Abstract
Periaxin (Prx), a PDZ domain protein expressed preferentially in myelinating Schwann cells and lens fibers, plays a key role in membrane scaffolding and cytoarchitecture. Little is known, however, about how Prx is anchored to the plasma membrane. Here we report that ankyrin-B (AnkB), a well-characterized adaptor protein involved in linking the spectrin-actin cytoskeleton to integral membrane proteins, is required for membrane association of Prx in lens fibers and colocalizes with Prx in hexagonal fiber cells. Under AnkB haploinsufficiency, Prx accumulates in the soluble fraction with a concomitant loss from the membrane-enriched fraction of mouse lenses. Moreover, AnkB haploinsufficiency induced age-dependent disruptions in fiber cell hexagonal geometry and radial alignment and decreased compressive stiffness in mouse lenses parallel to the changes observed in Prx null mouse lens. Both AnkB- and Prx-deficient mice exhibit disruptions in membrane organization of the spectrin-actin network and the dystrophin-glycoprotein complex in lens fiber cells. Taken together, these observations reveal that AnkB is required for Prx membrane anchoring and for maintenance of lens fiber cell hexagonal geometry, membrane skeleton organization, and biomechanics.
Keywords: ankyrin-B, periaxin, lens fibers, cytoarchitecture, stiffness, dystrophin
tissue architecture and function rely on specialized cell adhesive interactions, cytoarchitectural stability, and establishment of membrane protein assemblies/domains, which collectively enable cells to withstand the mechanical stresses encountered during organ development and function. Various membrane scaffolding proteins are thought to play a key role in linking membrane-spanning proteins, including ion channels, transporters, receptors, and cell adhesive molecules, to the underlying spectrin-actin cytoskeleton and in linking the actin cytoskeleton to the extracellular matrix required for maintaining tissue architecture and biomechanical stability (4–6, 18). Periaxin (Prx) is one such protein, believed to serve as a membrane scaffolding protein in myelinating Schwann cells of the peripheral nervous system (14, 15). Although Prx was initially thought to be expressed preferentially in the peripheral nervous system and to regulate myelin sheath stabilization and organization (15), this protein is also reported to be expressed in lens fibers (23, 28). Prx is a ∼150-kDa protein containing a PDZ (an acronym combining the first letters of three proteins: PSD95/Dlg1/Zo-1) -like domain at the NH2-terminal region, followed by a basic domain, a long repeat region, and an acidic domain, with the repeat region showing some sequence homology with desmoyokin (11, 26). Prx exists together with dystrophin-related protein-2 (Drp-2) and dystroglycan, as part of a large complex that links the actin cytoskeleton to the extracellular matrix and is required for assembly of appositions between the abaxonal surface of the myelin sheath and the Schwann cell plasma membrane (7, 27).
Although Prx is believed to play a membrane scaffolding role in both Schwann cells and lens fibers (23, 27), little is known about how Prx associates with the plasma membrane. Earlier work from our laboratory and others has demonstrated that, in lens, Prx coexists in a large complex with desmoyokin, ezrin, spectrin, periplakin, ankyrin-B (AnkB), neuron-glia cell adhesion molecule (NgCAM)-related cell adhesion molecule (NrCAM), and plectin (23, 28). Absence of Prx resulted in destabilization of the lens fiber cell membrane, disruption of fiber cell hexagonal symmetry and radial alignment, and destabilization of the spectrin-actin cytoskeleton in the mouse lens (23). Since our earlier data revealed that AnkB and its interacting proteins, including NrCAM and β-spectrin, co-immunoprecipitate with Prx from the mouse lens (23), we asked whether AnkB is required for Prx membrane association and to lens fiber cell cytoarchitecture and mechanics. The ankyrins are a family of metazoan adaptor proteins that play a key role in linking membrane-spanning proteins, including ion channels, transporters, receptors, and cell adhesive molecules, to the underlying spectrin-actin cytoskeleton (5, 6). The ankyrin family of proteins consists of three well-characterized subtypes, including ankyrin-R (AnkR), AnkB, and ankyrin-G (AnkG), encoded by three different genes, ANK1, ANK2, and ANK3, respectively (5, 6). Ankyrins consist of three well-conserved functional domains, including the membrane-binding domain, spectrin binding domain, and COOH-terminal regulatory domain (6). The lens is known to express AnkB abundantly relative to AnkG and AnkR, but its role in adult lens is not known (24). To explore whether AnkB is required for membrane anchoring of Prx and maintenance of lens fiber cell shape and cytoarchitecture, we investigated membrane localization of Prx, fiber cytoarchitecture, and lens stiffness in AnkB haploinsufficient mouse lenses in parallel with lens changes observed in Prx null mouse lenses. These studies reveal that AnkB is required for association of Prx with the plasma membrane, and that AnkB regulates lens fiber cell shape, alignment, membrane organization, and biomechanical properties, which are critical determinant of lens architecture and function.
MATERIALS AND METHODS
Mice.
AnkB haploinsufficient (AnkB−/+) mice used in this study have been backcrossed (>10 generations) into a C57/BL6 background (30). Prx null (−/−) mice were on a C57/BL6 genetic background and developed originally in the Brophy laboratory (15). All animal procedures were conducted in compliance with the recommendations in the “Care and Use of Laboratory Animals, National Institutes of Health,” and were approved and enforced by the Institutional Animal Care and Use Committee at the Duke University Medical Center.
Histological analysis.
Freshly enucleated eyes from AnkB−/+, Prx−/−, and wild-type (WT) mice were fixed in 3.7% buffered formalin, as our laboratory described earlier (23). The specimens were embedded in paraffin, and 5-μm-thick sections were cut and stained with hematoxylin and eosin. Images were captured using a Zeiss Axio Imager, as our laboratory described earlier (23).
