Abstract
Activation of Notch1 in cells of the osteoblastic lineage inhibits osteoblast differentiation/function and causes osteopenia, whereas its activation in osteocytes causes a distinct osteopetrotic phenotype. To explore mechanisms responsible, we established the contributions of canonical Notch signaling (Rbpjκ dependent) to osteocyte function. Transgenics expressing Cre recombinase under the control of the dentin matrix protein-1 (Dmp1) promoter were crossed with Rbpjκ conditional mice to generate Dmp1-Cre+/−;RbpjκΔ/Δ mice. These mice did not have a skeletal phenotype, indicating that Rbpjκ is dispensable for osteocyte function. To study the Rbpjκ contribution to Notch activation, RosaNotch mice, where a loxP-flanked STOP cassette is placed between the Rosa26 promoter and the NICD coding sequence, were crossed with Dmp1-Cre transgenic mice and studied in the context (Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ) or not (Dmp1-Cre+/−;RosaNotch) of Rbpjκ inactivation. Dmp1-Cre+/−;RosaNotch mice exhibited increased femoral trabecular bone volume and decreased osteoclasts and bone resorption. The phenotype was reversed in the context of the Rbpjκ inactivation, demonstrating that Notch canonical signaling was accountable for the phenotype. Notch activation downregulated Sost and Dkk1 and upregulated Axin2, Tnfrsf11b, and Tnfsf11 mRNA expression, and these effects were not observed in the context of the Rbpjκ inactivation. In conclusion, Notch activation in osteocytes suppresses bone resorption and increases bone volume by utilization of canonical signals that also result in the inhibition of Sost and Dkk1 and upregulation of Wnt signaling.
Keywords: Notch, Rbpjκ, Sost, osteocytes, bone remodeling
bone remodeling is a coordinated process dependent on the activity of bone-forming osteoblasts, bone-resorbing osteoclasts, and osteocytes (7, 40, 44). Osteocytes are osteoblasts that become embedded in the bone matrix, acquiring a dendritic morphology in specialized lacunae and distinct functions (11). Through a canalicular network, osteocytes communicate their signals to other skeletal cells in the bone microenvironment. Osteocytes play a fundamental role in bone remodeling and mechanotransduction and respond to mechanical loading by converting extracellular forces into intracellular signals that regulate specific pathways (27, 35, 52, 55).
Notch (1 to 4) are single-pass transmembrane receptors that play a critical role in cell fate decisions (58, 59). Notch regulates skeletal development and homeostasis and osteoblast and osteoclast differentiation (17, 23, 62). Notch is activated by Notch-ligand interactions, resulting in the release of the Notch intracellular domain (NICD). In the canonical signaling pathway, NICD translocates to the nucleus, displacing transcriptional repressors and interacting with recombination signal binding protein for immunoglobulin κJ region (Rbpjκ) and with Mastermind-like (Maml) proteins to regulate transcription (29, 36, 54). Classic targets of Notch canonical signaling include hairy enhancer of split (Hes)1, -5, and -7 and Hes related with YRPW motif (Hey)1, -2, and -L (24, 25, 34).
Studies on the activation of Notch1 and on the inactivation of Notch1 and Notch2 in cells of the osteoblastic lineage at various stages of differentiation reveal an inhibitory role of Notch in osteoblastogenesis (8, 57). Inactivation of Notch1 and Notch2 in the developing skeleton causes a transient increase in trabecular bone volume due to increased osteoblast number/function followed by osteopenia, due to an increase in osteoclastogenesis and bone resorption (23). These and related findings suggest that Notch1 has the potential to inhibit both osteoblast and osteoclast differentiation (2, 13, 61).
Recently, we explored the effects of Notch1 activation in cells of the osteoblastic lineage at various stages of cell differentiation and confirmed that activation of Notch1 impairs osteoblast differentiation/function (8). In contrast, Notch1 activation in osteocytes had distinct effects causing a pronounced increase in cancellous bone due to a suppression of bone resorption and an increase of cortical bone formation (6, 8). The mechanism of action of Notch in osteocytes included suppression of Sost expression and consequently enhanced Wnt signaling. Notch also induced Tnfrsf11b, encoding for osteoprotegerin, either directly or through the enhancement of Wnt activity (5).
The intent of the present study was to explore pathways responsible for the actions of Notch in osteocytes and determine whether Notch canonical signaling (Rbpjκ dependent) had a function in osteocytes and was responsible for the effects of Notch activation in these cells. To determine whether Rbpjκ had a function in osteocytes under basal conditions, Rbpjκ conditional mice were crossed with transgenic mice expressing the Cre recombinase under the control of the dentin matrix protein-1 (Dmp1) promoter (Dmp1-Cre). To activate Notch1 preferentially in osteocytes, RosaNotch mice, where a loxP-flanked STOP cassette was cloned downstream of the Rosa26 promoter and upstream of the coding sequence of the Notch1 NICD, were crossed with Dmp1-Cre transgenic mice (31, 33). To determine whether the canonical pathway was responsible for the effects of Notch signaling under conditions of Notch activation, Dmp1-Cre+/−;RosaNotch mice were studied in the context of an Rbpjκ deletion and the skeletal phenotype established.
MATERIALS AND METHODS
RosaNotch mice.
RosaNotch mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and studied in a C57BL/6 genetic background (33, 47). In these mice, the Rosa26 locus is targeted with a DNA construct encoding the Notch1 NICD, preceded by a STOP cassette flanked by loxP sites, cloned downstream of the Rosa26 promoter, so that NICD is expressed following the excision of the STOP cassette by Cre recombination (4, 43). To study the activation of Notch1 preferentially in osteocytes, homozygous RosaNotch mice were mated with hemizygous Dmp1-Cre transgenics in a C57BL/6 background to create Dmp1-Cre+/−;RosaNotch experimental and RosaNotch littermate controls. Dmp1-Cre transgenics express Cre under the control of a ∼14-kilobase (kb) regulatory fragment of Dmp1 containing a 9.6-kb Dmp1 promoter region (Dr. Feng, Texas A&M Health Science Center, Dallas, TX) (31).
Rbpjκ conditional mice.
To determine whether Notch canonical signaling is responsible for Notch effects in osteocytes, Notch activation was studied in the context of Rbpjκ inactivation (15). Rbpjκ conditional mice, with loxP sites placed upstream of exon 3 and downstream of exon 7 so that Cre recombination results in the deletion of the DNA binding domain, were obtained in a C57BL/6 background from Riken BioResource Center (Tsukuba, Ibaraki, Japan) (22). To inactivate Rbpjκ in osteocytes, RbpjκloxP/loxP mice were back-crossed into Dmp1-Cre+/− mice to create Dmp1-Cre+/−;RbpjκloxP/loxP mice to breed with RbpjκloxP/loxP mice. Rbpjκ-null and control male and female mice were studied at 1 and 3 mo of age. To inactivate Rbpjκ in the context of the Notch activation, Rbpjκ conditional alleles were introduced into RosaNotch homozygous mice (RosaNotch/Notch;RbpjκloxP/WT) and into Dmp1-Cre homozygous mice (Dmp1-Cre+/+;RbpjκloxP/WT), and these mice were crossed so that the progeny were heterozygous for Dmp1-Cre+/−;RosaNotch alleles, 25% in a wild-type background, and 25% in the context of the Rbpjκ deletion. This mating scheme does not allow for a direct comparison between Dmp1-Cre+/−;RosaNotch and control RosaNotch mice in the same litter. Therefore, the data obtained from the experimental litters were pooled with data comparing Dmp1-Cre+/−;RosaNotch with RosaNotch control littermates of the same sex and age collected over a period of ∼4 yr. Male and female mice were studied at 1 mo of age.
Genotyping of the Dmp1-Cre transgene and the RosaNotch and RbpjκloxP/loxP alleles was carried out by polymerase chain reaction (PCR) in tail DNA extracts (Table 1). Deletion of loxP-flanked sequences by the Cre recombinase was documented by PCR in DNA from tibiae or femurs, and changes in gene expression were documented in total RNA from calvariae or tibiae by quantitative reverse transcription-PCR (qRT-PCR).
Table 1.
