Abstract
Our recent studies in an inbred swine model demonstrated that both peripheral and intra-graft regulatory cells were required for the adoptive transfer of tolerance to a second, naïve donor-matched kidney. Here, we have asked whether both peripheral and intra-graft regulatory elements are required for adoptive transfer of tolerance when only a long-term tolerant (LTT) kidney is transplanted. Nine highly-inbred swine underwent a tolerance-inducing regimen to prepare LTT kidney grafts which were then transplanted to histocompatible recipients, with or without the peripheral cell populations required for adoptive transfer of tolerance to a naïve kidney. In contrast to our previous studies, tolerance of the LTT kidney transplants alone was achieved without transfer of additional peripheral cells and without strategies to increase the number/potency of regulatory T cells in the donor. This tolerance was systemic, since most subsequent, donor-matched challenge kidney grafts were accepted. These results confirm the presence of a potent tolerance-inducing and/or tolerance-maintaining cell population within LTT renal allografts. They suggest further that additional peripheral tolerance mechanisms, required for adoptive transfer of tolerance to a naïve donor-matched kidney, depend on peripheral cells that, if not transferred with the LTT kidney, require time to develop in the adoptive host.
INTRODUCTION
Adoptive transfer models provide an invaluable tool to investigate the mechanisms underlying transplantation tolerance, which remains one of the ultimate goals in the field of solid organ transplantation (1;2). Successful adoptive transfer of transplantation tolerance has only been demonstrated previously in inbred mice, perhaps because the regulatory cells thought to be responsible for this phenomenon, only survive if adoptively transferred to syngeneic recipients (1;2). Using inbred miniature swine with >94% coefficient of inbreeding, we have recently reported adoptive transfer of tolerance to a kidney transplanted across a class I MHC mismatch (3). However, successful transfer of this tolerance required treatment of naïve recipients with: 1) 150 cGy whole body irradiation (WBI); 2) an infusion of leukapheresed PBMCs from a syngeneic recipient already tolerant of a similarly class I-mismatched kidney and primed with donor-matched DST 8 days prior to PBMC harvest; and 3) simultaneous transplantation of the long-term tolerant (LTT) kidney along with the naïve class I-mismatched kidney. These results implied that both peripheral and intra-graft regulatory cells were required for transfer of tolerance to a naïve donor-matched kidney (3). In the present study, we have attempted to determine whether all off these elements were required for adoptive transfer of tolerance to the LTT kidney or whether intra-graft regulatory cells alone would suffice in the absence of an additional naïve, donor-matched kidney transplant.
MATERIALS AND METHODS
Animals
The characteristics of the Massachusetts General Hospital (MGH) Miniature Swine herd have been previously described (4;5). All the animals were in the 3-8 months age range, and donors and recipients were matched for size. The primary organ donors were from the Swine Leukocyte Antigen (SLA) gg line (class I SLA c/c, class II SLA d/d). Adoptive transfer donors and adoptive transfer recipients were from the G10 or the G11 highly-inbred sublines of the SLAdd herd (class I SLA d/d, class II SLA d/d). The G10 and G11 subline have been produced by sequential brother-sister mating and have coefficients of inbreeding greater than 96% (6). All the animals were cared for according to the guidelines of the MGH Institutional Animal Care and Use Committee and conducted in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press.
Surgical Procedures
Renal transplantation was carried out according to our previously published technique (7). Briefly, after bilateral nephrectomy, kidneys were transplanted in an orthotopic fashion. The donor's renal artery and vein were anastomosed end-to-side to the recipient's abdominal aorta and vena cava, respectively. Renal transplantation was completed by performing a vesicoureteral anastomosis. An identical procedure was repeated for the re-transplantation of the LTT kidney into the adoptive transfer recipient.
Semi-permanent indwelling silastic catheters were inserted into the recipient's internal and external jugular veins to facilitate blood collection, administration of drugs and cells and PBMC apheresis.
Immunosuppression
CyA (Novartis Pharmaceutical Corp., Hanover, NJ) was administered to the recipients of primary grafts (adoptive transfer donors) for 12 consecutive days, starting on the day of the primary transplant. CyA was administered intravenously at a daily dose of 10-13 mg/kg, adjusted to maintain a nadir whole blood level of 400-800 ng/dl. Whole blood drug levels were assessed by a monoclonal radioimmunoassay using an Architect i1000SR analyzer (Abbott Diagnostics, Abbott Park, IL).