Immunofluorescence and imaging.
Equatorial plane sections (5 μm) derived from paraffin embedded mouse eyes were used for immunofluorescence analyses. The tissue sections were deparaffinized and rehydrated using xylene and absolute ethyl alcohol, respectively, as our laboratory described earlier (23). Specimens were then placed in preheated antigen retrieval solution (0.1 M sodium-citrate buffer, pH 6.0) and heated for 20 min at 100°C in water bath. After rinsing, these sections were blocked for 10 min with the medical background Sniper reducing solution (BiocareMedical, Concord, CA) in a humidified chamber. Primary anti-AnkB antibodies (rabbit polyclonal and goat polyclonal, gift from Vann Bennett), anti-β-dystroglycan (monoclonal from Developmental Studies Hybridoma Bank, Iowa, IA), anti-NrCAM (rabbit polyclonal from Abcam), anti-NrCAM (monoclonal from Antibodies, Davis, CA), anti-Prx (rabbit polyclonal from Sigma-Aldrich), and anti-β-spectrin antibodies (monoclonal from BD Biosciences, San Jose, CA, and rabbit polyclonal, gift from Vann Bennett) were used (at 1:200 dilution in 1% fatty acid free bovine serum albumin Tris-buffered saline), either individually or in combination with other primary antibodies (for double labeling) and incubated overnight at 4°C in a humidified chamber. The slides were washed and incubated in the dark for 2 h at room temperature, with either Alexa fluor 488 or 568 conjugated secondary antibodies (Invitrogen; at 1:200 dilution) or both (for double labeling). Wherever applicable, fluorescein isothiocyanate or tetramethylrhodamine-conjugated wheat germ agglutinin (WGA; Sigma-Aldrich) was incubated, along with the secondary antibodies, as described above. Slides were then washed and mounted using Vecta mount (Vector Laboratories, Burlingame, CA), and images captured using Zeiss 780 inverted microscope and Nikon Eclipse 90i confocal laser scanning microscope. For all immunofluorescence analyses described in this study, a minimum of three independent specimens with three serial sections from each specimen were analyzed.
Imaging and three-dimensional reconstruction.
For colocalization measurements and three-dimensional (3D) reconstruction, the immunostained sections described in the section Immunofluorescence and imaging were imaged using a Zeiss 780 inverted confocal microscope equipped with Argon/2, 561-nm diode lasers, and a ×100 1.40 numerical aperture oil objective lens (zoom 3; see Fig. 2) at room temperature. Images were acquired with ZEN Black 2011 imaging software. For viewing the fiber cells, Z-stack images were captured at a 0.2-μm step interval, and 25–30 optical sections were collected. The captured Z-stacks were processed using Volocity 6.3.1 software (Perkin Elmer, Waltham, MA). Pearson's correlation coefficient for colocalization of AnkB and Prx with the other proteins described above was analyzed using single-optical images (see Fig. 2, A and B) and Volocity 6.3.1 software. Deconvoluted images were used for the 3D reconstruction (see Fig. 2, C and D) and processed using Adobe Photoshop version CS4.
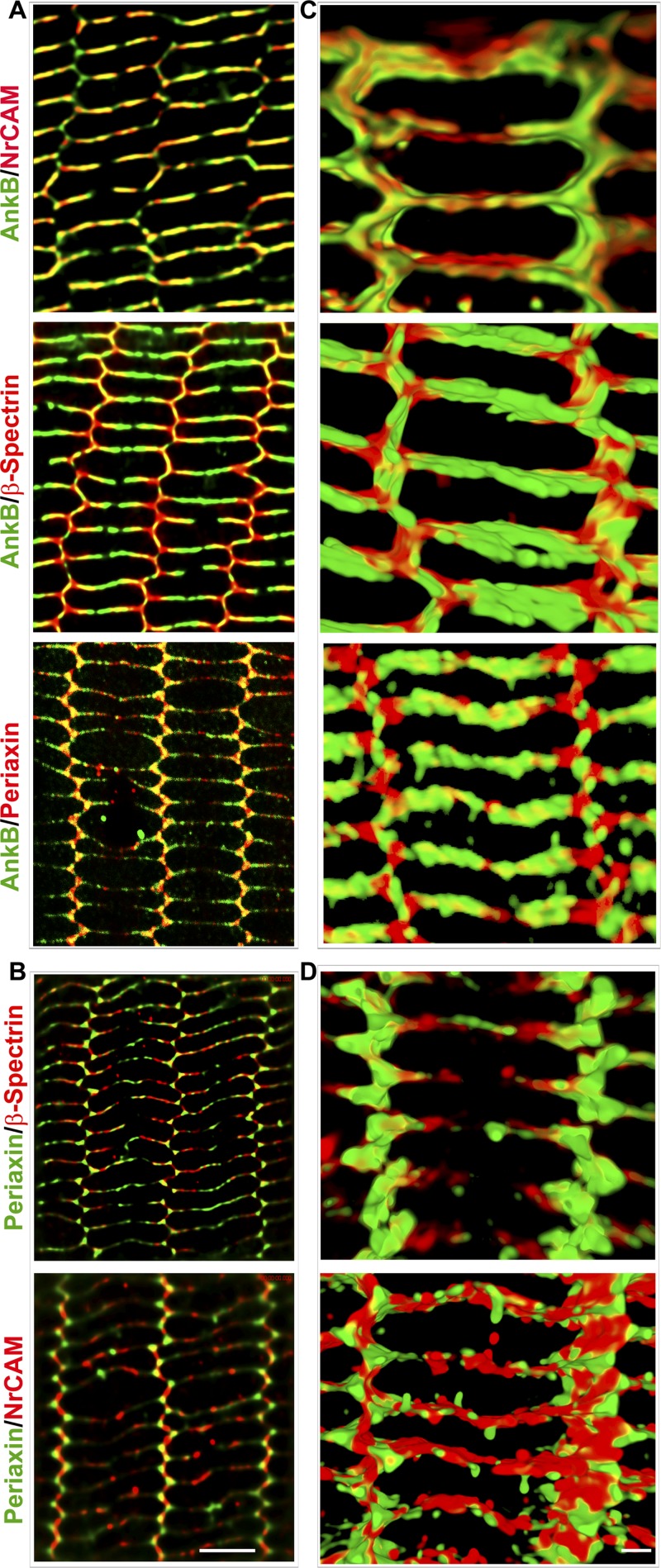
Fig. 2.
