Abstract
Adenosine triphosphate (ATP) is a ubiquitous extracellular messenger elevated in the tumor microenvironment. ATP regulates cell functions by acting on purinergic receptors (P2X and P2Y) and activating a series of intracellular signaling pathways. We examined ATP-induced Ca2+ signaling and its effects on antiapoptotic (Bcl-2) and proapoptotic (Bax) proteins in normal human airway epithelial cells and lung cancer cells. Lung cancer cells exhibited two phases (transient and plateau phases) of increase in cytosolic [Ca2+] ([Ca2+]cyt) caused by ATP, while only the transient phase was observed in normal cells. Removal of extracellular Ca2+ eliminated the plateau phase increase of [Ca2+]cyt in lung cancer cells, indicating that the plateau phase of [Ca2+]cyt increase is due to Ca2+ influx. The distribution of P2X (P2X1-7) and P2Y (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11) receptors was different between lung cancer cells and normal cells. Proapoptotic P2X7 was nearly undetectable in lung cancer cells, which may explain why lung cancer cells showed decreased cytotoxicity when treated with high concentration of ATP. The Bcl-2/Bax ratio was increased in lung cancer cells following treatment with ATP; however, the antiapoptotic protein Bcl-2 demonstrated more sensitivity to ATP than proapoptotic protein Bax. Decreasing extracellular Ca2+ or chelating intracellular Ca2+ with BAPTA-AM significantly inhibited ATP-induced increase in Bcl-2/Bax ratio, indicating that a rise in [Ca2+]cyt through Ca2+ influx is the critical mediator for ATP-mediated increase in Bcl-2/Bax ratio. Therefore, despite high ATP levels in the tumor microenvironment, which would induce cell apoptosis in normal cells, the decreased P2X7 and elevated Bcl-2/Bax ratio in lung cancer cells may enable tumor cells to survive. Increasing the Bcl-2/Bax ratio by exposure to high extracellular ATP may, therefore, be an important selective pressure promoting transformation and cancer progression.
Keywords: purinergic receptor, calcium signaling, Bcl-2, Bax, cell apoptosis, cell proliferation
there is growing interest in the role of ATP in the development of cancer. ATP, well known as an intracellular molecular energy source, also functions as an extracellular messenger (2). ATP receptors are purinergic receptors (P2 receptors) and include the ligand-gated ion channel family of P2 receptors (P2X1-7) as well as the G protein-coupled receptor (GPCR) family of P2 receptors (P2Y1-2, P2Y4, P2Y6 and P2Y11-14) (3, 31). Activation of P2X receptors, which are nonselective cation channels formed by three homomeric or heteromeric P2X subunits, directly results in Na+ and Ca2+ influx through the cell plasma membrane, leading to membrane depolarization, which in turn activates voltage-gated Na+ and Ca2+ channels and causes the firing of action potentials. Activation of P2Y receptors, which are GPCRs, increases cytosolic free Ca2+ concentration ([Ca2+]cyt) by inducing Ca2+ release from intracellular stores (e.g., sarcoplasmic or endoplasmic reticulum) and Ca2+ influx through store-operated (SOC) and/or receptor-operated (ROC) Ca2+ channels. P2X and P2Y signaling is not only responsible for inducing action potentials in excitable cells (e.g., neurons), but also has been implicated in cell proliferation, differentiation, and apoptosis in nonexcitable cells (e.g., epithelial cells) (5). In a lung cancer cell line, A549, extracellular ATP, UTP, and UDP have been shown to stimulate proliferation that is dependent on the P2Y2 and P2Y6 receptors (44).
Extracellular or intercellular ATP concentration is reported to be very low (1–5 μM) in normal healthy tissues; however, it is significantly increased (to >100 μM) in the tumor microenvironment (26). The effect of increased extracellular ATP on cancer cell function is, however, dependent of the expression of different P2 receptors and changes in cytosolic [Ca2+]cyt due to activation of the different P2 receptors. Recent observations imply that differential expression of P2X and P2Y receptors may determine the kinetically distinct patterns of ATP-mediated increases in [Ca2+]cyt; the different patterns of Ca2+ signaling may lead to differential effects on cancer cells (e.g., proliferation vs. apoptosis) (34). This underscores the necessity of further characterization of P2 receptors in tumor cells, which could lead to novel therapeutic strategies that target purinergic signaling in the treatment of cancer.
Intracellular Ca2+ plays a critical role in the regulation of processes relevant to tumorigenesis, including cell proliferation, migration, and apoptosis (1, 22, 34). The role of Ca2+ channels and Ca2+ pumps in tumorigenesis is achieved through altered global or local changes in [Ca2+]cyt. The remodeling of Ca2+ signals in cancer cells is due potentially to aberrant expression of different Ca2+ channels, Ca2+ pumps, and Ca2+ transporters (22, 30). For example, it has been shown that P2Y2 and P2Y4 receptor expression in A549 cells promotes an increase in [Ca2+]cyt and induces Ca2+-dependent release of ATP and UTP as part of a positive feedback loop (4).
The Bcl-2 protein family consists of both antiapoptotic and proapoptotic members, which are traditionally considered to reside in or translocate to mitochondria and function as apoptotic regulators by modulating mitochondrial membrane permeability. The ratio between anti- and proapoptotic Bcl-2 proteins determines whether cells survive or die (13). The Bcl-2 family of proteins has also been detected in other subcellular compartments including the endoplasmic reticulum, nuclear membrane, plasma membrane and within the cytosol. Furthermore, the Bcl-2 family of proteins has been implicated in regulating [Ca2+] homeostasis in these cellular compartments (15, 28, 41), while changes in [Ca2+]cyt also exert effects on the expression of the anti- or proapoptotic Bcl-2 proteins. The Bcl-2 family of proteins also functions to relay Ca2+ signals to regulate cell apoptosis and proliferation. We hypothesize that there is a feedback loop in which Ca2+ signaling regulates the expression of Bcl-2 family members, resulting in an increase of the Bcl-2/Bax ratio.
In this report, we show that extracellular ATP induces only a transient increase in [Ca2+]cyt in normal lung epithelial cells; however, ATP induces a transient increase in [Ca2+]cyt followed by a plateau phase (or a slowly declining phase) increase of [Ca2+]cyt in lung cancer cells (H23 and A549) that is dependent on extracellular Ca2+. Furthermore, extracellular treatment with ATP increases the ratio of the antiapoptotic protein, Bcl-2, to the proapoptotic protein, Bax (Bcl-2/Bax ratio) in lung cancer cells, but not in normal lung epithelial cells. We also demonstrate that chelation of intracellular Ca2+ (with BAPTA-AM) or removal of extracellular Ca2+ inhibits the ATP-induced increase of Bcl-2/Bax ratio in lung cancer cells. These data imply that extracellular ATP, by selectively inducing a sustained or long-lasting increase in [Ca2+]cyt in lung cancer cells (but not in normal lung epithelial cells), promotes cell survival and enhances lung tumor growth through increase of the Bcl-2/Bax ratio. Inhibiting ATP-induced Ca2+ influx in lung cancer cells may lead to novel therapeutic approaches for lung cancer.
MATERIALS AND METHODS
Chemicals and drugs.
Adenosine 5-triphosphate disodium salt hydrate (ATP), uridine 5-triphosphate trisodium salt hydrate (UTP), α,β-methylene adenosine 5-triphosphate lithium salt (α,β-meATP), β,γ-methyleneadenosine 5-triphosphate disodium salt (β,γ-meATP), and TNP-ATP hydrate were prepared as concentrated stock solutions in distilled water. U-73122 hydrate, BAPTA-AM, and cyclopiazonic acid (CPA) were prepared as concentrated stock solutions in dimethyl sulfoxide (DMSO). All stock solutions (in water, DMSO) were aliquoted and kept frozen at −20°C until use. On the day of experiments, aliquots of the stock solutions were diluted in HEPES-buffered bath solution to the final concentrations for each drug. The pH values of all solutions were measured after addition of the drugs and adjusted to 7.4. All drugs were from Sigma Chemical, unless otherwise indicated.
Cell culture.
Human non-small cell lung cancer cell lines A549 and H23 and human airway epithelial cell line BEAS-2B (American Type Culture Collection, Manassas, VA) were cultured in DMEM medium (Corning Cellgro, NY) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Grand Island, NY), 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO). Cells were routinely cultured in 20% O2 and 5% CO2 at 37°C.
Measurement of [Ca2+]cyt.
Human non-small cell lung cancer cell lines A549 and H23 and human airway epithelial cell line were grown at 50%–60% confluence on 25-mm diameter circular glass coverslips. Cells were loaded for 60 min in room temperature (23°C) with 4 μM fura-2 acetoxymethyl ester (fura-2/AM; Invitrogen/Molecular Probes, Eugene, OR) in HEPES-buffered solution. Coverslips with cells were placed into the recording chamber of an inverted fluorescent microscope (Eclipse Ti-E; Nikon, Tokyo, Japan) equipped with an objective lens (S Plan Fluor ×20/0.45 ELWD; Nikon), an EM-CC camera (Evolve; Photometrics, Tucson, AZ) and the NIS Elements 3.2 software (Nikon), and superfused with HEPES-buffered solution for 30 min to wash out residual extracellular fura-2/AM and allow sufficient time for intracellular esterase to convert fura-2/AM to fura-2. With excitation at 340 and 380 nm, the emitted fluorescence was acquired at 520 nm by a fluorescent objective lens and an EM-CCD camera using NIS Elements 3.2 software. Intracellular Ca2+ concentration was expressed as 340/380 fluorescence ratio within an area of interest in a cell recorded every 2 s. The HEPES-buffered solution contained (in mM) 137 NaCl, 5.9 KCl, 1.8 CaCl2, 1.2 MgCl2, 14 glucose, and 10 HEPES (pH was adjusted to 7.4 with 10 N NaOH). The Ca2+-free solution was prepared by replacing 1.8 mM CaCl2 with equimolar MgCl2 and adding 1 mM EGTA to chelate residual Ca2+. Measurement of [Ca2+]cyt was carried out at room temperature (23°C). For each experimental replicate we quantitated the fluorescence ratios for at least 20 cells.