Animal conditioning prior to adoptive transfer
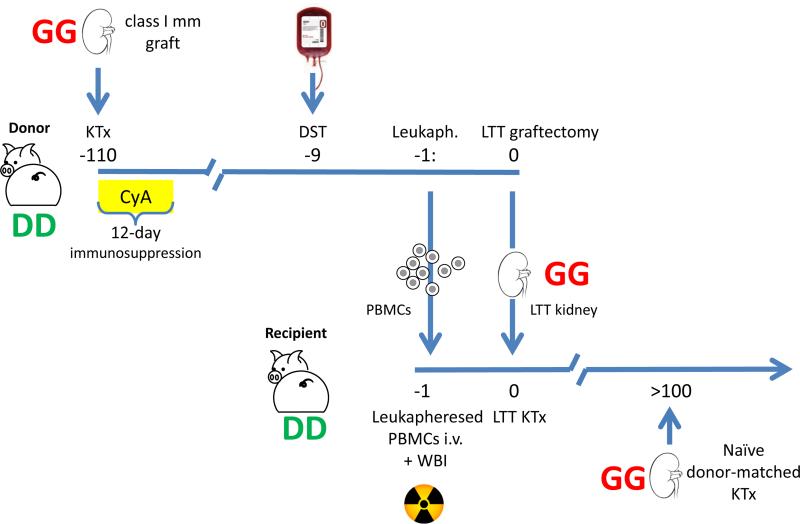
Prior to adoptive transfer, both the donor animal and the recipient underwent certain pre-treatment strategies in order to facilitate successful adoptive transfer of class I MHC tolerance. The general experimental design is depicted in Figure 1. Eight days prior to transfer of the LTT kidney (day −8), adoptive transfer donors in groups A and B underwent a DST, consisting of 10 ml/kg of donor-matched (SLAgg) non-irradiated whole blood administered intravenously. In group A, on day −1 the adoptive transfer donors underwent extensive leukapheresis to remove peripheral circulating cells (8). Following apheresis collection, PBMCs were immediately infused IV to the adoptive transfer recipient. Adoptive transfer recipients in groups A, B and C were also conditioned with 150 cGy WBI, administered on day −1 using a cobalt irradiator. Transplantation of the LTT kidney was performed on day 0, after bilateral nephrectomy. After at least 100 days with stable renal function, the LTT kidneys were explanted and a fresh, primary donor MHC-matched (SLAgg) kidney was transplanted in order to assess the stability of tolerance induced by adoptive transfer.
Figure 1. Schematic of the adoptive transfer experimental protocol.
On day −110, adoptive transfer donors (highly-inbred SLAdd) underwent bilateral nephrectomy and received a class I-mismatched (SLAgg) kidney graft, followed by 12 days of high-dose CyA. After 100 days with stable renal function and in vitro unresponsiveness, these animals were deemed LTT, and underwent a DST (day −9), and an extensive leukapheresis to remove PBMCs (day −1). These cells were immediately transferred to a syngeneic recipient (highly-inbred SLAdd), who underwent 150 cGy WBI just prior transfusion. On day 0, the donor animal was sacrificed and the LTT kidney transferred to the adoptive transfer recipient after bilateral native kidney nephrectomy. Recipients did not receive immunosuppression after transplant, and were followed for at least 100 days with serial creatinine measurements and biopsies on PODs 30, 60 and 90. Accepted LTT kidneys were removed after day 100 and replaced with naïve SLAgg kidney grafts. DD: SLAdd, GG: SLAgg, DST: donor-specific transfusion, KTx: kidney transplant, Leukaph: leukapheresis, LTT: long-term tolerant, mm: mismatched, PBMC: peripheral blood mononuclear cells, WBI: whole-body irradiation.
Rejection monitoring
Renal function was assessed by serial creatinine measurements. Routine renal wedge biopsies were performed through a flank incision at pre-determined time intervals (post-operative day (POD 30, POD 60, POD 100), during periods of rejection and/or at time of death. A senior transplant pathologist scored rejection. Immunohistochemical analysis was performed as previously described (3).
