Graphical Abstract
Transfer Hydrogenative Cycloaddition. Ruthenium(0) complexes modified by CyJohnPhos or RuPhos catalyze the successive C-C coupling of acetylenic aldehydes with α-ketols to form [4+2] cycloadducts as single diastereomers. This method enables convergent construction of type II polyketide ring systems of the angucycline class.
Keywords: Cycloaddition, Ruthenium, Transfer Hydrogenation, Angucycline, Polyketide
In the course of developing C-C bond forming hydrogenations and transfer hydrogenations,[1] we recently found that zero-valent ruthenium complexes derived from Ru3(CO)12 and phosphine ligands catalyze a diverse array of C-C couplings between vicinally dioxygenated hydrocarbons (e.g. diols, ketols, diones) and π-unsaturated reactants, including dienes,[2a–c] acrylates,[2d] α-olefins,[2e] alkynes[2f] and vinyl acetates.[2g] As in related ruthenium(0) catalyzed Pauson-Khand type reactions reported by Chatani,[3] these transformations proceed through catalytic mechanisms involving C=C/C=O oxidative coupling to form oxaruthenacycles.[2c] However, rather than carbonylating, as in Pauson-Khand type reactions, the oxaruthenacycles undergo hydrogenonlysis by dehydrogenating alcohol reactants,[4] releasing product and regenerating the carbonyl partner required for oxidative coupling. This pattern of reactivity served as the basis for the design of novel [4+2] cycloadditions of 1,2-diols with dienes[5a] and α-ketols with 3,4-benzannulated 1,5-diynes.[5b] Here, we report that zero-valent ruthenium catalysts promote the [4+2] cycloaddition of α-ketols with ortho-acetylenic benzaldehydes and demonstrate how this transformation enables concise construction of ring systems found in type II polyketides of the angucycline class.[6] This cycloaddition is unique among metal catalyzed reactions of ortho-acetylenic benzaldehydes (Scheme 1).[7–17]
Scheme 1.
Metal catalyzed C-C couplings of ortho-acetylenic benzaldehydes.
Type II polyketides natural products such as the tetracyclines, daunorubicin and doxorubicin have found broad use in human medicine.[18] Although total synthesis potentially offers entry to otherwise inaccessible analogues, current manufacturing routes rely on fermentation or semi-synthesis, suggesting improved methods for de novo chemical synthesis are necessary. The most common methods used for the construction of type II polyketides are stoichiometric benzannulations,[19] such as the Hauser reaction,[20] Diels-Alder benzannulations,[21] the Dötz reaction,[22] and aryne-mediated benzannulation.[23] To further broaden access to type II polyketides, we have begun to develop convergent methods for their assembly that target ubiquitous substructures via redox-triggered cycloaddition. The studies presented here are aimed at the rapid formation of angucycline ring systems (Figure 1).[6]
Figure 1.
Selected type II polyketides of the angucycline class.
Our initial experiments were guided by earlier studies conducted from our laboratory on the ruthenium(0)-catalyzed [4+2] cycloadditions of α-hydroxyketones with 3,4-benzannulated 1,5-diynes,[5b] which involved exposure of the reactants to the ruthenium(0) catalyst generated in situ from Ru3(CO)12 (2 mol%) and CyJohnPhos (12 mol%) (Figure 2) in m-xylene-H2O at 60–85 oC. Gratifyingly, upon application of these conditions to the cycloaddition of ortho-acetylenic arylaldehyde 1a with the para-fluorophenyl α-ketol 2a, the targeted product of [4+2] cycloaddition 3a could be isolated as a single stereoisomer in 75% yield (Table 1). The syn-diol and (E)-olefin stereochemistry were corroborated by single crystal X-ray diffraction analysis. Analysis of the crude reaction mixture by 19F NMR revealed the presence of one major byproduct, which was identified as the anti-diol (10:1 dr), as well as trace quantities of the 5-membered ring ketol.[24]Use of an aqueous organic reaction solvent (m-xylene-H2O) was crucial in terms of enforcing syn-diastereoselectivity and minimizing formation of numerous byproducts. Additionally, precise control of reaction temperature was required to preserve (E)-alkene geometry and mitigate 1,2-ketol rearrangement.[24]
Figure 2.