Primers used for allele identification and Cre-mediated recombination by PCR
| Allele | Strand | Sequence 5′-3′ | Amplicon Size, bp |
|---|---|---|---|
| Genotyping | |||
| Dmp1-Cre transgene | F | CCCGCAGAACCTGAAGATG | 534 |
| R | GACCCGGCAAAACAGGTAG | ||
| RbpjκloxP allele | FloxRbpjκ_F | GTTCTTAACCTGTTGGTCGGAACC | WT = 500 Rbpjκ = 610 |
| WT R | GCGAAGAGTTTGTCCTCAACC | ||
| FloxRbpjκ R | GCAATCCATCTTGTTCAATGGCC | ||
| RosaNotch allele | F | GGAGCGGGAGAAATGGATATG | WT = 600 RosaNotch = 250 |
| WT R | AAAGTCGCTCTGAGTTGTTATTG | ||
| RosaNotch R | GCGAAGAGTTTGTCCTCAACC | ||
| LoxP recombination | |||
| RbpjκloxP | F | TGCTGACATCTGAGAAGGCTAGGT | WT allele = 1918 |
| R | AATGTTAACAGCTGAGAGACAAGCCTAGA | Recombined allele = 700 | |
| RosaNotch STOP | F | TTCGCGGTCTTTCCAGTGG | Not recombined = 492 |
| R absent loxP recombination | AGCCTCTGAGCCCAGAAAGC | ||
| R present loxP recombination | GCCGACTGAGTCCTCGCC | Recombined = 296 | |
WT, wild type; R, reverse; F, forward.
Animal experiments were approved by the Animal Care and Use Committees of Saint Francis Hospital and Medical Center and of UConn Health.
Microcomputed tomography.
Bone microarchitecture of femurs from experimental and control mice was determined using a microcomputed tomography instrument (μCT 40; Scanco Medical, Bassersdorf, Switzerland), which was calibrated weekly using a phantom provided by the manufacturer (3, 20). Femurs were scanned in 70% ethanol at high resolution, energy level of 55 peak kV, intensity of 145 μA, and integration time of 200 ms. Trabecular bone volume fraction and microarchitecture were evaluated starting 1.0 mm proximal from the femoral condyles. A total of 160 consecutive slices were acquired at an isotropic voxel dimension of 216 μm3 and a slice thickness of 6 μm and chosen for analysis. Contours were manually drawn every 10 slices a few voxels away from the endocortical boundary to define the region of interest for analysis. The remaining slice contours were iterated automatically. Trabecular regions were assessed for total volume, bone volume, bone volume fraction (bone volume/total volume, BV/TV), trabecular thickness (Tb.Th), number (Tb.N), and separation, connectivity density (Conn.D), and structure model index (SMI), using a Gaussian filter (σ = 0.8) and user-defined thresholds (3, 20). For analysis of femoral cortical bone, contours were iterated across 100 slices along the cortical shell of the femoral midshaft, excluding the marrow cavity. Analyses of bone volume/total volume (BV/TV), porosity, cortical thickness, cross-sectional tissue (TA) and bone (BA) areas, periosteal (P.peri) and endocortical (P.endo) perimeters, and material density were performed using a Gaussian filter (σ = 0.8) and user-defined thresholds. The terminology and units used are in accordance with guidelines published by the Journal of Bone and Mineral Research (3).
Bone histomorphometric analysis.
Static and dynamic histomorphometry were carried out on mice injected with calcein (20 mg/kg) and demeclocycline (50 mg/kg) at an interval of 2 days in 1-mo-old or of 7 days in 3-mo-old animals. Five-micrometer longitudinal sections of femurs and cross sections at the middiaphysis were cut on a microtome (Microm; Richards-Allan Scientific, Kalamazoo, MI) and stained with 0.1% toluidine blue. Static parameters of cancellous bone histomorphometry were measured in a defined area between 360 and 2,160 μm from the growth plate, using an OsteoMeasure morphometry system (Osteometrics, Atlanta, GA). Bone area/tissue area (BA/TA) is expressed as percent trabecular bone over the defined tissue area measured, and trabecular number (Tb.N) as number of trabeculae/mm; osteoblast (NOb/BPm) and osteoclast (NOc/BPm) number per bone perimeter are expressed as the number of cells per millimeter of trabecular perimeter; osteoblast (ObS/BS), osteoclast (OcS/BS), and eroded (ES/BS) bone surfaces are expressed as percentage of the traced bone surface occupied by osteoblasts, osteoclasts, or resorbed surface, respectively. For dynamic histomorphometry, fluorescent labels were visualized on unstained sections under ultraviolet light, using a triple diamidino-2-phenylindole/fluorescein/Texas red set long-pass filter. Osteoclast number/endocortical perimeter was measured in cross sections at middiaphysis and expressed as osteoclasts per millimeter of endocortical surface. The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (12, 41).
qRT-PCR.
Total RNA was extracted from calvariae or tibiae of 1-mo-old control and experimental mice of both sexes, and mRNA levels were determined by qRT-PCR (37, 38). For this purpose, equal amounts of RNA were reverse-transcribed using the iScript RT-PCR kit (Bio-Rad), according to the manufacturer's instructions and amplified in the presence of specific primers (Table 2) and iQ SYBR Green Supermix (Bio-Rad) at 60°C for 35 cycles. Transcript copy number was estimated by comparison with a serial dilution of cDNA for Axin2 (GE Healthcare Dharmacon, Lafayette, CO) Dkk1 (encoding for Dickkopf-related protein-1, from C. Niehrs, Heidelberg, Germany), Hey1, Hey2 (both from T. Iso, Los Angeles, CA), HeyL (from D. Srivastava, Dallas, TX), Notch1, Rbpjκ, Sost (all three from Thermo Scientific, Waltham, MA), Tnfrsf11b [encoding for osteoprotegerin, from American Type Tissue Culture Collection (ATCC), Manassas, VA], and Tnfsf11 (encoding for receptor activator of nuclear factor-κB ligand, Rankl; Source BioScience, Nottingham, UK) (21, 25, 34). Reactions were conducted in a CFX96 qRT-PCR detection system (Bio-Rad), and fluorescence was monitored during every PCR cycle at the annealing step. Data are expressed as copy number corrected for ribosomal protein L38 (Rpl38) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) copy number, estimated by comparison with a serial dilution of cDNA for Rpl38 (ATCC) or Gapdh (R. Wu, Ithaca, NY) (50).
Table 2.
Primers used for qRT-PCR determinations
| Gene | Strand | Sequence 5′-3′ | GenBank Acc. No. |
|---|---|---|---|
| Axin2 | Forward | CAAGCCCCATAGTGCCCAAA | NM_015732 |
| Reverse | CAGACTCCAATGGGTAGCTCT | ||
| Dkk1 | Forward | CCCTCCCTTGCGCTGAAGATGAGGAGT | NM_010051 |
| Reverse | CGCTTTCGGCAAGCCAGAC | ||
| Gapdh | Forward | CCCCTCTGGAAAGCTGTGGCGT | NM_008084 |
| Reverse | AGCTTCCCGTTCAGCTCTGG | ||
| Hey1 | Forward | ATCTCAACAACTACGCATCCCAGC | NM_010423 |
| Reverse | GTGTGGGTGATGTCCGAAGG | ||
| Hey2 | Forward | AGCGAGAACAATTACCCTGGGCAC | NM_013904 |
| Reverse | GGTAGTTGTCGGTGAATTGGACCT | ||
| HeyL | Forward | CAGTAGCCTTTCTGAATTGCGAC | NM_013905 |
| Reverse | AGCTTGGAGGAGCCCTGTTTC | ||
| Notch1 | Forward | GTGCTCTGATGGACGACAAT | NM_008714 |
| Reverse | GCTCCTCAAACCGGAACTTC | ||
| Rbpjκ | Forward | ACAGACAAGGCAGAATACAC | NM_001080928 NM_009035 NM_001080927 |
| Reverse | CAACTGAAGACTTCTAGGA | ||
| Rpl38 | Forward | AGAACAAGGATAATGTGAAGTTCAAGGTTC | NM_001048057 NM_023372 NM_001048058 |
| Reverse | CTGCTTCAGCTTCTCTGCCCTTT | ||
| Sost | Forward | AGGAATGATGCCACAGAGGTC | NM_024449 |
| Reverse | CTGGTTGTTCTCAGGAGGAGGCTC | ||
| Tnfrsf11b | Forward | CAGAAAGGAAATGCAACACATGACAAC | NM_008764 |
| Reverse | GCCTCTTCACACAGGGTGACATC | ||
| Tnfsf11 | Forward | TATAGAATCCTGAGACTCCATGAAAAC | NM_011613 |
| Reverse | CCCTGAAAGGCTTGTTTCATCC |
GenBank accession number identifies transcript recognized by primer pairs.