Western blotting.
Total protein was isolated from human non-small cell lung cancer cell lines A549 and H23 and human airway epithelial cell lines that were lysed in 1× RIPA buffer (Bio-Rad, Hercules, CA) at 4°C. Protein was loaded on a 15% acrylamide gel, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA), and immunoblotted with anti-Bcl-2 and (SAB4500003; 1:1,000; Sigma-Aldrich) and anti-Bax monoclonal antibody (B3428; 1:4,000; Sigma-Aldrich). Signals were detected using a Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA). The protein levels were normalized to β-actin (sc-9104, 1:1,000; Santa Cruz Biotechnology) and are expressed in arbitrary units.
RT-PCR and real-time RT-PCR.
Total RNA was isolated from human non-small cell lung cancer cell line A549 and H23 and human airway epithelial cell line by the TRIzol method. The extracted RNA was quantified by spectrophotometry at 260 nm. The synthesis and polymerase chain reaction were carried out using a Platinum PCR SuperMix (Invitrogen, Grand Island, NY) with 3-step cycling. The relative expression levels of P2X and P2Y receptors were normalized against the amount of GAPDH mRNA in the same RNA extract. Quantitative real-time RT-PCR was performed on a Bio-Rad iQ5 real-time PCR detection system (Bio-Rad) using an iTaq Universal SYBR Green Supermix (Bio-Rad) following the instructions of the manufacturer. All samples were run in triplicate, and GAPDH was used as an internal control. The expression level of P2YR mRNA or P2XR mRNA was normalized to GAPDH and is expressed as a ratio relative to GAPDH.
Cell viability assay.
Viability of human non-small cell lung cancer cell line A549 and H23 and normal human airway epithelial cell line after treatments with DMEM with 10% FBS, 100 μM ATP in DMEM with 10% FBS, and 1 mM ATP DMEM with 10% FBS was determined by the Trypan blue dye exclusion assay. Control and treated cells were incubated with Trypan blue solution for 3–5 min and then counted on Countess (Invitrogen, Grand Island, NY). Cell numbers per milliliter cell suspension were determined in each preparation, and the percentage of cells that excluded Trypan blue stain is indicated as a measure of cell viability, which is expressed in percentage (%).
Statistical analysis.
Pooled data are shown as means ± SE. The statistical significance between two groups was determined by Student's t-test. The statistical significance among groups was determined by Scheffé's test after one-way analysis of variance. Significant difference is expressed in the figures or figure legends as P < 0.05.
RESULTS
Extracellular ATP induces a prolonged [Ca2+]cyt response in lung cancer cells but not in normal lung epithelial cells.
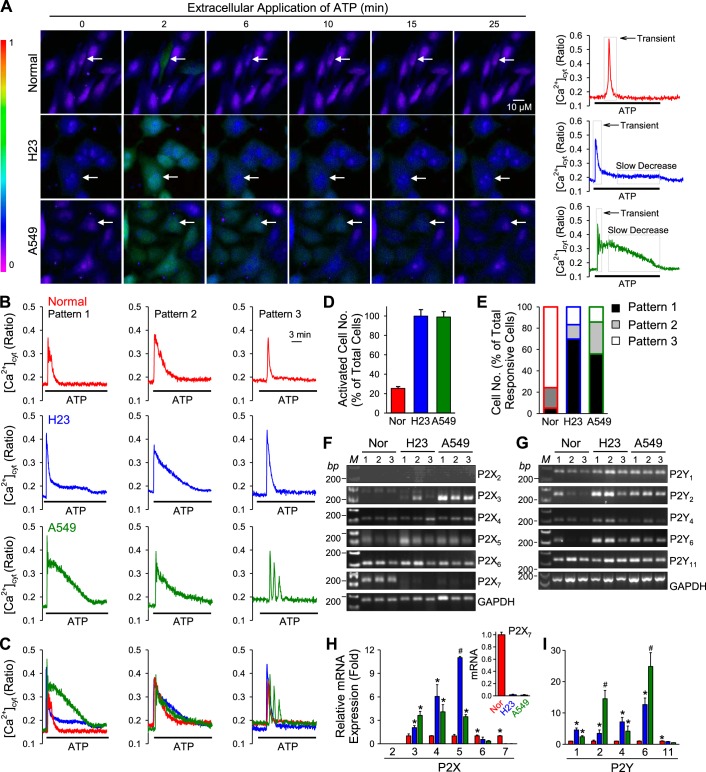
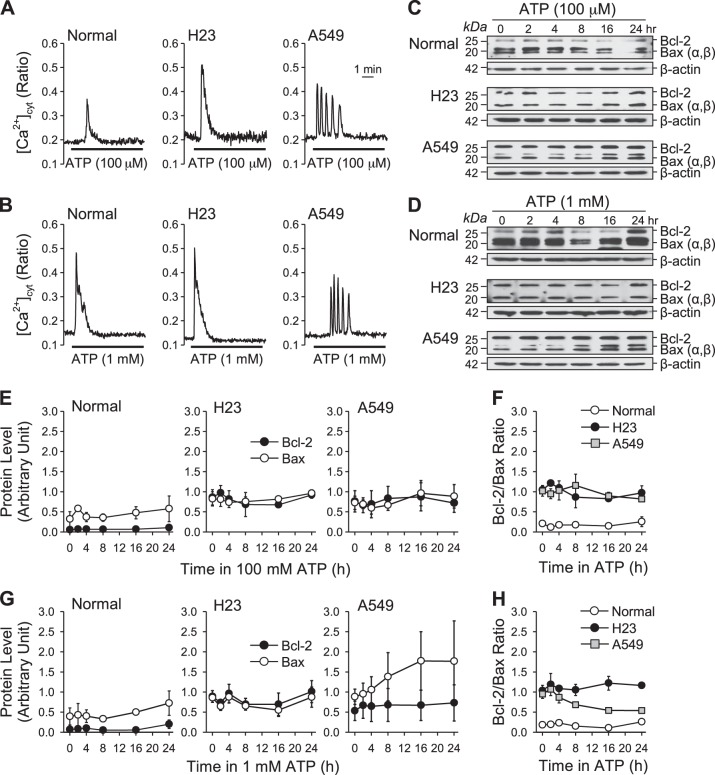
Extracellular ATP increases [Ca2+]cyt through activation of different P2 receptors. To determine whether extracellular application of ATP induces different patterns of intracellular Ca2+ signaling in normal and lung cancer cells, we treated cells with 100 μM of ATP and measured [Ca2+]cyt using the Ca2+ indicator fura-2. We compared a normal lung airway epithelial cell line (BEAS-2B) to two lung cancer cell lines (H23 and A549) that reflect the heterogeneity of human lung cancer (see Table 1 for details). Two different patterns ATP-induced increases in [Ca2+]cyt were observed in normal and lung cancer cells: a transient increase or slowly declining increase (Fig. 1, A–C). ATP induced a transient increase in [Ca2+]cyt in normal cells with a mean duration of 120 s (Fig. 1, A–C). In lung cancer cells, ATP induced the same rapid increase in [Ca2+]cyt; however, the [Ca2+]cyt rise that followed transient increase was sustained for a longer period of time (a plateau increase) in H23 (mean duration of 660 s) and A549 cells (mean duration of 700 s). While 95% and 99% of H23 and A549 lung cancer cells responded to ATP, only 30% of normal cells responded (Fig. 1D). Of the responding cells, we observed three patterns: 1) Pattern 1, a transient increase followed by a plateau increase; 2) Pattern 2, a transient increase followed by a slowly declining increase; and 3) Pattern 3, only a transient increase (Fig. 1B). Pattern 3 represents the highest proportion of normal responding cells (78%) (Fig. 1E). In contrast, Pattern 1 represents the highest proportion of responding lung cancer cells (H23, 70%; A549, 55%) (Fig. 1E). The [Ca2+]cyt increase during the plateau phase was higher in A549 cells [mean of 0.06 (ratio 340/380)] compared with H23 cells [mean of 0.18 (ratio of 340/380)]. The results demonstrate that lung cancer cells respond to extracellular ATP with a prolonged increase in [Ca2+]cyt. Since the ATP-induced increase in [Ca2+]cyt was mainly caused by activation of different P2 receptors, the ATP-induced plateau phase of increase in [Ca2+]cyt in lung cancer cells (but not in normal cells) suggests that ATP receptors (P2X or P2Y receptors) may be expressed differently in lung cancer cells versus normal cells.
Table 1.
Characteristics of the human lung cancer cell lines
| Cell Line | Cell Type | Disease | myc* Expression | src* Expression | ras* Expression | Keratin† Expression |
|---|---|---|---|---|---|---|
| Beas-2b | Epithelial | Normal | − | − | − | − |
| H23 (18, 45, 47) | Epithelial | Adenocarcinoma; non-small cell lung cancer | + | + | + | − |
| A549 | Epithelial | Carcinoma | − | − | + (47) | + (17, 32) |
Gene.
Protein. References in parentheses.
Fig. 1.
