to make insentient and perfused arterially. The perfusion pressure (PP), heart rate (HR), thoracic sympathetic nerve activity (tSNA) and phrenic nerve activity (PN) were measured during the basal conditions, peripheral chemoreceptor and baroreceptor reflexes were assessed, two sequential spinal transections were performed and the nerves were recorded as well.
Goldblatt model of hypertension (two kidney one clip, 2K1C)
The rats were anesthetized with ketamine (60 mg/kg) and medetomidine (250 μg/kg) intramuscularly. The level of anaesthesia was checked frequently by testing limb withdrawal reflexes to noxious pinching and additional doses of 0.1 mL were administered (i.m.) as required. The temperature of the rat was maintained at 37°C using a feedback controlled heating blanket. Via a midline laparotomy, the left renal artery was obstructed partially with a silver clip of 0.1 mm width. The SHAM animals were submitted to the same surgical procedure without clip placement and partial renal artery occlusion. Anesthesia was reversed with atipamezole (1 mg/kg).
In vivo blood pressure recording
Pulsatile arterial blood pressure was measured exactly 3 weeks after renal artery clipping via an arterial catheter in conscious rats. Rats, now weighing 60–90 g, were anaesthetized with halothane (3%) until loss of their paw withdrawal reflex and the femoral artery cannulated so that the catheter tip lay in the descending aorta. Blood pressure was measured 10 min after termination of anesthesia for 5 minutes when the animals remained sedated but not anesthetised. In all cases rats responded to a mild pinch of the tail or rear hind paw. This was done to ensure a similar behavioural state across all rats. The data were digitized using a CED 1401 A–D interface (CED, Cambridge Electronic Design, Cambridge, UK) and a computer running Spike 2 software (CED).
In situ working heart–brainstem preparation (WHBP)
After measuring arterial pressure, recordings of the thoracic sympathetic chain activity were performed as described previously (Paton, 1996; Antunes et al. 2006; Colombari et al. 2010). Briefly, rats were heparinized (1000 units, given i.p.) and subsequently re-anaesthetized deeply with halothane (5%) until loss of their paw withdrawal reflex. They were transected sub-diaphragmatically, immersed in carbogenated (95% O2 and 5% CO2) modified Ringer’s solution containing in mM: NaCl 120, NaHCO3 24, KCl 3, CaCl2 2.5, MgSO4 1.25, KH2PO4 1.25, glucose 10; pH 7.3 at 10°C, and decorticated, making them insentient. The phrenic nerve was cut distally. Preparations were transferred to a recording chamber. The descending aorta was perfused via a double-lumen cannula (DLR-4, Braintree Scientific, MA, USA) at a flow rate of 21 ml/min of Ringer solution containing Ficoll (2.5%) and carbogen (95% O2, 5% CO2) gassed at 32°C. The second lumen of the catheter monitored aortic perfusion pressure. Perfusion was supplied via a peristaltic roller pump (Watson Marlow 505D). Vasopressin was added to the perfusate to elevate perfusion pressure; an equivalent amount (400 pM) was used in all groups. The phrenic nerve and thoracic sympathetic chain (t; T8–T10 spinal level) were recorded simultaneously via bipolar glass suction electrodes; neural signals were amplified and filtered (100 Hz-1.5 kHz and 50 Hz-1.5 kHz, respectively, Neurolog). Nerve recordings were rectified, integrated and smoothed (50 ms). To standardize the data across preparations, noise levels were removed from the raw signal by applying lidocaine (2%) to the sympathetic chain at the end of the experiment; the noise level was measured and subtracted digitally.
Peripheral chemoreceptor and baroreceptor reflexes
Peripheral chemoreceptors were stimulated using sodium cyanide (NaCN; 0.03%, 25, 50 and 75μL bolus) injected into the aorta. The peripheral chemoreceptor reflex consisted of an increase in central respiratory drive, bradycardia, increased tSNA and a pressor response. The baroreflex was activated by perfusion pressure challenges (generated by altering the perfusate flow). Initial studies used flow steps to increase perfusion pressure (by 30 mmHg over 1 s) from a range of different baseline pressures (30–80 mmHg, by adjusting the baseline flow) comparable to that described previously (Paton & Kasparov, 1999).
Brain transection in situ
Two sequential transections were performed using a scapel blade: (i) pre-collicular transection (to disconnect the hypothalamus) and (ii) medulla oblongata–spinal cord junction. Transections were confirmed histologically.
Data Analysis
Data were digitized using a CED 1401 A–D interface (CED, Cambridge Electronic Design, Cambridge, UK) and a computer running Spike 2 software (CED) with custom-written scripts for data acquisition and on- and off-line analyses. tSNA changes were expressed as absolute levels and peak amplitude. Phrenic-triggered averaging (30 consecutive cycles) of integrated tSNA was carried out off-line as per Simms et al. (2009) (Simms et al. 2009). The respiratory cycle was divided into inspiratory and expiratory periods to assess the area under the curve of tSNA during these phases. To analyse the peripheral chemoreceptors reflex the tSNA changes were expressed as percentage difference from baseline, peak amplitude and duration. The time constant of 1 s was used to calculate the integrated SNA. From the dynamic biphasic pressure ramps (over 30 s) a baroreflex–function curve was constructed for SNA activity by fitting a logistic sigmoid (Prism5, Graphpad, CA, USA): y = P4 + P1/1 + exp(P2(x − P3)) where y =SNA, P1 =range; P2 =slope coefficient; P3 =perfusion pressure at 50% (PP50%); P4 =minimum; x =perfusion pressure. The curves were fitted to integrated SNA (time constant of 1 s) averaged into 1 s bins (n =30), measured over the extent of the ramp. The peak baroreflex gain was calculated from the first derivative.
Data presented are the mean ± Standard Error (S.E.M.). Data were compared using One-way ANOVA followed by Newman Keuls’s for comparisons (statistical software Graphpad Prism 5.0). The level of statistical significance was defined as P < 0.05.