Ligands used in this study.
Table 1.
Ruthenium(0) catalyzed [4+2] cycloaddition of ortho-acetylenic benzaldehydes 1a–1c with α-ketols 2a–2f.a
Yields are of material isolated by silica gel chromatography. See Supporting Information for further experimental details.
Ru3(CO)12 (3 mol%), RuPhos (18 mol%).
To establish the scope and limitation of this method, acetylenic benzaldehydes 1a–1c were reacted with a diverse set of α-ketols 2a–2f, which incorporate both aryl (2a–2e) and alkyl (2f) substituents (Table 1). In each case, minor variation of temperature was required to suppress formation of undesired isomers. For acetylenic benzaldehydes 1a and 1b, the cycloadducts 3a–3f and 4a–4f were formed in moderate to good yields and were isolated in diastereomerically pure form. The ortho,ortho-dimethoxy substituted acetylenic benzaldehyde 1c proved to be less reactive, requiring enhanced loadings of Ru3(CO)12 (3 mol%) and use of RuPhos (18 mol%) as ligand (Figure 2). Under these conditions, cycloadducts 5a–5f were isolated as single diastereomers in moderate to good yields. Among the α-ketols 2a–2f, the ortho-substituted aryl ketol 2e provided the lowest isolated yields of cycloadduct (3e, 4e, 5e). Analysis of crude reaction mixtures by 1H NMR indicated complete consumption of ketol 2e in reactions with acetylenic benzaldehydes 1a–1c, however, significant quantites of the undesired 5-membered cycloadduct were formed. Reexposure of the 6-membered cycloadducts 3e, 4e and 5e to the reaction conditions resulted in conversion to the same distribution of 5- and 6-membered ring products obtained from initial cycloaddition reactions, suggesting cycloadducts 3e, 4e and 5e have a lower barrier to 1,2-ketol rearrangement.[24]
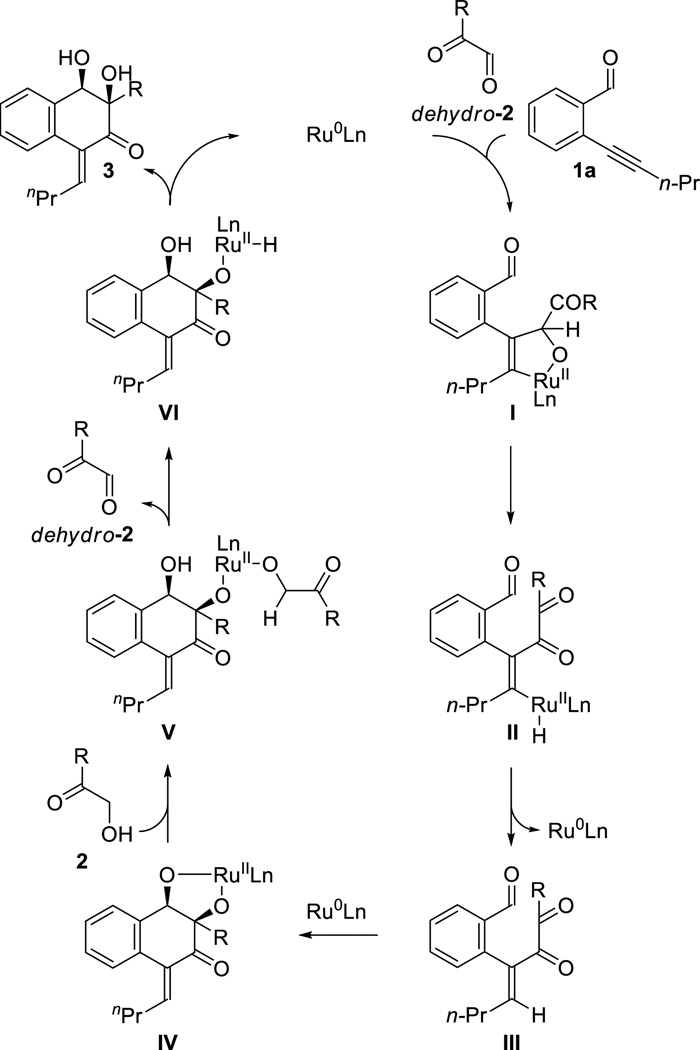
A plausible catalytic mechanism, illustrated for the coupling of acetylenic aldehyde 1a with α-ketol 2, is as follows (Scheme 2). A mononuclear ruthenium(0) complex[25] promotes alkyne-carbonyl oxidative coupling of acetylenic aldehyde 1a with α-ketoaldehyde dehydro-2 to form oxaruthenacycle I.[2f] For the first turnover of the catalytic cycle, the dicarbonyl partner required for oxidative coupling, dehydro-2, may be formed through Ru3(CO)12 catalyzed dehydrogenation of α-ketol 2 with hydrogen transfer to alkyne 1a.