Preparation of peripheral blood mononuclear cells (PBMCs)
Freshly heparinized whole blood was diluted approximately 1:2 with HBSS (Life Technologies, Grand Island, NY), and the PBMCs were obtained by means of gradient centrifugation with Histopaque (Sigma, St. Louis, MO). PBMCs were washed once with HBSS, and contaminating red cells were lysed with ammonium chloride potassium lysing buffer (Bio Whittaker, Inc, Walkersville, MD). Cells were then washed with HBSS and resuspended in tissue culture medium. All cell suspensions were kept at 4°C until used in cellular assays.
Cell-mediated lympholysis (CML) assay
CML assays with porcine cells have been described previously (9;10). The tissue culture media used for the CML assays consisted of RPMI-1640 (Life Technologies) supplemented with 6% fetal bovine serum (Sigma Chemical Co, St Louis, MO), 100 U/mL penicillin, 135 mg/mL streptomycin (Life Technologies), 50 mg/mL gentamicin (Life Technologies), 10 mmol/L N –2-hydroxyethylpiperazine-N -2-ethanesulfonic acid (HEPES; Fisher Scientific, Pittsburgh, PA), 2 mmol/L L -glutamine (Life Technologies), 1 mmol/L sodium pyruvate (Bio Whittaker, Inc), nonessential amino acids (Bio Whittaker, Inc), and 5 × 10−5 mol/L β2-mercaptoethanol (Sigma Chemical). The effector phase of the CML assay was performed with Basal Medium Eagle (Life Technologies) supplemented with 6% controlled processed serum replacement 3 (Sigma Chemical) and 10 mmol/L HEPES. Briefly, lymphocyte cultures containing 4 × 106/mL responder and 4 × 106/mL stimulator PBMCs (irradiated with 2500 cGy) were incubated for 6 days at 37° in 5% carbon dioxide and 100% humidity in CML medium. Bulk cultures were harvested, and effectors were tested for cytotoxic activity on chromium 51–labeled (ILPerkin Elmer, Waltham MA) lymphoblast targets generated from phytohemagglutinin (M-form; Life Technologies, Gaithersburg, MD) stimulation. Effector cells were incubated for 5.5 hours with target cells at effector/target ratios of 100:1, 50:1, 25:1, and 12.5:1. Two target cells were tested in each assay: (1) PBMCs SLA-matched to the donor and (2) third-party PBMCs. Supernatants were then harvested by using the Skatron collection system (Skatron, Sterling, VA), and 51Cr release was determined on a gamma counter (ALCobra 5010, Perkin Elmer). The results were expressed as a percentage of specific lysis and calculated as follows:
RESULTS
Pharmacological induction of tolerance and establishment of LTT donors
Nine highly-inbred swine received class I-mismatched kidney grafts followed by 12 days of high-dose CyA. We have previously reported that this regimen uniformly induces tolerance across a class I-mismatched barrier in miniature swine, and have therefore used it as a standard protocol for the induction of transplantation tolerance in our model over the last 20 years (11). All nine animals maintained stable renal function after transplantation, and developed donor-specific unresponsiveness in vitro (data not shown). One hundred days after primary transplantation, these animals were deemed LTT and used as donors of LTT kidneys and/or cells. Experimental groups and organ graft survivals are summarized in Table 1.
Table 1.
Treatment protocols and graft survival times for adoptive transfer recipients.
| Group | DST | WBI | PBMCs | Kidney graft | Animal ID | LTT graft survival (days) | Survival of LTT graft + naïve kidney (Okumi et al.) |
|---|---|---|---|---|---|---|---|
| A | + | + | + | LTT | 19678 20314 |
>130 >118 |
>150, >150, 28 |
| B | + | + | - | LTT | 20319 20791 |
>125 >161 |
9, 10, 73 |
| C | - | + | - | LTT | 21254 21713 21957 |
>130 >105 72 |
4, 10 , 46 |
| D | - | - | - | LTT | 21586 21666 |
14 13 |
|
| E | + | + | + | naïve | 15938 20107 20189 |
9 10 30 |
|
DST: donor-specific transfusion, WBI: whole-body irradiation, LTT: long-term tolerant.