Colocalization of Prx with AnkB in lens fiber cells. Codistribution is shown of AnkB with Prx, neuron-glia cell adhesion molecule-related cell adhesion molecule (NrCAM), and β-spectrin (A and C), and Prx with β-spectrin and NrCAM (B and D) in the P21 mouse lens mature fiber cells based on high-resolution confocal imaging and three-dimensional (3D) reconstruction. A and B images were based on single-optical sections (scale bar: 5 μm). C and D images were acquired with a Z-series and converted to maximum intensity projection and shown in the XY-plane. Scale bar: 2 μm.
Fiber cell width.
To measure changes in the internal width of fiber cells in AnkB−/+ lenses (see Fig. 3), ×100 images (×1 magnification; single optical images) of β-actin-labeled tissue sections were captured using a Nikon Eclipse C90i confocal laser scanning microscope, and cell width was measured as our laboratory described earlier (23), with data represented as a dot plot.
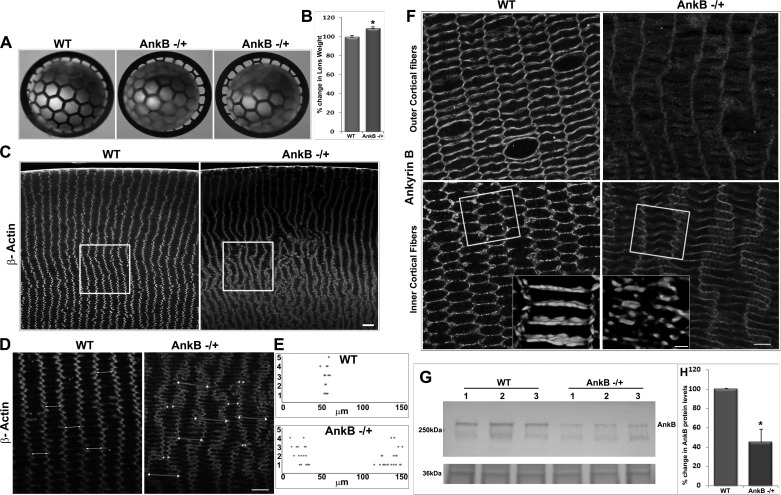
Fig. 3.
AnkB haploinsufficiency disrupts the hexagonal shape and radial arrangement of mouse lens fibers. A and B: 5-mo-old AnkB−/+ mouse lenses photographed against a black background grid under dark illumination show a slight haziness (A) and a marginal but significant increase (by ∼5%) in weight compared with WT (B). C: AnkB−/+ lenses exhibit disruptions in the radial row organization of fiber cells with pronounced changes in the inner cortical region (indicated with square box) compared with the outer cortical region. Scale bar, 40 μm. D and F: paraffin embedded, equatorial plane lens sections were immunostained either for β-actin (D) or AnkB (F), and confocal images of single optical sections were captured with a ×100 oil objective. Scale bars: 40 and 20 μm, respectively. Fiber cells from the inner cortical region of WT lens exhibit a perfect hexagonal shape with 2 long and 4 short arms. E: in contrast, the fibers from the AnkB−/+ lens show disruption of hexagonal shape together with width (in μm) alterations that result in either very long or short arm width (dot plot), marked with double-headed arrows in D. F: fiber cells from both outer and inner cortical regions reveal reduced immunostaining for AnkB in the AnkB−/+ lenses compared with WT. Insets: altered fiber cell shape in the AnkB−/+ lenses was associated with disruptions in membrane organization of AnkB (3D images). The boxed area represents the region of area that was shown in 3D images using different specimens. G and H: AnkB−/+ lenses show a ∼60% decrease in AnkB protein levels in the membrane-enriched fraction compared with WT, based on immunoblot analysis (G; data are shown for three individual specimens) and subsequent densitometry-based quantification (H). G, bottom: protein (36 kDa) staining of membrane-enriched fraction for normalization of loading. Values in B and H are means ± SE of 8–12 independent specimens. *P < 0.05.
Immunoblotting.