[4] β-Hydride elimination converts oxaruthenacycle I to the vinylruthenium hydride II, which upon C-H reductive elimination delivers the tricarbonyl intermediate III, regenerating ruthenium(0). The second C-C bond formation may occur via pinacol-type carbonyl-carbonyl oxidative coupling3 to deliver dioxaruthenacycle IV. Protonolytic cleavage of the complex IV mediated by α-ketol 2 forms the ruthenium alkoxide V. β-Hydride elimination from alkoxide V forms alkoxyruthenium hydride VI with concomittant release of α-ketoaldehyde dehydro-2. Finally, O-H reductive elimination delivers the product of cycloaddition, returning rutheium to its zero-valent form to close the catalytic cycle. An alternate pathway for formation of the second C-C bond involves aldol reaction of a ruthenium enediolate derived from tricarbonyl intermediate III to form dioxaruthenacycle IV. Remarkably, the secondary benzylic alcohol of the cycloadducts are not oxidized under these conditions, suggesting ketol dehydrogenation is more rapid. Once the acetylenic aldehyde is consumed, which serves as hydrogen acceptor, no further oxidation occurs.
Scheme 2.
General catalytic mechanism for ruthenium(0) catalyzed [4+2] cycloaddition of ortho-acetylenic benzaldehydes 1a–1c with α-ketols 2a–2f.
To illustrate how this new [4+2] cycloaddition methodology broadens access to type II polyketides, cycloadducts 3f, 4f and 5f were converted to the respective tetracyclic naphthoquinones 10a, 10b and 10c bearing the bridgehead diol motif found in many angucycline natural products (Scheme 3).[6]Diols 3f, 4f and 5f were first treated with triphosgene to furnish the respective cyclic carbonates 6a, 6b and 6c which were then subjected to ruthenium-catalyzed oxidative cleavage[26] to form diones 7a, 7b and 7c. Although diones 7a, 7b and 7c could be observed by 1H NMR analysis of crude reaction mixtures, attempted isolation by silica-gel chromatography triggered generation of the indicated 5-membered α-ketols.[24]Therefore, direct Friedel-Crafts-type cyclization of the crude diones 7a, 7b and 7c was attempted. To our delight, exposure of diones 7a, 7b and 7c to FeCl3[27] led to the formation of the desired cyclization products 8a, 8b and 8c as single diastereomers. The structural assignment of compounds 8a and 8b was confirmed by single crystal X-ray diffraction analysis. Basic hydrolysis of the carbonate provided the syn-triols 9a, 9b and 9c, which upon IBX-mediated oxidation[28] furnished the tetracyclic naphthoquinones 10a, 10b and 10c.
Scheme 3.
Conversion of cycloadducts 3f, 4f and 5f to tetracyclic naphthoquinones 10a, 10b and 10c, respectively, bearing the bridgehead diol motif found in many angucycline natural products.
