Abstract
Second mitochondria-derived activator of caspase (Smac) mimetics are considered as promising anticancer therapeutics that are currently under investigation in early clinical trials. They induce apoptosis by antagonizing inhibitor of apoptosis proteins, which are frequently overexpressed in cancer. We previously reported that Smac mimetics, such as BV6, additionally exert non-apoptotic functions in glioblastoma (GBM) cells by stimulating migration and invasion in a nuclear factor kappa B (NF-κB)-dependent manner. Because NF-κB target genes mediating these effects are largely unknown, we performed whole-genome expression analyses. Here, we identify chemokine (C-C motif) ligand 2 (CCL2) as the top-listed NF-κB-regulated gene being upregulated upon BV6 treatment in GBM cells. BV6-induced upregulation and secretion of CCL2 are required for migration and invasion of GBM cells because knockdown of CCL2 in GBM cells abolishes these effects. Co-culture experiments of GBM cells with non-malignant astroglial cells reveal that BV6-stimulated secretion of CCL2 by GBM cells into the supernatant triggers migration of astroglial cells toward GBM cells because CCL2 knockdown in BV6-treated GBM cells impedes BV6-stimulated migration of astroglial cells. In conclusion, we identify CCL2 as a BV6-induced NF-κB target gene that triggers migration and invasion of GBM cells and exerts paracrine effects on the GBM's microenvironment by stimulating migration of astroglial cells. These findings provide novel insights into the biological functions of Smac mimetics with important implications for the development of Smac mimetics as cancer therapeutics.
Abbreviations: cIAP, cellular inhibitor of apoptosis; CCL2, chemokine (C-C motif) ligand 2; EV, empty vector; FACS, fluorescence-activated cell sorting; GBM, glioblastoma; hrCCL2, human recombinant chemokine (C-C motif) ligand 2; IAP, inhibitor of apoptosis; IκBα-SR, IκBα superrepressor; MMP-9, matrix metallopeptidase 9; NF-κB, nuclear factor kappa B; PBS, phosphate-buffered saline; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; Smac, second mitochondria-derived activator of caspase; XIAP, x-linked inhibitor of apoptosis
Introduction
Gliomas are the most common primary tumors of the central nervous system and represent a heterogeneous group of neoplasms, among which glioblastoma (GBM) is the most malignant and most frequent tumor entity [1]. Treatment failure of GBM, its poor prognosis, and its recurrence are largely due to resistance to programmed cell death [2], [3] as well as to its migratory and invasive phenotype [4]. Inhibitor of apoptosis (IAP) proteins are a family of antiapoptotic proteins that are frequently overexpressed in cancers including GBM and confer resistance to cell death by blocking programmed cell death [5]. Furthermore, IAP proteins are involved in the regulation of additional signal transduction pathways, including the control of cell motility, migration, invasion, and metastasis [6]. Small-molecule second mitochondria-derived activator of caspases (Smac) mimetics bind to and neutralize IAP proteins and are therefore considered as promising novel therapeutic agents [7]. Several studies documented that inhibition of IAP proteins by Smac mimetics sensitizes GBM cells to temozolomide- or radiotherapy-induced apoptosis [8], [9], [10]. Furthermore, there is mounting evidence showing that Smac mimetics can also exert additional functions besides regulating cell death. We previously reported that the Smac mimetic BV6 promotes migration and invasion of GBM cells in an nuclear factor kappa B (NF-κB)-dependent manner [11]. However, the NF-κB target genes that mediate this BV6-stimulated migration and invasion of GBM cells remain largely unknown. Therefore, the aim of the present study is to identify key NF-κB target genes that mediate migration and invasion of GBM cells upon treatment with Smac mimetics.
Material and Methods
Cell Culture and Chemicals
Human GBM cell lines A172, T98G, and U87MG and human pediatric astroglial cell lines NHA-E6/E7/hTERT and SVG were obtained from ATCC (Manassas, VA) and grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Karlsruhe, Germany) supplemented with 1% penicillin/streptomycin, 1% sodium pyruvate (both from Invitrogen), and 10% fetal calf serum (Invitrogen) at 37°C in a humidified atmosphere with 5% CO2. NHA-E6/E7/hTERT and SVG cells have previously been characterized [12], [13]. Smac mimetic BV6, which neutralizes x-linked inhibitor of apoptosis (XIAP), cellular inhibitor of apoptosis (cIAP)1, and cIAP2 [14], was kindly provided by Genentech, Inc. (South San Francisco, CA). Human recombinant chemokine (C-C motif) ligand 2 (hrCCL2 279-MC-010) was provided from R&D Systems (Minneapolis, MN). All chemicals were obtained from Sigma (Deisenhofen, Germany) unless indicated otherwise.
Transduction and siRNA Transfection
Overexpression of the dominant-negative IκBα superrepressor (IκBα-SR) was performed by retroviral transduction as described previously [15]. For transient knockdown by siRNA, cells were reversely transfected with 5 nM (T98G) or 10 nM (U87MG) control siRNA (SilencerSelect siRNA 4390843, Invitrogen) or targeting siRNAs (SilencerSelect siRNA 142542 and 44845 for chemokine (C-C motif) ligand 2 [CCL2], Invitrogen) using Lipofectamine RNAi Max (Invitrogen) and OptiMEM (Life Technologies, Darmstadt, Germany).