Statistical analysis.
Data are expressed as medians, means, and range because of the limited number of observations. Statistical differences between groups were determined by Mann-Whitney U-test for pairwise comparisons or by Kruskal-Wallis test for multiple comparisons. Data for mRNA levels when two groups were compared are expressed as means ± SE, and statistical differences were determined by unpaired t-test.
RESULTS
General appearance and femoral microarchitecture of Dmp1-Cre+/−;RbpjκΔ/Δ conditional null mice.
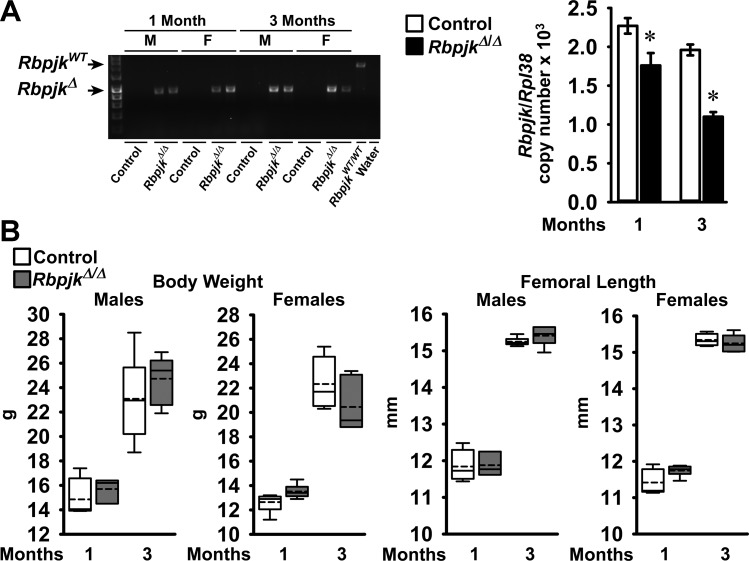
To study the conditional inactivation of Rbpjκ preferentially in osteocytes, hemizygous Dmp1-Cre transgenic mice in an Rbpjκ conditional background, Dmp1-Cre+/−;RbpjκloxP/loxP mice, were crossed with RbpjκloxP/loxP to generate Dmp1-Cre+/−;RbpjκΔ/Δ experimental and RbpjκloxP/loxP littermate controls. In preliminary experiments we documented that Dmp1-Cre transgenic and RbpjκloxP/loxP mice were not appreciably different from wild-type mice by femoral μCT at 1 mo of age (Ref. 8 and E. Canalis unpublished observations). Cre-mediated recombination of loxP sequences was documented in tibiae from Dmp1-Cre+/−;RbpjκΔ/Δ male and female mice, and Rbpjκ mRNA levels were 20–45% lower in tibiae from Dmp1-Cre+/−;RbpjκΔ/Δ mice than in RbpjκloxP/loxP controls (Fig. 1). The appearance of Rbpjκ conditional null mice and their weight and femoral length were not different from controls (Fig. 1). μCT of the distal femur revealed that male and female 1- and 3-mo-old Dmp1-Cre+/−;RbpjκΔ/Δ mice did not have an obvious phenotype in either trabecular or cortical bone compared with littermate RbpjκloxP/loxP mice (Tables 3 and 4). Minor differences in selected parameters of the μCT analysis were noted, but overall, Dmp1-Cre+/−;RbpjκΔ/Δ mice were not different from controls, indicating that Rbpjκ is dispensable in osteocytes.
Fig. 1.
A: PCR demonstration of Dmp1-Cre-mediated recombination of RbpjκloxP sequences and Rbpjκ mRNA levels in tibiae from 1- and 3-mo-old control RbpjκloxP/loxP and RbpjκΔ/Δ deleted mice. Recombination is shown for male (M) and female (F) mice independently and mRNA levels in samples from both sexes. Values for mRNA are means ± SE; n = 3–7. *Significantly different from control by unpaired t-test, P < 0.05. B: weight and femoral length of male and female Dmp1-Cre+/−;RbpjκΔ/Δ (dark gray boxes) and littermate RbpjκloxP/loxP controls (open boxes). Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate minimum and maximum values; n = 3–6.
Table 3.
Femoral microarchitecture assessed by μCT of 1- and 3-mo-old male conditional Dmp1-Cre+/−;RbpjκΔ/Δ null mice (RbpjκΔ/Δ) and RbpjκloxP/loxP controls of the same sex (Control)
| 1 Month |
3 Months |
|||
|---|---|---|---|---|
| Males | Control | RbpjκΔ/Δ | Control | RbpjκΔ/Δ |
| Trabecular bone | ||||
| Bone volume/tissue volume (%) | 4.9 (3.7–6.5) | 4.7 (3.3–5.0) | 8.5 (6.7–13.1) | 8.2 (7.3–12.6) |
| Trabecular separation (μm) | 201 (179–231) | 190 (188–278) | 200 (181–213) | 212 (204–219)* |
| Trabecular number (1/mm) | 5.0 (4.3–5.6) | 5.3 (3.6–5.3) | 4.9 (4.6–5.3) | 4.6 (4.5–4.8) |
| Trabecular thickness (μm) | 20 (20–22) | 21 (20–23) | 33 (31–41) | 34 (33–41) |
| Connectivity density (1/mm3) | 207 (71–386) | 136 (94–175) | 225 (198–281) | 206 (162–253) |
| Structure model index | 3.1 (3.0–3.2) | 3.1 (2.9–3.2) | 2.3 (2.0–2.5) | 2.4 (2.0–2.7) |
| Density of material (mg HA/cm3) | 893 (865–907) | 899 (867–916) | 933 (929–940) | 922 (906–936)* |
| Cortical bone | ||||
| Bone volume/tissue volume (%) | 87.9 (87.6–88.8) | 87.1 (86.2–87.6) | 88.6 (87.5–91.1) | 89.0 (87.2–90.0) |
| Cortical thickness (μm) | 88.5 (80.0–90.0) | 83.0 (80.0–87.0) | 145.0 (129.0–173.0) | 142.5 (128.0–162.0) |
| Periosteal perimeter (mm) | 4.1 (4.0–4.4) | 4.3 (4.3–4.4) | 4.3 (4.2–4.6) | 4.6 (4.3–4.8) |
| Endosteal perimeter (mm) | 3.4 (3.3–3.7) | 3.6 (3.6–3.7) | 3.2 (3.1–3.3) | 3.4 (3.3–3.5)* |
| Total area (mm2) | 1.3 (1.3–1.5) | 1.5 (1.4–1.5) | 1.5 (1.4–1.7) | 1.7 (1.5–1.9) |
| Bone area (mm2) | 0.39 (0.36–0.45) | 0.41 (0.39–0.43) | 0.66 (0.58–0.81) | 0.70 (0.61–0.85) |
Values are medians (minimum and maximum values for each parameter); n = 3–6. μCT was performed on femurs from Dmp1-Cre+/−;RbpjκΔΔ and control RbpjkloxP/loxP littermates.
Significantly different from controls by Mann-Whitney U-test, P < 0.05.
Table 4.