Results
In vivo measurements
Arterial blood pressure and heart rate measured 3 weeks after renal artery clipping
In 15 rats with a clipped renal artery, we observed after 3 weeks that just some animals (n = 6) exhibited an increase in SBP (i.e. 184 ± 11 mmHg; P<0.0001). In the other 9 animals, SBP was 106 ± 3 mmHg and not different to SHAM controls (98 ± 3 mmHg) (Figure 1A). So, we separated rats into 3 groups: hypertensive 2K-1C rats (2K1C-H, n=6), normotensive 2K1C (2K1C-N, n=9) and SHAM (n=7). In all rat groups operated, we verified that the clip was securely on the renal artery with no notable difference in its position or orientation. A significant increase in the diastolic blood pressure (DBP) also occurred at 3 weeks post clipping in 2K1C-H versus both the SHAM and 2K1C-N groups (158 ± 22 vs 70 ± 4 vs 78 ± 4 mmHg, respectively; P<0.05; Figure 1B). The values of HR were unchanged in all groups (SHAM: 312 ± 8; 2K1C-N: 302 ± 6; 2K1C-H: 314 ± 6 bpm), Figure 1C.
Figure 1.
Cardiovascular parameters measured in SHAM and 2 Kidney-1Clip (2K-1C) rats in vivo. [A] Systolic blood pressure (SBP), [B] diastolic blood pressure (DBP) and [C] heart rate (HR) measured before the experimental protocol by the direct method via an arterial catheter. *P<0.05 different from SHAM group; † P<0.05 different from Normotensive 2K1C (One-way ANOVA followed by the Newman Keuls’s post-test). [D] Perfusion pressure (PP) measured during the in situ working heart brainstem preparation of SHAM rats (n = 7) and 2K1C (3 weeks) of which some were hypertensive (2K1C-H, n = 6) or and others normotensive (2K1C-N, n = 9). PP was measured at same perfusion flow rates in all 3 groups. Values are expressed as the means ± SEM.
In situ measurements
Perfusion pressure
Since flow rate and vasopressin used were similar in all three rat groups, differences in PP can be expected to reflect alterations in peripheral vascular resistance directly. The PP was significantly higher in both 2K1C groups (2K1C-H: 76 ± 0.4 mmHg; 2K1C-N: 74 ± 3.0 mmHg) versus SHAM (62 ± 3 mmHg, P<0.05, Figure 1D).
Sympathetic activity is raised 3 weeks after renal artery clipping independent of the hypertension and has increased respiratory modulation in situ
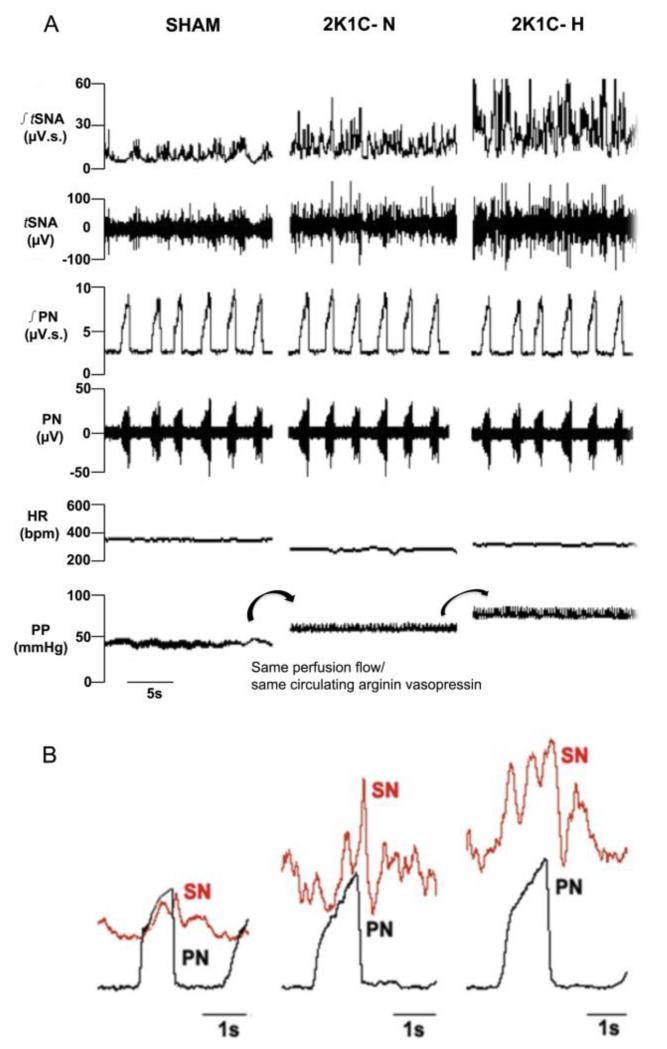
Figure 2A shows representative recordings from a SHAM, 2K1C-N and 2K1C-H rats indicating elevated perfusion pressure in the 2K1C-N rat and further elevated in 2K1C-H rat compared to SHAM indicative of increased peripheral vascular resistance in the 2K1C groups.
Figure 2.
In situ sympathetic activity 3 weeks after renal clipping. [A] Representative recordings from SHAM, normotensive 2 Kidney-1Clip (2K1C-N) and hypertensive 2 Kidney-1Clip (2K1C-H) rat, showing basal values in integrated thoracic sympathetic nerve activity (∫tSNA), frequency of tSNA, integrated phrenic nerve (∫PN), heart rate (HR) and perfusion pressure (PP). [B] Representative phrenic nerve cycle-triggered averages (CTA) of sympathetic nerve (SN) activity in SHAM, 2K1C-H and 2K1C-N groups. Each CTA panel comprises 30 PN cycles.
