Abstract
Platelets have been long postulated to play a critical role in the pathogenesis of prostate cancer, although relatively little is known regarding the precise mechanisms involved. Androgen deprivation therapy (ADT) for prostate cancer eventually fails with relapse occurring in the form of castration-resistant prostate cancer (CRPC). CRPC tumors typically overexpress androgen receptor (AR), demonstrating continued dependence upon AR signaling. Platelets have been previously demonstrated to contain androgens, and we sought to explore the contribution of platelet-derived androgens in CRPC. In this study, we examined the role of platelet-derived androgens in vitro using platelets from men with CRPC, men with high-risk prostate cancer, and healthy male donors. A series of in vitro assays was performed to elucidate the impact of platelet-derived androgens on androgen-sensitive prostate tumor cells. By examining platelet-derived androgen effects on AR signaling in prostate tumor cells, we found that platelets, from men with CRPC and on ADT, strongly induce AR target genes and tumor cell proliferation. Moreover, we show a fully intact testosterone (T) biosynthetic pathway within platelets from its precursor cholesterol and demonstrate that platelets of CRPC patients with ADT resistance are able to generate T. Overall, our findings reveal an unknown capacity of platelets to synthesize T at functionally relevant levels in patients with lethal prostate cancer. Importantly, it suggests a novel paracrine mechanism of T production that may act to sustain CRPC state and potentiate therapeutic resistance.
Abbreviations: ADT, androgen deprivation therapy; AR, androgen receptor; CRPC, castration-resistant prostate cancer; DHT, dihydrotestosterone; HR, high-risk disease; MS, mass spectrometry; T, testosterone
Introduction
The androgen receptor (AR)–mediated cellular program is a driving force in the process of prostate carcinogenesis [1]. For men with advanced disease, initial therapy consists of androgen-ablative strategies. Unfortunately, despite an initial response, most tumors relapse in the form of castration-resistant prostate cancer (CRPC), a lethal condition that accounts for approximately 30,000 deaths in the United States annually [2]. Notably, despite systemic androgen ablation, CRPC tumors exhibit continued cellular dependence on AR signaling. A variety of mechanisms have been demonstrated to support the castrate-resistant state including amplification and mutations of the AR gene, ligand-independent activation of AR, and dysregulation of AR gene coactivators and repressors, among others [3], [4]. In addition, intratumoral prostate cancer cell production of androgens has also been shown to play an important role in the sustenance of CRPC tumors [5], [6]. It is important to note that the current generation of androgen-ablative therapies (e.g., abiraterone) seeks to block this source of androgen synthesis [7].
Platelets have been postulated to perform a critical function in the pathogenesis of prostate cancer for decades [8], [9], yet the precise nature of this interaction remains poorly understood. Proposed ways platelets may induce prostate cancer progression include the local delivery of growth factors and the protection of circulating tumor cells from immune surveillance [10], [11], [12]. Intriguingly, platelets have also been shown to produce the androgen dehydroepiandrosterone [13], raising the possibility of an additional mechanism through which platelets might support prostate cancer growth. This observation motivated us to assess intraplatelet testosterone (T) levels in men with advanced prostate cancer; interrogate the capacity of platelets to produce T from its precursor cholesterol, specifically in the castrate-resistant state; and test the ability of platelet-derived T to induce androgen signaling within prostate cancer cells. The findings presented in this work provide novel insight into a previously unknown role for platelets in the pathogenesis of prostate cancer and importantly identify platelets as both potentially new therapeutic targets as well as markers of extragonadal androgen biosynthesis.
Material and Methods
Reagents
All metabolites were from Sigma-Aldrich (St. Louis, MO): 13C-T (cat no. 73610), 13C-cholesterol (cat no. 749478), and 13C-pregnenolone (cat no. 740985). Bicalutamide was a kind gift of Dr. Chinnaiyan (University of Michigan). Abiraterone acetate (cat no. SC-207240) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture
Human prostate cancer cell lines LNCaP and PC3 were obtained from the American Type Culture Collection (Manassas, VA), and LNCaP-AR cells were a kind gift from Dr. C. Sawyers (Memorial Sloan Kettering Cancer Center, New York, NY). All cells were authenticated by the University of Michigan DNA Sequencing Core using short tandem repeat DNA fingerprinting. Cells were maintained in the RPMI 1640 medium (Gibco; Life Technologies) supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate.