To determine changes in levels of selected proteins in the soluble and membrane-enriched fractions of AnkB−/+ and Prx−/− lenses; 5 mo-old AnkB−/+ or Prx−/− and WT mouse lenses (4 lenses/pooled sample) were homogenized using a Dounce glass homogenizer and cold (4°C) hypotonic buffer containing 10 mM Tris buffer, pH 7.4, 0.2 mM MgCl2, 5 mM N-ethylmaleimide, 2.0 mM sodium orthovanadate, 10 mM sodium fluoride, 60 μM phenylmethylsulfonyl fluoride, 0.4 mM iodoacetamide, protease inhibitor cocktail (complete, Mini, ethylenediaminetetraacetic acid-free) and PhosSTOP phosphatase inhibitor cocktail (one each/10 ml buffer; obtained from Roche, Manheim, Germany). Homogenates were processed for separation of soluble and membrane-enriched fractions as our laboratory described earlier (23). Protein concentrations were estimated in both the soluble and membrane-enriched fractions using protein assay reagent (Bio-Rad, Hercules, CA). Protein samples were separated by SDS-PAGE (8% and 10% acrylamide gels) using 1× SDS gel running buffer, followed by electrophoretic transfer to nitrocellulose membrane, which were blocked and incubated with primary antibodies, as described earlier (22). Antibodies generated against AnkB, β-spectrin, Prx, anti-dystrophin (rabbit polyclonal from Abcam, Cambridge, MA), NrCAM, voltage-gated calcium channels CaV1.3, and CaV1.2 (mouse monoclonal from Antibodies, Davis, CA), α-dystroglycan (mouse monoclonal from Millipore, Temecula, CA), and glyceraldehyde 3-phosphate dehydrogenase (mouse monoclonal from Imagenex, Port Coquitlam, BC, Canada), were used at 1:1,000 dilution. Immunoblots were developed by enhanced chemiluminescence and scanned using a Foto Dyne Gel Doc scanner equipped with TL100 software (Hartland, WI), and densitometry was performed with ImageJ software. Total soluble lens fractions prepared in 8 M urea were used for analysis of α-dystroglycan levels (mouse monoclonal from Millipore, Temecula, CA). Changes in the levels of phosphorylated myosin light chain (pMLC) and total MLC were determined by immunoblot analysis using urea/glycerol-polyacrylamide gels and the respective polyclonal antibodies obtained from Cell Signaling Technology, as our laboratory described previously (22). For normalizing protein loading, membrane-rich protein fractions resolved by SDS-PAGE and transferred to nitrocellulose were stained with MemCode reversible protein stain (Thermo Fisher Scientific), followed by densitometric scanning-based quantification using ImageJ software, as described earlier (22).
Biomechanical compression analysis.
Changes in the compressive stiffness of AnkB−/+, Prx−/−, and WT mouse lenses were analyzed using an RSA III micro-strain analyzer (TA Instruments, New Castle, DE) equipped with parallel plate tools. Briefly, lenses from the 1- and 5 mo-old AnkB−/+, Prx−/−, and WT mice were dissected into organ culture medium containing Dulbecco's modified Eagle's medium (low glucose, 298 mosM; Life Technology, Grand Island, NY), penicillin (100 U/ml), and streptomycin (100 mg/ml) and incubated at 37°C under 5% CO2 until further analysis (<2 h). Lens compression was carried out between two 8-mm plates, which were attached to the parallel plate tools and mounted on actuator shafts. All measurements were performed while the lens was submerged in culture medium, at room temperature. The samples were strained at a constant rate of 0.05 mm/s for a total of 35 s until sample rupture occurred. Data were acquired and plotted in real time using TA Orchestrator software. Applied stress was calculated by dividing measured changes in applied force by the area. Slope values before lens rupture were calculated from the linear range of slope and plotted in Microsoft excel. Percent change in Young's modulus between control and Prx−/− or AnkB−/+ lenses is shown as a histogram output.
Statistical analysis.
Wherever required, the Student's t-test was performed to determine significance of differences (P < 0.05) between the AnkB−/+, Prx−/−, and WT lens specimens. Values are represented as means ± SE of 4–10 samples.
RESULTS
Prx requires AnkB for its membrane localization.
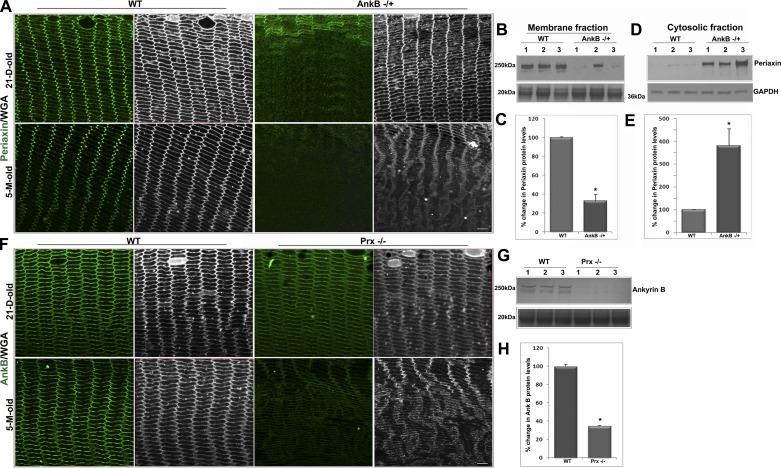
To determine whether AnkB and Prx functionally interact in lens, we evaluated the membrane organization of Prx in AnkB−/+ haploinsufficient mouse lens fibers (Fig. 1) using immunofluorescence analysis. Protein levels of Prx were also monitored in the lens membrane-enriched fraction by immunoblotting analyses. Membrane organization of Prx was completely disrupted in lens fibers of both postnatal day 21 (P21) and 5 mo-old AnkB−/+ mice. As shown in Fig. 1A, while Prx is localized primarily to the fiber cell membrane with intense clustering at the vertices in WT lenses, this organization was dramatically disrupted in the AnkB−/+ lenses. The same lens sections were counterstained with WGA (shown in gray scale) to monitor cell shape changes and organization. Moreover, unlike in WT lenses, in which Prx is present mostly in the membrane-rich fraction of fiber cells (Fig. 1, B and C), immunoblot analysis of AnkB−/+ lenses revealed that Prx accumulated in the soluble lens fraction (Fig. 1, D and E; data from 5-mo-old lenses). In contrast, membrane organization of AnkB appears to be normal in P21 Prx−/− lenses based on immunofluorescence (Fig. 1F) and immunoblotting analyses (data not shown). Only in 5-mo-old Prx−/− null mouse lenses was there a decrease in AnkB protein levels in the membrane-enriched fraction, revealing an acquired rather an intrinsic influence of Prx on AnkB (Fig. 1, F, G, and H). Moreover, unlike Prx, which accumulates in the soluble fraction in the AnkB−/+ lenses, AnkB was not found to accumulate in the soluble fraction of Prx−/− mouse lenses (unpublished data). Based on WGA labeling, both AnkB−/+ and Prx−/− lenses from 5-mo-old mice reveal disrupted fiber cell shape and organization compared with the respective WT controls. These results reveal that a 50% deficiency in AnkB protein results in a major dissociation of Prx from the plasma membrane. In contrast, complete absence of Prx had little to no effect on the membrane localization of AnkB in young and clear lenses, indicating AnkB is upstream in a pathway required to target Prx to the lens plasma membrane. The bottom immunoblots in Fig. 1, B and G, show MemCode blue staining of the same samples for assessment of protein loading.