Quantitative Real-Time polymerase chain reaction (PCR)
Total RNA was extracted using the peqGOLD Total RNA kit from Peqlab Biotechnologie GmbH (Erlangen, Germany) according to the manufacturer’s protocol. Total RNA (300 ng) was used to synthesize the corresponding cDNA using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific First Strand cDNA synthesis kit, Germany). To quantify gene expression levels, SYBR-Green-based quantitative PCR was performed using the 7900HT fast real-time PCR system from Applied Biosystems (Darmstadt, Germany). Data were normalized to 28S rRNA expression as reference gene. Primer sequences are listed in Supplementary Table 1. Melting curves are depicted to verify the specificity of the amplified products. The relative expression of the target gene transcript and reference gene transcript was calculated as ΔΔCt.
Gene Expression Profiling
Gene expression profiling was performed as previously described [16]. Briefly, A172 empty vector (EV) and IκBα-SR cells were treated for 9 hours with 2 μM BV6 or DMSO as solvent. Total RNA was extracted using peqGOLD Total RNA kit from Peqlab Biotechnologie GmbH (Erlangen, Germany) according to the manufacturer’s instructions, including DNase digestion. Gene expression profiling was performed using Illumina Whole-Genome Expression Beadchips Human HT12v4. For target gene identification, genes were ranked according to their fold upregulation in vector control cells. Genes that were similarly regulated in IκBα-SR cells or did not show significant differences in expression levels in both treated conditions were removed from the data set (P < 1.25 × 10− 6).
Determination of Cell Viability
Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany).
Western Blotting
Western blot analysis was performed as described previously [17] using the following antibodies: anti-cIAP1 (R&D Systems, Inc., Wiesbaden-Nordenstadt, Germany), anti-cIAP2 (Epitomics, Burlingam, CA), anti-XIAP from BD Biosciences, anti–phospho-p65 from Cell Signaling (Beverly, MA), anti-phospho-IκBα and anti-IκBα (Cell Signaling), anti-β-actin (Sigma), anti-p65, and anti-p52 from Santa Cruz Biotechnology (Santa Cruz, CA). Donkey anti-mouse IgG, donkey anti-rabbit IgG, or donkey anti-goat IgG labeled with IRDye infrared dyes was used for fluorescence detection at 680 or 800 nm (LI-COR Biotechnology, Bad Homburg, Germany).
Cytokine Quantification
CCL2 concentrations in glioma cell supernatants were quantified using CCL2 Flex Sets (BD Biosciences). Samples were analyzed by fluorescence-activated cell sorting (FACS) and processed with BD Biosciences FCAP software.
Determination of Migration and Invasion
A total of 0.2 × 105 cells were seeded onto 8-mm Transwell migration chambers (Corning Inc., Wiesbaden, Germany) and stimulated by adding 2.5 μM BV6 (GBM and NHA-E6/E7/hTERT cells) or 1 μM BV6 (SVG cells) to both the lower and the upper chambers to avoid any gradient effect. Following incubation for 24 hours, cells on the upper part of the membrane were scraped using a cotton swab. Migrated cells on the membrane were fixed in paraformaldehyde (4% in cold phosphate-buffered saline [PBS]) and stained with 4’-6-diamidino-2-phenylindole (Life Technologies, Carlsbad, CA). Migrated cells were counted on the underside of the membrane using a fluorescent microscope. The total average number of migrated/invaded cells in five predefined fields of view per insert was taken to quantify the total number of migrated cells. CCL2 stimulation experiments were performed by adding increasing concentrations of CCL2 (1-10 ng/ml) to the lower chamber of a 24-well plate to establish a CCL2 gradient for astroglial attraction. For CCL2 stimulation of GBM cells, cells were preincubated with 1 ng/ml CCL2 for 10 minutes prior to migration start. Here, CCL2 was added to the upper and lower chambers of a 24-well plate to avoid any gradient. In vitro invasiveness of glioma cell lines was examined using 8-mm transwell migration chambers (Corning Inc., Wiesbaden, Germany) that were coated with diluted matrigel (1:3 in cold PBS). Glioma cells were seeded at a density of 0.6 × 105 cells onto the matrigel-coated upper chamber of the transwell culture plate. After incubation for 24 hours, cells were fixed and stained using the same protocol as described above for the transwell migration assay.
Co-culture Experiments
T98G or U87MG glioma cells were seeded at 0.2 × 105 cells/ml in the bottom chamber of transwell inserts, treated with 2.5 μM BV6 to stimulate CCL2 release, and co-cultured with astroglial SVG and NHA-E6/E7/hTERT cells (density NHA-E6/E7/hTERT: 0.2 × 105 cells and SVG: 0.6 × 105 cells per insert) for 24 hours. For CCL2 knockdown experiments, T98G or U87MG cells in the bottom chamber were transiently transfected by siRNA against CCL2 on day 1. On day 2, medium was changed; and on day 3, cells were pretreated with 2.5 μM BV6 for 4 hours to stimulate CCL2 release. After 4 hours, medium was changed; GBM cells were washed with PBS twice; and fresh medium was added. After additional 5 hours (U87MG) or 11 hours (T98G), co-culture with astroglial cell lines SVG and NHA-E6/E7/hTERT was started for 24 hours.
Statistical Analysis
Statistical significance was assessed by two-sided Student’s t test (two-tailed distribution, two samples, unequal variance) using Microsoft Excel (Microsoft Deutschland GmbH, Unterschleißheim, Germany).