In summary, we report a novel [4+2] cycloaddition of ortho-acetylenic benzaldehydes 1a–1c with α-ketols 2a–2f via ruthenium(0) catalyzed C-C bond forming transfer hydrogenation. The dense functional group arrays embodied by cycloadducts 3a–3f, 4a–4f and 5a–5f offer new possibilities for the synthesis of type II polyketides, as demonstrated through the conversion of cycloadducts 3f, 4f and 5f to tetracyclic naphthoquinones 10a, 10b and 10c, respectively, bearing the bridgehead diol substructure often found in angucycline natural products.[6] More broadly, the transformations reported in this account contribute to a growing body of redox-neutral C-C bond formations that emanate from the merger of carbonyl addition and transfer hydrogenation.[1a]
Supplementary Material
Footnotes
Acknowledgment is made to the Robert A. Welch Foundation (F-0038) and the NIH (RO1-GM093905) for partial support of this research. Rusan Pharma Ltd. is acknowledged for partial financial support of Dr. Aakarsh Saxena. Dr. Felix Perez is an NIH-IRACDA Postdoctoral Fellow (NIH 1K12GM102745). Kyle Bauernschmitt is acknowledged for skillful technical assistance.
References
- 1.For reviews, see: Ketcham JM, Shin I, Montgomery TP, Krische MJ. Angew. Chem. 2014;126:9294–9302. doi: 10.1002/anie.201403873. Angew. Chem. Int. Ed.2014, 53, 9142–9150; Hassan A, Krische MJ. Org. Process Res. Dev. 2011;15:1236–1242. doi: 10.1021/op200195m. Bower JF, Krische MJ. Top. Organomet. Chem. 2011;34:107–138. doi: 10.1007/978-3-642-15334-1_5.
- 2.a) Leung JC, Geary LM, Chen T-Y, Zbieg JR, Krische MJ. J Am. Chem. Soc. 2012;134:15700–15703. doi: 10.1021/ja3075049. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen T-Y, Krische MJ. Org. Lett. 2013;15:2994–2997. doi: 10.1021/ol401184k. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Park BY, Montgomery TP, Garza V, Krische MJ. J Am. Chem. Soc. 2013;135:16320–16323. doi: 10.1021/ja4087193. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) McInturff EL, Mowat J, Waldeck AR, Krische MJ. J Am. Chem. Soc. 2013;135:17230–17235. doi: 10.1021/ja410533y. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Yamaguchi E, Mowat J, Luong T, Krische MJ. Angew. Chem. 2013;125:8586–8589. doi: 10.1002/anie.201303552. Angew. Chem. Int. Ed.2013, 52 8428–8431; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) McInturff EL, Nguyen KD, Krische MJ. Angew. Chem. 2014;126:3296–3299. doi: 10.1002/anie.201311130. Angew. Chem. Int. Ed.2014, 53, 3232–3235; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Park BY, Luong T, Sato H, Krische MJ. J Am. Chem. Soc. 2015;137:7652–7655. doi: 10.1021/jacs.5b04688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Chatani N, Tobisu M, Asaumi T, Fukumoto Y, Murai S. J Am. Chem. Soc. 1999;121:7160–7161. [Google Scholar]; (b) Tobisu M, Chatani N, Asaumi T, Amako K, Ie Y, Fukumoto Y, Murai S. J Am. Chem. Soc. 2000;122:12663–12674. [Google Scholar]
- 4.For early examples of Ru3(CO)12 catalyzed alcohol dehydrogenation, see: Blum Y, Reshef D, Shvo Y. Tetrahedron Lett. 1981;22:1541–1544. Shvo Y, Blum Y, Reshef D, Menzin M. J Organomet. Chem. 1982;226:C21–C24.
- 5.a) Geary LM, Glasspoole BW, Kim MM, Krische MJ. J Am. Chem. Soc. 2013;135:3796–3799. doi: 10.1021/ja400691t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Saxena A, Perez F, Krische MJ. J Am. Chem. Soc. 2015;137:5883–5886. doi: 10.1021/jacs.5b02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For reviews of angucycline natural products, see: Kharel MK, Pahari P, Shepherd MD, Tibrewal N, Nybo SE, Shaaban KA, Rohr J. Nat. Prod. Rep. 2012;29:264–325. doi: 10.1039/c1np00068c. Carreño MC, Urbano A. Synlett. 2005:1–25. Krohn K, Rohr J. Top. Curr. Chem. 1997;188:127–195.