ATP-induced increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) and P2X and P2Y purinergic receptor expression in normal airway epithelial cell line and lung cancer cell lines. A: representative images showing different patterns of [Ca2+]cyt changes in normal (top) and lung cancer cells (middle and bottom) before, during, and after application of 100 μM ATP. B: representative traces showing different patterns of [Ca2+]cyt changes in normal (top) and lung cancer cells (middle and bottom) before, during, and after application of 100 μM ATP. C: representative traces from B were overlaid to show the differences of [Ca2+]cyt changes in normal and lung cancer cells in each pattern. D: summarized data showing the proportion of activated cells in each of normal and lung cancer cells (n = 4–14). E: summarized data showing the proportion of each pattern of changes of [Ca2+]cyt induced by ATP in normal and lung cancer cells (n = 4–14 separated experiments). F: RT-PCR data showing the expression level of P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 purinergic receptors in normal and lung cancer cells (n = 5). G: RT-PCR data showing the expression level of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 purinergic receptors in normal and lung cancer cells (n = 4). H: summarized data (means ± SE) from real-time RT-PCR showing the expression level of P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 purinergic receptors in normal (Nor) and lung cancer cells (n = 3, *P < 0.05 vs. Normal; #P < 0.05 vs. Normal and A549, Normal and H23). I: summarized data (means ± SE) from real-time PCR showing the expression level of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 purinergic receptors in normal and lung cancer cells (n = 3, *P < 0.05 vs. Normal; #P < 0.05 vs. Normal and A549, Normal and H23).
In the next set of experiments, we performed RT-PCR and real-time RT-PCR to examine and compare the relative expression levels of P2X receptors (P2X2, P2X3, P2X4, P2X5, P2X6, P2X7) and P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6 P2Y11) in normal and lung cancer cells. We observed a statistically significant (P < 0.05) increase in expression of several of the P2X receptors and the P2Y receptors in lung cancer cells compared with normal cells (P2X3: H23 = 2.05-fold, A549 = 3.02-fold; P2X4: H23 = 4.05-fold, A549 = 4.08-fold; P2X5; H23 = 11.00-fold, A549 = 3.45-fold. P2Y1: H23 = 4.55-fold, A549 = 2.37-fold; P2Y2: H23 = 3.44-fold, A549 = 14.53-fold; P2Y4: H23 = 7.12-fold, A549 = 4.18-fold; P2Y6: H23 = 12.64-fold, A549 = 24.84-fold) (Fig. 1, F–I). Our findings suggest that the prolonged increase in [Ca2+]cyt induced by ATP in lung cancer cells may be due to upregulated expression of P2 receptors.
Lung cancer cells are more sensitive to extracellular ATP than normal cells.
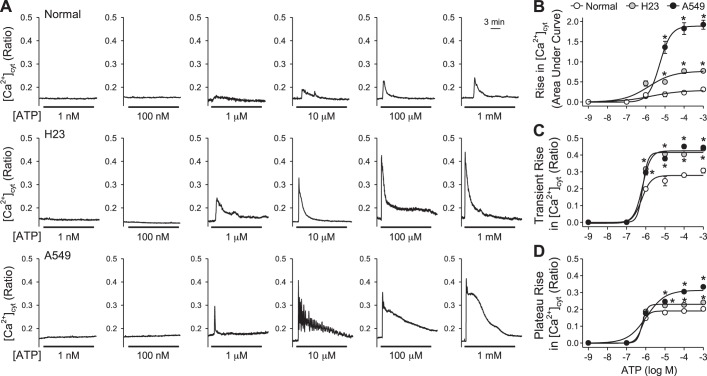
To determine whether the pattern of ATP-induced increase in [Ca2+]cyt is concentration-dependent, we treated normal (BEAS-2B) and lung cancer cells (H23 and A549) with different concentrations of ATP. In lung cancer cells, ATP induced an increase in [Ca2+]cyt starting at 1 μM; however, ATP induced an increase in [Ca2+]cyt that was detectable at a higher concentration of 10 μM in normal cells (Fig. 2A). Both H23 and A549 cells that respond to ATP have a plateau phase of increase in [Ca2+]cyt when treated with higher concentrations of ATP (100 μM). While H23 cells also respond to lower concentrations of ATP (1 μM and 10 μM) with a plateau phase of increase in [Ca2+]cyt, A549 cells respond to the lower concentrations of ATP with Ca2+ oscillations. To determine the total amount of increase in [Ca2+]cyt over time, we calculated the area under the curve (AUC). Lung cancer cells exhibited a dose-dependent increase in the AUC starting at 10 μM compared with normal cells (P < 0.05) (Fig. 2B). We observed that the transient phase of increase (induced by ATP starting at 1 μM) and plateau phase of increase (induced by ATP starting at 10 μM) in [Ca2+]cyt were significantly higher in H23 and A549 cells than in normal cells (Fig. 2, C and D). Furthermore, we observed that in all cell types the amplitude of the transient increase (Fig. 2C) and the amplitude of the plateau increase (Fig. 2D) maximized at the ATP concentration of 10−6 to 10−5 M. Further increasing ATP concentration from 10−5 M to 10−3 M did not further increase the amplitude of both transient and plateau increases in [Ca2+]cyt (Fig. 2, C and D). These observations suggest that lung cancer cells are more sensitive to extracellular ATP and that the downstream intracellular Ca2+ signaling may be of higher intensity with a more prolonged duration in lung cancer cells than in normal cells.
Fig. 2.
Dose-response curves of area under the curve, transient, and plateau amplitude of increase in [Ca2+]cyt induced by ATP in normal and lung cancer cells. A: representative traces showing changes in [Ca2+]cyt before, during, and after application of 1 nM, 100 nm, 1 μM, 10 μM, 100 μM, and 1 mM ATP in normal (top) and lung cancer cells (middle and bottom). B: summarized data (means ± SE) showing the dose-response curve of area under the curve of increase in [Ca2+]cyt induced by ATP in normal and lung cancer cells (n = 3, *P < 0.05 vs. Normal). C: summarized data (means ± SE) showing dose-response curve of transient amplitude of increase in [Ca2+]cyt induced by ATP in normal and lung cancer cells (n = 3, *P < 0.05 vs. Normal). D: summarized data (means ± SE) showing dose-response curve of plateau amplitude of increase in [Ca2+]cyt induced by ATP in normal and lung cancer cells (n = 3, *P < 0.05 vs. Normal).
Ca2+ influx is required for ATP-induced plateau phase of increase of [Ca2+]cyt in lung cancer cells.
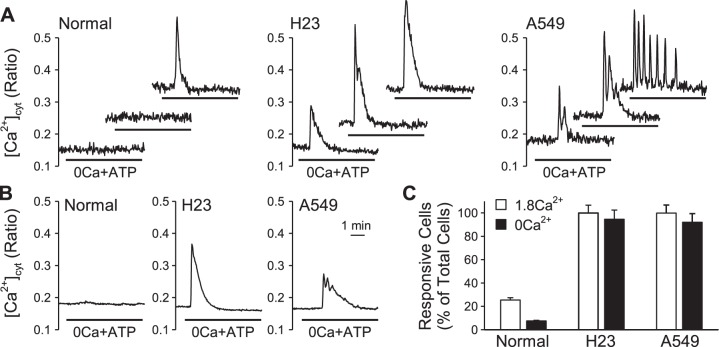
It is known that ATP can increase [Ca2+]cyt by inducing Ca2+ release from intracellular Ca2+ stores and/or Ca2+ influx from extracellular source via the GPCR P2Y receptors. To determine whether the observed ATP-induced plateau phase of increase of [Ca2+]cyt is dependent on extracellular Ca2+, we treated cells with ATP in the absence of extracellular Ca2+. In the absence of extracellular Ca2+, only 5% of the normal cells (BEAS-2B) responded to ATP with a transient increase in [Ca2+]cyt (Fig. 3, A–C). In H23 cells, ATP induced a transient increase in [Ca2+]cyt in 95% of the cells; however, the plateau phase increase of [Ca2+]cyt was no longer observed (Fig. 3, A–C). In A549 cells, ATP induced either transient increases in [Ca2+]cyt or Ca2+ oscillations in 97% of the cells and, similar to H23 cells, the plateau phase of increase in [Ca2+]cyt was no longer observed (Fig. 3, A–C). Extracellular ATP induced transient increases in [Ca2+]cyt in both H23 and A549 cells superfused either with Ca2+-containing (99% of H23 and A549 cells) solution or with Ca2+-free solution (95% of H23 and A549 cells) (Fig. 3C). These data suggest that normal cells require extracellular Ca2+ for ATP to induce a transient increase in [Ca2+]cyt. In contrast, cancer cells do not require extracellular Ca2+ for ATP to induce a transient increase in [Ca2+]cyt. Our results also suggest that Ca2+ influx from extracellular source is responsible for the ATP-induced plateau phase of increase in [Ca2+]cyt observed in lung cancer cells.
Fig. 3.
ATP-induced changes in [Ca2+]cyt in the absence of extracellular Ca2+ in normal and lung cancer cells. A: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 100 μM ATP when depriving extracellular Ca2+ in individual cells. B: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 100 μM ATP when depriving extracellular Ca2+ in average. C: summarized data showing the proportion of activated cells in 1.8 mM and 0 mM extracellular Ca2+ (OCa2+) in normal and lung cancer cells, respectively (n = 3–14).