Results
Identification of CCL2 as a Key NF-κB Target Gene that Is Upregulated and Secreted upon BV6 treatment in GBM Cells
Based on our previous findings showing that the Smac mimetic BV6 stimulates migration and invasion of GBM cells in an NF-κB-dependent manner ([11] and Supplementary Figure 1), in the present study, we aimed to identify key NF-κB target genes mediating these effects. To address this question, we performed whole-genome expression profiling in an NF-κB-proficient and -deficient system to filter for Smac mimetic-induced NF-κB-regulated genes. Interestingly, we identified CCL2, a member of the CC chemokine family, as the top-listed NF-κB-regulated gene that is differentially upregulated upon treatment with BV6 in vector control compared to IκBα-SR-overexpressing cells (Table 1). Quantitative real-time PCR analysis confirmed that IκBα-SR overexpression prevented BV6-stimulated increase in CCL2 mRNA expression (Figure 1A), demonstrating that BV6 triggers upregulation of CCL2 in an NF-κB-dependent manner. Additional validation experiments showed that non-toxic concentrations of BV6 (Supplementary Figure 2A) significantly increased CCL2 mRNA levels in two other GBM cell lines (Figure 1B). Furthermore, we determined whether this upregulation of CCL2 mRNA expression also translates into increased CCL2 protein expression. Notably, BV6 significantly enhanced the secretion of CCL2 protein into the supernatant of GBM cells (Figure 1C). These experiments demonstrate that BV6 upregulates CCL2 mRNA levels and significantly increases CCL2 secretion by GBM cells in an NF-κB-dependent manner.
Table 1.
Top Five BV6-Induced Genes
| Gene Symbol | Reference Sequence | Fold Increase | SD |
|---|---|---|---|
| CCL2 | NM_002982.3 | 8.6 | ± 0.3 |
| VCAM1 | NM_001078.2 | 5.7 | ± 0.2 |
| BIRC3 | NM_001165.3 | 4.2 | ± 0.2 |
| CCL5 | NM_002985.2 | 3.0 | ± 0.2 |
| IRAK2 | NM_001570.3 | 2.9 | ± 0.2 |
A172 cells transfected with EV or IκBα were treated for 9 hours with 2 μM BV6. Gene expression profiling was performed using Illumina Whole-Genome Expression Beadchips Human HT12v4. Expression data were ranked according to their fold upregulation comparing EV transfected cells with and without BV6 treatment. Genes showing upregulation in IκBα-SR transfected cells (with and without BV6 treatment) served as background expression and were excluded from the analysis (P < 1.25 × 10− 6). Top five BV6-induced genes are shown. Fold increase in mRNA levels is shown with mean and SD of three independent experiments. VCAM1, vascular cell adhesion molecule; BIRC3, baculoviral IAP repeat containing 3, also called cIAP2; CCL5, chemokine (C-C motif) ligand 5; IRAK2, interleukin-1 receptor-associated kinase 2.
Figure 1.
BV6-induced, NF-κB-mediated upregulation and secretion of CCL2 in GBM cells. (A) A172 cells expressing SR or EV were treated with 2 μM BV6 for 9 hours. mRNA expression of CCL2 was analyzed by quantitative reverse transcriptase (qRT)-PCR. Fold increase in mRNA levels is shown with mean and SEM of three independent experiments performed at least in duplicate. (B) T98G and U87MG cells were treated for indicated times with 2.5 μM BV6. mRNA expression of CCL2 was analyzed by qRT-PCR. Fold increase in mRNA levels is shown with mean and SEM of three to six independent experiments performed at least in duplicate. * P < .05, ** P < .01, and *** P < .001. (C) T98G and U87MG cells were treated with 2.5 μM BV6 (12 hours for T98G; 9 hours for U87MG). CCL2 protein expression in supernatants was determined by FACS analysis. Mean and SD of three to four independent experiments are shown. * P < .05 and ** P < .01.
BV6-Induced Upregulation of CCL2 Triggers Migration and Invasion in GBM Cells
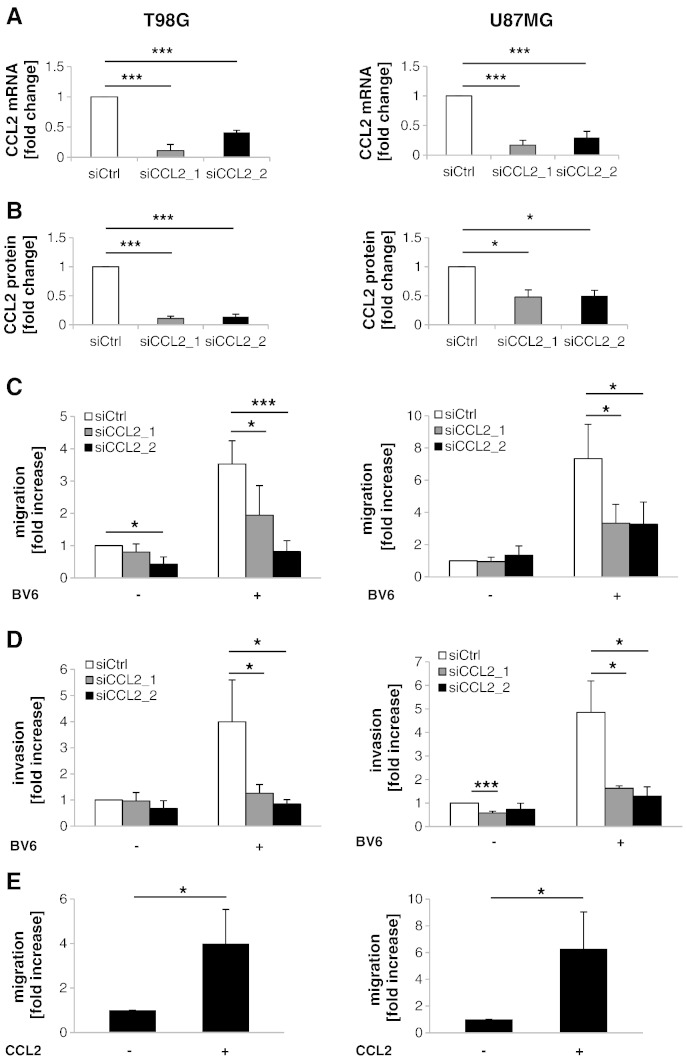
As CCL2 has been described to regulate migration and invasion in several cancer types [18], [19], we analyzed whether BV6-induced upregulation of CCL2 alters migration and invasion of GBM cells. To genetically block CCL2 signaling, we created CCL2 knockdown cells, in which CCL2 mRNA expression and protein secretion were significantly suppressed (Figure 2A and B). Importantly, CCL2 silencing significantly reduced the BV6-stimulated migration and invasion of GBM cells compared to control siRNA (siCtrl) (Figure 2C and D). In addition, we used exogenous application of hrCCL2 protein as a positive control to demonstrate that CCL2 stimulates migration of GBM cells. Addition of hrCCL2 significantly increased migration of GBM cells in comparison to non-treated cells (Figure 2E). These results demonstrate that BV6-mediated upregulation of CCL2 contributes to the increased migration and invasion of GBM cells.