- 7.For selected gold catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Asao N, Takahashi K, Lee S, Kasahara T, Yamamoto Y. J Am. Chem. Soc. 2002;124:12650–12651. doi: 10.1021/ja028128z. Dyker G, Hildebrandt D, Liu J, Merz K. Angew. Chem. 2003;115:4536–4538. doi: 10.1002/anie.200352160. Angew. Chem., Int. Ed.2003, 42, 4399–4402; Asao N, Aikawa H, Yamamoto Y. J Am. Chem. Soc. 2004;126:7458–7459. doi: 10.1021/ja0477367. Asao N, Sato K, Menggenbateer, Yamamoto Y. J Org. Chem. 2005;70:3682–3685. doi: 10.1021/jo0500434. Yao X, Li C-J. Org. Lett. 2006;8:1953–1955. doi: 10.1021/ol060645p. Beeler AB, Su S, Singleton CA, Porco JA., Jr J Am. Chem. Soc. 2007;129:1413–1419. doi: 10.1021/ja0674744. Patil NT, Mutyala AK, Lakshmi PGVV, Raju PVK, Sridhar B. Eur. J. Org. Chem. 2010:1999–2007. Handa S, Slaughter LM. Angew. Chem. 2012;124:2966–2969. doi: 10.1002/anie.201107789. Angew. Chem., Int. Ed.2012, 51, 2912–2915; Patil NT, Mutyala AK, Konala A, Tella RB. Chem. Commun. 2012;48:3094–3096. doi: 10.1039/c2cc17450b. Malhotra D, Liu L-P, Mashuta MS, Hammond GB. Chem. Eur. J. 2013;19:4043–4050. doi: 10.1002/chem.201203841. Zhu S, Guo Z, Huang Z, Jiang H. Chem. Eur. J. 2014;20:2425–2430. doi: 10.1002/chem.201304839.
- 8.For selected silver catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Ding Q, Wu J. Org. Lett. 2007;9:4959–4962. doi: 10.1021/ol7020669. Ding Q, Yu X, Wu J. Tetrahedron Lett. 2008;49:2752–2755. Yu X, Ding Q, Wang W, Wu J. Tetrahedron Lett. 2008;49:4390–4393. Ye S, Wu J. Tetrahedron Lett. 2009;50:6273–6275. Lou H, Ye S, Zhang J, Wu J. Tetrahedron. 2011;67:2060–2065. Wang H, Ye S, Jin H, Liu J, Wu J. Tetrahedron. 2011;67:5871–5877. Dell’Acqua M, Castano B, Cecchini C, Pedrazzini T, Pirovano V, Rossi E, Caselli A, Abbiati G. J Org. Chem. 2014;79:3494–3505. doi: 10.1021/jo5002559. Wang X, Qiu G, Zhang L, Wu J. Tetrahedron Lett. 2014;55:962–964. Liang R, Ma T, Zhu S. Org. Lett. 2014;16:4412–4415. doi: 10.1021/ol5017299. Mariaule G, Newsome G, Toullec PY, Belmont P, Michelet V. Org. Lett. 2014;16:4570–4573. doi: 10.1021/ol5021256. Reddy V, Jadhav AS, Anand RV. Org. Biomol. Chem. 2015;13:3732–3741. doi: 10.1039/c4ob02641a.