Fig. 1.
Ankyrin-B (AnkB) regulates periaxin (Prx) membrane localization in lens fibers. A: membrane organization of Prx in fiber cells of AnkB−/+ lenses shows a complete disruption relative to the characteristic pattern associated with wild-type (WT) lenses, in both postnatal day 21 (P21) and 5-mo-old mice, based on confocal imaging (single optical sections) of equatorial paraffin-embedded lens sections immunolabeled for Prx. The specimens were also costained with wheat germ agglutinin (WGA) to follow changes in cell morphology (in gray scale). B and C: membrane-enriched fraction from AnkB−/+ mouse lenses (5 mo old) shows significantly decreased levels of Prx based on immunoblot analysis (B) and quantification (C) relative to WT controls. To normalize for protein loading (B and G, bottom), samples resolved by SDS-PAGE and transferred to nitrocellulose membrane were stained with MemCode reversible stain and densitometrically scanned for quantification. D and E: significantly increased levels of Prx in the soluble fraction (100,000 g supernatant) of AnkB−/+ lenses compared with WT controls, with GADPH being used as a loading control. F–H: only in 5-mo-old, but not in P21, Prx−/− mouse lenses was AnkB immunofluorescence found to be moderately (F) decreased in the fiber cells, with protein levels (G and H) being reduced significantly in the membrane-enriched fraction compared with WT. Scale bars: 20 μm. Values in C, E, and H are means ± SE of 8 independent samples. *P < 0.05.
In a previous study using mouse lens homogenates, we noted that Prx co-immunoprecipitates with AnkB and AnkB-interacting proteins, including NrCAM and β-spectrin (23). To seek further evidence for a plausible functional interaction between Prx and AnkB in the lens, we examined the colocalization pattern of Prx with AnkB compared with NrCAM and β-spectrin, using confocal microscopy-based images derived from the single optical sections and 3D rendering (acquired with Z-series and converted to maximum intensity projections) of P21 mouse lens specimens. Figure 2, A and B, shows single-optical section-derived colocalization images of AnkB with Prx, β-spectrin, or NrCAM, and of Prx with β-spectrin or NrCAM, respectively. The corresponding Pearson correlation coefficient values for the colocalization analyses were 0.60, 0.68, and 0.85 (Fig. 2A), and 0.59 and 0.30 (Fig. 2B), respectively. Figure 2, C and D, shows high magnification images taken at a single-fiber cell level (shown in XY-plane) from the inner cortical region.
AnkB haploinsufficiency induces age-dependent loss of lens fiber cell hexagonal geometry and radial alignment.
Since the role of AnkB in cytoarchitecture of the mature lens is not known (24), we explored whether AnkB haploinsuffiency impacts lens architecture and fiber cell phenotype and asked whether the phenotype of the AnkB−/+ lens overlaps with changes noted in the Prx null lens. This was done by comparing fiber cell phenotype in AnkB-deficient lenses with the phenotypic data generated in our earlier work with the Prx−/− mouse lens (23). More et. al (24) had previously reported the effects of AnkB complete deficiency in neonatal mouse lenses; however, since AnkB null mice survive only for <1 day after birth (24, 30), the role of AnkB in adult lenses, which exhibit the stereotypical hexagonal geometry and radial arrangement of fiber cells, is unknown. Therefore, we characterized AnkB−/+ mouse lenses in detail to investigate the role of AnkB in maintenance of cell shape and alignment in mature lens fibers. Unlike AnkB null mice (24, 30), the AnkB−/+ mice breed and age normally, and adult AnkB−/+ mice do not exhibit any obvious ocular phenotype. AnkB protein levels in the young AnkB−/+ mouse (P21) lenses were reduced as expected by ∼50% compared with WT lenses, but lens fiber cell shape and arrangement appeared to be largely normal (data not shown). However, assessment of lens fiber cell architecture in older AnkB−/+ mice (5 mo old) revealed some obvious and major differences in fiber cell shape and alignment compared with WT littermates (Fig. 3). Lenses derived from 5-mo-old AnkB−/+ mice exhibited a slight but uniform haziness throughout when examined under dark illumination against a black lined grid (Fig. 3A) and weighed slightly (8 ± 0.1 mg/lens; n = 12) but significantly more (by ∼5%) compared with WT lenses (7.6 ± 0.12 mg/lens; n = 12; Fig. 3B). Levels of AnkB protein in the membrane-enriched fraction of the 5-mo-old AnkB−/+ lenses were reduced by ∼60% compared with WT controls (Fig. 3, G and H). The global lens protein profile of both soluble and membrane-enriched fractions from these adult AnkB−/+ mice, however, was found to be comparable to WT lenses, based on SDS-PAGE and Coomassie blue staining profiles (data not shown). While hematoxylin- and eosin-stained equatorial plane sections derived from the 5-mo-old AnkB−/+ mouse lenses showed no obvious changes in the epithelium, fiber cell shape and alignment were altered and progressively disrupted in the inner cortical region compared with WT lenses. These morphological changes were more noticeable in the equatorial plane sections immunostained either for β-actin or AnkB (Fig. 3, D and F). As shown in Fig. 3, C, D, and F, while lens fibers from the inner cortical region exhibited a perfect radial organization with cells maintaining a typical hexagonal shape and close cell-cell contacts in WT controls, AnkB−/+ lenses were characterized by extensive disruptions in fiber cell radial alignment and hexagonal shape, with some cells exhibiting either much longer or much shorter widths.