Femoral microarchitecture assessed by μCT of 1- and 3-mo-old female conditional Dmp1-Cre+/−;RbpjκΔ/Δ null mice (RbpjκΔ/Δ) and RbpjκloxP/loxP controls of the same sex (Control)
| 1 Month |
3 Months |
|||
|---|---|---|---|---|
| Females | Control | RbpjκΔ/Δ | Control | RbpjκΔ/Δ |
| Trabecular bone | ||||
| Bone volume/tissue volume (%) | 4.4 (2.9–5.5) | 5.6 (4.4–6.1) | 5.4 (3.9–6.2) | 6.4 (4.1–6.9) |
| Trabecular separation (μm) | 227 (189–231) | 203 (187–225) | 281 (267–298) | 261 (249–291)* |
| Trabecular number (1/mm) | 4.8 (3.9–5.3) | 4.9 (4.5–5.3) | 3.6 (3.4–3.7) | 3.8 (3.4–4.0)* |
| Trabecular thickness (μm) | 21 (19–22) | 23 (21–25) | 37 (34–37) | 40 (37–42)* |
| Connectivity density (1/mm3) | 183 (40–225) | 250 (145–359) | 112 (47–155) | 107 (44–125) |
| Structure model index | 3.1 (3.0–3.2) | 2.8 (2.6–3.0)* | 2.8 (2.6–3.2) | 3.0 (2.6–3.4) |
| Density of material (mg HA/cm3) | 890 (876–909) | 887 (863–896) | 949 (938–961) | 954 (939–964) |
| Cortical bone | ||||
| Bone volume/tissue volume (%) | 87.3 (86.0–93.5) | 87.0 (86.2–89.0) | 89.2 (88.4–89.8) | 89.4 (88.5–90.2) |
| Cortical thickness (μm) | 77.0 (75.0–84.0) | 84.0 (77.0–90.0) | 151.0 (148.0–160.0) | 151.5 (142.0–158.0) |
| Periosteal perimeter (mm) | 4.07 (3.9–4.2) | 4.13 (4.0–4.35) | 4.4 (4.3–4.6) | 4.3 (4.2–4.5) |
| Endosteal perimeter (mm) | 3.5 (3.3–3.6) | 3.52 (3.3–3.6) | 3.2 (3.1–3.4) | 3.2 (3.0–3.3) |
| Total area (mm2) | 1.3 (1.2–1.4) | 1.4 (1.3–1.4) | 1.6 (1.5–1.7) | 1.5 (1.4–1.6) |
| Bone area (mm2) | 0.34 (0.33–0.38) | 0.38 (0.35–0.42) | 0.73 (0.69–0.74) | 0.69 (0.67–0.77) |
Values are medians (minimum and maximum values for each parameter); n = 3–6. μCT was performed on femurs from Dmp1-Cre+/−; RbpjκΔ/Δ and control RbpjκloxP/loxP littermates.
Significantly different from controls by Mann-Whitney U-test, P < 0.05.
Rescue of Dmp1-Cre+/−;RosaNotch skeletal phenotype by Rbpjκ inactivation.
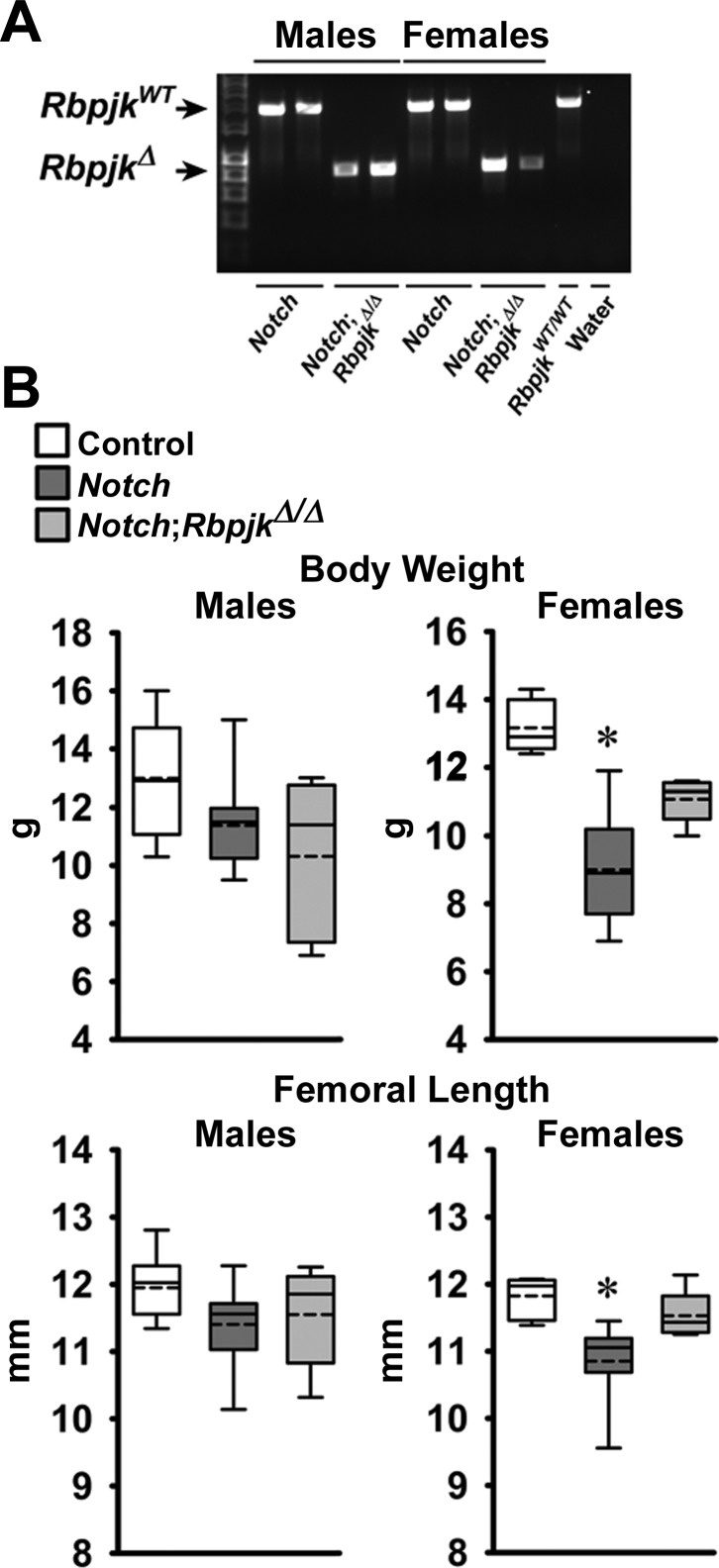
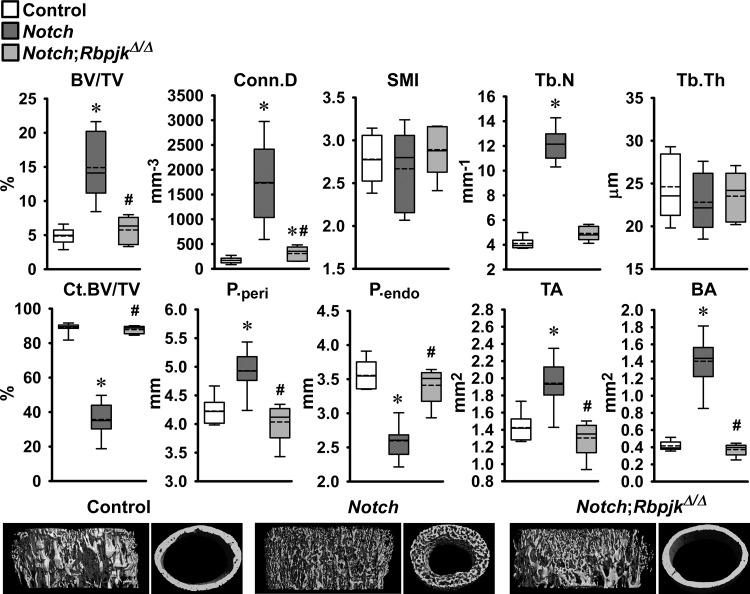
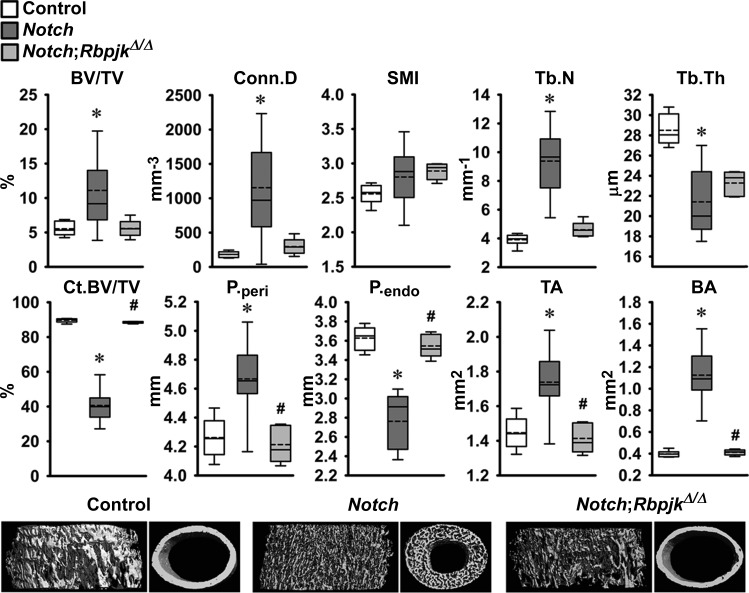
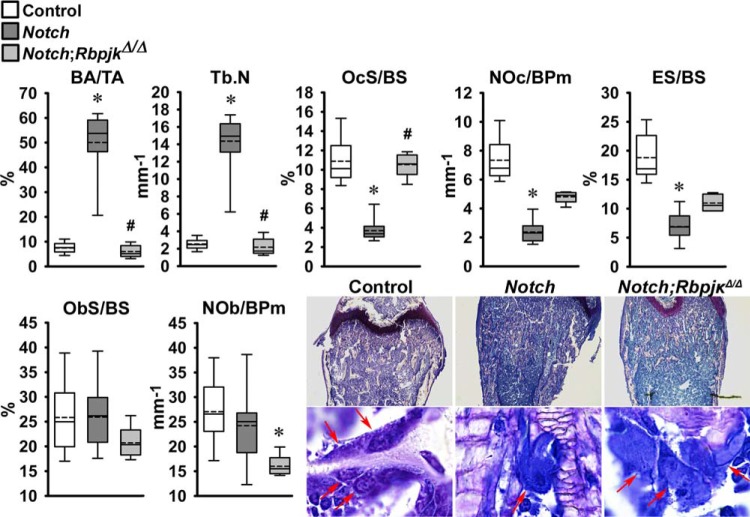
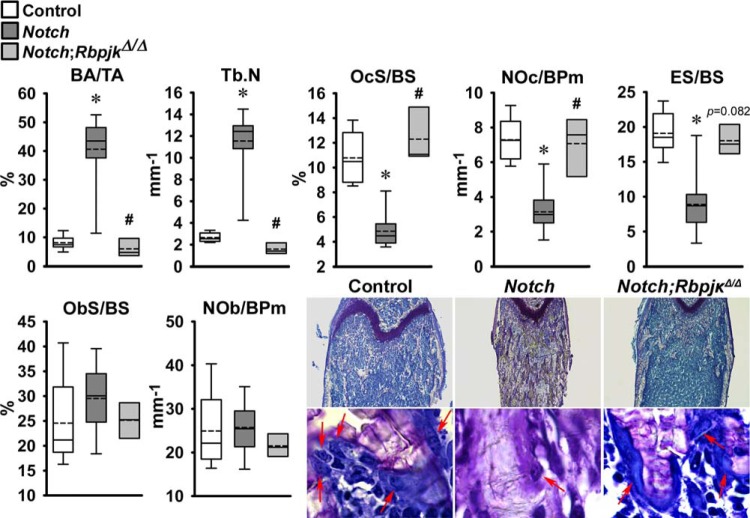
The skeletal phenotype of Dmp1-Cre+/−;RosaNotch mice expressing the Notch1 NICD preferentially in osteocytes was described in previous reports from this laboratory (6, 8). To determine whether the phenotype was secondary to activation of Notch canonical signaling, Dmp1-Cre+/−;RosaNotch mice were studied in the context (Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ) or not of Rbpjκ inactivation. Cre-mediated recombination of Rbpjκ loxP sequences was documented in tibiae from Rbpjκ deleted mice of both sexes (Fig. 2). Preferential activation of Notch in osteocytes resulted in a decrease in weight and femoral length in female but not in male mice, and the effect was reversed in the context of the Rbpjκ inactivation (Fig. 2). In agreement with former studies, femoral μCT revealed that 1-mo-old male Dmp1-Cre+/−;RosaNotch mice had a marked increase in trabecular bone volume/tissue volume compared with RosaNotch controls because of an increase in trabecular number (Fig. 3) (6). Cortical total and bone area were increased, but bone was porous, so that cortical bone volume/total volume was decreased in Dmp1-Cre+/−;RosaNotch mice. The effects of Notch in osteocytes were secondary to activation of the canonical pathway since they were reversed in the context of the Rbpjκ inactivation, and skeletal microarchitecture parameters of Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ mice were not different from control RosaNotch mice except for a slight increase in connectivity density (Fig. 3). A similar phenotype of the Notch activation in osteocytes was observed in female mice, and the phenotype also was reversed in the context of the Rbpjκ inactivation (Fig. 4). Bone histomorphometric analysis confirmed the increase in cancellous bone in Dmp1-Cre+/−;RosaNotch mice due to a decrease in osteoclast number and eroded surface with no changes in osteoblast number in male (Fig. 5) and female (Fig. 6) mice. The animals studied by μCT and histomorphometry overlapped by 30% for male and 70–75% for female mouse cohorts. Changes in osteoclast