- 9.For selected copper catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Asao N, Kasahara T, Yamamoto Y. Angew. Chem. 2003;115:3628–3630. doi: 10.1002/anie.200351390. Angew. Chem. Int. Ed.2003, 42, 3504–3506; Asao N, Nogami T, Lee S, Yamamoto Y. J Am. Chem. Soc. 2003;125:10921–10925. doi: 10.1021/ja036927r. Patil NT, Yamamoto Y. J Org. Chem. 2004;69:5139–5142. doi: 10.1021/jo049416b. Asao N, Aikawa H. J Org. Chem. 2006;71:5249–5253. doi: 10.1021/jo060597m. Ding Q, Wang B, Wu J. Tetrahedron. 2007;63:12166–12171. Gao K, Wu J. J Org. Chem. 2007;72:8611–8613. doi: 10.1021/jo7016839. Ye Y, Ding Q, Wu J. Tetrahedron. 2008;64:1378–1382. Yu M, Wang Y, Li C-J, Yao X. Tetrahedron Lett. 2009;50:6791–6794.
- 10.For selected palladium catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Asao N, Nogami T, Takahashi K, Yamamoto Y. J Am. Chem. Soc. 2002;124:764–765. doi: 10.1021/ja017366b. Asao N, Chan CS, Takahashi K, Yamamoto Y. Tetrahedron. 2005;61:11322–11326. Tang R-Y, Li J-H. Chem. Eur. J. 2010;16:4733–4738. doi: 10.1002/chem.201000133. Yu S-Y, Zhang H, Gao Y, Mo L, Wang S, Yao Z-J. J Am. Chem. Soc. 2013;135:11402–11407. doi: 10.1021/ja405764p.
- 11.For selected platinum catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Kusama H, Funami H, Takaya J, Iwasawa N. Org. Lett. 2004;6:605–608. doi: 10.1021/ol0364024. Hildebrandt D, Hüggenberg W, Kanthak M, Plöger T, Müller IM, Dyker G. Chem. Commun. 2006:2260–2261. doi: 10.1039/b602498j. Kusama H, Funami H, Iwasawa N. Synthesis. 2007;13:2014–2024. Oh CH, Lee JH, Lee SJ, Kim JI, Hong CS. Angew. Chem. 2008;120:7615–7617. Angew. Chem. Int. Ed.2008, 47, 7505–7507; Shu X-Z, Zhao S-C, Ji K-G, Zheng Z-J, Liu X-Y, Liang Y-M. Eur. J. Org. Chem. 2009:117–122. Hsu Y-C, Ting C-M, Liu R-S. J Am. Chem. Soc. 2009;131:2090–2091. doi: 10.1021/ja809560c. Oh CH, Lee SM, Hong CS. Org. Lett. 2010;12:1308–1311. doi: 10.1021/ol100255z. Oh CH, Yi HJ, Lee JH, Lim DH. Chem. Commun. 2010;46:3007–3009. doi: 10.1039/c001495h.
- 12.For selected indium catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Obika S, Kono H, Yasui Y, Yanada R, Takemoto Y. J Org. Chem. 2007;72:4462–4468. doi: 10.1021/jo070615f. Yanada R, Hashimoto K, Tokizane R, Miwa Y, Minami H, Yanada K, Ishikura M, Takemoto Y. J Org. Chem. 2008;73:5135–5138. doi: 10.1021/jo800474c. Selvi T, Srinivasan K. Org. Biomol. Chem. 2013;11:2162–2167. doi: 10.1039/c3ob26284g. Sakthivel K, Srinivasan K. Org. Biomol. Chem. 2014;12:269–277. doi: 10.1039/c3ob41439f.
- 13.For zinc catalyzed C–C couplings of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Fang X-L, Tang R-Y, Zhang X-G, Zhong P, Deng C-L, Li J-H. J Organomet. Chem. 2011;696:352–356. Zhao X, Zhang X-G, Tang R-Y, Deng C-L, Li J-H. Eur. J. Org. Chem. 2010:4211–4217.
- 14.For selected rhenium catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Umeda R, Kaiba K, Morishita S, Nishiyama Y. ChemCatChem. 2011;3:1743–1746.
- 15.For selected tungsten catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Kusama H, Funami H, Shido M, Hara Y, Takaya J, Iwasawa N. J Am. Chem. Soc. 2005;127:2709–2716. doi: 10.1021/ja044194k.