Transplantation of LTT kidneys and PBMCs into naïve syngeneic animals
Relevant data from our previous series are included in Table 1 for comparison. Animals in group A (n=2) received the full adoptive transfer preparatory regimen, consisting of DST priming of the donor, PBMC and LTT kidney transplantation. Both animals accepted the LTT kidney (Figure 2A shows a POD 118 biopsy from animal 20314, showing no signs of rejection) and developed donor-specific unresponsiveness in vitro (Figure 3A), confirming adoptive transfer of tolerance by transplantation of LTT kidney and cells.
Figure 2. Histology of the adoptively transferred LTT kidneys after transplantation.
A: POD 118 biopsy from animal 20314, showing no interstitial infiltrate or cellulitis. B: POD 125 biopsy from animal 20319, showing mild glomerulitis without cellular rejection. C: POD 105 biopsy from animal 21713, consistent with ACR1, signs of glomerulopathy, no cellular rejection. D: POD 14 biopsy from animal 21586 showing massive lymphocyte infiltration and severe hemorrhagic changes. POD: Post-operative day; ACR: acute cellular rejection.
Figure 3. In vitro responsiveness after adoptive transfer.
Representative day ~100 CMLs for animals in group A (19678), B (20319), and C (21713) showed hypo-/unresponsiveness to donor-type cell targets. Panel D shows responsiveness for animal 21666 in group D, who failed to achieve tolerance and rejected the adoptively transferred kidney (day 14 CML, at the time of sacrifice). PBMCs from a naïve haploidentical animal (SLAdd) were used as control in all assays (naïve DD); all animals maintained in vitro reactivity to third party (class I-mismatched) PBMCs (data not shown), suggesting that unresponsiveness was donor-specific. CML: cellular mediated lympholysis.
Transplantation of LTT kidneys
We next assessed whether PBMC transplantation was required for successful adoptive transfer of tolerance when only the LTT kidney was transplanted. Two animals in group B received LTT kidneys from DST-primed syngeneic donors, without co-transplantation of peripheral blood cells. These animals accepted the LTT grafts and showed evidence of donor-specific unresponsiveness in CML assays, confirming the successful transfer of tolerance. Figure 2B shows a day 125 biopsy from animal 20319 (group B) showing a kidney with no evidence of rejection, consistent with tolerance.
Considering the fact that we have previously demonstrated DST to have a major effect on boosting of Treg in the PBMCs (3), we next asked whether PBMC transfusion was required at all when only the LTT kidney was transferred. Three animals received LTT kidneys from unprimed tolerant animals with no immunosuppression other than the standard 150 cGy WBI administered on day −1. Of these three animals, two went on to become long-term tolerant (Figure 2C shows a POD 105 biopsy from animal 21713 (group C) consistent with acceptance of the kidney), while one (#21957, group C) showed fluctuations in creatinine levels from day 14 post-transplantation, and eventually rejected the kidney on post-operative day 73. On analyzing the pedigree of this animal, we learned that it was from the subline in which a mutation of a gene required for tolerance induction with CyA has been reported (6). Since it remains unclear whether this same gene might be operative in the case of re-transplantation of a LTT kidney, we did not remove this animal from the final analysis, although this might provide a reason why this animal did not achieve tolerance.
Low-dose WBI is required for acceptance of LTT kidneys
To determine whether low dose WBI is required when only the LTT kidney was transferred, we next attempted to transplant non-DST primed LTT kidneys into naïve syngeneic recipients, without low-dose WBI. Thus, two animals received LTT kidneys without WBI or immunosuppression (group D). Both rejected the LTT grafts abruptly, in 13 and 14 days, respectively, suggesting that low-dose irradiation was required in the previous series, not only for cellular therapy but also to achieve tolerance of the adoptively transferred LTT kidney (Table 1). Finally, to ensure that low-dose irradiation alone was not responsible for achieving tolerance in all three groups, we treated 3 animals with 150 cGy WBI, no cells and only a naïve class-I mismatched kidney (group E). All of these control animals rejected their transplanted grafts acutely, on days 7, 7 and 8 (Table 1).