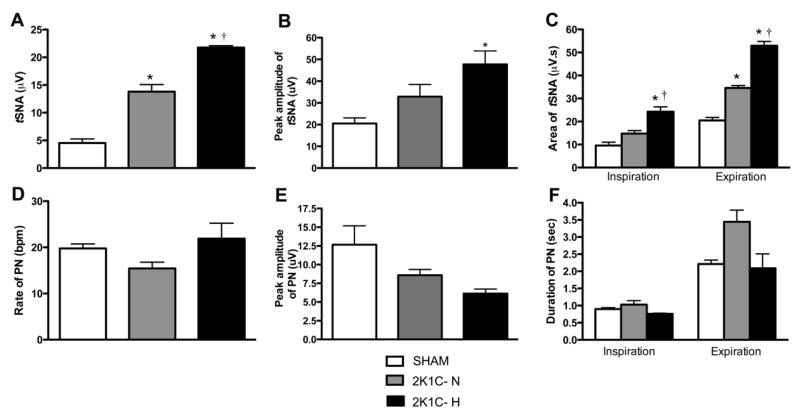
An elevation in tSNA preceded the hypertension recorded in vivo in the Goldblatt model. However, there were qualitative differences between hypertensive and normotensive 2K1C groups. tSNA was significantly raised in both 2K1C groups relative to SHAM (2K1C-H: 21.7 ± 0.3 vs SHAM: 4.5 ± 0.7 μV, P<0.05, 2K1C-N: 13.8 ± 1.2 vs. SHAM, P<0.05, Figure 3A). As shown in Figure 3A, the tSNA was higher in the hypertensive 2K1C-H (i.e. 21.7 ± 0.3 vs 2K1C-N: 13.8 ± 1.2, P<0.05). Correspondingly, both 2K1C groups exhibited a higher peak burst amplitude than the SHAM group (2K1C-H: 47.7 ± 6.1; 2K1C-N: 32.8 ± 5.6 vs SHAM: 20.5 ± 02.5 μV, P<0.05, Figure 3B). tSNA showed clear respiratory modulation with peak activity coincident at the end of inspiration and start of expiration in all three rat groups (Figure 2B). However, the respiratory modulation of tSNA was increased in both Goldblatt rat groups. For example, there was a significant increase in the area under the curve of tSNA during inspiration in both 2K1C rat groups relative to SHAM and the hypertensive Goldblatt group showed greater modulation compared to the normotensive 2K1C group too: (Inspiratory modulation: 2K1C-H: 24.2 ± 2.1 vs. 2K1C-N: 14.7 ± 1.2 μV.s, P<0.05; 2K1C-H vs SHAM: 9.5 ± 1.4 μV.s−1, P<0.05 and 2K1C-N vs SHAM, P<0.05). Differences during early expiration were also exaggerated (Expiratory modulation: 2K1C-H: 52.9 ± 1.8 vs. 2K1C-N: 34.5 ± 1.0 μV.s−1, P<0.05; 2K1C-H and 2K-1C-N vs. SHAM: 20.4 ± 1.3 μV.s−1, P<0.05, Figure 3C).
Figure 3.
Sympathetic activity is raised 3 weeks after renal artery clipping and shows increased respiratory modulation. [A] Graphs show thoracic sympathetic nerve activity (tSNA), [B] peak of amplitude of tSNA burst, [C] area of tSNA burst during inspiration and expiration, [D] phrenic nerve activity (PN), [E] peak of amplitude of PN burst and [F] duration of phrenic bursts (inspiration) and period between bursts (expiration). Values are expressed as mean ± SEM. *P<0.05 different from SHAM group; † P<0.05 different from 2K1C-N. (One-way ANOVA followed by the Newman Keuls’s post-test).
Phrenic Nerve Motor Output in Goldblatt rats
The frequency of phrenic nerve discharge was unchanged in all groups (2K1C-H: 21.9 ± 3.3; 2K1C-N: 15.4 ± 1.3 vs SHAM: 19.7 ± 0.9 bursts per min, Figure 3D). Additionally, inspiratory time did not differ significantly among groups (2K1C-H: 0.7 ± 0.02; 2K1C-N: 1.0 ± 0.1; SHAM: 0.9 ± 0.03 s), and expiratory time was unchanged too (2K1C-N: 3.0 ± 0.2 vs SHAM: 2.2 ± 0.1 sec P<0.05, Figure 3F). In contrast, the peak amplitude of phrenic activity was lower in hypertensive animals (2K1C-H: 6.1 ± 0.5; 2K1C-N: 8.5 ± 0.7; SHAM: 12.6 ± 2.5 μV, Figure 3E).
Peripheral chemoreceptor and arterial baroreceptor reflexes
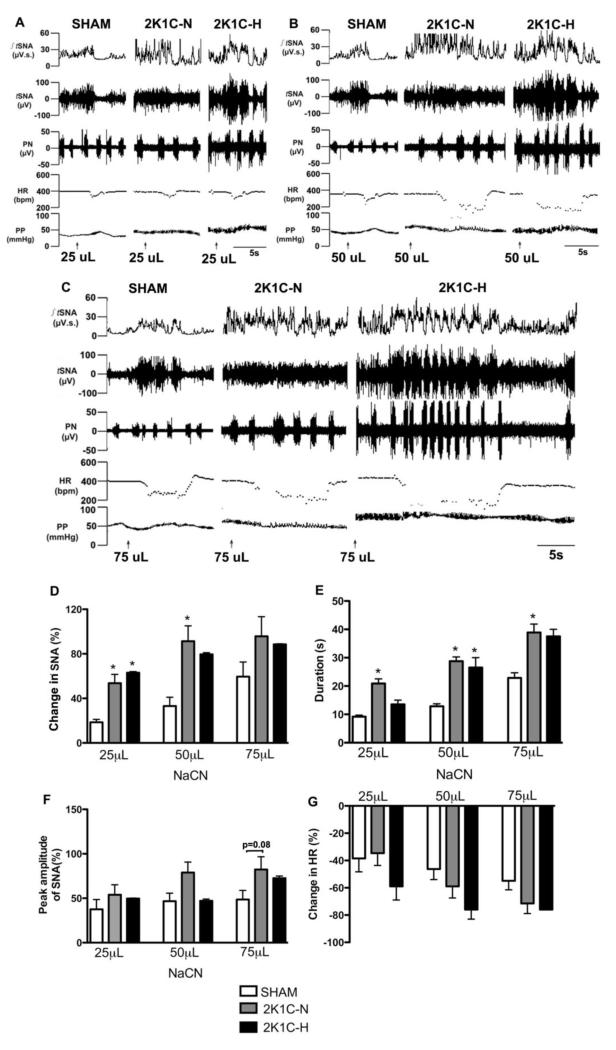
Figure 4A,B,C shows the representative recordings of the peripheral chemoreceptor reflex in SHAM, 2K1C-N and 2K1C-H rats. The chemoreflex evoked increased the percentage difference from baseline and duration of SNA in the 2K1C rat groups compared to SHAM (P<0.05, Figure 4D,E). However, the chemoreflex induced change in peak amplitude of SNA and bradycardia did not differ among the three rat groups (Figure 4F and 4G), only 2K1C-H vs. SHAM at 75uL showed a tendency of different statistically (p=0.08).