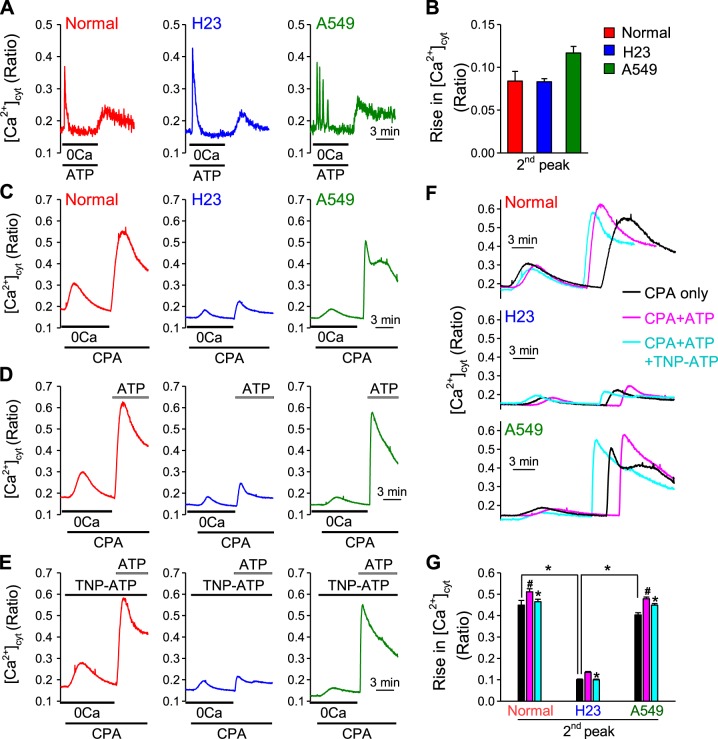
Next, we wanted to investigate the potential mechanism that mediates Ca2+ influx in lung cancer cells (H23 and A549). Upon activation of P2Y receptors, ATP may mobilize Ca2+ from intracellular Ca2+ stores by inositol trisphosphate (IP3) and activation of the IP3 receptor (a Ca2+ release channel) on the sarcoplasmic (SR) or endoplasmic (ER) reticulum membrane. To determine whether depletion or partial depletion of intracellular Ca2+ stores (SR) by ATP could induce store-operated Ca2+ entry (SOCE), we first treated cells with ATP in Ca2+-free solution and observed a transient increase in [Ca2+]cyt (due apparently to Ca2+ leak from the intracellular stores) in both normal and lung cancer cells. We then superfused the cells with 1.8 mM Ca2+-containing solution and observed that restoration of extracellular Ca2+ induced a second increase in [Ca2+]cyt which was due apparently to Ca2+ influx through store-operated Ca2+ channels (Fig. 4A). In both normal and lung cancer cells, the second increases in [Ca2+]cyt induced by ATP-mediated SOCE showed no statistically significant differences [0.08 ± 0.010 (340/380 ratio) for normal cells; 0.08 ± 0.003 for H23 cells; and 0.11 ± 0.01 for A549 cells] (Fig. 4B) (19, 44). Next, we treated cells with cyclopiazonic acid (CPA), a reversible inhibitor of the SR/ER Ca2+-pump (SERCA), passively depletes Ca2+ from the SR and induces SOCE. We observed that the second increase in [Ca2+]cyt due to extracellular Ca2+ restoration-mediated SOCE was significantly lower in H23 cells (0.10 ± 0.004) than in normal cells (0.45 ± 0.022, P < 0.05); however, we did not observe a significant difference in A549 cells (0.40 ± 0.010) compared with normal cells (Fig. 4, C and G). When ATP was then introduced to the cells in the presence of extracellular Ca2+, we observed that the second increase in [Ca2+]cyt was significantly higher than that in the presence of extracellular Ca2+ without ATP in both normal and lung cancer cells (0.51 ± 0.010 for normal cells; 0.13 ± 0.003 for H23 cells; 0.47 ± 0.008 for A549 cells; P < 0.05 vs. the value in the absence of ATP) (Fig. 4, D, F, and G).
Fig. 4.
ATP-induced store-operated Ca2+ entry (SOCE) and extracellular Ca2+ influx in normal and lung cancer cells. A: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells treated with 100 μM ATP in the absence of extracellular Ca2+ and subsequent [Ca2+]cyt changes during and after application of 1.8 mM extracellular Ca2+. B: summarized data (means ± SE) showing the amplitude of increase in [Ca2+]cyt induced by 1.8 mM extracellular Ca2+ after application of 100 μM ATP in the absence of extracellular Ca2+ (n = 5, *P < 0.05 vs. Normal). C: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 10 μM cyclopiazonic acid (CPA, an inhibitor of SERCA) and subsequent [Ca2+]cyt changes during and after application of 1.8 mM extracellular Ca2+. D: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 10 μM CPA and subsequent [Ca2+]cyt changes during and after application of 100 μM ATP in 1.8 mM extracellular Ca2+. E: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 10 μM CPA and subsequent [Ca2+]cyt changes during and after application of 100 μM ATP in 1.8 mM extracellular Ca2+ during treatment with 1 μM TNP-ATP (P2XR blocker). F: overlaid curves of C, D, and E. G: summarized data (means ± SE) showing the amplitude of second peak of [Ca2+]cyt increases in normal and lung cancer cells induced by 1.8 mM extracellular Ca2+ or 1.8 mM extracellular Ca2+ plus 100 μM ATP after the application of 10 μM CPA, and amplitude of second peak of [Ca2+]cyt increases in normal and lung cancer cells induced by 1.8 mM extracellular Ca2+ plus 100 μM ATP after the application of 10 μM CPA during TNP-ATP treatment (n = 3–10, *P < 0.05 vs. Control, #P < 0.05 vs. Normal).
To determine whether P2X receptors are involved, we pretreated cells with TNP-ATP (P2X receptor inhibitor). TNP-ATP treatment significantly attenuated the amplitude of the second increase in [Ca2+]cyt induced by extracellular Ca2+ restoration with ATP in both normal and lung cancer cells (0.46 ± 0.012 for normal cells; 0.01 ± 0.003 for H23 cells; 0.44 ± 0.0047 for A549 cells; P < 0.05 vs. the value in the absence of TNP-ATP) (Fig. 4, E, F, and G). Taken together, these data suggest that ATP-induced Ca2+ influx is at least partially mediated through store-operated Ca2+ channels (SOCC) and P2X receptors.
P2X and P2Y receptors are both required for ATP-induced increases in [Ca2+]cyt in lung cancer cells.
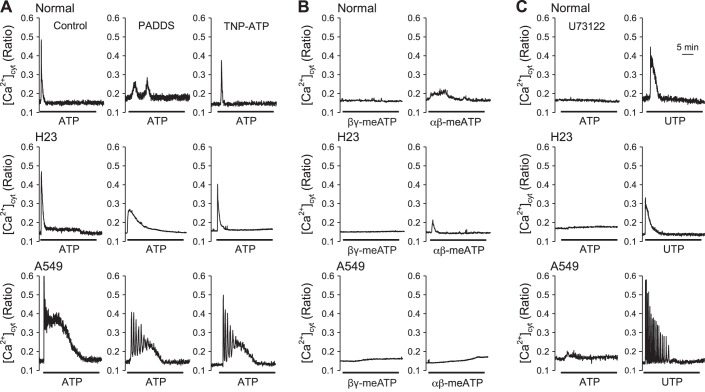
Activation of P2X and P2Y receptors is known to mediate changes in intracellular Ca2+ through different mechanisms. For example, activation of P2X receptors, nonselective cation channels, directly mediate Ca2+ influx through the receptors, while activation of P2Y receptors, GPCRs, mediates Ca2+ influx through receptor-operated and store-operated Ca2+ channels and/or Ca2+ mobilization from the SR/ER as a result of G protein-mediated increases in diacylglycerol (DAG) and IP3. To determine whether P2X receptors are responsible for the ATP-induced plateau phase of [Ca2+]cyt increase in lung cancer cells (H23 and A549), we treated cells with the P2X receptor inhibitors PPADS and TNP-ATP (14, 16). We observed that both PPADS and TNP-ATP decreased the ATP-induced transient phase of [Ca2+]cyt increase in normal cells and the ATP-induced transient and plateau phases of [Ca2+]cyt increases in lung cancer cells (Fig. 5A). To determine whether P2X receptor activation can directly increase [Ca2+]cyt, by Ca2+ influx through the receptors, we treated cells with P2X receptor activators β,γ-meATP and α,β-meATP (11, 23). We observed that α,β-meATP induced a small increase in [Ca2+]cyt in both normal and lung cancer cells, while β,γ-meATP only induced a small increase in [Ca2+]cyt in A549 cells but not in normal cells and H23 cells (Fig. 5B).
Fig. 5.
Effect of P2X and P2Y inhibitors on ATP-induced changes of [Ca2+]cyt and intracellular Ca2+ changes induced by P2X and P2Y activators. A: representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM ATP in normal and lung cancer cells (n = 4–14) (left); representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM ATP in the present of 10 μM PPADS in normal and lung cancer cells (n = 4–5) (middle); representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM ATP in the present of 100 nM TNP-ATP in normal and lung cancer cells (n = 3) (right). B: representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM β,γ-meATP in normal and lung cancer cells (n = 3) (left); representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM α,β-meATP in normal and lung cancer cells (n = 3) (right). C: representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM ATP in the presence of 10 μM U-73122 in normal and lung cancer cells (n = 3) (left); representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM UTP (n = 3) (right).
It is known that P2Y receptors are GPCRs that can activate phospholipase C (PLC) to increase [Ca2+]cyt via increasing IP3 and DAG. U-73122, a PLC inhibitor, attenuated ATP-induced transient phase of [Ca2+]cyt increase in both normal and lung cancer cells. Only a small increase in [Ca2+]cyt was induced by ATP in H23 and A549 cells, but not in normal cells, after U-73122 treatment (Fig. 5C) (42).
We next examined whether the P2Y receptor activator UTP can increase [Ca2+]cyt (35). We observed that only transient or oscillatory increases of [Ca2+]cyt (without the plateau phase) were observed in normal and lung cancer cells in response to UTP (Fig. 5C). These observations suggest that both P2X and P2Y receptors are involved in the ATP-induced increase in [Ca2+]cyt in normal and lung cancer cells, of which P2X receptors are involved in Ca2+ influx from extracellular source and P2Y receptors are involved in Ca2+ release from intracellular Ca2+ stores. The specific subtypes of P2X receptors or P2Y receptors involved cannot be discriminated due to the nonspecificity of the inhibitors and activators. Future studies are needed to identify the specific subunits of P2X and P2Y receptors involved in ATP-mediated increases in [Ca2+]cyt.