Figure 2.
BV6-induced upregulation of CCL2 triggers migration and invasion in GBM cells. (A) Cells were transiently transfected with siRNA against CCL2 or siCtrl. CCL2 mRNA expression in GBM cells was analyzed by qRT-PCR. Fold change in mRNA is shown with mean and SEM of three independent experiments performed at least in duplicate. * P < .05 and *** P < .001. (B) CCL2 protein expression in supernatants was assessed by FACS analysis. Mean and SD of four independent experiments performed in duplicate are shown. * P < .05 and *** P < .001. (C and D) GBM cells were transiently transfected with siRNA against CCL2 or control siRNA and treated with 2.5 μM BV6 or DMSO for 24 hours. Migration was assessed by transwell migration assay (C); and invasion, by matrigel-precoated Transwell migration chamber (D). Fold increase in migration or invasion relative to untreated cells transfected with control siRNA with mean and SD of four independent experiments performed at least in duplicate is shown. * P < .05 and *** P < .001. (E) GBM cells were preincubated for 10 minutes with 1 ng/ml recombinant CCL2 and then CCL2 was added to the upper and lower migration chamber at same concentration. Migration was assessed after 24 hours by transwell migration assay. Fold increase in migration relative to untreated cells with mean and SD of three independent experiments performed at least in duplicate is shown. * P < .05.
BV6 Causes Depletion of IAP Proteins and NF-κB Activation in Astroglial Cells
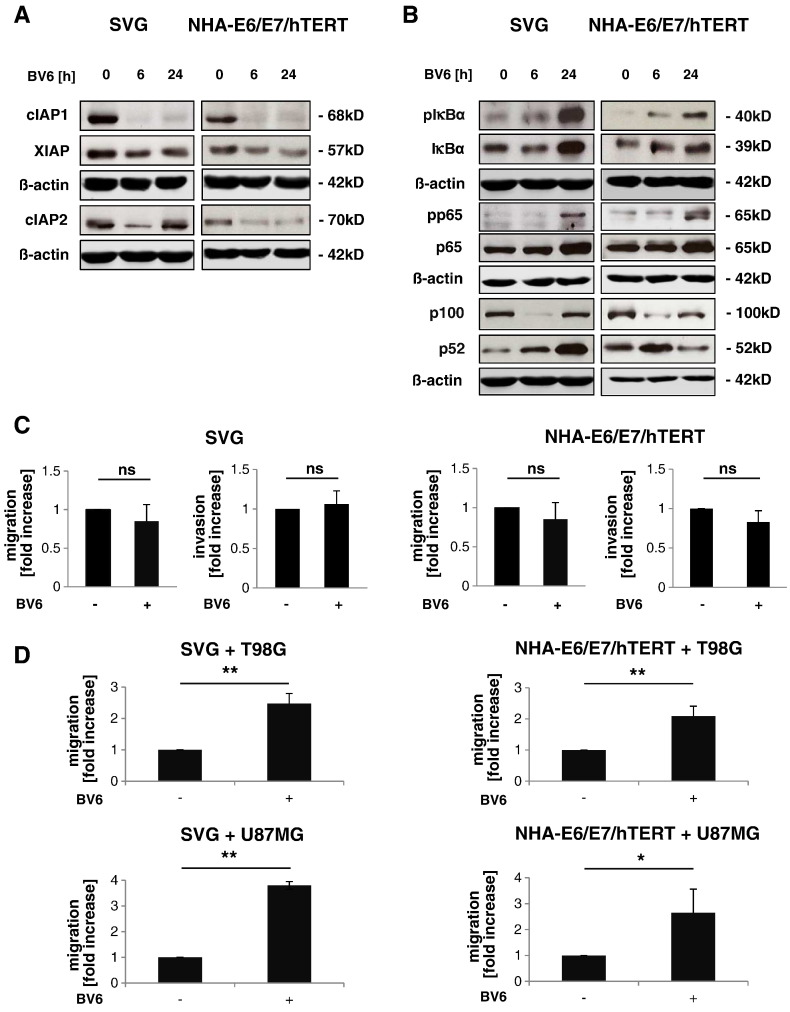
Next, we investigated the effect of BV6 on non-malignant cells of the tumor microenvironment using the astroglial cell lines SVG and NHA-E6/E7/hTERT. Concentrations of BV6 up to 1 μM for SVG cells and 2.5 μM for NHA-E6/E7/hTERT cells had little effect on cell viability of astroglial cells (Supplementary Figure 2B). As Smac mimetics stimulate autoubiquitination of IAP proteins followed by their proteasomal degradation [14], [20], [21], we examined IAP protein levels upon BV6 treatment. BV6 treatment caused downregulation of cIAP1, cIAP2, and XIAP in SVG and NHA-E6/E7/hTERT cells (Figure 3A). We then examined the effect of BV6 on NF-κB pathway activity in SVG and NHA-E6/E7/hTERT cells, as BV6-mediated depletion of IAP proteins has previously been reported to induce NF-κB activation [14], [20], [21]. To assess the activity status of the canonical NF-κB pathway, we analyzed phosphorylation of IκBα and p65. BV6 stimulated phosphorylation of IκBα and p65 in a time-dependent manner (Figure 3B). Furthermore, BV6 triggered processing of p100 to p52, known as a marker for non-canonical NF-κB activation (Figure 3B). These experiments show that BV6 stimulates depletion of IAP proteins and activates NF-κB signaling in astroglial cells.