Figure 4.
The chemoreflex evoked increase in both amplitude and duration of SNA were both potentiated in the 2K1C rat groups compared to SHAM. Representative recordings from SHAM, 2K1C-N and 2K1C-H rat, showing values in integrated thoracic sympathetic nerve activity (∫tSNA), frequency of tSNA, frequency of phrenic nerve (PN), heart rate (HR) and perfusion pressure (PP) during infusion of 25 uL [A], 50 uL [B] and 75 uL of NaCN [C]. Mean percent changes from control sympathetic chain activity (tSNA) during chemoreflex activation induced by increasing doses of NaCN in SHAM, 2K1C-N and 2K1C-H [D]; Duration of responses [E]; Changes in peak amplitude of tSNA [F]; Changes in heart rate (HR) [G]. *P<0.05 different from SHAM group (One-way ANOVA followed by the Newman Keuls’s).
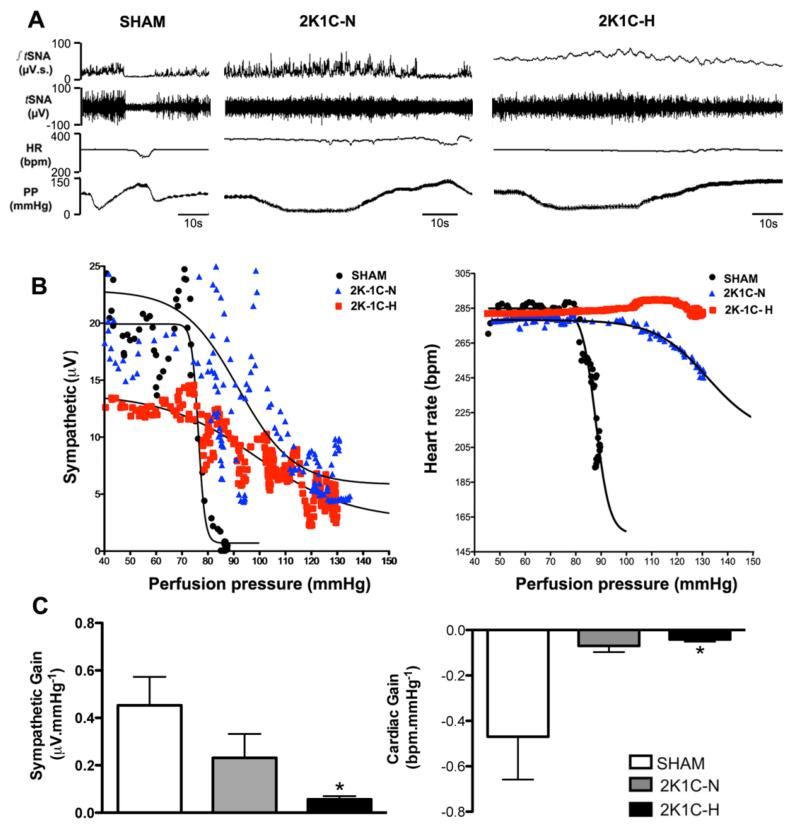
Figure 5A shows representative recordings from SHAM, 2K1C-N and 2K1C-H rats during baroreflex testing. Logistic sigmoid baroreflex function curves were fitted to the integrated sympathetic and HR data and showed an impaired baroreflex in the hypertensive animals (Figure 5B). The sympathetic gain decreased in the 2K1C-H and 2K-1C-N groups when compared to the SHAM group (2K1C-H:0.05 ± 0.01; 2K1C-N: 0.23 ± 0.1 and SHAM:0.45 ± 0.11 μV.mmHg−1); the difference between 2K1C-H vs SHAM was significant (p<0.05). In addition, the cardiac baroreflex gain was reduced in both 2K1C groups compared to the SHAM group (2K1C-H:-0.04 ± 0.009; 2K1C-N: −0.06 ± 0.02 and SHAM:−0.47 ± 0.1 bpm.mmHg−1; 2K1C-H vs. SHAM, p<0.05) (Figure 5C) .
Figure 5.
The sympathetic gain decreased in the hypertensive 2 Kidney-1Clip (2K1C-H) and normotensive 2 Kidney-1Clip (2K-1C-N) groups when compare to the SHAM group. [A] Representative recordings from SHAM, 2K1C-N and 2K1C-H rat, showing values in integrated thoracic sympathetic nerve activity (tSNA), frequency of tSNA, heart rate (HR) and perfusion pressure (PP) during baroreflex. [B] Logistic sigmoid baroreflex function curves were fitted to the sympathetic and heart rate data from one representative rat of each group SHAM, 2K1C-N and 2K1C-H. [C] Sympathetic and cardiac gain of SHAM, 2K1C-N and 2K1C-H groups. *P<0.05 different from SHAM group (One-way ANOVA followed by the Newman Keuls’s post-test).
Neural Circuits Involved in Generating Sympathetic Activity in Goldblatt rats
Pre-collicular transection reduced tSNA in all rat groups (2K1C-H: −32.5 ± 7.5; 2K1C-N: −48.0 ± 6.9 vs SHAM: −13.2 ± 1.0 % P<0.05). A subsequent transection at the medullary-spinal cord junction abolished tSNA in both SHAM and 2K1C-N groups. In contrast, tSNA in 2K1C-H rats was not completely abolished (−57.5 ± 5.5%, P<0.05, Figure 6A). Figure 4B shows a representative example of baseline sympathetic activity of SHAM, 2K1C-N and 2K1C-H rats. At the end of the experiment, we compared tSNA levels to those observed after lidocaine applied to the sympathetic chain (Figure 6B).