Platelet Isolation
Written informed consent in accordance with the Declaration of Helsinki was received from all participants before inclusion in the study. All patient samples were obtained under an institutional review board–approved protocol at the University of Michigan following informed consent. Human platelets were isolated from venous blood from healthy subjects or prostate cancer patients not taking antiplatelet drugs (e.g., aspirin, long-term nonsteroidal anti-inflammatory drugs, clopidogrel). Separation of platelets from whole blood was achieved by centrifugation (1000 rpm for 20 minutes at room temperature). Platelet-rich plasma was then transferred into a separate tube, and prostaglandin-1 (500 ng/ml) was added to prevent platelet activation. Platelets were then precipitated by centrifugation at room temperature for 5 minutes at 2200 rpm and washed extensively in wash buffer (140 mM NaCl, 5 mM KCl, 12 mM trisodium citrate, 10 mM glucose, 12.5 mM sucrose, pH 6.0). Harvested platelets were then counted with a Coulter counter (Beckman Coulter, Fullerton, CA) and resuspended in platelet buffer (PB) (10 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid, 140 mM NaCl, 3 mM KCl, 0.5 mM MgCl2, 5 mM NaHCO3, 10 mM glucose) before use.
Cell Proliferation
Cells were seeded into Poly-l-Lysine (Sigma-Aldrich)–coated 12-well plates at a density of 5 × 103 cells per well in RPMI 1640 containing 10% FBS. Medium was replaced 18 hours later with platelet buffer alone (control) or with human platelets (10 × 106/ml) resuspended in platelet buffer (treatment). Cell counts were done using a Coulter counter (Beckman Coulter) at indicated time points in triplicates. At least three independent experiments were performed.
Quatitative reverse transcriptase polymerase chain reaction
Cells were seeded into six-well plates (CellBIND) from Corning (Corning, NY) at a density of 5 × 105 cells per well in RPMI 1640 containing 10% FBS. Approximately 18 hours later, attached cells were androgen deprived for the next 48 hours by replacing the media with phenol red–free RPMI supplemented with 1% charcoal-stripped serum (CSS) from Life Technologies (Grand Island, NY). CSS containing media was replaced every 12 hours for the next 48 hours. For bicalutamide experiments, drug (10 μM) was added for 10 hours in 10% CSS phenol red–free RPMI and then added again in platelet buffer immediately before platelet addition. Either platelet buffer alone or platelets (150,000/μl) in 3 ml of platelet buffer were added to the cells.
After 24-hour incubation with platelets in a cell incubator, cells were washed 2 × with phosphate-buffered saline (PBS) and lysed in Trizol (Life Technologies). RNA extraction from Trizol was performed according to the manufacturer’s protocol; BioRad iScript cDNA synthesis kit was used for reverse transcriptase polymerase chain reaction (PCR) (BioRad, Hercules, CA). Quantitative PCR was performed using BioRAD SYBR Green Mastermix on an Applied Biosystems 7300 Real-Time PCR system. All reactions were performed in triplicates. Fold mRNA expression was calculated using 2− ΔΔCT method [14]. Primers were synthesized by Integrated DNA Technologies (Coralville, IA). Primers used were as follows:
PSA: (forward) 5’-TGACCAAGTTCATGCTGTGT-3’ and (reverse) 5’-GTCATTTCCAAGGTTCCAAG-3’; TMPRSS2: (forward) 5’-CAGGAGTGTACGGGAATGTGATGGT-3’ and (reverse) 5’-GATTAGCCGTCTGCCCTCATTTGT-3’; GAPDH: (forward) 5’-GCACCGTCAAGGCTGAGAAC-3’ and (reverse) 5’-TGGTGAAGACGCCAGTGGA-3’.
Immunoblot
Platelet protein extracts were prepared using cell lysis buffer (Cell Signaling; cat no. 9803S) with protease inhibitors (Thermo Scientific; cat no. 78410). Protein concentrations were determined by BCA assays (BioRAD). Samples were boiled in Laemmli sample buffer (BioRAD) with β-ME and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. One hundred micrograms of protein was loaded per lane. Proteins were transferred to a nitrocellulose membrane, blocked in 5% nonfat milk (wt/vol in PBS), and immunoblotted with indicated primary antibodies and appropriate horseradish peroxidase–conjugated secondary antibodies. Immunoblots were developed using Western Lighting Chemiluminescence Reagent (Milipore, Billerica, MA).
Antibodies anti-CYP17A1 (cat no. SAB1300941), anti-HSD3B2 (cat no. SAB2101087), and anti–actin-HRP (cat no. A3854) were from Sigma-Aldrich; anti-CYP11A1 (cat no. 12491) was from Cell Signaling Technology (Danvers, MA).