Stability of tolerance induced by means of adoptive transfer
We followed the adoptively transferred LTT kidneys for at least 100 days after transplantation, evaluating the graft function by serial serum creatinine measurements, monthly CML assays and monthly biopsies. In order to assess the stability of tolerance induced by transplantation of a LTT kidney, animals were tested in vivo with a challenge graft. Thus, if an animal reached day 100 with stable renal function, no evidence of rejection on histological analysis and donor-specific hyporesponsiveness in vitro, we then removed the adoptively transferred LTT graft and replaced it with a fresh, MHC-matched kidney (SLAgg), without any additional immunosuppression. Six animals were re-transplanted (2 animals each in groups A, B, and C; results are summarized in Table 2). Of these 6 animals, one in each group accepted the second renal graft and maintained stable renal function for more than 100 days (Figure 4 A-B). Survival of the second graft could not be determined in two of the other animals: Animal 19678 in group A developed fluctuating creatinine levels after re-transplantation due to ureteral stenosis, which did not improve after multiple attempts of surgical correction. The animal eventually became uremic and was sacrificed on day 48, at which point it was still unresponsive in vitro. Histological analysis of the biopsies collected prior to sacrifice showed severe reflux, but no signs of rejection (data not shown). Animal 21254 (group C) died on day 25 after renal transplantation, from a pulmonary embolism, with stable creatinine (1.0 mg/dl) and no histological evidence of rejection. The third animal, 20791, (group B) maintained stable renal function for the first 30 days after re-transplantation, although histology at one month after transplantation already showed signs of rejection (vasculitis, interstitial infiltrate). This animal eventually regained donor-responsiveness in vitro (Figure 4D) and went on to reject the re-transplanted kidney on day 53, with histologic evidence of acute cellular rejection (Figure 4C). As discussed below, these observations suggest that tolerance induced by adoptive transfer of a LTT kidney may be less stable than the tolerance induced by our standard 12-day course of high-dose CyA.
Table 2.
Summary of challenge grafts survival.
| Group | DST | WBI | PBMCs | Kidney Graft | Animal ID | Challenge graft survival (days) |
|---|---|---|---|---|---|---|
| A | + | + | + | LTT | 19678 20314 |
>48(*) >129 |
| B | + | + | - | LTT | 20319 20791 |
> 135 53 |
| C | - | + | - | LTT | 21254 21713 |
>25(**) >273 |
Two animals were lost to follow-up for reasons other than rejection (19678: recurrent ureteral reflux with pyelonephritis
21254, pulmonary embolism.
Figure 4. Outcomes of donor-type challenge grafting.
Panels A and B show histology and in vitro unresponsiveness on day 121 after re-transplantation from animal 20319, one of the three who accepted the re-transplanted challenge graft. Panel C shows arteritis and acute cellular rejection in the challenge graft of swine 20791 at the time of sacrifice (day 53 after re-transplantation). At that time the animal also regained responsiveness in vitro (panel D). PBMCs from a naïve haploidentical animal (SLAdd) were used as control in all assays (naïve DD).
DISCUSSION
“Infectious tolerance” was a term introduced by Gershon in 1971 (12). In the original definition provided by Gershon, this term defined “the ability to transfer a state of unresponsiveness from one animal to another”. In the recent years, this concept has been used to describe one of the major mechanisms underlying immunological tolerance. Qin et al. were the first to demonstrate that tolerance could be adoptively transferred in a small animal model. After inducing tolerance of a skin graft via anti-CD4 treatment, they demonstrated that naïve syngeneic CD4 cells transplanted into the tolerant animal would acquire a regulatory phenotype. The “re-educated” regulatory T cells could then be transplanted to a naïve animal and induce donor-specific tolerance to a skin graft (1). In another experiment, Chen et al. were able to demonstrate that CD4+ T cells transfused from a mouse tolerant of a fully mismatched vascularized heart were able to induce donor-specific tolerance in a syngeneic recipient (2). In the same study, Chen et al. also showed that this process could be repeated for up to 10 subsequent generations of adoptively transferred cell recipients (2). Since the publication of these seminal studies, several authors have developed adoptive transfer models in mice and rats, using different cells populations to transfer tolerance, such as PBMCs, splenocytes, lymph nodes or graft infiltrating cells (GILs) (1;13;14).
Of note, all of these experiments demonstrating adoptive transfer of tolerance require the use of inbred animals as donors and recipients to avoid rejection of the transferred cells. It is likely for this reason that adoptive transfer had never been demonstrated in a large animal model until our recent report on the successful induction of tolerance through adoptive transfer in miniature swine (3). As in the studies reported here, we made use of our highly inbred, histocompatible subline of our SLAdd miniature swine in order to avoid loss of the adoptively transferred cells by rejection. In fact, we had attempted similar adoptive transfer experiments several years ago, before the histocompatible line was available, and had been unsuccessful, presumably because the recipient animals had rejected the transferred cells. Consistent with this possibility, the recipients become sensitized to minor antigens at the cellular level (Yamada et al., unpublished data).