number appeared limited to cancellous bone, since number of osteoclast/endocortical perimeter were (means ± SE; n = 4 to 6) 0.60 ± 0.35/mm in control and 0.52 ± 0.30/mm (NS) in Dmp1-Cre+/−;RosaNotch male mice at 1 mo of age (6). The presence of osteoclasts in periosteal surfaces could not be determined with certainty. The inactivation of Rbpjκ in the context of the preferential activation of Notch in osteocytes (Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ) reversed the phenotype so that, in cancellous bone, osteoclast number, eroded surface, and bone area/tissue area were not different between Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ and control mice (Figs. 5 and 6). Calcein and demeclocycline labels were detected only in a limited number of mice, such that dynamic histomorphometry parameters could not be established.
Fig. 2.
A: PCR demonstration of Dmp1-Cre-mediated recombination of RbpjκloxP sequences in tibiae from 1-mo-old male and female Dmp1-Cre+/−;RosaNotch and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ mice. B: weight and femoral length of male and female 1-mo-old RosaNotch controls (open boxes), Dmp1-Cre+/−;RosaNotch (dark gray boxes), and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ mice (light gray boxes). Horizontal continuous lines represent the median, and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate minimum and maximum values; n = 5–12. *Significantly different from RosaNotch control mice by Kruskal-Wallis test.
Fig. 3.
Femoral microarchitecture assessed by μCT analysis of the distal femur trabecular compartment (top) and femoral mid-shaft cortical bone (bottom) of 1-mo-old male Dmp1-Cre+/−;RosaNotch mice in an Rbpjκ intact background (Notch, dark gray boxes) and in the context of Rbpjκ deletion, Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ, light gray boxes), and control RosaNotch (control, open boxes) mice. Trabecular μCT parameters: bone volume/total volume (BV/TV), connectivity density (Conn.D), structure model index (SMI), and trabecular number (Tb.N) and thickness (Tb.Th). Cortical μCT parameters: cortical BV/TV (Ct.BV/TV), periosteal (P.peri) and endocortical perimeter (P.endo), and total (TA) and bone (BA) area. Dmp1-Cre+/−;RosaNotch and control mice were obtained from litters pooled over a period of ∼4 yr and included Dmp1-Cre+/−;RosaNotch matched to Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ littermate mice (n = 5–12). Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate minimum and maximum values. *Significantly different from control RosaNotch mice; #Signficantly different between Dmp1-Cre+/−;RosaNotch and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ by Kruskal-Wallis test, P < 0.05. Representative μCT of cancellous and cortical bone from Dmp1-Cre+/−;RosaNotch (Notch) and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ) and RosaNotch controls are shown on the bottom.
Fig. 4.
Femoral microarchitecture assessed by μCT analysis of the distal femur trabecular compartment (top) and femoral midshaft cortical bone (bottom) of 1-mo-old female Dmp1-Cre+/−;RosaNotch in an Rbpjκ intact background (Notch, dark gray boxes) and in the context of Rbpjκ deletion, Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ, light gray boxes), and control RosaNotch (control, open boxes) mice. Trabecular μCT parameters: BV/TV, Conn.D, SMI, and Tb.N and Tb.Th. Cortical μCT parameters: Ct.BV/TV, P.peri and P.endo, and TA and BA. Dmp1-Cre+/−;RosaNotch and control mice were obtained from litters pooled over a period of ∼4 yr and included Dmp1-Cre+/−;RosaNotch matched to Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ littermate mice (n = 5–11). Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate minimum and maximum values. *Significantly different from control mice; #Signficantly different between Dmp1-Cre+/−;RosaNotch and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ by Kruskal-Wallis test, P < 0.05. Representative μCT of cancellous and cortical bone from Dmp1-Cre+/−;RosaNotch (Notch) and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ) and RosaNotch controls are shown on the bottom.