Measurements of fiber cell width in the AnkB−/+ lenses shown as a dot plot revealed a 2.5-fold increase or a 50% decrease in width relative to WT controls (Fig. 3E). As shown in the insets to Fig. 3F, membrane organization (based on 3D reconstruction of Z-stack images) was disrupted and discontinuous in the AnkB−/+ lens fibers compared with WT controls. Significantly, the altered fiber cell phenotype (including shape and arrangement) noted in the AnkB−/+ lenses was found to be closely similar to the changes our laboratory previously reported in adult Prx−/− mouse lens fibers (23), revealing that the two proteins manifest somewhat similar but noncompensatory role(s) within the fiber cell. In Fig. 3G, the bottom immunoblot represents a protein loading control.
AnkB deficiency causes age-dependent loss of lens stiffness.
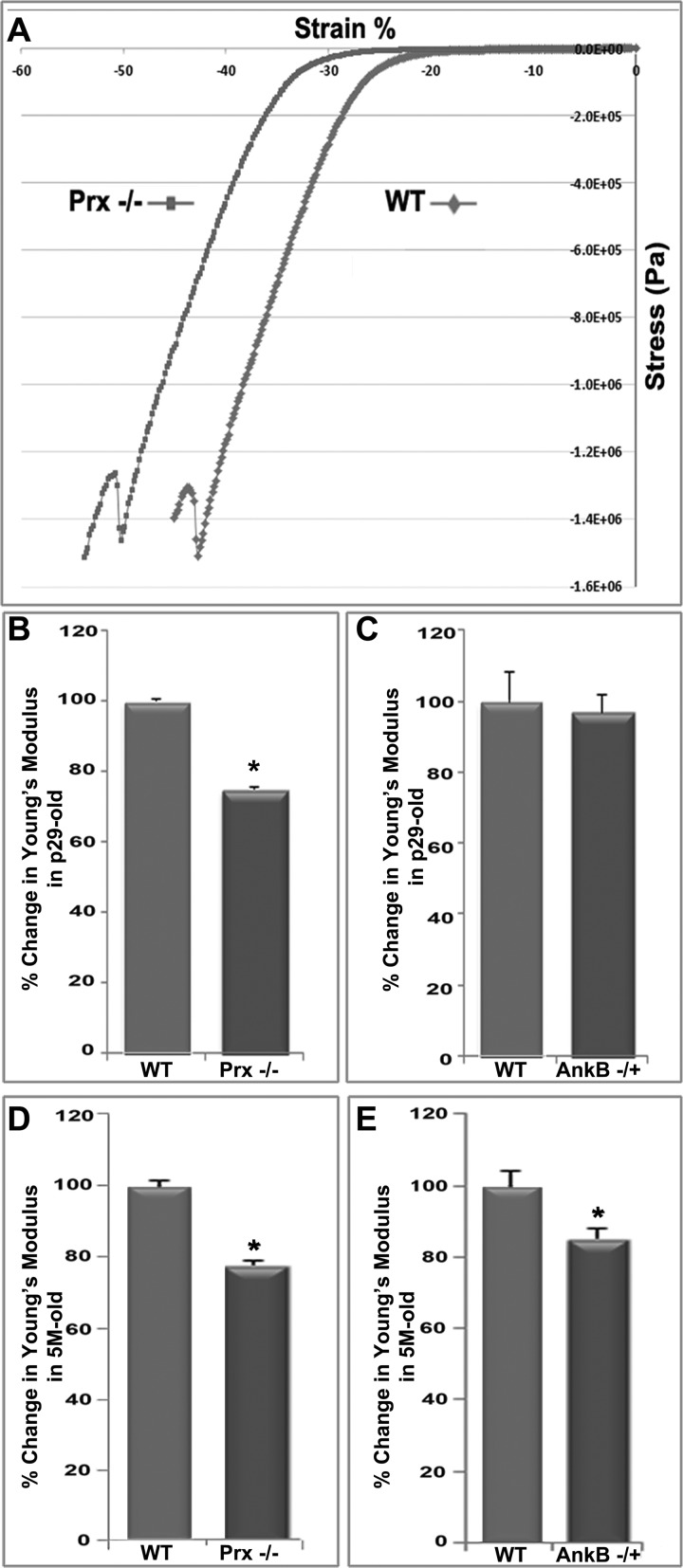
Given the effects of AnkB deficiency on lens fiber cell shape, including disruption of fiber cell hexagonal geometry and alignment, and in light of our laboratory's previous finding that Prx−/− lenses were soft (23), we evaluated lens stiffness in AnkB−/+ mice to address a potential role for AnkB in maintenance of biomechanical properties compared with Prx−/− mouse lenses. To evaluate this aspect, we performed stress/strain analysis of AnkB−/+ and Prx−/− mouse lenses using a microstrain analyzer and the respective WT controls, as described in the materials and methods section (Fig. 4). Figure 4A shows a representative real-time tracing of lens compressive stress/strain analysis. Using 8–10 lenses in each group, compressive stress/strain analyses of intact lenses (∼5 mo old) revealed a significant decrease in Young's modulus in both AnkB−/+ and Prx−/− lenses (by ∼18 and 23%, respectively) compared with WT lenses (Fig. 4, D and E), implying a direct role for AnkB and Prx in maintenance of tensile properties of the lens. A significant decrease (by ∼22%) in stiffness of younger lens specimens (1 mo old), however, was only noted in Prx−/− mice, and not in AnkB−/+ mice, as shown in Fig. 4, B and C, indicating the stronger effect of Prx complete absence on lens stiffness compared with AnkB haploinsuffiency.