Mass Spectrometry
Platelets from healthy donors or prostate cancer patients were isolated from venous blood as described above. For platelet transfer of T to tumor cells, LNCaP cells were plated on the Poly-l-Lysine (Sigma-Aldrich)–coated 10-cm dishes in 10% FBS RPMI. The next day, the medium was replaced with 10% CSS phenol-free RPMI for the next 24 hours. Washed platelets (~ 6 × 106) were resuspended in 6 ml of platelet buffer and incubated with 10 μl of 13C-T (1 mg/ml in ethanol) for 24 hours. After incubation, platelets were washed 2 × with wash buffer and then added to the cells in 10-cm dishes. After 24-hour incubation, control (untreated cells) and platelet-treated cells were washed with PBS 2 ×; cells were then fixed by the addition of ~ 5 ml of liquid nitrogen. Frozen dishes were then stored at − 80°C before being transferred for mass spectrometry (MS) analysis.
For metabolite conversion studies, washed platelets (~ 8 × 104) were resuspended in 320 μl of platelet buffer. Equal volume of platelets was then transferred into separate tubes for control (untreated) and the experiment. One microliter of 13C-cholesterol (2 mg/Ml in ethanol) and 1 μl of 13C-pregnenolone (1 mg/Ml in ETHANOL) were then added to the platelet suspension.
To establish the intraplatelet T and dihydrotestosterone (DHT) concentrations, platelets from patients (5.2 × 106 - 39 × 106 platelets) were resuspended in 200 μl of platelet buffer. Platelets were then activated with thrombin (1 U/μl) and centrifuged at 14,000 rpm for 1 minute. Androgen-containing supernatant was then transferred into a fresh tube and frozen at − 80°C before being transferred for MS analysis. The lower limit of quantification for each androgen using the MS assay was 10 pg/ml using 100 μl of serum. The intraassay coefficients of variation for T and DHT were < 5%.
For the abiraterone studies, platelets were pretreated with 10 μg of the drug for 8 hours before either 13C-cholesterol or 13C-pregenenolone was added. After 24-hour incubation at room temperature, platelets were briefly mixed and thrombin (1 U/μl) (Sigma-Aldrich) was added to activate platelets. Samples were then stored at − 80°C until ready for MS analyses. The metabolite extraction method used for platelets and cells was performed as previously described [15], [16], [17]. Estrone was used as an internal standard.
Statistics
For the immunoassay experiment, a two-tailed unpaired t test was used to compare the T values of controls versus patients; for all other experiments, two-tailed paired t test was used to compare the samples. P values less than .05 were considered significant (*); and P values less than .001, very significant (***).
Results
Platelet De Novo Synthesis of Testosterone
We first assessed whether platelets can synthesize T de novo. In these studies, we co-incubated platelets from healthy donors with the T lipid precursors cholesterol and pregnenolone labeled with carbon-13 (13C). We then measured levels of 13C-T in platelets after 24 hours using MS. We found that platelets were able to successfully convert both cholesterol and pregnenolone into T as demonstrated in Figure 1A and DHT (Supplementary Figure S1A).
Figure 1.
Platelet de novo synthesis of T. (A) Ratio of 13C-T to an internal standard (isotopically labeled estrone) in healthy donor platelets (n = 4) preincubated with cholesterol or pregnenolone. “Platelets”: untreated platelets; “Platelets + 13C-Cholesterol”: platelets incubated with 13C-cholesterol; “Platelets + 13C-Pregnenolone”: platelets incubated with 13C-pregnenolone. Significance: ***P < .001. (B) Western blot of platelet lysates demonstrating the presence of T biosynthetic enzymes (black arrows) in washed platelets of “patients” (men with prostate cancer) (from Table 1) and “controls” (healthy donors). Actin was used as loading control. (C) Ratio of 13C-T to an internal standard in healthy donor platelets (n = 4) preincubated with 13C-pregnenolone and abiraterone acetate at 10 μg/ml. “Platelets”: untreated platelets; “Platelets + 13C-Pregnenolone”: platelets incubated with 13C-pregnenolone. (D) Graph representing the ratio of 13C-T to an internal standard in platelets from abiraterone-resistant men with CRPC (n = 3). “Platelets”: untreated platelets; “Platelets + 13C-Cholesterol”: incubated with 13C-cholesterol. Significance: *P < .05. (E) Platelets from a man currently receiving abiraterone (castration-sensitive disease). “Platelets”: untreated platelets; “Platelets + 13C-Cholesterol”: incubated with 13C-cholesterol.
To further characterize the androgen biosynthetic pathway within platelets, we interrogated the intraplatelet expression of the T synthetic enzymes CYP11A1, HSD3B2, and CYP17A1 at the protein level. Western blot analyses of six healthy donor and six prostate cancer patient platelet lysates (Table 1) revealed the expression of all three enzymes required for de novo generation of T within platelets (Figure 1B). Interestingly, platelets of three CRPC patients (#1, #4, and #5; Table 1) currently undergoing androgen deprivation therapy (ADT) and platelets from two patients with high-risk disease showed elevated levels of CYP17A1 protein as compared with platelets of healthy controls.