In our previous study using highly inbred, histocompatible donors and recipients, we transplanted a naïve donor kidney at the same time as the LTT kidney from the tolerant donor animal (3). When we analyzed the serial biopsies from animals in the previous series, we noted that in the animals that did not accept the grafts, rejection appeared to occur in the naïve transplant first, involving the LTT graft only later. We therefore questioned whether tolerance of the LTT kidney might have been more readily transferred if it had been transplanted without a simultaneous naïve kidney. In the present study, we have indeed been able to adoptively transfer tolerance more readily when the LTT kidney was transplanted alone (Table 1, Group B). Furthermore, when it was transplanted without a naïve kidney, neither PBMCs nor DST-priming of the donor animal were required to induce this “infectious tolerance” (Table 1, Group C). To our knowledge, this is the first report of tolerance induced by transplantation of a vascularized allograft alone.
Interestingly, when the kidney in these tolerant animals was replaced with a second donor-matched graft, clear evidence for rejection was observed in one of the four “informative” animals (table 2, Group B). On the other hand, we have performed more than 20 cases of re-transplantation in the standard CyA model, and have observed no instances of rejection. Indeed, only following extensive manipulation (removal the LTT kidney and of a large number of PBMCs via leukapheresis) prior to re-transplantation, were we able to induce rejection in the CyA model (8). This comparison suggests that tolerance induced by adoptive transfer of a LTT kidney may be less robust than that induced by the standard CyA protocol. More animals will be needed to substantiate this hypothesis.
These results may have both theoretical and practical implications. From a theoretical viewpoint, these results suggest the presence of a potent cell population within the tolerated organ of an LTT animal, that is capable not only of protecting the graft from rejection but also of transferring tolerance adoptively when the organ is transplanted into a naïve, syngeneic animal. One might ask whether the ease of tolerance induction might be due to decreased antigenicity of the LTT graft. However, this possibility is made unlikely by the failure of similar adoptive transfer experiments carried out previously, using non-highly inbred animals (see above). In addition, DST priming of the LTT donor and transfer of PBMC were required for adoptive transfer of tolerance when both a naïve and an LTT class I-mismatched kidney were transplanted, but not when the LTT kidney was transplanted alone. These results are most consistent with the presence of a cell population with strong tolerogenic properties in LTT kidneys. Several tolerogenic cells have been identified in tolerant kidneys, including Tregs, renal tubular epithelial cells, plasmacytoid dendritic cells (15;16) and current studies in our laboratory are directed toward determining whether these elements are involved in the adoptive transfer of tolerance we have observed. Of note, we consistently observed a number of perivascular lymphoid aggregates in tolerant kidneys in this model. The localization of these aggregates suggest that they may constitute Treg-rich organized lymphoid structures (TOLS), which have previously been reported in tolerated renal allografts in a murine model (17). However we were not able to perform a FoxP3 immunohistochemical staining with an anti-swine antibody in order to confirm the nature of the cells forming these aggregates.
From a practical standpoint, these results suggest a cautionary note with regard to clinical trials utilizing cell therapy in attempts to induce regulatory tolerance (18;19). These trials have been based on observations made both in vitro and in vivo in small animal models. However, as discussed above, adoptive transfer of tolerance has been achieved using cell transfer alone in murine models, while in the present large animal model, adoptive tolerance of a naïve kidney was only achieved when a kidney containing this tolerogenic cell population was also transplanted. Our data therefore suggest that in more complex large animal models (and presumably in human beings), the localization and the nature of the regulatory cell populations is likely to play an essential role in tolerance induction. Therefore, success of Treg-based tolerance strategies may require a better understanding of the role of intra-graft regulatory T cells and their homing to the transplanted organ more than is currently available.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R37AI031046-24 and C06RR020135-01. Cyclosporine A was kindly provided by Novartis. JRS is a recipient of a Basic Science Fellowship Grant from the American Society of Transplantation. We thank Drs. Maria Lucia Madariaga and Isabel M. Hanekamp for critical review of the manuscript, and Rebecca Brophy for her expert editorial assistance.