Fig. 5.
Bone histomorphometry performed on femurs from 1-mo-old Dmp1-Cre+/−;RosaNotch male mice in an Rbpjκ intact background (Notch, dark gray boxes) or in the context of Rbpjκ deletion, Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ, (Notch;RbpjκΔ/Δ, light grey boxes) and control RosaNotch (control, open boxes) mice. Trabecular bone histomorphometric parameters: bone area/tissue area (BA/TA), trabecular number (Tb.N), osteoclast surface/bone surface (OcS/BS), number of osteoclasts/bone perimeter (NOc/BPm), eroded surface/bone surface (ES/BS), osteoblast surface/bone surface (ObS/BS), and number of osteoblasts/bone perimeter (NOb/BPm). Dmp1-Cre+/−;RosaNotch and RosaNotch control mice shown were obtained from litters pooled over an ∼ 4-yr period and included Dmp1-Cre+/−;RosaNotch matched to Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ littermate mice, n = 5–12. Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate minimum and maximum values. *Significantly different from controls; #Significantly different between Dmp1-Cre+/−;RosaNotch and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ, by Kruskal-Wallis test, P < 0.05. Representative histological femoral sections from 1-mo-old Dmp1-Cre+/−;RosaNotch (Notch), Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ), and RosaNotch controls are shown on the bottom. Sections were stained with toluidine blue; final magnification ×40 and ×630. Arrows point to osteoclasts on trabecular surfaces.
Fig. 6.
Bone histomorphometry performed on femurs from 1-mo-old Dmp1-Cre+/−;RosaNotch female mice in an Rbpjκ intact background (Notch, dark grey boxes) or in the context of the Rbpjκ deletion, Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ, (Notch;RbpjκΔ/Δ, light gray boxes) and control RosaNotch (control, open boxes) mice. Trabecular bone histomorphometric parameters: BA/TA, Tb.N, OcS/BS, NOc/BPm, ES/BS, ObS/BS, and NOb/BPm. Dmp1-Cre+/−;RosaNotch and RosaNotch control mice shown were obtained from litters pooled over an ∼4-yr period and included Dmp1-Cre+/−;RosaNotch matched to Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ littermate mice; n = 3–14. Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate maximum and minimum values. *Significantly different from controls; #Significantly different between Dmp1-Cre+/−;RosaNotch and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ, by Kruskal-Wallis test, P < 0.05. Representative histological femoral sections from 1-mo-old Dmp1-Cre+/−;RosaNotch (Notch), Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ), and RosaNotch controls are shown on the bottom. Sections were stained with toluidine blue; final magnification ×40 and ×630. Arrows point to osteoclasts on trabecular surfaces.
Mechanisms responsible for the effect of Notch in osteocytes.
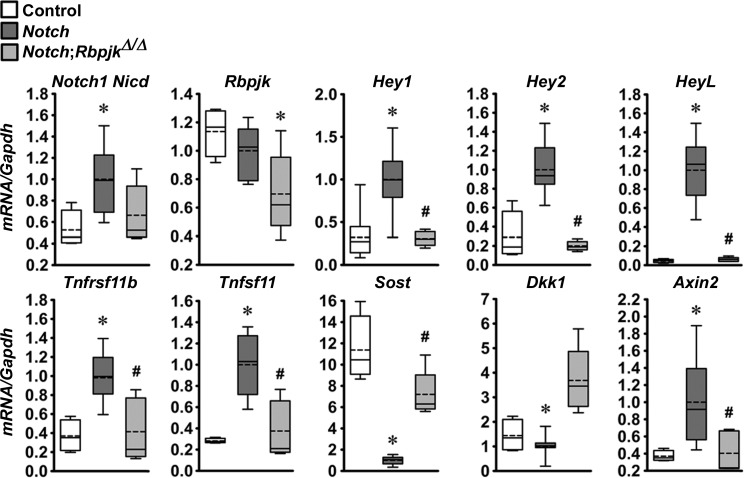
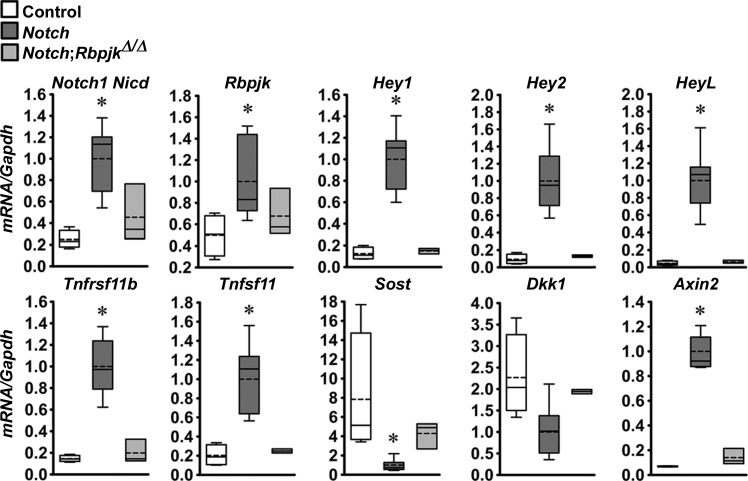
In previous work, we demonstrated that the preferential activation of Notch in osteocytes caused a marked suppression of Sost and, to a lesser extent, Dkk1, genes encoding for the Wnt antagonists sclerostin and dickkopf1 (6). As a consequence, there was an increase in Wnt signaling. To determine whether these effects, as well as the upregulation of Tnfrsf11b (encoding for osteoprotegerin), were mediated by Notch canonical signaling, calvariae and tibiae extracts were obtained from Dmp1-Cre+/−;RosaNotch mice in the context or not of the Rbpjκ inactivation. The preferential activation of Notch in osteocytes resulted in an induction of the Notch target genes Hey1, Hey2, and HeyL, and the induction of Tnfrsf11b and Tnfsf11 and suppression of Sost and Dkk1 with a consequent increase in Axin2 mRNA in calvariae (Fig. 7) and tibiae (Fig. 8); the suppression of Dkk1 reached statistical significance only in calvariae. In the context of Notch activation in osteocytes, deletion of Rbpjκ reversed the stimulatory effect of Notch on Hey1, Hey2, HeyL, Tnfrsf11b, and Tnfsf11 transcripts, and the inhibition of Sost and stimulation of Axin2 mRNA levels in calvariae (Fig. 7). The same trend was observed in mRNA levels obtained in tibiae (Fig. 8). However, due to the limited number of samples (n = 3) available from Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ mice, the results did not reach a statistically significant difference when this group of mice was compared with Dmp1-Cre+/−;RosaNotch mice. mRNA levels in Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ mice were not different from control mice, indicative of a normalization of transcript expression by the Rbpjκ inactivation. Both Tnfrsf11b and Tnfsf11 were induced by the Notch activation, so that the ratio of Tnfrsf11b/Tnfsf11 transcripts was not affected. Neither induction was observed in the context of the Rbpjκ deletion. The results indicate that the suppression of the Wnt antagonists Sost and Dkk1 and upregulation of Tnfrsf11b are mediated by the Notch canonical pathway. The reversal of the suppression of Sost and Dkk1 and to some extent that of the induction of Tnfrsf11b may explain the reversal of the Notch phenotype by the downregulation of Rbpjκ.
Fig. 7.