Table 1.
Prostate Cancer Patients’ Data
| Patient ID | Age | Cancer Type | PSA | Gleason Sum | Therapy | Abiraterone Resistance | Platelet [T] Levels (pg/ml) | Platelet [DHT] Levels (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| #1 | 60 | CRPC | 28.4 | – | ADT | Yes | 20.5 | 27.7 |
| #2 | 79 | HR | 20.2 | N.A. | Radiation | – | 46.1 | 76.8 |
| #3 | 65 | HR | 7.9 | 9 | Surgery | – | 13 | 18.6 |
| #4 | 76 | CRPC | 8.1 | – | ADT | Yes | 12.3 | 15.3 |
| #5 | 80 | CRPC | 6.1 | – | ADT | Yes | 9.2 | 11.8 |
| #6 | 67 | HR | 18 | 9 | Surgery | – | 49 | 81.7 |
Cancer type: CRPC, castration-resistant prostate cancer; HR, high-risk prostate cancer defined as Gleason sum > 8 and/or PSA > 20 ng/ml. “PSA”: PSA levels at the time platelets were collected. Gleason sum at the time of platelet collection or not available (N.A.). “Therapy”: type of treatment at the time of platelet collection. “Platelet [T] levels”: concentration of T pg/ml in 1 × 106 platelets. “Platelet [DHT] levels”: concentration of dehydrotestosterone pg/ml in 1 × 106 platelets. Patients #1, #4, and #5 have abiraterone resistance as indicated by their rising PSA levels (not shown).
To test whether the T biosynthetic pathway in platelets is accessible to agents like abiraterone, a CYP17A1-blocking drug (half-life ~ 15 hours) commonly used in ADT in CRPC patients, we pretreated platelets from healthy donors with abiraterone before adding 13C-cholesterol. We then measured the ability of platelets to convert cholesterol into T and DHT by MS analyses and observed that abiraterone significantly inhibited platelet production of T and DHT, indicating that platelet T converting enzymes are accessible to CYP17A1 inhibitors (Figure 1C and Supplementary Figure S1B).
Next, we assessed the capacity of platelets from men with CRPC and known clinical abiraterone resistance to convert cholesterol into T. We found that platelets from three of three men with CRPC and known abiraterone resistance (and undergoing abiraterone treatment at the time of platelet collection) were able to generate 13C-T (Figure 1D and Supplementary Figure S1C), signifying that platelets from these men are able to metabolize cholesterol into T despite systemic CYP17A1 blockade. Interestingly, MS analyses of platelets from a CRPC patient with abiraterone-sensitive disease on abiraterone treatment at the time of platelet collection revealed that his platelets were unable to convert 13C-cholesterol into 13C-T (Supplementary Figure S1C).
Platelet-Derived Androgens Stimulate AR Signaling and Promote Tumor Cell Proliferation
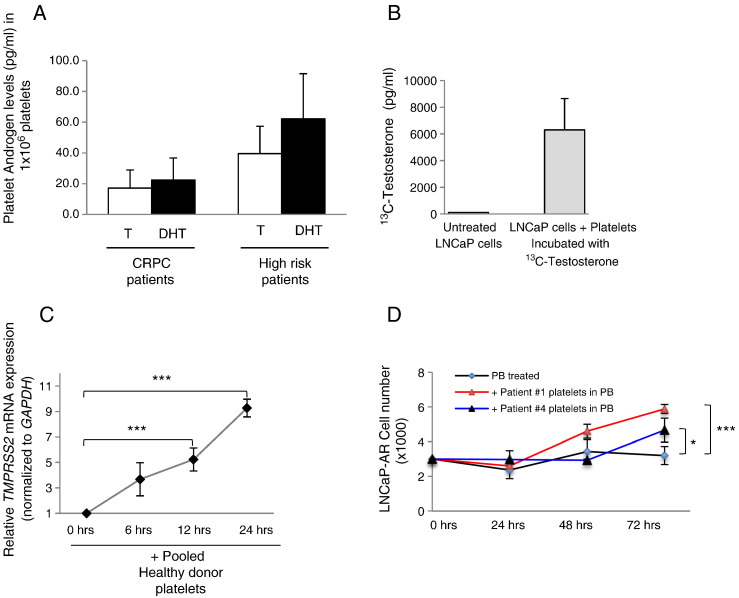
To gain a better understanding of the landscape of intraplatelet androgen levels, we measured the levels of T and DHT in platelets from men with prostate cancer using MS analysis. We found detectable levels of both androgens in platelets of all men studied (Figures 2A and S2A), and no significant difference between the intraplatelet levels of T and DHT in men with CRPC (n = 10) or in men with high-risk (n = 8) disease was observed.