Lung cancer cells have a decreased cytotoxic response to extracellular ATP compared with normal cells.
RT-PCR data have previously shown that the expression level of P2X7 mRNA was very low in lung cancer cells (H23 and A549) compared with normal cells (BEAS-2B) (20, 21, 39). The P2X7 receptor is known to be involved in non-Bcl-2/Bax-mediated cell apoptosis (29, 44). The loss of P2X7 in lung cancer cells is surprising, given that numerous studies have reported that ATP can exert a cytotoxic effect on several tumor cell types (6, 12, 33, 43). Therefore, we investigated whether extracellular ATP has a differential effect on cell growth processes (including cell apoptosis and cell proliferation) in normal cells versus lung cancer cells.
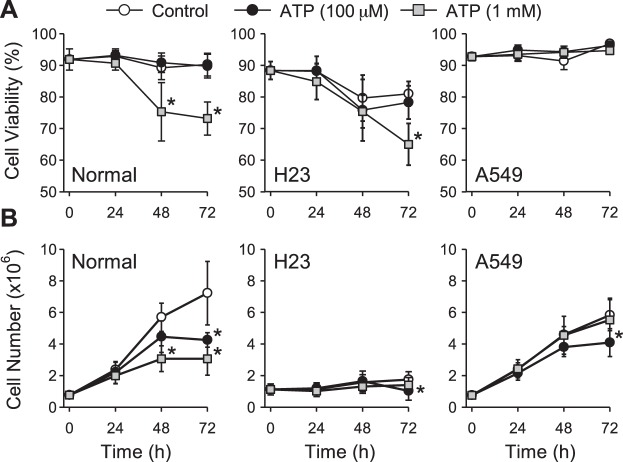
There was no effect of 100 μM ATP on cell viability in both normal and lung cancer cells in comparison to their control during ATP stimulation at 24, 48, and 72 h, respectively (Fig. 6A). However, a decrease in cell viability was observed when normal cells were treated with 1 mM ATP for 48 h (75.33% ± 9.23 vs. control of 89.25% ± 3.74, P < 0.05) and 72 h (73.16% ± 5.23 vs. control of 90.23% ± 3.61, P < 0.05). Decreased cell viability was also observed in H23 lung cancer cells at 72 h (65.00% ± 6.61 vs. control of 81.00% ± 3.88, P < 0.05) with 1 mM ATP, while no change of cell viability was observed in A549 lung cancer cells (Fig. 6A).
Fig. 6.
Effect of ATP on cell viability and cell proliferation in normal and lung cancer cells. A: summarized data (means ± SE) showing cell viability of normal (left) and lung cancer cells (middle and right) treated with ATP (0 μM, 100 μM, 1 mM; n = 4 separated experiments, *P < 0.05 vs. Control). B: summarized data (means ± SE) showing cell proliferation of normal (left) and lung cancer cells (middle and right) treated with ATP (0 μM, 100 μM or 1 mM; n = 4 separated experiments, *P < 0.05 vs. Control).
We next examined the ability of ATP to affect cell proliferation. Cell proliferation was inhibited in normal cells treated with 100 μM ATP for at 72 h (4.25 × 106 ± 0.45 vs. control of 7.22 × 106 ± 2.00, P < 0.05) and with 1 mM ATP for 48 h (3.06 × 106 ± 0.81 vs. control of 5.69 × 106 ± 0.89, P < 0.05) and 72 h (3.06 × 106 ± 1.03 vs. control of 7.22 × 106 ± 2.00, P < 0.05) (Fig. 6B). There was also a small decrease in cell proliferation in lung cancer cells at 72 h when cells were treated with 100 μM ATP (1.03 × 106 ± 0.59 vs. control of 1.74 × 106 ± 0.50 for H23 cells; 4.08 × 106 ± 0.88 vs. control of 5.82 × 106 ± 0.97 for A549 cells, P < 0.05). However, no change in cell proliferation was observed in lung cancer cells when treated with 1 mM ATP (Fig. 6B).
These data indicate that relatively low concentrations of ATP (micromole) negatively modulate cell proliferation but not cell viability in lung cancer cells. Higher concentrations of ATP (in the millimolar range), however, do not affect cell proliferation in lung cancer cells, but induce decreased cell viability in H23 cells. In normal cells, in which P2X7 receptors are expressed at a higher level, we observed consistent effects of ATP on cell viability and cell proliferation. Future studies are needed to determine whether the absence of P2X7 uncouples cell viability and proliferation in lung cancer cells.
ATP increases the Bcl-2/Bax ratio in lung cancer cells.
Since the lung cancer cells (H23 and A549) express low levels of the P2X7 receptor, we considered whether ATP could modulate cell apoptosis by other means. Antiapoptotic Bcl-2 is well known for its classic role in blocking cytochrome c release from mitochondria and thereby inhibiting apoptosis; its expression is upregulated in many tumor cells (9, 10). Bax, a member of the Bcl-2 family of proteins, has a proapoptotic role that opposes Bcl-2; as such, high Bcl-2/Bax ratio represents an antiapoptotic cell state. Therefore we examined the expression levels of both Bcl-2 and Bax proteins to determine whether extracellular ATP affects Bcl-2/Bax ratio in normal and lung cancer cells.
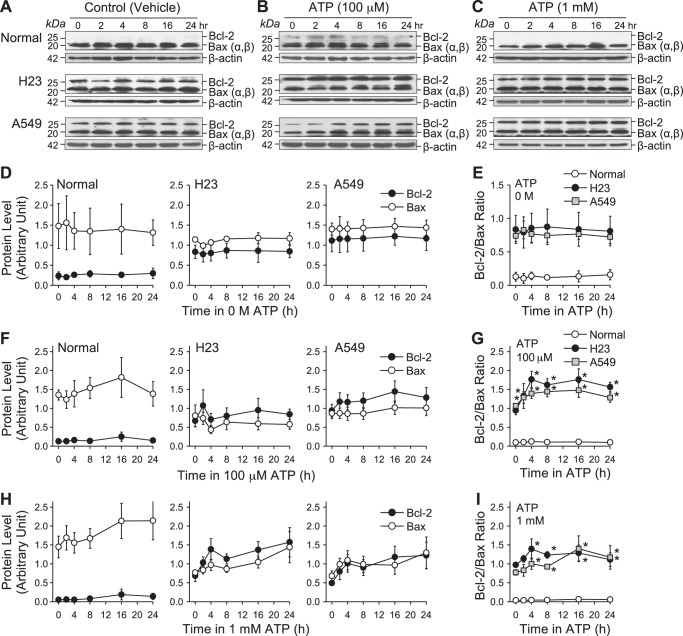
The cells were first treated with vehicle (water) for 0, 2, 4, 8, 16, and 24 h; Bcl-2 and Bax protein expression levels, as well as Bcl-2/Bax ratio, showed no change in both normal and lung cancer cells (Fig. 7, A, D, and E). We observed that Bcl-2 protein expression level was higher in basal conditions (0 h) in lung cancer cells than in normal cells, while the Bax protein expression level showed no difference between normal and lung cancer cells (Fig. 7, A and D). We next treated cells with ATP (100 μM and 1 mM) for 0, 2, 4, 8, 16, 24 h. In normal cells, both Bcl-2 and Bax protein expression levels, as well as Bcl-2/Bax ratio, were not changed following treatment with ATP (100 μM and 1 mM) (Fig. 7, B, C, F, G, H, and I). In lung cancer cells (H23 and A549), Bcl-2 protein expression was upregulated following treatment with ATP (100 μM and 1 mM) from 2 h to 24 h, while Bax protein level was negligibly changed or slightly (but not significantly) increased (Fig. 7, B, C, F, and H). The Bcl-2/Bax ratio was significantly increased following treatment of lung cancer cells (H23 and A549) with ATP (100 μM and 1 mM) (Fig. 7, G and I). We observed that Bcl-2, rather than Bax, was more sensitive to ATP in lung cancer cells H23 and A549. In normal cells, because of the low expression level of Bcl-2 protein, the Bcl-2/Bax ratio was much lower and less affected by ATP than in lung cancer cells. However, consistent with the decreased viability observed in normal cells, Bax protein expression was upregulated in these cells following treatment with ATP. These data suggest that an ATP-induced increase of the Bcl-2/Bax ratio in lung cancer cells may be the mechanism by which lung cancer cells escape from ATP-induced cytotoxic effects.
Fig. 7.
Effect of ATP on Bcl-2 and Bax expression and Bcl-2/Bax ratio in normal and lung cancer cells. A: Western blot analysis of Bcl-2 and Bax expression in normal (top) and lung cancer cells (middle and bottom) treated with vehicle (water) for 0, 2, 4, 8, 16, and 24 h. β-Actin was used as a control. B: Western blot analysis of Bcl-2 and Bax expression in normal (top) and lung cancer cells (middle and bottom) treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h. β-Actin was used as a control. C: Western blot analysis of Bcl-2 and Bax expression in normal (top) and lung cancer cells (middle and bottom) treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h. β-Actin is used as a control. D: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with vehicle for 0, 2, 4, 8, 16, and 24 h. E: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio level in normal and lung cancer cells treated with vehicle for 0, 2, 4, 8, 16, and 24 h (n = 3). F: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h. G: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio level in normal and lung cancer cells treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h (n = 8, *P < 0.05 vs. Normal). H: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h. I: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio level in normal and lung cancer cells treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h (n = 7, *P < 0.05 vs. Normal).
Intracellular Ca2+ is required for extracellular ATP to increase Bcl-2/Bax ratio in lung cancer cells.