Figure 6.
Pre-collicular transection reduced SNA in all rat groups. Subsequent transection at the medullary-spinal cord abolished SNA in SHAM and normotensive 2 Kidney-1Clip (2K1C-N) but not hypertensive 2 Kidney-1Clip (2K1C-H) rats. [A] Graph shows changes in tSNA after pre-collicular and medullary-spinal cord transections in SHAM (n=5), 2K1C-N (n=5) and 2K1C-H group (n=2). *P<0.05 different from SHAM group; † P<0.05 different from 2K1C-N (One-way ANOVA followed by the Newman Keuls’s post-test. [B] Representative recordings baseline values and effect of pre-collicular transection and subsequent medullary-spinal cord transection and following lidocaine on integrated thoracic sympathetic nerve activity (∫tSNA) and frequency of tSNA from SHAM, 2K1C-N and 2K1C-H rats.
Discussion
The three major new findings of this study are: (i) on the 3rd week after clipping the renal artery, SNA is raised relative to SHAM operated animals which can either occur prior to the onset of hypertension or accompany it; (ii) the increased SNA is generated, in part, from enhanced respiratory modulation during late inspiratory and early expiratory phases; (iii) in hypertensive Goldblatt rats ~40% of sympathetic activity remains after dis-connecting the spinal cord from the brainstem.
Our aim was to determine when SNA increased relative to the onset of hypertension in the Goldblatt rat model. Based on our previous spectral density analysis of systolic blood pressure, we detected an increase in SNA around the fourth week post-clipping in conscious, radio-telemetered rats, a time coincident with the mid-point rise in arterial pressure (Oliveira-Sales et al. 2014). Herein, we studied rats exactly three weeks post-clipping, a time closer to the initiation point of the rise in arterial pressure, which occurred between the second and third week post-clipping (Oliveira-Sales et al. 2014). In 15 rats with a clipped renal artery, 6 were hypertensive and 9 normotensive relative to SHAM operated animals three weeks after clipping. Given that there was no obvious difference between rats that were hypertensive versus normotensive in terms of both the clip position/orientation and degree of sedation post-anaesthesia (see Methods), our interpretation is that three weeks post-clipping reflects the approximate time threshold for the elevation of blood pressure. However, we didn’t measure levels of circulating angiotensin. In fact, we believe that those animals that were clipped and did not show hypertension may well go on to develop hypertension.
We believe the WHBP is more physiological than an anaesthetised preparation, which the bulk of relevant literature is based on. Importantly, we have measured brain tissue oxygen and CO2 and pH (Wilson et al. 2001) and when these are within physiological limits eupneic breathing is generated (St-John & Paton, 2003). This has been critical in ensuring real time monitoring of the physiological viability of our preparation. Our lab has recorded sympathetic activity from conscious rats previously (McBryde et al. 2013) and this activity shows both baroreflex and respiratory modulated discharge, as we find in the in situ rat preparation (present study). The in situ preparation has shown nicely that the baroreflex is pressure threshold dependent as known in vivo and re-set in hypertension as seen in vivo and humans (Simms et al. 2007). The carotid chemoreflex evoked sympathetic activity is heightened in hypertension as reported in vivo (McBryde et al. 2013, Tan et al. 2010) and humans (Somers et al. 1988). Numerous studies of ours have shown that the activity in the preparation is dependent on drives from hypothalamus and RVLM as seen in vivo (Colombari et al. 2010; Oliveira-sales et al. 2010). The hypothalamic PVN responds to hyperosmolarity to increase sympathetic activity (Antunes et al. 2006). These are just some of the comparable data that support our contention that the WHBP model is usefully physiological. Most recent studies on chemoreflex in hypertension have translated directly across to conscious rats.
SNA elevation is independent of hypertension at onset of Goldblatt hypertension
In our in situ studies there was a significant elevation in SNA in all renal artery clipped rats relative to SHAM rats that was highest in those animals that had already developed hypertension. This leads to two deductions: first, SNA can be elevated ahead of Goldblatt hypertension. This finding supports the notion of a causative role of sympathetic activity in the initiation as well as the development and maintenance of the high blood pressure (Oliveira-Sales et al. 2014) and is consistent with data from the spontaneously hypertensive rat (Simms et al. 2009; Zoccal et al. 2008) and humans (Grassi, 2004a e 2004b; Smith et al. 2002; 2004). A further study will be needed to assess exactly when the SNA first shows elevated levels post-renal artery clipping. Second, the finding that SNA was higher in the 2K1C-H than the 2K1C-N rat group suggests that substantial levels of activity are needed to generate a rise in arterial pressure. If not contributing to high blood pressure this raises the issue as to the functional importance of the raised SNA in the 2K1C-N rat. In our in situ studies the latter group did show an elevated perfusion pressure relative to SHAM (and not different to 2K-1C-H group) suggesting that vascular resistance was already elevated, perhaps due to sympathetically mediated re-modelling/stiffening. This finding is of interest because in vivo, no hypertension was recorded in the 2K1C-N group. Reasons for this may include differences in: (i) cardiac output; (ii) circulating plasma levels of renin and angiotensin II; (iii) noradrenaline and/or co-transmitter released; (iv) sensitivity of receptors in the smooth muscle end-plate (McElroy & Zimmerman, 1989). An additional possibility is that the baroreceptor reflex was less attenuated in the 2K1C-N relative to 2K1C-H rats thereby capable of maintaining pressure within normal levels. The data in Figure 6 shows that whilst the baroreflex is partially attenuated compared to SHAM controls, both its sympathetic and cardiac limbs remain fully functional, which is not the case in 2K1C-H rats. It is clear from previous studies that angiotensin II (peripheral and/or central), which becomes elevated in the Goldblatt model, can attenuate baroreflex function (Polson et al. 2007; Tan et al. 2007; Kasparov et al. 1998; Paton & Kasparov, 1999) this mechanism could explain the data we report herein.