Figure 2.
Platelet-derived androgens stimulate AR and promote tumor cell proliferation. (A) MS analysis of intraplatelet levels of androgens in platelets of men with prostate cancer. Concentrations of T and DHT in 1 × 106 platelets from men with CRPC (n = 10) and from men with high-risk disease (n = 8). (B) MS analyses demonstrate 13C-T in LNCaP cells after addition of washed platelets preincubated with 13C-T for 24 hours in vitro. (C) Relative mRNA levels of TMPRSS2 (normalized to GAPDH) in LNCaP cells after the addition of pooled platelets from three healthy donors under androgen-deprived cell culture conditions for 6, 12, and 24 hours (significance: ***P < .001). (D) LNCaP cell proliferation over 72 hours in response to healthy donor platelets under androgen- and serum-deprived cell culture conditions. “PB treated”: cells incubated in platelet buffer only; “+ Healthy donor platelets in PB”: cells incubated with healthy donor’s platelets in platelet buffer (significance: ***P < .001). (E) LNCaP-AR (AR overexpressing) cell proliferation over 72 hours in response to prostate cancer patient platelets under androgen- and serum-deprived cell culture conditions. “PB treated”: cells incubated in platelet buffer only; “+ Patient # platelets in PB”: cells incubated with CRPC patient (Table 1) platelets in platelet buffer (significance: *P < .05; ***P < .001).
Given the known ability of platelets to interact and deliver content directly to tumor cells [11], [12], we sought to investigate platelet capacity to deliver T to prostate cancer cells. We first co-incubated platelets with 13C-labeled T and then incubated these loaded platelets with the androgen-dependent prostate cancer cell line LNCaP under androgen-deprived cell culture conditions. MS cellular analysis revealed prostate cancer cell uptake of platelet-derived 13C-T after a 24-hour incubation period (Figure 2B) indicating that platelet-derived T can be delivered by platelets and is able to enter prostate cancer cells.
The mechanisms responsible for maintaining the castrate-resistant state following ADT failure include, but are not limited to, AR gene amplification and overexpression. In this condition, even low levels of androgens are capable of inducing the AR-associated cellular program [3], [4]. Thus, we treated androgen-dependent LNCaP cells with healthy donor platelets for 6, 12, and 24 hours under serum- and androgen-deprived cell culture conditions. We found that pooled platelets from healthy male donors induce activation of the AR-dependent gene TMPRSS2 (a prostate-specific, androgen-responsive, transmembrane serine protease gene) in a time-dependent manner (Figure 2C). Because TMPRSS2 has been also implicated in cell proliferation, we then examined the effect of platelets on prostate cancer cell proliferation and observed that, in serum- and androgen-deprived cell culture conditions, platelets induced cell proliferation of androgen-responsive LNCaP cells (Supplementary Figure S2B) but had no effect on the AR-negative prostate cancer cell line PC3 (Supplementary Figure S2C), suggesting that, under these conditions, platelet influence on prostate cancer cells may be mediated via androgens rather than the delivery of other platelet-derived growth factors.
We confirmed our proliferation studies using platelets from men with prostate cancer (Table 1) and LNCaP cells genetically engineered to overexpress AR (LNCaP-AR). After 72 hours of platelet incubation under serum- and androgen-deprived conditions, we observed a significant induction of proliferation of LNCaP-AR cells by platelets from men with prostate cancer (Figure 2D). These studies indicate that platelet-derived androgens are capable of stimulating CRPC-like (i.e., AR overexpressing) cells.
Platelet-Derived Testosterone Induces AR Target Genes in CRPC-Like Cells
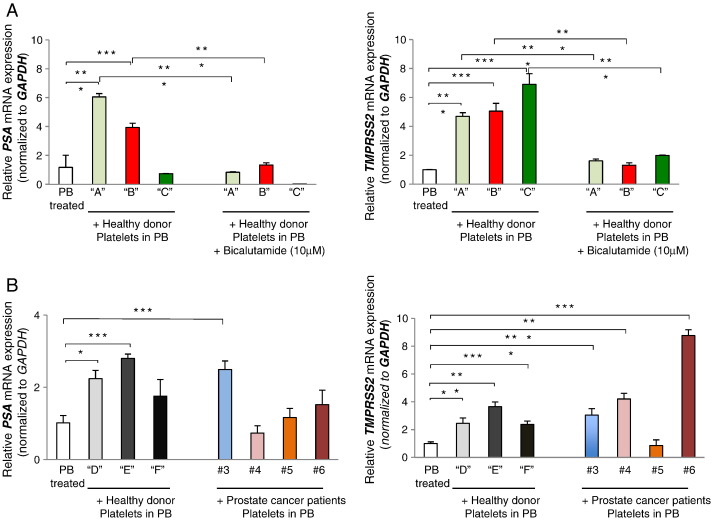
To assess the ability of platelet androgens to drive AR signaling in prostate cancer cells, we treated CRPC-like LNCaP-AR cells with platelets in the presence of the AR antagonist bicalutamide. Under these conditions, healthy donor platelets were unable to activate AR, indicating that platelet induction of both prostate-specific antigen (PSA) and TMPRSS2 gene expression in prostate cancer cells requires functional AR (Figure 3A, left and right panels).