- 16.For selected rhodium catalyzed reactions of ortho-acetylenic benzaldehydes via benzopyrylium intermediates, see: Shin S, Gupta AK, Rhim CY, Oh CH. Chem. Commun. 2005:4429–4431. doi: 10.1039/b506003f. Brown LE, Cheng KC-C, Wei W-G, Yuan P, Dai P, Trilles R, Ni F, Yuan J, MacArthur R, Guha R, Johnson RL, Su X-Z, Dominguez MM, Snyder JK, Beeler AB, Schaus SE, Inglese J, Porco JA., Jr Proc. Natl. Acad. Sci. U.S.A. 2011;108:6775–6780. doi: 10.1073/pnas.1017666108.
- 17.For selected rhodium catalyzed reactions of ortho-acetylenic benzaldehydes via hydroacylation, see: Tanaka K, Hagiwara Y, Noguchi K. Angew. Chem. 2005;117:7426–7429. doi: 10.1002/anie.200502380. Angew. Chem. Int. Ed.2005, 44, 7260–7263; Tanaka K, Hagiwara Y, Hirano M. Angew. Chem. 2006;118:2800–2803. doi: 10.1002/anie.200504470. Angew. Chem. Int. Ed.2006, 45, 2734–2737; Tanaka K, Hagiwara Y, Hirano M. Eur. J. Org. Chem. 2006:3582–3595. Hojo D, Noguchi K, Hirano M, Tanaka K. Angew. Chem. 2008;120:5904–5906. doi: 10.1002/anie.200801642. Angew. Chem. Int. Ed.2008, 47, 5820–5822; Tanaka K, Tanaka R, Nishida G, Noguchi K, Hirano M. Chem. Lett. 2008;37:934–935. Hojo D, Noguchi K, Tanaka K. Angew. Chem. 2009;121:8273–8276. doi: 10.1002/anie.200904024. Angew. Chem. Int. Ed.2009, 48, 8129–8132; Tanaka K, Mimura M, Hojo D. Tetrahedron. 2009;65:9008–9014. Hojo D, Tanaka K. Org. Lett. 2012;14:1492–1495. doi: 10.1021/ol300234g.
- 18.Dechert-Schmitt A-MR, Schmitt DC, Gao X, Itoh T, Krische MJ. Nat. Prod. Rep. 2014;31:504–513. doi: 10.1039/c3np70076c. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For a review on benzannulation, see: Kotha S, Misra S, Halder S. Tetrahedron. 2008;64:10775–10790.
- 20.For selected examples of the use of the Hauser-Kraus annulation in the synthesis of Type II polyketides, see: Hauser FM, Rhee RP. J Am. Chem. Soc. 1979;101:1628–1629. Hauser FM, Mal DJ. J Am. Chem. Soc. 1983;105:5688–5690. Hauser FM, Gauuan PJF. Org. Lett. 1999;1:671–672. doi: 10.1021/ol990758r. Matsumoto T, Yamaguchi H, Tanabe M, Yasui Y, Suzuki K. Tetrahedron Lett. 2000;41:8393–8396. Nicolaou KC, Zhang H, Chen JS, Crawford JJ, Pasunoori L. Angew. Chem. 2007;119:4788–4791. doi: 10.1002/anie.200700917. Angew. Chem. Int. Ed.2007, 46, 4704–4707; Gibson JS, Andrey O, Brimble MA. Synthesis. 2007:2611–2613. Nicolaou KC, Becker J, Lim YH, Lemire A, Neubauer T, Montero A. J Am. Chem. Soc. 2009;131:14812–14826. doi: 10.1021/ja9073694. Mal D, De SR. Org. Lett. 2009;11:4398–4401. doi: 10.1021/ol901817r. Yang X, Fu B, Yu B. J Am. Chem. Soc. 2011;133:12433–12435. doi: 10.1021/ja205339p. Liau BB, Milgram BC, Shair MD. J Am. Chem. Soc. 2012;134:16765–16772. doi: 10.1021/ja307207q. Adachi S, Watanabe K, Iwata Y, Kameda S, Miyaoka Y, Onozuka M, Mitsui R, Saikawa Y, Nakata M. Angew. Chem. 2013;125:2141–2145. doi: 10.1002/anie.201209205. Angew. Chem. Int. Ed.2013, 52, 2087–2091.