Notch1 Nicd, Rbpjκ, Hey1, Hey2, HeyL, Tnfsrf11b, Tnfsf11, Sost, Dkk1, and Axin2 mRNA levels in calvarial extracts from 1-mo-old Dmp1-Cre+/−;RosaNotch mice in an Rbpjκ intact background (Notch, dark gray boxes) or in the context of the Rbpjκ deletion, Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ, light gray boxes), and control RosaNotch (control, open boxes) mice. mRNA levels obtained from RosaNotch control and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ were compared with littermate-matched Dmp1-Cre+/−;RosaNotch mice. To compare the 3 groups, mRNA levels from Dmp1-Cre+/−;RosaNotch mice were normalized to 1. Data from Dmp1-Cre+/−;RosaNotch and RosaNotch control littermate mice were obtained from litters pooled over an ∼4-yr period and included Dmp1-Cre+/−;RosaNotch matched to Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ littermate mice; n = 4–20. Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate maximum and minimum values. *Significantly different from controls; #Significantly different between Dmp1-Cre+/−;RosaNotch and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ, by Kruskal-Wallis test, P < 0.05.
Fig. 8.
Notch1 Nicd, Rbpjκ, Hey1, Hey2, HeyL, Tnfsrf11b, Tnfsf11, Sost, Dkk1 and Axin2 mRNA levels in tibiae of 1-mo-old Dmp1-Cre+/−;RosaNotch mice in an Rbpjκ intact background (Notch, dark gray boxes) or in the context of Rbpjκ deletion, Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ (Notch;RbpjκΔ/Δ, light gray boxes), and control RosaNotch (control, open boxes) mice. mRNA levels obtained from RosaNotch control and Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ were compared with littermate-matched Dmp1-Cre+/−;RosaNotch mice. To compare the 3 groups, mRNA levels from Dmp1-Cre+/−;RosaNotch mice were normalized to 1. Data from Dmp1-Cre+/−;RosaNotch and RosaNotch control littermate mice were obtained from litters pooled over an ∼4-yr period and included Dmp1-Cre+/−;RosaNotch matched to Dmp1-Cre+/−;RosaNotch;RbpjκΔ/Δ littermate mice; n = 3–7. Horizontal continuous lines represent the median and interrupted lines represent the mean; upper and lower limits of the box represent 75th and 25th percentiles. Whiskers (bars) indicate maximum and minimum values. *Significantly different from controls; by Kruskal-Wallis test, P < 0.05.
DISCUSSION
Osteocytes are cells embedded in the bone matrix and communicate through a canalicular network with each other and with osteoblasts and lining cells. Osteocytes play a fundamental role in mechanotransduction, and osteocyte-ablated mice exhibit bone loss and microstructural deterioration (49). Osteocytes and osteoblasts do not have redundant functions, and the impact of a regulatory signal may result in different biological events when it operates in osteoblasts or when it is activated in osteocytes. Whereas Notch activation in immature and mature osteoblasts causes osteopenia, its preferential activation in osteocytes causes osteopetrosis (8). This is because the effects of Notch are cell context dependent.
Although the effects of Notch in cancellous bone are not sexually dimorphic, Notch activation in osteocytes resulted in lower weight and shorter femurs in female but not in male Dmp1-Cre+/−;RosaNotch mice (8). There is no clear explanation for this difference, which is observed as early as 1 mo of age, suggesting independence from hormonal influences. This does not discount potential sex hormone-Notch interactions in adult mice. Since ovariectomy enhances bone resorption and Notch activation in osteocytes suppresses this process, it is possible for estrogen deficiency to oppose the Notch phenotype. Conversely, Notch activation in osteocytes may serve to protect from the bone loss of estrogen deficiency. Our findings confirm that the activation of Notch1 canonical signaling preferentially in osteocytes causes an increase in cancellous bone volume. This osteopetrotic phenotype can be explained by an induction of osteoprotegerin, and a suppression of Sost and Dkk1 leading to enhanced Wnt signaling and an inhibition of bone resorption. It is of interest to note that Notch activation in osteoblasts and ST-2 stromal cell lines inhibits Wnt signaling directly, explaining the osteopenic phenotype observed following the activation of Notch in osteoblasts (13, 62). Since the expression of Sost in osteoblasts is minimal, it cannot be downregulated by Notch so that Wnt signaling cannot be enhanced by this mechanism in osteoblasts, as it can in osteocytes. The downregulation of Wnt antagonists by Notch with the consequent increase in Wnt signaling may serve as a positive feedback autocrine regulatory mechanism, since Wnt itself can activate the Notch pathway in osteocytes (51).
Wnt signaling induces osteoblastogenesis and inhibits osteoclastogenesis and bone resorption, and the constitutive activation of β-catenin in the osteoblast and osteoclast lineages causes osteopetrosis (5, 19, 32, 53). The inhibitory effect of Wnt signaling on bone resorption resembles the effect of the Notch activation in osteocytes and has been explained by an increase in osteoprotegerin expression by cells of the osteoblastic lineage and by direct effects of Wnt on osteoclast precursors (1, 9, 42). Increases in bone volume are reported in conditions where suppressed bone resorption and decreased remodeling are the dominant events, such as the inactivation of Tnfrsf11a, encoding for the receptor activator of NF-κB (Rank) and the transgenic overexpression of Tnfrsf11b (encoding for osteoprotegerin) (16, 30, 39, 46).
Although Notch activation in osteocytes causes a cancellous bone osteopetrotic phenotype, the cortical bone remains porous. This appearance resembles murine cortical bone during embryonic development and may represent failure to form a fully mature and compact cortical bone under conditions of Notch activation (45). The cortical bone phenotype is more apparent when Notch is activated in osteocalcin-expressing cells; and under these conditions cortical bone fails to form as a compact structure (8). Examination of von Kossa-stained histological sections does not suggest a failure to mineralize but to form a compact cortex (E. Canalis, unpublished observations). Moreover, the cortical porosity could not be attributed to an increased number of osteoclasts on cortical bone, since osteoclast number in the endocortical surface did not differ between Dmp1-Cre+/−;RosaNotch and control mice. The suppression of bone resorption by Notch1 activation in osteocytes is in accord with the previously reported inhibitory effect of Notch on osteoclastogenesis. Notch1 inhibits osteoclastogenesis by direct effects on osteoclast precursors and indirectly through actions in cells of the osteoblastic lineage by altering the osteoprotegerin/Rankl ratio (2). However, in former (6) and in the present studies, we did not detect differences in the osteoprotegerin/Rankl ratio at the mRNA level between Notch-activated and control mice. It is important to note that other Notch receptors may have distinct effects on the skeleton, and activation of Notch2 in cells of the osteoclast lineage causes an induction of nuclear factor of activated T cells (Nfat)c1 and enhanced osteoclastogenesis (18). It is of interest that this effect is accompanied by an increase in the expression of the Notch target gene Hes1, and Hes1 expression in skeletal cells causes osteopenia secondarily to decreased bone formation and enhanced bone resorption (18, 60).
Inactivation of Rbpjκ in osteocytes did not result in a skeletal phenotype, and this is in agreement with prior observations following its inactivation in cells expressing a 2.3-kb fragment of the type Iα1 collagen promoter (48). These findings indicate that Rbpjκ is dispensable for the function of mature osteoblasts and osteocytes. The reason for this is not entirely clear, but Rbpjκ under basal conditions is an inhibitor of gene transcription, suggesting that this function is not essential for osteoblast/osteocyte maturation or activity. In contrast, inactivation of Notch1 and Notch2 in osteocytes causes an increase in cancellous bone secondary to an increase in osteoblast number, suggesting that these effects of Notch1 and Notch2 do not overlap with those of Rbpjκ and are independent of Rbpjκ-mediated signaling (6). If this is the case, Notch noncanonical signaling may play an undiscovered role in osteocyte function, but under conditions of pronounced Notch activation, as occurring in the RosaNotch mouse model, canonical signaling prevails. It is also possible that the deletion of Notch1 and -2 by the Dmp1-driven Cre recombinase was more efficient than the deletion of Rbpjκ, since Rbpjκ mRNA levels in bone extracts were suppressed by 20–45%. Whereas noncanonical signals can mediate Notch actions, their role is poorly understood, and this pathway has not been characterized in cells of the osteoblastic lineage, adding difficulties to the interpretation of the discrepant results between the deletion of Notch1 and -2 and of Rbpjκ in osteocytes. Deltex is known to mediate noncanonical signals of Notch, and Deltex2 and -3 are expressed by cells of the osteoblastic lineage, and their selective deletion in osteocytes may help clarify their function and the role of noncanonical Notch signaling in these cells (14).