Figure 3.
Platelet-derived T regulates AR target genes in LNCAP-AR cells. (A) Relative mRNA expression levels of PSA (left panel) and TMPRSS2 (right panel) normalized to GAPDH in LNCAP-AR cells after treatment with platelets for 24 hours under androgen- and serum-deprived cell culture conditions in the presence or absence of bicalutamide (10 μM). Control cells were treated with platelet buffer alone - “PB treated”; cells treated with platelets from individual healthy donors: “A”, “B” and “C” “+ healthy donor platelets in PB” - resuspended in platelet buffer; and cells treated with Bicalutamide and platelets (significance: *P < .05, ***P < .001). (B) Relative mRNA expression levels of PSA (left panel) and TMPRSS2 (right panel) normalized to GAPDH in LNCAP-AR cells after addition of washed platelets for 24 hours under androgen- and serum-deprived cell culture conditions. Control cells were treated with platelet buffer alone: “PB treated”; cells treated with platelets from individual healthy donors “D”, “E,” and “F” resuspended in platelet buffer: “+ healthy donors’ platelets in PB”; and cells treated with platelets from individual prostate cancer patients #3, #4, #5, and #6 (from Table 1) resuspended in platelet buffer: “+ Prostate cancer patients’ platelets in PB” (significance: *P < .05, ***P < .001).
To further investigate the impact of platelet androgens on AR gene transcription, we assessed the effect of single-donor platelets on AR gene targets. Using individual platelets from healthy donors (“D,” “E,” “F”) and prostate cancer patients (#3, #4, #5, and #6; Table 1), we evaluated platelet effect on PSA and TMPRSS2 expression in LNCaP-AR cells. We selected at random patients whose platelets contained detectable levels of T and found that platelets from two of three healthy donors and three of four CRPC patients contained sufficient levels of androgens to significantly induce activation of PSA and/or TMPRSS2 (Figure 3B, left and right panels) in AR-dependent cells.
Discussion
Platelets have been demonstrated to modulate tumor growth through direct delivery of their content to cancer cells [11], [12]. Nevertheless, to date, the precise role of platelets in prostate cancer progression remains unclear; and furthermore, an examination of platelet-derived androgens in prostate cancer biology has not been described. Previous reports profiling intraplatelet mRNA content demonstrated the expression of steroidogenic dehydrogenases involved in the production of androgens [18]. Moreover, prior findings that platelets are capable of producing the androgen dehydroepiandrosterone [13] and that platelets from healthy men possess detectable levels of androgens [19] led us to further assay the intraplatelet T biosynthetic pathway in the context of prostate cancer. The data presented in this report demonstrate (a) that the full intact T biosynthetic pathway is present within platelets of healthy men and men with CRPC; (b) de novo T synthesis from cholesterol within platelets from men with CRPC exhibiting clinical abiraterone resistance, despite ADT; and (c) that platelets from men with prostate cancer contain a pool of T sufficient to induce AR signaling in prostate cancer cells (Supplementary Figure S3). These findings constitute a novel mechanism through which platelets may promote and sustain the growth of lethal prostate cancer.
We found that platelets from healthy men and men undergoing ADT for advanced prostate were able to metabolize T from its precursor cholesterol. Furthermore, platelets from all healthy men (n = 10) and from men with prostate cancer (n = 18) men, including 10 men with CRPC and currently undergoing ADT, contained detectable levels of T. These data suggest that platelets possess T biosynthetic activity that is reflective of the disease state. That is to say, a known mechanism of castrate resistance involves prostate cancer cell de novo androgen biosynthesis. Here we show that platelets also possess this ability and hence may serve as a new platform to assay, study, and target extragonadal steroid biosynthesis in advanced prostate cancer. Further study of how platelets are capable of producing T in the setting of CRPC, inclusive of abiraterone resistance, may shed important light on similar processes at work in the tumor itself.