Novel mechanisms driving SNA in Goldblatt model of hypertension
There are likely to be multiple mechanisms involved in increasing SNA in the Goldblatt model and these are likely to change with time as the pathology matures. For example, many studies have demonstrated the role of renal afferents (Katholi et al. 1982; Campese et al. 2004), neurohumoral activation including elevated renin and angiotensin II activity for increasing SNA following clipping of a renal artery (Oliveira-Sales et al. 2014). There is also evidence for angiotensin II acting both via the circumventricular organs (to activate the subfornical organ) and via area postrema to modulate the other downstream cardiovascular control regions of the brain, such as nucleus of the solitary tract, paraventricular nucleus of the hypothalamus and rostral ventrolateral medulla resulting in elevated SNA and BP (Ku et al. 1999; Ferguson 2009; de Oliveira-Sales et al. 2010; Nishi et al. 2013; Biancardi et al. 2014). Certainly the present finding of a reduction in SNA after pre-collicular transection is consistent with excitatory drives from the diencephalon. However, in the in situ preparation there are no kidneys nor circulating renin or angiotensin II yet SNA remains elevated. This observation indicates that the role of renal afferents or forebrain structures is not necessarily important for the maintenance of high SNA post renal artery clipping (at least for several hours while the preparation remains viable). However, renal afferents and angiotensin II may play major roles in triggering the change in the central set point of SNA and its baroreflex control through long lasting mechanisms such as change in gene expression (Johnson et al. 2015).
Previously, we demonstrated a vital role for the rostral ventrolateral medulla in elevating arterial pressure in the Goldblatt rat and that this was entirely dependent upon a local increase in reactive oxygen species (Oliveira-Sales et al. 2008, 2010). In the normotensive 2K1C rat group, disconnection of the spinal cord from the medulla oblongata abolished all SNA suggesting a dependence on medullary sites such as the rostral ventrolateral medulla. In contrast, in the 2K1C-H rat, dis-connection of the medulla from the spinal cord did not abolish SNA; our data indicated that at least 40% of SNA could be maintained by neural circuitry in the spinal cord in the 2K1C-H group. How spinal circuitry is recruited in the 2K1C-H but not 2K1C-N group is not clear but may relate to the absolute level of SNA and an elevated intrinsic excitability of pre- and post- ganglionic sympathetic neurones. This could come about through sympathetic and /or angiotensin II mediated activation of the immune system affecting neurones within both the spinal cord and sympathetic chain (Marvar et al. 2010; Zubcevic et al. 2011; Zubcevic et al. 2014).
Given the recent interest in the carotid body and hypertension (Ratcliffe et al. 2014; McBryde et al. 2013; Tan et al. 2010; Peng, et al. 2014) we postulate that in the 2K1C rat groups the carotid bodies become sensitized, which is generally supported by our findings (Figure 5). In the spontaneously hypertensive rat, the carotid body generates aberrant discharge, which drives sympathetic nerve activity. Preliminary data suggest that carotid body resection stunts the development of hypertension in the Goldblatt rat (W. Pijacka and J.F.R. Paton – unpublished data). Why the peripheral chemoreceptors become activated in renovascular hypertension is unknown but could occur because of raised sympathetically mediated vasoconstriction in the carotid body itself, activation of glomus cells by angiotensin II (Allen, 1998; Lam & Leung, 2008) or local/systemic inflammation (Ackland et al. 2013).
Renal denervation as an anti-hypertensive strategy in renovascular hypertension
Radio frequency ablation of renal nerves has been used to interrupt both afferents from and sympathetic innervation to the kidney as an interventional anti-hypertensive therapy in drug-resistant hypertensive patients (Krum et al. 2014). Given the inability to select patient responders (Mahfoud et al. 2014; Persu et al. 2014; Barnes et al. 2014) it has been suggested to consider patients with renovascular or chronic kidney disease related hypertension (Papademetriou et al. 2014). However, based on our findings caution should be aired since SNA persisted in the absence of both renal nerves and the kidneys albeit only for several hours during the viability of the preparation. Nevertheless, the observation that a pathological condition can persist in the absence of the triggering mechanism is currently most pertinent and should be considered in any interventional approach. Moreover, elucidation of increased ‘layers’ of neural circuitry (diencephalon, pons-medulla and spinal cord) that become recruited as hypertension develops in renovascular hypertension informs that the target(s) may move and become increasingly more complex.
New Findings.
1. What is the central question of this study?
To evaluate whether elevated sympathetic nerve activity (SNA) occurs before the onset of arterial hypertension in a rat renovascular model of hypertension, the two kidney one clip (2K-1C) Goldblatt model, and to determine possible mechanisms and origins.
2. What is the main finding and its importance?
Sympathetic activity (SNA) can be raised prior to the onset of hypertension by the 3rd week after renal artery clipping and this originates, in part, from enhanced respiratory modulation. Spinal circuits contribute to the elevation of SNA but only when after hypertension has developed.
Acknowledgments
Funding
This work was supported by the Coordenação de Aperfeiçoamento de pessoal de Nivel Superior CAPES (Brazil) (#3496/07-4), the British Heart Foundation (RG/12/6/29670) and the National Institutes of Health (RO1 NS069220). EC was supported by (CNPq) - Conselho Nacional de Desenvolvimento Cientifico e Tecnologico and CAPES from Brazil and a Benjamin Meaker Fellowship awarded from the University of Bristol. JFRP was in receipt of a Royal Society Wolfson Research Merit Award.
Footnotes
Publisher's Disclaimer: This is an Accepted Article that has been peer-reviewed and approved for publication in the Experimental Physiology, but has yet to undergo copy-editing and proof correction. Please cite this article as an Accepted Article; doi: 10.1113/EP085390.
Competing Interests
None declared.