Figure 3.
BV6-stimulated GBM cells induce astroglial cell migration in a co-culture model. (A and B) SVG or NHA-E6/E7/hTERT cells were treated for indicated times with 1 μM (SVG) or 2.5 μM (NHA-E6/E7/hTERT) BV6. Expression levels of cIAP1, cIAP2, and XIAP (A) and expression and/or phosphorylation of p100, p52, p65, and IκBα (B) were analyzed by Western blotting; β-actin served as loading control. Representative blots of four independent experiments are shown. (C) SVG or NHA-E6/E7/hTERT cells were treated for 24 hours with 1 μM (SVG) or 2.5 μM (NHA-E6/E7/hTERT) BV6. Migration was assessed after 24 hours by transwell migration assay. Fold increase in migration relative to untreated cells with mean and SD of three to four independent experiments performed at least in duplicate is shown; ns, not significant. (D) SVG or NHA-E6/E7/hTERT cells in the upper chamber of the transwell plate were co-cultured with T98G or U87MG cells in the bottom chamber that were pretreated with 2.5 μM BV6 for 4 hours to stimulate cytokine expression. Migration was assessed after 24 hours by transwell migration assay. Fold increase in migration relative to untreated cells with mean and SD of three independent experiments performed at least in duplicate is shown. * P < .05 and ** P < .01.
BV6-Stimulated GBM Cells Induce Astroglial Cell Migration in a Co-culture Model
We then asked whether BV6-stimulated NF-κB activation in astroglial cells also increases their motility. However, treatment with BV6 did not result in enhanced migration or invasion of SVG and NHA-E6/E7/hTERT cells (Figure 3C). Next, we determined whether BV6 upregulates CCL2 expression in astroglial cells. Although BV6 treatment increased CCL2 mRNA levels in SVG and NHA-E6/E7/hTERT cells, it did not enhance CCL2 protein secretion (Supplementary Figure 3A and B). Subsequently, we set up a co-culture model to find out whether BV6-treated GBM cells, which secrete CCL2 as shown in Figure 1C, trigger migration of astroglial cells. Here, T98G or U87MG glioma cells were pretreated for 4 hours with BV6 to stimulate release of CCL2 as shown in Supplementary Figure 4A. Medium was changed after stimulation, GBM cells were washed with PBS to remove residual BV6, and SVG or NHA-E6/E7/hTERT cells were seeded in transwell inserts and allowed to migrate toward GBM cells in the lower compartment for 24 hours. Interestingly, this co-culture model revealed a significant increase in migration of SVG and NHA-E6/E7/hTERT cells toward BV6-pretreated GBM cells compared to untreated GBM cells (Figure 3D). Together, these experiments suggest that CCL2 secreted from BV6-treated GBM cells stimulates astroglial cell migration toward GBM cells, whereas direct treatment of astroglial cells with BV6 does not alter the migratory phenotype of astroglial cells.
BV6-Induced Upregulation of CCL2 in GBM Cells Induces Astroglial Cell Migration
To investigate whether BV6-stimulated upregulation and secretion of CCL2 in GBM cells are required for migration of astroglial cells toward GBM cells, we performed co-culture experiments after silencing of CCL2 in GBM cells by RNA interference. Importantly, knockdown of CCL2 in T98G and U87MG cells significantly prevented BV6-induced migration of astroglial cells toward GBM cells (Figure 4A and B, Supplementary Figure 4B). In addition, we used increasing concentrations of exogenous hrCCL2 protein as a positive control to demonstrate that CCL2 increases migration of astroglial cells. Of note, SVG and NHA-E6/E7/hTERT cells migrated toward a CCL2 gradient in a concentration-dependent manner (Supplementary Figure 4C). Together, these results indicate that BV6-induced upregulation and secretion of CCL2 in GBM cells affect astroglial cells in a paracrine manner by increasing their migratory phenotype.
Figure 4.
BV6-stimulated CCL2 release by GBM cells induces astroglial cell migration in a co-culture model. (A and B) SVG cells in the upper chamber of the transwell plate were co-cultured with T98G or U87MG cells in the bottom chamber that were transiently transfected with siRNA against CCL2 or siCtrl and pretreated with 2.5 μM BV6 for 4 hours to stimulate cytokine expression. Migration was assessed after 24 hours by transwell migration assay. Fold increase in migration relative to untreated cells with mean and SD of three to four independent experiments performed at least in duplicate is shown. * P < .05, ** P < .01, and *** P < .001. (C) Scheme of BV6-induced upregulation of CCL2 and its influence on GBM cells and the tumor microenvironment. Smac mimetic BV6 activates NF-κB signaling pathway in GBM and astroglial cells. BV6-induced NF-κB activation in GBM cells induces CCL2 upregulation and triggers migration and invasion of GBM cells in an autocrine/paracrine manner. CCL2 secretion of BV6-pretreated GBM cells increases migration of cells of astroglial cells in a paracrine manner.