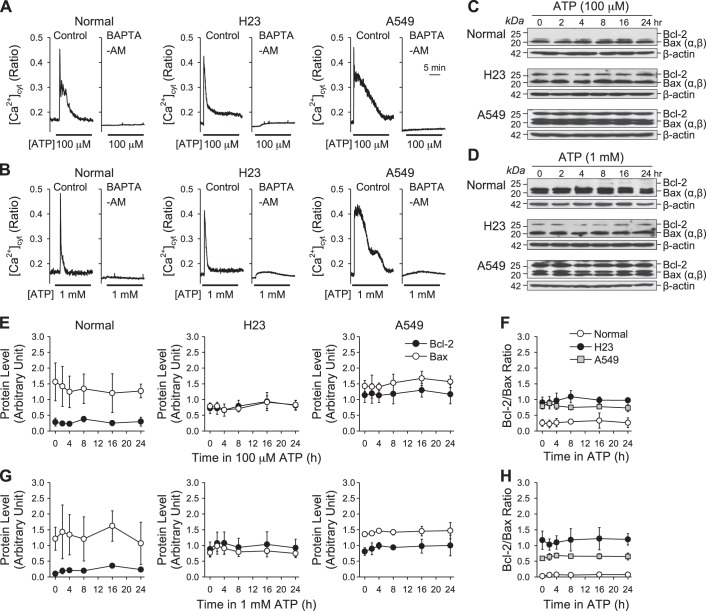
To understand the potential mechanism by which ATP increases Bcl-2/Bax ratio, we focused on ATP-induced [Ca2+]cyt increase, which is one of the major downstream messengers of P2 receptor (P2X and P2Y) activation. Cells were treated with BAPTA-AM, a membrane-permeable Ca2+ chelator, for 30 min before treatment with ATP (100 μM and 1 mM) to determine whether intracellular Ca2+ is required for regulation of Bcl-2 and Bax protein expression (37). BAPTA-AM pretreatment blocked the ATP-induced increase of [Ca2+]cyt in both normal and lung cancer cells (H23 and A549) (Fig. 8, A and B). We also treated cells with ATP (100 μM and 1 mM) for 0, 2, 4, 8, 16, 24 h, respectively, after BAPTA-AM treatment to determine whether intracellular Ca2+ is required for ATP-induced increase in Bcl-2/Bax ratio. In both normal and lung cancer cells, chelation of intracellular Ca2+ with BAPTA-AM precluded the ATP-induced changes in Bcl-2 and Bax protein expression and Bcl-2/Bax ratio (Fig. 8, C–H). These data suggest that an increase of intracellular Ca2+ is required for extracellular ATP to increase Bcl-2/Bax ratio in lung cancer cells.
Fig. 8.
Effect of ATP on Bcl-2 and Bax expression and Bcl-2/Bax ratio in normal and lung cancer cells after application of BAPTA-AM. A: representative traces showing changes in [Ca2+]cyt before, during, and after application of 100 μM ATP after treatment with 25 μM BAPTA-AM in normal and lung cancer cells. B: representative traces showing changes in [Ca2+]cyt before, during, and after application of 1 mM ATP after treatment with 25 μM BAPTA-AM in normal and lung cancer cells. C: Western blot analysis of Bcl-2 and Bax expression in normal and lung cancer cells treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h after application of 25 μM BAPTA-AM. β-Actin was used as a control. D: Western blot analysis of Bcl-2 and Bax expression in normal and lung cancer cells treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h after application of 25 μM BAPTA-AM. β-Actin was used as a control. E: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h after application of 25 μM BAPTA-AM. F: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio in normal and lung cancer cells treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h after application of 25 μM BAPTA-AM (n = 5). G: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h after application of 25 μM BAPTA-AM. H: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio in normal and lung cancer cells treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h after application of 25 μM BAPTA-AM (n = 5).
Ca2+ influx is required for ATP-induced increase of Bcl-2/Bax ratio in lung cancer cells.
Because ATP induces two phases of increase in [Ca2+]cyt in lung cancer cells (H23 and A549), the transient phase (due to Ca2+ release) and the plateau phase (due to Ca2+ influx), we investigated which phase is responsible for the ATP-induced increase of Bcl-2/Bax ratio. To determine the role of Ca2+ influx, we decreased the extracellular Ca2+ concentration in the media to 80 nM to inhibit Ca2+ influx while avoiding cell death. Under these conditions, we observed that ATP (100 μM and 1 mM) induced only transient or oscillatory increases in [Ca2+]cyt without a plateau phase in both normal and lung cancer cells (Fig. 9, A and B). We also treated cells with ATP (100 μM and 1 mM) for 0, 2, 4, 8, 16, 24 h in 80 nM Ca2+ media and then measured expression levels of Bcl-2 and Bax proteins. In both normal and lung cancer cells, Bcl-2 and Bax protein expression levels, as well as Bcl-2/Bax ratio, showed no change during ATP treatment (100 μM and 1 mM) in the extracellular media containing low (80 nM) Ca2+ concentration (Fig. 9, C–H). These data suggest that Ca2+ influx and the resulting plateau phase of increase, or sustained increase, in [Ca2+]cyt are required for the enhancement of Bcl-2/Bax ratio in lung cancer cells.
Fig. 9.
Effect of ATP on Bcl-2 and Bax expression and Bcl-2/Bax ratio in normal and lung cancer cells in low extracellular Ca2+ culture conditions. A: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 100 μM ATP in media containing 80 nM extracellular Ca2+. B: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 1 mM ATP in media containing 80 nM extracellular Ca2+. C: Western blot analysis of Bcl-2 and Bax expression in normal and lung cancer cells treated with 100 μM ATP in media containing 80 nM extracellular Ca2+ for 0, 2, 4, 8, 16, and 24 h. β-Actin was used as a control. D: Western blot analysis of Bcl-2 and Bax expression in normal and lung cancer cells treated with 1 mM ATP in media containing 80 nM extracellular Ca2+ for 0, 2, 4, 8, 16, and 24 h. β-Actin was used as a control. E: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with 100 μM ATP in media containing 80 nM extracellular Ca2+ for 0, 2, 4, 8, 16, and 24 h. F: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio in normal and lung cancer treated with 100 μM ATP in media containing 80 nM extracellular Ca2+ for 0, 2, 4, 8, 16, and 24 h (n = 3). G: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer cells treated with 1 mM ATP in media containing 80 nM extracellular Ca2+ for 0, 2, 4, 8, 16, and 24 h. H: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio in normal and lung cancer cells treated with 1 mM ATP in media containing 80 nM extracellular Ca2+ for 0, 2, 4, 8, 16, and 24 h (n = 3).
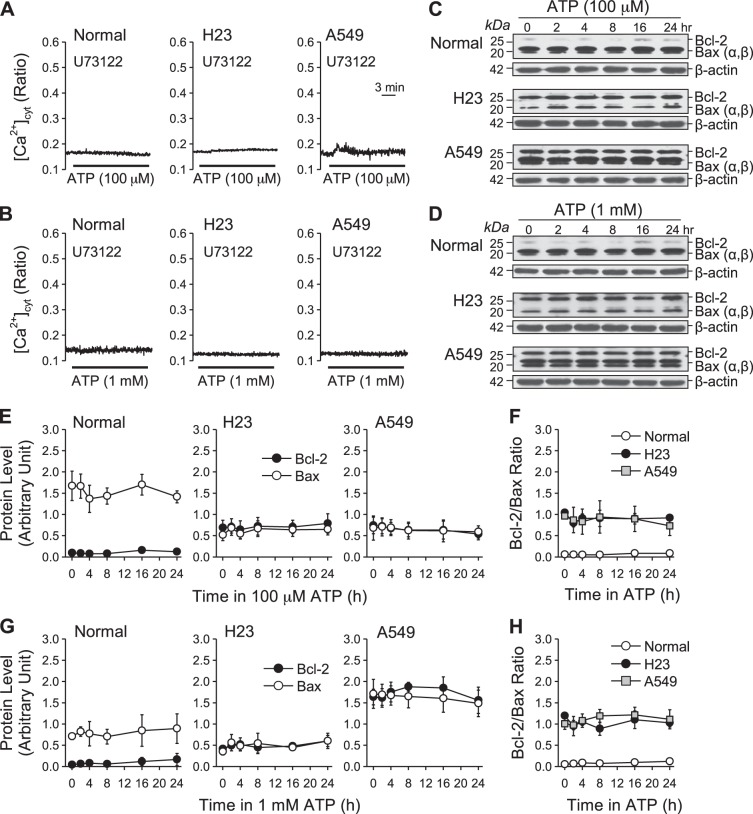
To further verify that Ca2+ influx is responsible for the ATP-induced increase of Bcl-2/Bax ratio in lung cancer cells, we performed experiments to maintain the ATP-induced plateau phase of increase (Ca2+ influx) in [Ca2+]cyt but to eliminate the transient phase of increase (Ca2+ release) in [Ca2+]cyt. To accomplish this, we treated cells with U-73122, an inhibitor of phospholipase C that is downstream of the P2Y receptors, before and during ATP (100 μM and 1 mM) treatment. Indeed, we observed that pretreatment of cells with U-73122 not only eliminated ATP-induced transient increase in [Ca2+]cyt as expected, but also inhibited Ca2+ influx (plateau phase) in both normal and lung cancer cells (Fig. 10, A and B). When we treated both normal and cancer cells with ATP (100 μM and 1 mM) following U-73122 treatment for 0, 2, 4, 8, 16, 24 h, we did not see any changes in the Bcl-2/Bax ratio (Fig. 10, C–H). Accordingly, blockade of P2Y receptors, which signal through the PLC/IP3/DAG signaling cascade to induce Ca2+ release and influx, made it possible to eliminate both the transient and plateau phases of increase in [Ca2+]cyt. These data suggest that the P2X receptors (nonselective cation channels) are not the major channels for ATP-induced Ca2+ influx in lung cancer cells. Furthermore, these data also suggest that the Ca2+-dependent regulation of Bcl-2/Bax ratios is not mediated by Ca2+ influx through P2X receptors. However, U-73122 has been shown to inhibit phospholipase A2 (PLA2) and 5-lipoxygenase as well. To our knowledge, these enzymes are not involved in Bcl-2/Bax protein regulation. Further studies are needed to focus on investigating the channels that mediate ATP-induced Ca2+ influx required for the plateau phase in lung cancer cells.