Fig. 4.
AnkB and Prx deficiency is associated with decreases in lens stiffness. Stiffness analyses of lenses derived from AnkB−/+, Prx−/− (1 and 5 mo old), and corresponding WT mice were carried out using a microstrain analyzer. A: representative real-time tracing of lens compressive stress/strain responses. Changes in Young's modulus were calculated from the lens prerupture stress/strain slope values shown in A. While both the AnkB−/+ (C and E) and Prx−/− (B and D) lenses derived from older (5 mo old; D and E) mice showed a significantly decreased Young's modulus compared with WT, confirming decreased stiffness, AnkB−/+ lenses from 1-mo-old mice did not show changes in lens stiffness relative to the Prx−/− mouse lenses (B and C). Values are means ± SE estimates from 8–10 independent analyses. *P < 0.05.
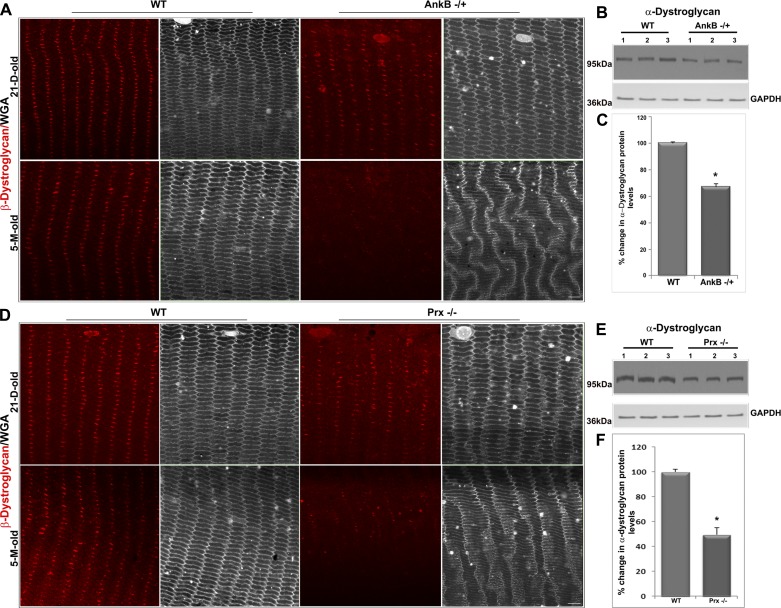
AnkB deficiency disrupts membrane organization of the dystrophin-glycoprotein complex in lens fibers.
The dystrophin-glycoprotein complex (DGC) is a multimeric transmembrane assembly that serves to link the extracellular matrix and actin cytoskeleton and plays a vital role in muscle cell physiology by providing mechanical support to the sarcolemma during muscle contraction (4, 17). AnkB interacts with the DGC complex through dystrophin-Dp71 to coordinate adhesive interactions at the costameres and neuromuscular junctions (1, 2). Similarly, Prx has been shown to interact with Drp-2 and maintain myelin sheath organization (27). Given the observation of impaired lens stiffness recorded in both AnkB−/+ and Prx−/− mice, we were interested in further exploring the possible influence of AnkB and Prx on membrane organization of the DGC in lens fibers. To pursue this objective, we initially examined the membrane organization pattern of β-dystroglycan (the transmembrane component of dystroglycan) compared with WGA labeling (in gray scale; Fig. 5) by immunofluorescence analysis of lens fibers from equatorial plane sections of young (P21) and old (5-mo-old) AnkB−/+ and Prx−/− mouse lenses. In WT lenses, β-dystroglycan localizes discretely in a large cluster at the center of the long arm of hexagonal fiber cells (Fig. 5). This clustered organization of β-dystroglycan was disrupted dramatically in 5-mo-old AnkB−/+ and Prx−/− mouse lens fibers (Fig. 5, A and D). In lens fibers of P21 AnkB−/+ and Prx−/− mice, however, the clustered pattern of β-dystroglycan appears to be only marginally altered relative to WT controls (Fig. 5, A and D). The disruption noted in membrane organization of β-dystroglycan in 5-mo-old lens fibers of both AnkB−/+ and Prx−/− mice was associated with a significant decrease in the protein levels of α-dystroglycan (in the total lens protein fraction) relative to WT controls, based on immunoblot analysis (Fig. 5, B, C, E, and F). Consistent with the stiffness changes noted in both P21 and 5-mo-old Prx−/− lenses, the DGC disruption is relatively severe in the Prx−/− lenses compared with AnkB−/+ lenses.
Fig. 5.