We examined the ability of the new-generation CYP17A1-blocking drug abiraterone (commonly used in ADT in CRPC patients) to inhibit T biosynthesis in platelets and found that abiraterone was able to inhibit platelet capacity to generate T from cholesterol but only in platelets from men with clinical sensitivity to the drug. Development of abiraterone resistance is a growing and common clinical problem, with many men manifesting signs and symptoms of disease progression within 6 to 12 months following abiraterone treatment initiation [7]. Hence, although further studies are needed, our preliminary findings that platelets from men with abiraterone resistance are capable of T generation from cholesterol suggest that platelet analysis of men with CRPC may potentially provide evidence of impending abiraterone failure and guide therapeutic decision making. IN addition, our data also indicate that molecular alterations that support CRPC may not be exclusive to the tumor but may also be present in other tissues (e.g., platelets), suggesting a more systemic CRPC potentiating phenotype than has been previously reported.
Intriguingly, we observed that platelets of five out of seven men with high-risk prostate cancer contained significantly higher levels of T compared to healthy controls (data not shown). Additional studies are needed to corroborate this observed association of relatively high intraplatelet T levels and aggressive disease. Still, in this setting where prostate cancer is androgen dependent, one might speculate that intratumoral delivery of high amounts of T by platelets could promote cancer growth.
Taken together, our findings provide evidence for a functional role for platelets in promoting CRPC through the paracrine production of androgens. Moreover, our work identifies platelets as a potentially important site of extragonadal androgen biosynthesis as well as a possible novel therapeutic target in advanced prostate cancer. Further work is needed to define the upstream causes and downstream effects of platelet-derived androgen production and delivery to tumor cells in prostate cancer.
Authors’ Contributions
Conception and design: A.Z., M.T., F.F., G.P.
Development of methodology: A.Z., N.P., T.M., A.S., G.P.
Acquisition of data A.Z., A.G.-K., M.A., T.M., N.P.
Writing, review, and/or revision of the manuscript: A.Z., F.F., M.T., G.P.
Administrative, technical, or material support: H.V., H.L.
Study supervision: A.Z., G.P.
The following are the supplementary data related to this article.
(A) Platelet de novo synthesis of DHT in healthy donors (n = 4). Ratio of DHT to an internal standard (isotopically labeled estrone) in healthy donor platelets preincubated with 13C-cholesterol (significance: **P < .01). (B) Platelet de novo synthesis of T is inhibited in an abiraterone-sensitive patient. Platelets from a man currently receiving abiraterone (castration-sensitive disease). “Platelets”: untreated platelets; “Platelets + 13C-Cholesterol”: incubated with 13C-cholesterol. (C) Platelet de novo synthesis of DHT in abiraterone-resistant patients (n = 3). Ratio of DHT to an internal standard (isotopically labeled estrone) in preincubated with 13C-cholesterol (Significance: *P < .05).
(A) MS analysis of intraplatelet levels of androgens in platelets. Relative abundance of T and DHT in ~ 300,000 platelets from healthy donors (n = 4). (B) Platelets promote LNCaP tumor cell proliferation. LNCaP cell proliferation over 72 hours in response to healthy donor platelets, under androgen- and serum-deprived cell culture conditions. “PB treated”: cells incubated in platelet buffer only; “+ Healthy donor platelets in PB”: cells incubated with healthy donor’s platelets in platelet buffer (significance: ***P < .001). (C) Platelets have no effect on PC3 tumor cell proliferation. PC3 cell proliferation over 72 hours in the presence of healthy donor platelets, under androgen- and serum-deprived cell culture conditions. “PB treated”: cells incubated in platelet buffer only; “+ Healthy donor platelets in PB”: cells incubated with healthy donor’s platelets in platelet buffer.
Overview of platelet-derived androgens in prostate cancer. Platelets are capable of taking in exogenous cholesterol and enzymatically converting this into T and DHT sufficient to induce AR-dependent gene expression in LNCaP prostate cancer cells. Abiraterone, a CYP17A1 inhibitor, inhibits this process in abiraterone-sensitive men but not in men with abiraterone resistance. Our findings suggest that, in the context of abiraterone resistance, platelets are capable of synthesizing levels of T and DHT that can promote androgen signaling.
Acknowledgements
We thank Dr. Chandan Kumar for critically reading the manuscript and Amy Gursky for assistance.
Footnotes
Grant Support
This work was supported by the University of Michigan Prostate Specialized Program of Research Excellence, a Prostate Cancer Foundation Young Investigator Award (T.M.), and a Department of Defense Physician Research Training Award (T.M.). M.T. received support from National Institutes of Health Transformative R01 grant DK-085714 and from a Prostate Cancer Foundation Creativity Award. A.S. and G.S.P. received support from NCI/NIH - UCA167234A and from the Department of Defense - W81XWH-12-1-0130. Some experimental data were obtained by the metabolomics Core Services supported by grant U24 DK097153 of National Institutes of Health Common Funds Project to the University of Michigan.