Fig. 10.
Effect of ATP on Bcl-2 and Bax expression and Bcl-2/Bax ratio in normal and lung cancer cells with treatment of U-73122. A: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before, during, and after application of 100 μM ATP after treatment with 10 μM U-73122. B: representative traces showing changes in [Ca2+]cyt in normal and lung cancer cells before and after application of 1 mM ATP after treatment with 10 μM U-73122. C: Western blot analysis of Bcl-2 and Bax expression in normal and lung cancer cells treated with 100 μM ATP for 0, 2, 4, 8, 16, and 24 h after treatment with 10 μM U-73122. β-Actin was used as a control. D: Western blot analysis of Bcl-2 and Bax expression in normal and lung cancer cells treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h after treatment with 10 μM U-73122. β-Actin was used as a control. E: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer treated 100 μM ATP for 0, 2, 4, 8, 16, and 24 h after treatment with 10 μM U-73122. F: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio in normal and lung cancer treated 100 μM ATP for 0, 2, 4, 8, 16, and 24 h after treatment with 10 μM U-73122 (n = 3). G: summarized data (means ± SE) showing the changes of Bcl-2 and Bax expression level in normal and lung cancer treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h after treatment with 10 μM U-73122. H: summarized data (means ± SE) showing the changes of Bcl-2/Bax ratio in normal and lung cancer treated with 1 mM ATP for 0, 2, 4, 8, 16, and 24 h after treatment with 10 μM U-73122 (n = 3).
DISCUSSION
In addition to functioning as a molecular energy source, ATP is also an important extracellular messenger and agonist. In this study, we have shown that ATP induces different Ca2+ signaling patterns in normal lung airway epithelial cells versus lung cancer cells. Purinergic receptors P2Xs and P2Ys are ATP receptors that are widely expressed on multiple cell types, including tumor cells. Our study showed that P2Xs and P2Ys exhibit different expression distribution in normal and lung cancer cells. Our data show that P2X3, P2X4 and P2X5 receptors and P2Y1, P2Y2, P2Y4 and P2Y6 receptors are expressed more highly in lung cancer cells than in normal cells. Indeed, P2Xs and P2Ys are known to respond to ATP to regulate tumorigenic behaviors including cell proliferation and apoptosis.
The Ca2+ signal is also known to be involved in many cellular processes that promote tumorigenesis (1, 22). Ca2+ signaling components that determine Ca2+ signaling homeostasis change their expression or function in the occurrence and development of disease (30). The remodeling of Ca2+ signaling that occurs in cancer cells is achieved through differential expression of specific Ca2+ pumps and channels (30). Although alternative Ca2+ signaling is not necessary for cancer initiation, the consequence of onset may contribute to tumor progression. Our study showed that ATP induces markedly Ca2+ influx in lung cancer cells but not in normal cells. The most probable candidate channels for Ca2+ influx are P2Xs, because P2Xs per se are Ca2+-permeable cation channels (31). As discussed earlier, H23 and A549 lung cancer cells had significantly higher levels of P2X3, P2X4, and P2X5 expression compared with normal cells. Studies have shown that P2X receptors desensitize ATP fast and mediate short-time inward current (36), which may underlie the limited Ca2+ influx induced by the activation of P2X receptors shown in P2X inhibitor and agonist data.
Store-operated Ca2+ entry (SOCE) through GPCR-activated store-operated Ca2+ channels (SOC) become the next most plausible overfunctioning Ca2+ influx pathway in consideration of overexpressed P2Y receptors (GPCRs) in lung cancer cells. P2Ys function as G protein-coupled receptors and can cause Ca2+ release from the intracellular Ca2+ stores to increase cytosolic [Ca2+] upon ATP activation (3, 31, 44). ATP (by active depletion of Ca2+ from the intracellular stores) and CPA (by passive depletion of Ca2+ from the intracellular stores) both induced SOCE, but ATP/CPA-induced SOCE in cancer cells was not enhanced, but rather reduced, in H23 lung cancer cells. This result was surprising because aggressive cancer cells would be expected to show stronger Ca2+ signaling intensity in consideration of sustained proliferation and migration. Although it has been demonstrated in proliferative pulmonary artery smooth muscle cells that enhanced SOCE promotes cell proliferation and growth, its effect on tumor growth is less clear (38, 46). Several studies have shown that, in cancer, decreased SERCA2 expression leads to low ER Ca2+ capacity, which then lowers the risk of mitochondrial Ca2+ overload that triggers cell apoptosis (27, 40). In addition, overexpressed antiapoptotic protein Bcl-2 was shown to reduce ER Ca2+ content in part by interaction with IP3 receptors (25). However, other studies have shown that overexpressed Bcl-2 protein conserved SR/ER Ca2+ content by upregulating SERCA2 (7). These conflicting results suggest that the relationship between Bcl-2 protein and ER Ca2+ content depends on cell context. It is of great interest that our results show that both H23 and A549 lung cancer cells had significantly higher levels of Bcl-2 protein expression compared with normal epithelial lung cells. Thus, the low SOCE in cancer cells may result from reduced ER Ca2+ capacity, which is due to overexpressed Bcl-2 protein. Therefore, it is possible that low SOCE is important to survival if it normally couples to reduced ER Ca2+ capacity to regulate mitochondrial Ca2+ loading. Both SOCE and P2X-mediated Ca2+ influx participate in the Ca2+ influx induced by ATP; however, they are not critically significant to the different kinetic Ca2+ signal in lung cancer cells compared with normal cells.
Cell viability results showing that lung cancer cells exhibit resistance to the cytotoxicity of high concentration of ATP in comparison to normal cells are consistent with published data that showed low (nearly undetectable) expression levels of P2X7R in lung cancer cells (20, 21, 39). P2X7 is considered to act as a proapoptotic factor through increasing cytomembrane permeability activated by a high concentration of ATP (9, 29). However, significantly decreased cell viability was observed in H23 lung cancer cells at late (72 h) culture but not in A549 lung cancer cells. These two lung cancer cell lines chosen in this study reflect the heterogeneity of the human lung cancer progression pathway, involving, for example, the expression of P53, Myc, and Ras. This allows us to speculate that, despite both being lung carcinoma, each has its own particular response to environmental stimuli. We also showed in both normal and lung cancer cells that ATP affected cell proliferation. It is interesting to note that a lower concentration of ATP reduced cell proliferation in both H23 and A549 lung cancer cells, while reduced cell proliferation was not observed with a higher concentration of ATP. These findings are inconsistent with cell viability results that indicated more cell death in a high concentration of ATP while the cells remained healthy in low concentration. These results suggest that ATP-induced cell apoptosis and cell proliferation are through separate pathways, and each sensitizes to a specific concentration range of ATP.
It is worth mentioning here that A549 lung cancer cells have been reported to secrete extracellular matrix components that have ATP binding sites. In our study, A549 lung cancer cells start to respond to ATP at 1 μM, which is the same as H23 lung cancer cells. A strong increase in intracellular Ca2+ increase was observed in A549 lung cancer cells after ATP stimulation, and P2X and P2Y receptor inhibitors weakened ATP-induced intracellular Ca2+ increase in A549 lung cancer cells. These observations suggest that although extracellular matrix components secreted by A549 lung cancer cells might bind ATP, the binding ability is not sufficient to eliminate ATP action on P2 receptors. Therefore, the lack of effect of ATP on cell viability of A549 lung cancer cells is not due to competing binding of ATP to extracellular matrix components.
There are data suggesting that very high intracellular Ca2+ levels can promote cell death through necrosis, whereas sustained and lower intracellular Ca2+ increases induced by milder insults promote cell death through apoptosis (8, 24). In this study, our data showed that ATP induces slow and long term phase of [Ca2+]cyt increase in lung cancer cells, but that ATP at both high and relatively lower levels rarely affected cell viability, which indicates cell apoptosis. This leads us to propose that ATP may enhance the ability of antiapoptosis of lung cancer cells in some way to desensitize the cytotoxicity of Ca2+ overload. Thus, we focused on the Bcl-2/Bax ratio, the change of which determines cell fate. Our data showed that ATP promotes antiapoptosis by increasing Bcl-2/Bax ratio in lung cancer cells. It is of great interest that cancer cells respond to extracellular ATP by increasing survival capacities via multiple anti-death pathways that include low P2X7 expression, high Bcl-2 expression, and an increased Bcl-2/Bax ratio.