In contrast to the lack of an Rbpjκ function under basal conditions, Rbpjκ inactivation reversed the phenotype observed following Notch activation. This is in accord with the reversal of the osteosclerotic phenotype by the Rbpjκ inactivation following Notch1 activation in type I collagen-expressing cells (48). Similarly, the effects of the Notch1 activation on limb development are Rbpjκ dependent, whereas the effects of Notch1 on committed chondrocytes are both Rbpjκ dependent and independent (10, 28).
A limitation of the present work is that the expression of Dmp1, used to direct Cre and activate Notch, is not circumscribed to osteocytes, and Dmp1 is also expressed by other cells of the same lineage, including terminally mature osteoblasts (26, 56). However, activation of Notch1 in osteocytes results in a unique osteosclerotic phenotype, suggesting that the impact of Dmp1-directed Notch activation occurred primarily in these cells. The RosaNotch model allows for the preferential activation of Notch in osteocytes. However, osteocytes contact neighboring cells through gap junctions that form on the tips of their dendritic processes (11). As a consequence, classic Notch activation by Notch-ligand interactions in neighboring cells might not occur (29, 58). Notch activation in osteocytes could occur in response to mechanical forces, as it has been reported for Wnt signal activation (27). Indeed, Notch signaling is activated by fluid shear stress in the osteocytic cell line MLO-Y4 (S. Zanotti and E. Canalis, unpublished observations). Another possible way of Notch activation in osteocytes might be in response to Wnt signaling, which has been shown to activate the Notch pathway in these cells (51).
In conclusion, the activation of Notch1 in osteocytes causes osteopetrosis by inducing Notch canonical signaling, which downregulates Sost and Dkk1 and upregulates osteoprotegerin expression.
GRANTS
This work was supported by Grant AR-063049 (E. Canalis) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.C. conception and design of research; E.C. and S.Z. analyzed data; E.C. and S.Z. interpreted results of experiments; E.C. drafted manuscript; E.C. and S.Z. edited and revised manuscript; E.C., D.B., L.S., and S.Z. approved final version of manuscript; D.B., L.S., and S.Z. performed experiments; S.Z. prepared figures.
ACKNOWLEDGMENTS
We thank J. Feng for the Dmp1-Cre transgenic mice, T. Iso for the Hey1 and Hey2 cDNAs, C. Niehrs for the Dkk1 cDNA, D. Srivastava for the HeyL cDNA, R. Wu for the Gapdh, Dorothy Wakefield for statistical analysis, and Mary Yurczak for secretarial support.
Glossary
- BA
Bone area
- BA/TA
Bone area/tissue area
- BV/TV
Bone volume/tissue volume
- ConnD
Connectivity density
- DMP1
Dentin matrix protein-1
- Dkk1
Dickkopf-related protein-1
- P.endo
endosteal perimeter
- ES/BS
Eroded surface/bone surface
- Gapdh
Glyceraldehyde-3-phosphate dehydrogenase
- Hes
Hairy enhancer of split
- Hey
Hes-related with YRPW motif
- Maml
Mastermind-like
- NICD
Notch intracellular domain
- NOb/BPm
Number of osteoblasts/bone perimeter
- NOc/BPm
Number of osteoclasts/bone perimeter
- Nfat
Nuclear factor of activated T cells
- ObS/BS
Osteoblast surface/bone surface
- OcS/BS
Osteoclast surface/bone surface
- P.peri
Periosteal perimeter
- RANK
Receptor activator of NF-κB
- RANK-L
RANK ligand
- Rbpjκ
Recombination signal binding protein for immunoglobulin κJ region
- Rpl38
Ribosomal protein L38
- SMI
Structure model index
- TA
Tissue area
- Tb.N
Trabecular number
- Tb.Th
Trabecular thickness
REFERENCES
- 1.Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, Amling M, Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol 200: 537–549, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 283: 6509–6518, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468–1486, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz F, Ringrose L, Angrand PO, Rossi F, Stewart AF. Different thermostabilities of FLP and Cre recombinases: implications for applied site-specific recombination. Nucleic Acids Res 24: 4256–4262, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 9: 575–583, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Canalis E, Adams DJ, Boskey A, Parker K, Kranz L, Zanotti S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem 288: 25614–25625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357: 905–916, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of Notch activation in the skeleton. Endocrinology 154: 623–634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Long F. Beta-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res 28: 1160–1169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Tao J, Bae Y, Jiang MM, Bertin T, Chen Y, Yang T, Lee B. Notch gain of function inhibits chondrocyte differentiation via Rbpj-dependent suppression of Sox9. J Bone Miner Res 28: 649–659, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell… and more. Endocr Rev 34: 658–690, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28: 2–17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem 281: 6203–6210, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Role of the RAM domain and ankyrin repeats on notch signaling and activity in cells of osteoblastic lineage. J Bone Miner Res 21: 1317–1326, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, O'Keefe RJ, Hilton MJ. RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development 137: 1461–1471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De ST, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev 13: 2412–2424, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 14: 299–305, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol 28: 6402–6412, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22: 1197–1207, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol 14: 637–645, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 14: 306–314, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194: 237–255, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol 21: 6071–6079, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalajzic I, Matthews BG, Torreggiani E, Harris MA, Divieti PP, Harris SE. In vitro and in vivo approaches to study osteocyte biology. Bone 54: 296–306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone 47: 872–881, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohn A, Dong Y, Mirando AJ, Jesse AM, Honjo T, Zuscik MJ, O'Keefe RJ, Hilton MJ. Cartilage-specific RBPjkappa-dependent and -independent Notch signals regulate cartilage and bone development. Development 139: 1198–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene 27: 5099–5109, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97: 1566–1571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res 86: 320–325, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene 492: 1–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 100: 14920–14925, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol 216: 72–84, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell 124: 973–983, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Nazarenko I, Lowe B, Darfler M, Ikonomi P, Schuster D, Rashtchian A. Multiplex quantitative PCR using self-quenched primers labeled with a single fluorophore. Nucleic Acids Res 30: e37, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazarenko I, Pires R, Lowe B, Obaidy M, Rashtchian A. Effect of primary and secondary structure of oligodeoxyribonucleotides on the fluorescent properties of conjugated dyes. Nucleic Acids Res 30: 2089–2195, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ominsky MS, Stolina M, Li X, Corbin TJ, Asuncion FJ, Barrero M, Niu QT, Dwyer D, Adamu S, Warmington KS, Grisanti M, Tan HL, Ke HZ, Simonet WS, Kostenuik PJ. One year of transgenic overexpression of osteoprotegerin in rats suppressed bone resorption and increased vertebral bone volume, density, and strength. J Bone Miner Res 24: 1234–1246, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res 16: 1583–1585, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987. [DOI] [PubMed] [Google Scholar]
- 42.Sato MM, Nakashima A, Nashimoto M, Yawaka Y, Tamura M. Bone morphogenetic protein-2 enhances Wnt/beta-catenin signaling-induced osteoprotegerin expression. Genes Cells 14: 141–153, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA 85: 5166–5170, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354: 2250–2261, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Sharir A, Stern T, Rot C, Shahar R, Zelzer E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 138: 3247–3259, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA 102: 12443–12448, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, Lee B. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res 25: 2175–2183, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 5: 464–475, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res 13: 2485–2502, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc Natl Acad Sci USA 112: E478–486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 3: 346–355, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol Cell Biol 31: 4706–4719, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson JJ, Kovall RA. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell 124: 985–996, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 17: 1235–1241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O'Brien CA. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 10: e0138189, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanotti S, Canalis E. Notch1 and Notch2 expression in osteoblast precursors regulates femoral microarchitecture. Bone 62: 22–28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanotti S, Canalis E. Notch and the skeleton. Mol Cell Biol 30: 886–896, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanotti S, Canalis E. Notch signaling in skeletal health and disease. Eur J Endocrinol 168: R95–R103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanotti S, Smerdel-Ramoya A, Canalis E. Hairy and enhancer of split (HES)1 is a determinant of bone mass. J Biol Chem 286: 2648–2657, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanotti S, Smerdel-Ramoya A, Canalis E. Reciprocal regulation of notch and nuclear factor of activated T-cells (NFAT)c1 transactivation in osteoblasts. J Biol Chem 286: 4576–4588, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology 149: 3890–3899, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]