Discussion
We previously reported that small-molecule IAP antagonists, such as the Smac mimetic BV6, can exert non-apoptotic functions in GBM cells in a context-dependent manner in addition to promoting cell death. Namely, BV6 at non-toxic concentrations stimulates migration and invasion of GBM cells via NF-κB activation [11]. However, the NF-κB target genes that mediate these BV6-triggered migration and invasion of GBM cells remained largely elusive. Therefore, the aim of the present study is to discover NF-κB-regulated genes that are upregulated upon BV6 treatment and required for BV6-stimulated migration and invasion of GBM cells. Using a genome-wide cDNA microarray analysis, we identify CCL2 as the top-listed NF-κB-regulated gene upon BV6 treatment. We report that BV6 upregulates CCL2 expression in GBM cells and its secretion into the supernatant, which in turn stimulates migration and invasion of GBM cells in an autocrine/paracrine manner (Figure 4C). In addition, CCL2 secreted from BV6-pretreated GBM cells exerts paracrine effects on cells of the tumor microenvironment and promotes migration of astroglial cells toward GBM cells (Figure 4C). These conclusions are supported by several findings. First, CCL2 mRNA levels are upregulated in an NF-κB-dependent manner upon treatment with BV6 because inhibition of NF-κB activation by IκBα-SR overexpression prevents BV6-stimulated increase in CCL2 expression. Second, this upregulation of CCL2 occurs at both mRNA and protein levels; and CCL2 protein is then secreted into the supernatant. Third, CCL2 is indispensable for BV6-induced migration and invasion, as siRNA-mediated knockdown of CCL2 significantly rescues BV6-imposed migration and invasion of GBM cells. In addition, the notion that CCL2 is a critical mediator of BV6-imposed migration of GBM cells is emphasized by our data showing that exogenous application of hrCCL2 similarly promotes GBM cell migration. Fourth, pretreatment of GBM cells with BV6 significantly increases migration of astroglial cells toward GBM cells in co-culture experiments in a CCL2-dependent manner because CCL2 silencing in GBM cells abolishes this effect. Taken together, the novelty of our study particularly resides in the demonstration that BV6-induced upregulation and secretion of CCL2 by GBM cells promote migration and invasion of both GBM and astroglial cells via autocrine and paracrine mechanisms. CCL2 stimulates migration and invasion of GBM cells via an autocrine/paracrine CCL2 loop. In addition, CCL2 affects the interaction of GBM cells with their microenvironment by stimulating astroglial cell migration toward GBM cells in a paracrine manner. CCL2, also called monocyte chemoattractant protein-1, is a member of the cytokine/chemokine superfamily [22] and a known NF-κB target gene [23]. CCL2 has previously been reported to be upregulated upon treatment with Smac mimetics [11], [14], depending on non-canoncial NF-κB signaling as shown by genetic silencing of NIK [11]. Although BV6 stimulated a much stronger upregulation of CCL2 expression in T98G cells than in U87MG cells, the increase in migration and invasion upon treatment with BV6 was comparable in both cell lines. As U87MG cells express much lower basal CCL2 levels than T98G cells, one possible explanation is that U87MG cells are more susceptible to BV6-induced CCL2 upregulation. Our discovery that CCL2 is a key mediator of BV6-induced migration and invasion of GBM cells is in line with previous studies underscoring the importance of CCL2 for the malignant phenotype of cancers including GBM. For example, increased CCL2 levels were documented in GBM tissue as compared to adjacent brain tissue [24], [25]. Also, cerebrospinal fluid samples from GBM patients were described to contain significantly higher levels of CCL2 compared to patients with no brain tumor [26]. Furthermore, CCL2 has been shown to function as a chemoattractant for glioma-infiltrating microglial cells [27]. In addition, antibody-mediated blockade of CCL2 has been reported to prolong survival in orthotopic glioma mouse models [28]. The observation that, in a study using one GBM cell line [29], overexpression of CCL2 was not accompanied by an increase in invasion suggests that additional factors are involved in the control of invasion and migration of GBM cells. This notion is consistent with our findings showing that treatment with BV6 results in upregulation of other cytokines besides CCL2, including tumor necrosis factor-α and interleukin-8 [11]. Mechanistically, CCL2-mediated migration has previously been linked to activation of CC chemokine receptor type 2, rat sarcoma/rapidly accelerated fibrosarcoma 1/mitogen-activated protein kinase/extracellular signal-regulated kinase and NF-κB pathways, as well as upregulation of matrix metallopeptidase 9 (MMP-9) in chondrosarcoma cells [19]. MMPs have been shown to exert an important role in cancer invasion through enzymatic degradation of the extracellular matrix [30]. MMP-9 may be involved in BV6-induced invasion of GBM cells, because we previously demonstrated that MMP-9 is upregulated upon BV6 treatment in GBM cells [11]. CCL2 has been reported in the past to act both on tumor cells and, as a chemoattractant, on cells of the tumor microenvironment. Several tumor types, including myeloma, breast cancer, and prostate cancer, have been described to express CC chemokine receptor type 2 and to secrete CCL2, thereby engaging an autocrine/paracrine loop that can trigger chemotactic migration and invasion of cancer cells [18], [19], [31], [32]. Moreover, it has been reported that CCL2 contributes to the development of a so-called metastatic niche in the bone marrow compartment by stimulating the recruitment of monocytes/macrophages and angiogenesis [33]. Consistently, we show that BV6-induced CCL2 expression and secretion affect not only GBM cells but also cells of the GBM's microenvironment in a paracrine manner. We demonstrate