Author Contributions
Conception and format of the paper was made by EBO, EC and JFRP. Acquisition, analysis, or interpretation of data was made by EBO, EC, APA, RRC and JFRP. EBO drafted the article and EC, APA, RRC and JFRP revised it critically for important intellectual content. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
REFERENCES
- Ackland GL, Kazymov V, Marina N, Singer M, Gourine AV. Peripheral neural detection of danger-associated and pathogen-associated molecular patterns. Crit Care Med. 2013;41:85–92. doi: 10.1097/CCM.0b013e31827c0b05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM. Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity. J Physiol. 1998;1:510. doi: 10.1111/j.1469-7793.1998.773bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JFR. A spinal vasopressinergic mechanism mediate hyperosmolality-induced sympathoexcitation. J. Physiology. 2006;576:569–583. doi: 10.1113/jphysiol.2006.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63:303–308. doi: 10.1161/HYPERTENSIONAHA.113.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–579. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody MJ, Johnson AK. Role of the anteroventral third ventricle region in fluid and electrolyte balance, arterial pressure regulation, and hypertension. In: Martini L, Ganong WF, editors. Frontiers in Neuroendocrinology. Raven Press; New York: 1980. p. 249. [Google Scholar]
- Burke SL, Evans RG, Head GA. Effects of chronic sympatho-inhibition on reflex control of renal blood flow and plasma renin activity in renovascular hypertension. Br J Pharmacol. 2010;159:438–48. doi: 10.1111/j.1476-5381.2009.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese VM, Ye SO, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol. 2004;287:H695–H703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- Colombari E, Colombari DS, Sumners C, Raizada MK, Murphy D, Paton JFR. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases symapthoexcitatory responses to hyperosmotic stimuli in situ. Hypertension. 2010;56:956–963. doi: 10.1161/HYPERTENSIONAHA.110.155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C Wistar rat model. Am J Hypertens. 2010;23:708–15. doi: 10.1038/ajh.2010.64. [DOI] [PubMed] [Google Scholar]
- Dibona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- Edmunds ME, Russel GI, Bing RF. Reversal of experimental renovascular hypertension. Hypertension. 1991;9:289–301. doi: 10.1097/00004872-199104000-00001. [DOI] [PubMed] [Google Scholar]
- Elliott WJ. Secondary hypertension: renovascular hypertension. In: Black, Elliott WG, editors. Hypertension: a companion to Braunwald’s heart disease. Sanders-Elsevier; Philadelphia: 2007. pp. 93–105. [Google Scholar]
- Esler M, Lambert E, Schlaich M. Point: Chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010;109:1996–1998. doi: 10.1152/japplphysiol.00182.2010. [DOI] [PubMed] [Google Scholar]
- Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension. 1. The production of persistent elevation systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–378. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G. Counteracting the sympathetic nervous system in essential hypertension. Curr Opin Nephrol Hypertens. 2004a;13:513–519. doi: 10.1097/00041552-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des. 2004b;10:3579–3589. doi: 10.2174/1381612043382756. [DOI] [PubMed] [Google Scholar]
- Grundy D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Experimental Physiology. 2015;100:755–758. doi: 10.1113/EP085299. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL. Sympathetic responses to stress and rilmenidine in 2K1C rabbits. Evidence of enhanced nonvascular effector mechanism. Hypertension. 2004;43:636–642. doi: 10.1161/01.HYP.0000116301.02975.aa. [DOI] [PubMed] [Google Scholar]
- Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Zhang Z, Clayton SC, Beltz TG, Hurley SW, Thunhort RL, Xue B. The roles of sensitization and neuroplasticity in the long-term regulation of blood pressure and hypertension. Am J Physiol Regul Integr Comp Physiol. 2015 doi: 10.1152/ajpregu.00037.2015. doi: 10.1152/ajpregu.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparov S, Butcher JW, Paton JFR. Angiotensin II receptors within the nucleus of the solitary tract attenuate the baroreceptor vagal reflex in pre-weaned rats. J Auton Nerv Syst. 1998;74:160–168. doi: 10.1016/s0165-1838(98)00149-0. [DOI] [PubMed] [Google Scholar]
- Katholi RE, Winternitz SR, Oparil S. Decrease in peripheral sympathetic nervous system activity following renal denervation or unclipping in the one-kidney one clip Goldblatt hypertensive rat. Clin Invest. 1982;69:55–62. doi: 10.1172/JCI110441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- Ku YH, Jia YF, Chang YZ. Mechanisms underlying pressor response of subfornical organ to angiotensin II. Peptides. 1999;20:171–176. doi: 10.1016/s0196-9781(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Lam SY, Leung PS. A locally generated angiotensin system in rat carotid body. Regul Pept. 2002;107:97–103. doi: 10.1016/s0167-0115(02)00068-x. [DOI] [PubMed] [Google Scholar]
- Lerman LO, Chade AR, Sica V, Napoli C. Animals models of hypertension: an overview. J Lab Clin Med. 2005;146:160–173. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mahfoud F, Tunev S, Ruwart J, Schulz-Jander D, Cremers B, Linz D, Zeller T, Bhatt DL, Rocha-Singh K, Böhm M, Melder RJ. Efficacy and Safety of Catheter-Based Radiofrequency Renal Denervation in Stented Renal Arteries. Circ Cardiovasc Interv. 2014;7:813–20. doi: 10.1161/CIRCINTERVENTIONS.114.001506. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- McElroy ND, Zimmerman BG. Characterization of intrarenal arterial adrenergic receptors in renovascular hypertension. Hypertension. 1989;13:851–8. doi: 10.1161/01.hyp.13.6.851. [DOI] [PubMed] [Google Scholar]
- Melaragno MG, Fink GD. Slow pressor effect of angiotensin II in normotensive rats with renal artery stenosis. Clin Exp Pharmacol Phisiol. 1996;23:140–144. doi: 10.1111/j.1440-1681.1996.tb02585.x. [DOI] [PubMed] [Google Scholar]