References
- 1.Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012;30:644–646. doi: 10.1200/JCO.2011.39.1300. [DOI] [PubMed] [Google Scholar]
- 2.Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588–595. doi: 10.7150/ijbs.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang K-H, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J. Vol. 154. Elsevier Inc.; 2013. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer; pp. 1074–1084. (Cell). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30:2362–2367. doi: 10.1161/ATVBAHA.110.207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 11.Zaslavsky A, Baek K-H, Lynch RC, Short S, Grillo J, Folkman J. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood. 2010;115:4605–4613. doi: 10.1182/blood-2009-09-242065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuznetsov HS, Marsh T, Markens BA, Castano Z, Greene-Colozzi A, Hay SA. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov. 2012;2:1150–1165. doi: 10.1158/2159-8290.CD-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido A, Munoz Y, Sierralta W, Valladares L. Metabolism of dehydroepiandrosterone sulfate and estrone-sulfate by human platelets. Physiol Res. 2012;61:381–388. doi: 10.33549/physiolres.932323. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Putluri N, Shojaie A, Vasu VT, Vareed SK, Nalluri S, Putluri V. Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011;71:7376–7386. doi: 10.1158/0008-5472.CAN-11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushik AK, Vareed SK, Basu S, Putluri V, Putluri N, Panzitt K. Metabolomic profiling identifies biochemical pathways associated with castration-resistant prostate cancer. J Proteome Res. 2014;13:1088–1100. doi: 10.1021/pr401106h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putluri N, Shojaie A, Vasu VT, Nalluri S, Vareed SK, Putluri V. Metabolomic profiling reveals a role for androgen in activating amino acid metabolism and methylation in prostate cancer cells. Vanacker J-M, editor. PLoS One. 2011;6:e21417. doi: 10.1371/journal.pone.0021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnatenko DV, Cupit LD, Huang EC, Dhundale A, Perrotta PL, Bahou WF. Platelets express steroidogenic 17beta-hydroxysteroid dehydrogenases. Distinct profiles predict the essential thrombocythemic phenotype. Thromb Haemost. 2005;94:412–421. doi: 10.1160/TH05-01-0037. [DOI] [PubMed] [Google Scholar]
- 19.Sarabia SF, Raya JL, Hoogeveen RC, Bray PF. Human platelets differentially concentrate estradiol, estrone and testosterone. J Thromb Haemost. 2008;6:703–705. doi: 10.1111/j.1538-7836.2008.02898.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Platelet de novo synthesis of DHT in healthy donors (n = 4). Ratio of DHT to an internal standard (isotopically labeled estrone) in healthy donor platelets preincubated with 13C-cholesterol (significance: **P < .01). (B) Platelet de novo synthesis of T is inhibited in an abiraterone-sensitive patient. Platelets from a man currently receiving abiraterone (castration-sensitive disease). “Platelets”: untreated platelets; “Platelets + 13C-Cholesterol”: incubated with 13C-cholesterol. (C) Platelet de novo synthesis of DHT in abiraterone-resistant patients (n = 3). Ratio of DHT to an internal standard (isotopically labeled estrone) in preincubated with 13C-cholesterol (Significance: *P < .05).
(A) MS analysis of intraplatelet levels of androgens in platelets. Relative abundance of T and DHT in ~ 300,000 platelets from healthy donors (n = 4). (B) Platelets promote LNCaP tumor cell proliferation. LNCaP cell proliferation over 72 hours in response to healthy donor platelets, under androgen- and serum-deprived cell culture conditions. “PB treated”: cells incubated in platelet buffer only; “+ Healthy donor platelets in PB”: cells incubated with healthy donor’s platelets in platelet buffer (significance: ***P < .001). (C) Platelets have no effect on PC3 tumor cell proliferation. PC3 cell proliferation over 72 hours in the presence of healthy donor platelets, under androgen- and serum-deprived cell culture conditions. “PB treated”: cells incubated in platelet buffer only; “+ Healthy donor platelets in PB”: cells incubated with healthy donor’s platelets in platelet buffer.
Overview of platelet-derived androgens in prostate cancer. Platelets are capable of taking in exogenous cholesterol and enzymatically converting this into T and DHT sufficient to induce AR-dependent gene expression in LNCaP prostate cancer cells. Abiraterone, a CYP17A1 inhibitor, inhibits this process in abiraterone-sensitive men but not in men with abiraterone resistance. Our findings suggest that, in the context of abiraterone resistance, platelets are capable of synthesizing levels of T and DHT that can promote androgen signaling.