that CCL2 secretion into the supernatant of BV6-treated GBM cells alters communication of GBM cells with non-malignant cells of the central nervous system by triggering the recruitment of astroglial cells toward GBM cells. BV6-induced CCL2 secretion is required for the GBM cell-mediated attraction of astroglial cells because CCL2 knockdown in GBM cells abolishes BV6-induced secretion of CCL2 by GBM cells and astroglial cell migration toward GBM cells. By comparison, treatment of astroglial cells with non-toxic concentrations of BV6 does not increase their migratory or invasive phenotype, although BV6 depletes IAP proteins and activates NF-κB in these cells. This finding is in line with our observation that BV6 treatment of astroglial cells does not result in secretion of CCL2 protein, whereas it upregulates CCL2 mRNA levels. One possible explanation for these findings is that astroglial cells may differentially respond to activation by CCL2 compared to GBM cells, for example, by increased proliferation and upregulation of cellular and molecular markers of activated astroglial cells [34]. In addition to the identification of CCL2 as an important mediator of BV6-induced migration and invasion of GBM cells, our study underscores that Smac mimetics are involved in the regulation of non-apoptotic pathways beyond the control of cell death. In this respect, we previously showed that the Smac mimetic BV6 induces astrocytic differentiation of cancer stem-like cells by activating NF-κB [35]. Furthermore, we demonstrated that BV6 stimulates cytokine secretion and monocyte recruitment via activation of interferon regulatory factor 1 [36]. In addition to CCL2, we reported that tumor necrosis factor-α autocrine/paracrine signaling contributes to BV6-induced migration and invasion of GBM cells [11]. Whereas depletion of IAP proteins has been documented by other investigators to result in increased migration [37], [38], consistent with our findings, IAP proteins have also been described to promote migration [39], [40], [41]. This indicates that IAP proteins play a complex role in the regulation of cancer cell migration. Smac mimetics are currently under evaluation in early clinical trials [42]. Therefore, further insights into the spectrum of their biological functions, including also potentially undesirable therapeutic effects, have important implications for the transfer of this approach into clinical application for the treatment of cancer. By identifying BV6-induced upregulation and secretion of CCL2 as key mediators of migration and invasion of GBM cells and their interaction with astroglial cells, our study contributes to a better understanding of Smac mimetic–mediated effects in GBM cells.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Acknowledgements
We thank D. Vucic (Genentech, South San Francisco, CA) for providing BV6, M. Burger (Goethe-University Frankfurt, Germany) for providing SVG and NHA-E6/E7/hTERT cell lines, D. Brücher for expert technical assistance, and C. Hugenberg for expert secretarial assistance. This work was supported by grants from the Medical Faculty of Goethe-University Frankfurt (to Carina Lindemann) and grants from the Deutsche Forschungsgemeinschaft and the BMBF (to Simone Fulda).
Footnotes
This work was supported by grants from the Medical Faculty of Goethe-University Frankfurt (to Carina Lindemann) and grants from the Deutsche Forschungsgemeinschaft and the BMBF (to Simone Fulda).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2015.05.002.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511–515. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 3.Fulda S., Wick W., Weller M., Debatin K.M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug–induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 4.Giese A., Bjerkvig R., Berens M.E., Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S., Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S. Regulation of cell migration, invasion and metastasis by IAP proteins and their antagonists. Oncogene. 2014;33:671–676. doi: 10.1038/onc.2013.63. [DOI] [PubMed] [Google Scholar]
- 7.Fulda S. Novel promising IAP antagonist on the horizon for clinical translation. J Med Chem. 2012;55:4099–4100. doi: 10.1021/jm300475b. [DOI] [PubMed] [Google Scholar]
- 8.Wagner L., Marschall V., Karl S., Cristofanon S., Zobel K., Deshayes K., Vucic D., Debatin K.M., Fulda S. Smac mimetic sensitizes glioblastoma cells to temozolomide-induced apoptosis in a RIP1- and NF-kappaB–dependent manner. Oncogene. 2013;32:988–997. doi: 10.1038/onc.2012.108. [DOI] [PubMed] [Google Scholar]
- 9.Houghton P.J., Kang M.H., Reynolds C.P., Morton C.L., Kolb E.A., Gorlick R., Keir S.T., Carol H., Lock R., Maris J.M. Initial testing (stage 1) of LCL161, a SMAC mimetic, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2012;58:636–639. doi: 10.1002/pbc.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger R., Jennewein C., Marschall V., Karl S., Cristofanon S., Wagner L., Vellanki S.H., Hehlgans S., Rodel F., Debatin K.M. NF-{kappa}B is required for Smac mimetic–mediated sensitization of glioblastoma cells for {gamma}-irradiation–induced apoptosis. Mol Cancer Ther. 2011;10:1867–1875. doi: 10.1158/1535-7163.MCT-11-0218. [DOI] [PubMed] [Google Scholar]
- 11.Tchoghandjian A., Jennewein C., Eckhardt I., Rajalingam K., Fulda S. Identification of non-canonical NF-kappaB signaling as a critical mediator of Smac mimetic–stimulated migration and invasion of glioblastoma cells. Cell Death Dis. 2013;4:e564. doi: 10.1038/cddis.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonoda Y., Ozawa T., Hirose Y., Aldape K.D., McMahon M., Berger M.S., Pieper R.O. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–4960. [PubMed] [Google Scholar]