- 21.For selected examples of the use of the Diels-Alder reaction in the synthesis of Type II polyketides, see: Kelly TR, Vaya J, Ananthasubramanian L. J Am. Chem. Soc. 1980;102:5983–5984. Danishefsky SJ, Uang BJ, Quallich G. J Am. Chem. Soc. 1985;107:1285–1293. Tamiya M, Ohmori K, Kitamura M, Kato H, Arai T, Oorui M, Suzuki K. Chem. Eur. J. 2007;13:9791–9823. doi: 10.1002/chem.200700863. Yamashita Y, Hirano Y, Takada A, Takikawa H, Suzuki K. Angew. Chem. 2013;125:6790–6793. doi: 10.1002/anie.201301591. Angew. Chem. Int. Ed.2013, 52, 6658–6661.
- 22.For selected examples of the use of the Dötz reaction in the synthesis of Type II polyketides, see: Semmelhack MF, Bozell JJ, Sato T, Wulff W, Spiess E, Zask A. J Am. Chem. Soc. 1982;104:5850–5852. Dötz KH, Popall M. Angew. Chem. 1987;99:1220–1221. Angew. Chem. Int. Ed.1987, 26, 1158–1160; Wulff WD, Xu Y-C. J Am. Chem. Soc. 1988;110:2312–2314. Boger DL, Hüter O, Mbiya K, Zhang M. J Am. Chem. Soc. 1995;117:11839–11849. Fernandes RA, Mulay SV. J Org. Chem. 2010;75:7029–7032. doi: 10.1021/jo101628m. Fernandes RA, Chavan VP, Mulay SV, Manchoju A. J Org. Chem. 2012;77:10455–10460. doi: 10.1021/jo3019939.
- 23.For selected examples of the use of aryne-mediated benzannulation in the synthesis of Type II polyketides, see: Hosoya T, Takashiro E, Matsumoto T, Suzuki K. J Am. Chem. Soc. 1994;116:1004–1015. Matsumoto T, Sohma T, Yamaguchi H, Kurata S, Suzuki K. Synlett. 1995:263–266. Chen C-L, Sparks SM, Martin SF. J Am. Chem. Soc. 2006;128:13696–13697. doi: 10.1021/ja0652619. O’Keefe BM, Mans DM, Kaelin DE, Jr, Martin SF. J Am. Chem. Soc. 2010;132:15528–15530. doi: 10.1021/ja107926f.
- 24.In our previously developed [4+2] cycloadditions of α-hydroxyketones with 3,4-benzannulated 1,5-diynes (ref. 5b), structurally related 5- and 6-membered ring ketols were found to exist in equilibrium at 130 °C.
- 25.Ru3(CO)12 reacts with dppe in benzene solvent to provide Ru(CO)3(dppe): Sanchez-Delgado RA, Bradley JS, Wilkinson G. J Chem. Soc. Dalton Trans. 1976:399–404.
- 26. Yang D, Zhang C. J Org. Chem. 2001;66:4814–4818. doi: 10.1021/jo010122p. For reviews, see: Spannring P, Bruijnincx PCA, Weckhuysen BM, Klein Gebbink RJM. Catal. Sci. Technol. 2014;4:2182–2209. Rajagopalan A, Lara M, Kroutil W. Adv. Synth. Catal. 2013;355:3321–3335.
- 27.Snyder SA, Brill ZG. Org. Lett. 2011;13:5524–5527. doi: 10.1021/ol2022406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.For IBX-mediated oxidation of vicinal diols, see: Moorthy JN, Singhal N, Senapati K. Org. Biomol. Chem. 2007;5:767–771. doi: 10.1039/b618135j. Frigerio M, Santagostino M. Tetrahedron Lett. 1994;35:8019–8022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.