- Mitchell KD, Navar LG. Hypertension: Pathophysiology, Diagnosis, and Management. Raven; New York: 1995. Intrarenal actions of Angiotensin II in the pathogenesis of experimental hypertension; pp. 1437–1450. [Google Scholar]
- Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension. 1991;17:1057–1062. doi: 10.1161/01.hyp.17.6.1057. [DOI] [PubMed] [Google Scholar]
- Moraes DJA, Machado BH, Paton JFR. Specific respiratory neuron types have increased excitability that drive pre-sympathetic neurones in neurogenic hypertension. Hypertension. 2014;63:1309–1318. doi: 10.1161/HYPERTENSIONAHA.113.02283. [DOI] [PubMed] [Google Scholar]
- Nakada T, Kubota Y, Suzuki H, Sasagawa I, Watanabe M, Ishigooka M. Supression of sympathetic nervous system attenuates the development of two kidney, one-clip Goldblatt hypertension. J Urol. 1996;156:1480–1484. [PubMed] [Google Scholar]
- Navar LG, Zou L, Thun AV, Wang CT, Iming JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- Navar LG. Counterpoint: Activation of the intrarenal renin-angiotensin system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010;109:1998–2000. doi: 10.1152/japplphysiol.00182.2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi EE, Bergamaschi CT, Oliveira-Sales EB, Simon KA, Campos RR. Losartan reduces oxidative stress within the rostral ventrolateral medulla of rats with renovascular hypertension. Am J Hypertens. 2013;26:858–65. doi: 10.1093/ajh/hpt037. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sales EB, Dugaich AP, Carillo BA, Abreu NP, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Oxidative stress contributes to renovascular hypertension. Am J Hypertens. 2008;21:98–104. doi: 10.1038/ajh.2007.12. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sales EB, Colombari DSA, Davisson RL, Kasparov S, Hirata AE, Campos RR, Paton JFR. Kidney-Induced Hypertension Depends on Superoxide Signaling in the Rostral Ventrolateral Medulla. Hypertension. 2010;56:290–296. doi: 10.1161/HYPERTENSIONAHA.110.150425. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sales EB, Toward MA, Campos RR, Paton JF. Revealing the role of the autonomic nervous system in the development and maintenance of Goldblatt hypertension in rats. Auton Neurosci. 2014;183:23–29. doi: 10.1016/j.autneu.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos RR, Oliveira-Sales EB, Nishi EE, Paton JF, Bergamaschi CT. Mechanisms of renal sympathetic activation in renovascular hypertension. Exp Physiol. 2015;100(5):496–501. doi: 10.1113/expphysiol.2014.079855. [DOI] [PubMed] [Google Scholar]
- Papademetriou V, Doumas M, Anyfanti P, Faselis C, Kokkinos P, Tsioufis C. Renal nerve ablation for hypertensive patients with chronic kidney disease. Curr Vasc Pharmacol. 2014;2:47–54. doi: 10.2174/15701611113119990143. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Differential effects of angiotensin II on cardiovascular reflexes mediated by nucleus tractus solitarii I. A microinjection study. J Physiol. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci. 2014;111:1174–9. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persu A, Jin Y, Baelen M, Vink E, Verloop WL, Schmidt B, Blicher MK, Severino F, Wuerzner G, Taylor A, Pechère-Bertschi A, Jokhaji F, Fadl Elmula FE, Rosa J, Czarnecka D, et al. European Network Coordinating research on Renal Denervation Consortium. Eligibility for renal denervation: experience at 11 European expert centers. Hypertension. 2014;63:1319–1325. doi: 10.1161/HYPERTENSIONAHA.114.03194. [DOI] [PubMed] [Google Scholar]
- Ploth DW. Angiotensin-dependent renal mechanisms in two-kidney one-clip renal vascular hypertension. Am J Physiol. 1983;245:131–141. doi: 10.1152/ajprenal.1983.245.2.F131. [DOI] [PubMed] [Google Scholar]
- Polson JW, Boscan P, Pickering AE, Dampney RAL, Paton JFR. Differential baroreflex control of sympathetic drive by angiotensin II in nucleus tractus solitarii. Am J Physiol. 2007;293:R1954–R1960. doi: 10.1152/ajpregu.00041.2007. [DOI] [PubMed] [Google Scholar]
- Ratcliffe LE, Pijacka W, McBryde FD, Abdala AP, Moraes DJ, Sobotka PA, Hart EC, Narkiewicz K, Nightingale AK, Paton JF. CrossTalk opposing view: Which technique for controlling resistant hypertension? Carotid chemoreceptor denervation/modulation. J Physiol. 2014;592:3941–3944. doi: 10.1113/jphysiol.2013.268227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE. Hierarchical recruitment of the sympathetic and parasympathetic limbs of the baroreflex in normotensive and spontaneously hypertensive rats. J Physiol. 2007;579:473–86. doi: 10.1113/jphysiol.2006.124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms AE, Paton JFR, Pickering A, Allen AM. Amplified respiratory-sympathetic coupling in neonatal and juvenile spontaneously hypertensive rats: does it contribute to hypertension? J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–12. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary A. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol. 2002;40:126–132. doi: 10.1016/s0735-1097(02)01931-9. [DOI] [PubMed] [Google Scholar]
- St -John WM, Paton JF. Defining eupnea. Respir Physiol Neurobiol. 2003;139:97–103. doi: 10.1016/s1569-9048(03)00193-9. [DOI] [PubMed] [Google Scholar]
- Tan PS, Killinger S, Horiuchi J, Dampney RA. Baroreceptor reflex modulation by circulation angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Remmers JE, Paton JF. Brain stem PO(2) and pH of the working heart-brain stem preparation during vascular with aqueous medium. Am J Physiol Regul Integr Comp Physiol. 2001;281:R528–38. doi: 10.1152/ajpregu.2001.281.2.R528. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LH, Braga VA, Pickering AE, Machado BH, Paton JFR. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Santisteban MM, Pitts T, Baekey DM, Perez PD, Bolser DC, Febo M, Raizada MK. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension. 2014;63:129–139. doi: 10.1161/HYPERTENSIONAHA.114.02440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Waki H, Raizada MK, Paton JFR. Autonomic-Immune-Vascular Interaction: An Emerging Concept for Neurogenic Hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]