- 13.Major E.O., Miller A.E., Mourrain P., Traub R.G., de Widt E., Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci U S A. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varfolomeev E., Blankenship J.W., Wayson S.M., Fedorova A.V., Kayagaki N., Garg P., Zobel K., Dynek J.N., Elliott L.O., Wallweber H.J. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Karl S., Pritschow Y., Volcic M., Hacker S., Baumann B., Wiesmuller L., Debatin K.M., Fulda S. Identification of a novel pro-apopotic function of NF-kappaB in the DNA damage response. J Cell Mol Med. 2009;13:4239–4256. doi: 10.1111/j.1582-4934.2009.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckhardt I., Roesler S., Fulda S. Identification of DR5 as a critical, NF-kappaB-regulated mediator of Smac-induced apoptosis. Cell Death Dis. 2013;4:e936. doi: 10.1038/cddis.2013.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogler M., Durr K., Jovanovic M., Debatin K.M., Fulda S. Regulation of TRAIL-induced apoptosis by XIAP in pancreatic carcinoma cells. Oncogene. 2007;26:248–257. doi: 10.1038/sj.onc.1209776. [DOI] [PubMed] [Google Scholar]
- 18.Loberg R.D., Day L.L., Harwood J., Ying C., St John L.N., Giles R., Neeley C.K., Pienta K.J. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang C.H., Tsai C.C. CCL2 increases MMP-9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF-kappaB signaling pathway. Biochem Pharmacol. 2012;83:335–344. doi: 10.1016/j.bcp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Vince J.E., Wong W.W., Khan N., Feltham R., Chau D., Ahmed A.U., Benetatos C.A., Chunduru S.K., Condon S.M., McKinlay M. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Du F., Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein–1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda A., Okuda K., Ohno S., Shirai A., Igarashi T., Matsunaga K., Fukushima J., Kawamoto S., Ishigatsubo Y., Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 24.Takeshima H., Kuratsu J., Takeya M., Yoshimura T., Ushio Y. Expression and localization of messenger RNA and protein for monocyte chemoattractant protein–1 in human malignant glioma. J Neurosurg. 1994;80:1056–1062. doi: 10.3171/jns.1994.80.6.1056. [DOI] [PubMed] [Google Scholar]
- 25.Desbaillets I., Tada M., de Tribolet N., Diserens A.C., Hamou M.F., Van Meir E.G. Human astrocytomas and glioblastomas express monocyte chemoattractant protein–1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 26.Kuratsu J., Yoshizato K., Yoshimura T., Leonard E.J., Takeshima H., Ushio Y. Quantitative study of monocyte chemoattractant protein–1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. J Natl Cancer Inst. 1993;85:1836–1839. doi: 10.1093/jnci/85.22.1836. [DOI] [PubMed] [Google Scholar]
- 27.Platten M., Kretz A., Naumann U., Aulwurm S., Egashira K., Isenmann S., Weller M. Monocyte chemoattractant protein–1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54:388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X., Fujita M., Snyder L.A., Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Sarkar S., Cua R., Zhou Y., Hader W., Yong V.W. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33:312–319. doi: 10.1093/carcin/bgr289. [DOI] [PubMed] [Google Scholar]
- 30.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 31.Vanderkerken K., Vande Broek I., Eizirik D.L., Van Valckenborgh E., Asosingh K., Van Riet I., Van Camp B. Monocyte chemoattractant protein–1 (MCP-1), secreted by bone marrow endothelial cells, induces chemoattraction of 5T multiple myeloma cells. Clin Exp Metastasis. 2002;19:87–90. doi: 10.1023/a:1013891205989. [DOI] [PubMed] [Google Scholar]
- 32.Youngs S.J., Ali S.A., Taub D.D., Rees R.C. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997;71:257–266. doi: 10.1002/(sici)1097-0215(19970410)71:2<257::aid-ijc22>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Craig M.J., Loberg R.D. CCL2 (monocyte chemoattractant protein–1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 34.Ridet J.L., Malhotra S.K., Privat A., Gage F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 35.Tchoghandjian A., Jennewein C., Eckhardt I., Momma S., Figarella-Branger D., Fulda S. Smac mimetic promotes glioblastoma cancer stem-like cell differentiation by activating NF-kappaB. Cell Death Differ. 2014;21:735–747. doi: 10.1038/cdd.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckhardt I., Weigert A., Fulda S. Identification of IRF1 as critical dual regulator of Smac mimetic-induced apoptosis and inflammatory cytokine response. Cell Death Dis. 2014;5:e1562. doi: 10.1038/cddis.2014.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dogan T., Harms G.S., Hekman M., Karreman C., Oberoi T.K., Alnemri E.S., Rapp U.R., Rajalingam K. X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nat Cell Biol. 2008;10:1447–1455. doi: 10.1038/ncb1804. [DOI] [PubMed] [Google Scholar]
- 38.Oberoi T.K., Dogan T., Hocking J.C., Scholz R.P., Mooz J., Anderson C.L., Karreman C., Meyer zu Heringdorf D., Schmidt G., Ruonala M. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 2012;31:14–28. doi: 10.1038/emboj.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., Ahn S., Ko Y.G., Boo Y.C., Chi S.G., Ni C.W., Go Y.M., Jo H., Park H. X-linked inhibitor of apoptosis protein controls alpha5-integrin-mediated cell adhesion and migration. Am J Physiol Heart Circ Physiol. 2010;299:H300–H309. doi: 10.1152/ajpheart.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehrotra S., Languino L.R., Raskett C.M., Mercurio A.M., Dohi T., Altieri D.C. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez J., John S.W., Tenev T., Rautureau G.J., Hinds M.G., Francalanci F., Wilson R., Broemer M., Santoro M.M., Day C.L. CARD-mediated autoinhibition of cIAP1's E3 ligase activity suppresses cell proliferation and migration. Mol Cell. 2011;42:569–583. doi: 10.1016/j.molcel.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Fulda S. Molecular pathways: targeting inhibitor of apoptosis proteins in cancer–from molecular mechanism to therapeutic application. Clin Cancer Res. 2014;20:289–295. doi: 10.1158/1078-0432.CCR-13-0227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.