Abbreviations
- cGy
centigray
- CML
cell-mediated lympholysis
- CyA
cyclosporine A
- DCs
dendritic cells
- DST
donor-specific transfusion
- IV
intravenous
- LTT
long-term tolerant
- MHC
major histocompatibility complex
- POD
post-operative day
- PBMCs
peripheral blood mononuclear cells
- SLA
swine leukocyte antigen
- Tregs
regulatory T cells
- WBI
whole-body irradiation
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Reference List
- 1.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–7. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZHK, Cobbold SP, Waldmann H, Metcalfe S. Amplification of natural regulatory immune mechanisms for transplantation tolerance. Transplantation. 1996;62:1200–6. doi: 10.1097/00007890-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Okumi M, Scalea JR, Gillon BC, Tasaki M, Villani V, Cormack T, et al. The induction of tolerance of renal allografts by adoptive transfer in miniature swine. Am J Transplant. 2013 May;13(5):1193–202. doi: 10.1111/ajt.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanekamp JS, Duran-Struuck R, Sachs DH. Transplantation in Miniature Swine. In: McAnulty PA, Dayan A, Hastings KH, Ganderup N-C, editors. The Minipig in Biomedical Research. Taylor & Francis Group Publication; 2011. [Google Scholar]
- 5.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–67. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Lee PW, Hanekamp JS, Villani V, Vagefi PA, Cina RA, Kamano C, et al. Evidence for a Gene Controlling the Induction of Transplantation Tolerance. Am J Transplant. 2014 Mar 4; doi: 10.1111/ajt.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okumi M, Fishbein JM, Griesemer AD, Gianello PR, Hirakata A, Nobori S, et al. Role of Persistence of Antigen and Indirect Recognition in the Maintenance of Tolerance to Renal Allografts. Transplantation. 2008 Jan 27;85(2):270–80. doi: 10.1097/TP.0b013e31815e8eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scalea JR, Okumi M, Villani V, Shimizu A, Nishimura H, Gillon BC, et al. Abrogation of Renal Allograft Tolerance in MGH Miniature Swine: The Role of Intra-Graft and Peripheral Factors in Long-Term Tolerance. Am J Transplant. 2014 Sep;14(9):2001–10. doi: 10.1111/ajt.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ierino FL, Yamada K, Lorf T, Arn JS, Sachs DH. Mechanism of tolerance to class I-mismatched allografts in miniature swine: Regulation of interleukin-2 receptor α-chain expression on CD8 peripheral blood lymphocytes of tolerant animals. Transplantation. 1998;66(4):454–60. doi: 10.1097/00007890-199808270-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999 Jan 1;162(1):550–9. [PubMed] [Google Scholar]
- 11.Rosengard BR, Ojikutu CA, Guzzetta PC, Smith CV, Sundt TM, III, Nakajima K, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–7. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–14. [PMC free article] [PubMed] [Google Scholar]
- 13.Kitade H, Kawai M, Rutgeerts O, Landuyt W, Waer M, Mathieu C, et al. Early presence of regulatory cells in transplanted rats rendered tolerant by donor-specific blood transfusion. J Immunol. 2005 Oct 15;175(8):4963–70. doi: 10.4049/jimmunol.175.8.4963. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka M, Margenthaler JA, Ku G, Flye MW. Development of infectious tolerance after donor-specific transfusion and rat heart transplantation. J Immunol. 2003 Jul 1;171(1):204–11. doi: 10.4049/jimmunol.171.1.204. [DOI] [PubMed] [Google Scholar]
- 15.Frasca L, Marelli-Berg F, Imami N, Potolicchio I, Carmichael P, Lombardi G, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998 Mar;53(3):679–89. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 16.Zheng D, Cao Q, Lee VW, Wang Y, Zheng G, Wang Y, et al. Lipopolysaccharide pretreated plasmacytoid dendritic cells ameliorate experimental chronic kidney disease. Kidney Int. 2012 May;81(9):892–902. doi: 10.1038/ki.2011.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della PP, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011 Apr;178(4):1635–45. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+C. Clin Immunol. 2009 Oct;133(1):22–6. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 19.The ONE Study UK Treg Trial (ONETreg1) 2015 3-5-2015. [Google Scholar]