To provide some insight on the mechanism of ATP-modulated Bcl-2/Bax ratio in lung cancer cells, we abolished ATP-induced [Ca2+]cyt increase by using BAPTA-AM and eliminated Ca2+ influx through treating cells in low extracellular Ca2+ media, and both ways led to unchanged Bcl-2/Bax ratio in lung cancer cells. Additionally, U-73122 was applied to abolish Ca2+ mobilizing from intracellular Ca2+ stores rather than extracellular Ca2+ influx. However, calcium image data showed that U-73122 also reduced Ca2+ influx significantly. Cells treated with U-73122 showed no change in the ratio of Bcl-2/Bax after ATP treatment. PLC inhibition leads to attenuation or blockade of two other Ca2+ influx pathways, store-operated Ca2+ entry through SOC and receptor-operated Ca2+ entry through ROC, even when P2Y receptors are activated by ATP. The U-73122 data indicate that, although the P2X family is one of the mechanisms that mediated ATP-induced Ca2+ influx, the P2X contribution to ATP-induced total Ca2+ influx is limited or, by itself, is insufficient to affect Bcl-2 expression and the Bcl-2/Bax ratio. These results suggest that a sustained increase in cytosolic Ca2+ due to Ca2+ influx from the extracellular environment is the critical mediator between extracellular ATP and an enhanced Bcl-2/Bax ratio in lung cancer cells. Thus, determining Ca2+ channels and transporters leading to sustained [Ca2+]cyt increase in lung cancer cells would provide an important therapeutic target eliminating ATP-mediated increase in the Bcl-2/Bax ratio and, therefore, attenuating the ability of antiapoptosis of lung cancer cells. In this study, we did not determine possible pathways that lead to enhanced Ca2+ influx in lung cancer cells compared with normal cells. Ca2+ influx through P2X channels or SOCE showed no difference between cancer cells and normal cells. Other channels like VDCC and ROCC or Ca2+ extrusion and sequestration mechanisms such as the Ca2+-Mg2+-ATPase in the plasma membrane or SERCA on the membrane of SR or ER might be the reasons, which would be of great interest in a future study.
This study has highlighted the role of ATP in the extracellular microenvironment with regard to tumor cell growth in vitro. Enhancing the antiapoptotic ability of ATP may be an important selective pressure promoting oncogene transformation and cancer progression. We have shown for the first time that ATP increases the Bcl-2/Bax ratio through a sustained increase in [Ca2+]cyt in lung cancer cells. Additionally, we have demonstrated that Ca2+ from the extracellular environment mediates this ATP-induced increase in Bcl-2/Bax ratio. This study has several implications for lung cancer biology. It suggests that tumor cells may adapt to high ATP in the tumor microenvironment through low P2X7 receptor expression to escape apoptosis and also turn ATP into a stimulus to inhibit apoptosis. This conversion of harm to benefit is important for the aggressive behavior of tumors. Despite the exogenous application of ATP having antineoplastic action on tumors, as implicated by several studies, because of its cytotoxicity on low-tolerance normal cells, this side effect weakens its therapeutic value. Thus, understanding this ATP-induced antiapoptotic pathway observed in lung cancer cells would be of therapeutic value to facilitate the development of cancer-specific targeted therapies.
GRANTS
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL-115014, HL-066012, and HL-098053).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S., K.N.J., and J.X.-J.Y. conception and design of research; S.S. performed experiments; S.S., K.N.J., K.M.M., S.P.R., A.E.C., H.T., S.M.D., S.M.B., J.G.G., A.M., and J.X.-J.Y. analyzed data; S.S., K.M.M., S.P.R., A.E.C., H.T., S.M.D., S.M.B., J.G.G., A.M., and J.X.-J.Y. interpreted results of experiments; S.S. and J.X.-J.Y. prepared figures; S.S., K.N.J., and J.X.-J.Y. drafted manuscript; S.S., K.N.J., K.M.M., S.P.R., A.E.C., H.T., S.M.D., S.M.B., J.G.G., A.M., and J.X.-J.Y. edited and revised manuscript; S.S., K.N.J., K.M.M., S.P.R., A.E.C., H.T., S.M.D., S.M.B., J.G.G., A.M., and J.X.-J.Y. approved final version of manuscript.
REFERENCES
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36: 1127–1139, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal 9: 491–540, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240: 31–304, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chahwala SB, Cantley LC. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J Biol Chem 259: 13717–13722, 1984. [PubMed] [Google Scholar]
- 7.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol 166: 193–203, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci 18: 58–60, 1995. [PubMed] [Google Scholar]
- 9.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 102: 13944–13949, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fickova M, Macho L, Brtko J. A comparison of the effects of tributyltin chloride and triphenyltin chloride on cell proliferation, proapoptotic p53, Bax, and antiapoptotic Bcl-2 protein levels in human breast cancer MCF-7 cell line. Toxicol In Vitro 29: 727–731, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Guzman M, Soto F, Laube B, Stuhmer W. Molecular cloning and functional expression of a novel rat heart P2X purinoceptor. FEBS Lett 388: 123–127, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Greig AV, Linge C, Healy V, Lim P, Clayton E, Rustin MH, McGrouther DA, Burnstock G. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol 121: 315–327, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hanson CJ, Bootman MD, Distelhorst CW, Maraldi T, Roderick HL. The cellular concentration of Bcl-2 determines its pro- or anti-apoptotic effect. Cell Calcium 44: 243–258, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Lewis CJ, Surprenant A, Evans RJ. 2′,3′-O-(2,4,6- trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP)–a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br J Pharmacol 124: 1463–1466, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majors BS, Betenbaugh MJ, Chiang GG. Links between metabolism and apoptosis in mammalian cells: applications for anti-apoptosis engineering. Metab Eng 9: 317–326, 2007. [DOI] [PubMed] [Google Scholar]
- 16.McLaren GJ, Lambrecht G, Mutschler E, Baumert HG, Sneddon P, Kennedy C. Investigation of the actions of PPADS, a novel P2x-purinoceptor antagonist, in the guinea-pig isolated vas deferens. Br J Pharmacol 111: 913–917, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meleady P, Clynes M. Bromodeoxyuridine increases keratin 19 protein expression at a posttranscriptional level in two human lung tumor cell lines. In Vitro Cell Dev Biol Anim 37: 536–542, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Miao LJ, Huang SF, Sun ZT, Gao ZY, Zhang RX, Liu Y, Wang J. MiR-449c targets c-Myc and inhibits NSCLC cell progression. FEBS Lett 587: 1359–1365, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Michaelis M, Nieswandt B, Stegner D, Eilers J, Kraft R. STIM1, STIM2, and Orai1 regulate store-operated calcium entry and purinergic activation of microglia. Glia 63: 652–663, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Mistafa O, Hogberg J, Stenius U. Statins and ATP regulate nuclear pAkt via the P2X7 purinergic receptor in epithelial cells. Biochem Biophys Res Commun 365: 131–136, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mistafa O, Stenius U. Statins inhibit Akt/PKB signaling via P2X7 receptor in pancreatic cancer cells. Biochem Pharmacol 78: 1115–1126, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7: 519–530, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Nichols CM, Povstyan OV, Albert AP, Gordienko DV, Khan O, Vasilikostas G, Khong TK, Wan A, Reddy M, Harhun MI. Vascular smooth muscle cells from small human omental arteries express P2X1 and P2X4 receptor subunits. Purinergic Signal 10: 565–572, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicotera P, Orrenius S. The role of calcium in apoptosis. Cell Calcium 23: 173–180, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA 102: 105–110, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 3: e2599, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J 20: 2690–2701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. A role for calcium in Bcl-2 action? Biochimie 84: 195–201, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Pizzo P, Murgia M, Zambon A, Zanovello P, Bronte V, Pietrobon D, Di Virgilio F. Role of P2z purinergic receptors in ATP-mediated killing of tumor necrosis factor (TNF)-sensitive and TNF-resistant L929 fibroblasts. J Immunol 149: 3372–3378, 1992. [PubMed] [Google Scholar]
- 30.Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med 16: 107–121, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 32.Schaafsma HE, Ramaekers FC. Cytokeratin subtyping in normal and neoplastic epithelium: basic principles and diagnostic applications. Pathol Annu 29: 21–62, 1994. [PubMed] [Google Scholar]
- 33.Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol Lung Cell Mol Physiol 285: L376–L385, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Schultze-Mosgau A, Katzur AC, Arora KK, Stojilkovic SS, Diedrich K, Ortmann O. Characterization of calcium-mobilizing, purinergic P2Y(2) receptors in human ovarian cancer cells. Mol Hum Reprod 6: 435–442, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Scodelaro Bilbao P, Boland R, Russo de Boland A, Santillan G. ATP modulation of mitogen activated protein kinases and intracellular Ca2+ in breast cancer (MCF-7) cells. Arch Biochem Biophys 466: 15–23, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Shen JB, Cronin C, Sonin D, Joshi BV, Gongora Nieto M, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy: implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol 292: H1077–H1084, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soliman EM, Rodrigues MA, Gomes DA, Sheung N, Yu J, Amaya MJ, Nathanson MH, Dranoff JA. Intracellular calcium signals regulate growth of hepatic stellate cells via specific effects on cell cycle progression. Cell Calcium 45: 284–292, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ 1: 84–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-beta1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci 125: 5051–5060, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Abeele F, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, Roudbaraki M, Mauroy B, Wuytack F, Prevarskaya N. Bcl-2-dependent modulation of Ca2+ homeostasis and store-operated channels in prostate cancer cells. Cancer Cell 1: 169–179, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol 1: E209–E216, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Vanoverberghe K, Mariot P, Vanden Abeele F, Delcourt P, Parys JB, Prevarskaya N. Mechanisms of ATP-induced calcium signaling and growth arrest in human prostate cancer cells. Cell Calcium 34: 75–85, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol 287: C1349–C1358, 2004. [DOI] [PubMed] [Google Scholar]
- 44.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci 27: 211–217, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Wu DW, Wu TC, Wu JY, Cheng YW, Chen YC, Lee MC, Chen CY, Lee H. Phosphorylation of paxillin confers cisplatin resistance in non-small cell lung cancer via activating ERK-mediated Bcl-2 expression. Oncogene 33: 4385–4395, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Yamamura A, Yamamura H, Zeifman A, Yuan JX. Activity of Ca2+-activated Cl− channels contributes to regulating receptor- and store-operated Ca2+ entry in human pulmonary artery smooth muscle cells. Pulm Circ 1: 269–279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zundelevich A, Elad-Sfadia G, Haklai R, Kloog Y. Suppression of lung cancer tumor growth in a nude mouse model by the Ras inhibitor salirasib (farnesylthiosalicylic acid). Mol Cancer Ther 6: 1765–1773, 2007. [DOI] [PubMed] [Google Scholar]