AnkB haploinsufficiency and Prx deficiency disrupt membrane organization of β-dystroglycan in mouse lens fibers. A: membrane organization of β-dystroglycan in mature fibers of AnkB−/+ lenses from P21 and 5-mo-old mice shows a dramatic disruption in 5-mo-old specimens relative to the characteristic pattern associated in WT lenses, based on confocal imaging (single-optical sections) of equatorial sections. These tissue specimens were also costained with WGA (shown in gray scale). B and C: the disruption of β-dystroglycan was associated with a significant decrease in protein levels of α-dystroglycan in the 800 g lens supernatant, based on immunoblot analysis (B shows data from 3 independent specimens) and densitometric quantification (C). GAPDH was immunoblotted as a loading control. D–F: similar to AnkB−/+ lenses, Prx−/− lenses also exhibit altered membrane organization of β-dystroglycan in 5-mo-old specimens compared with WT controls (D), correlating with a significant decrease in the protein levels of α-dystroglycan in the 800 g lens supernatant from 5-mo-old mice (E and F). Scale bars: 20 μm. Values in C and F are means ± SE of 8 independent specimens. *P < 0.05.
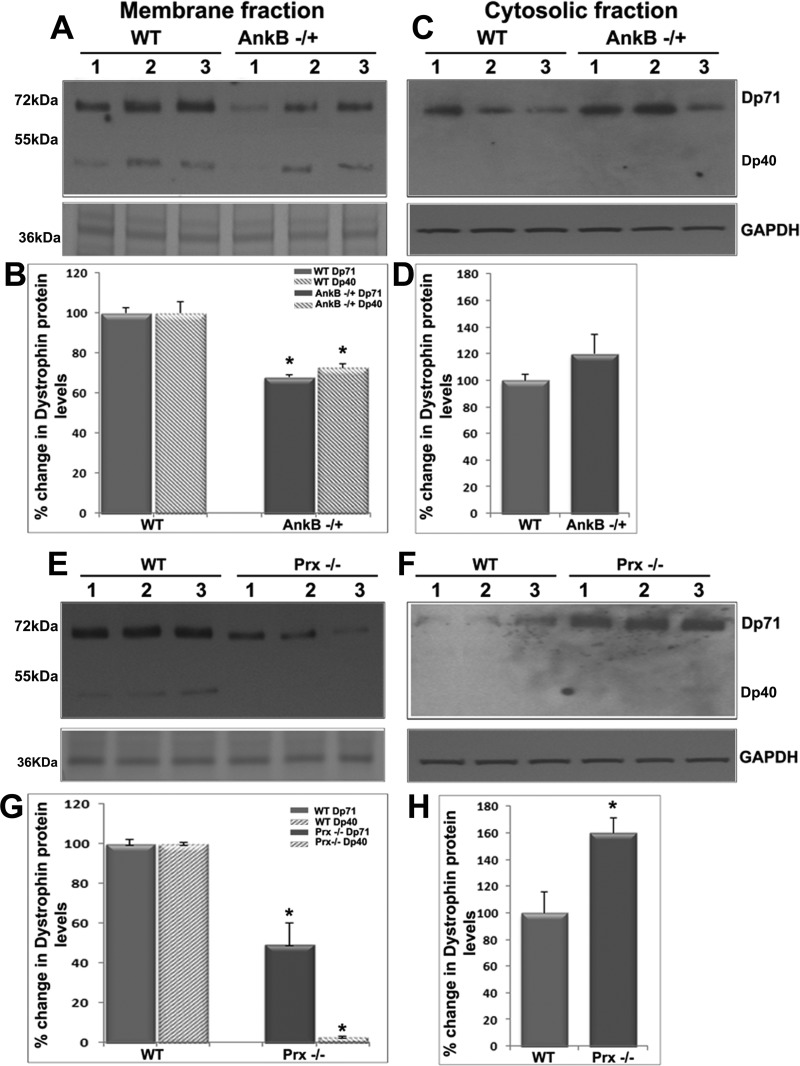
Moreover, since AnkB is known to interact with Dp71 in cardiomyocytes and skeletal muscle (1), and absence of Dp71 has been reported recently to result in cataract formation (12), we evaluated for changes in Dp71 protein levels in the soluble and membrane-enriched fractions of AnkB−/+ and Prx−/− mouse lenses compared with respective WT. In both AnkB−/+ and Prx−/− lenses, Dp71 protein levels were decreased significantly in the membrane-enriched fraction and simultaneous accumulation in the soluble fraction relative to WT lenses, based on immunoblot analysis (Fig. 6). Additionally, we also detected dystrophin isoform Dp40, which was found to be mostly membrane localized in normal lenses. Protein levels of Dp40 were decreased significantly in both AnkB−/+ and Prx−/− lenses relative to WT controls (Fig. 6). Collectively, the changes observed in both Dp71 and Dp40, together with those noted for dystroglycan, in both AnkB−/+ and Prx−/− mouse lenses, reveal a disintegration of the DGC and dystrophin-associated protein complex in the absence of AnkB and Prx. These changes appear to be age-dependent in both AnkB−/+ and Prx−/− mouse lenses.
Fig. 6.
AnkB haploinsufficiency and Prx deficiency disrupt membrane targeting of Dp71 in mouse lens fibers. The reciprocal changes in levels of Dp71 protein in the membrane-enriched fraction (decreases; A, B, E, G) vs. the soluble fractions (accumulation; C, D, F, H) of the AnkB−/+ (A–D) and Prx−/− (E–H) were recorded in mouse lenses. In AnkB−/+ and Prx−/− lenses, in addition to Dp71, the shorter form of dystrophin (Dp40) was also found to be significantly decreased in the membrane-enriched fractions compared with WT lenses. These changes were found to be more severe in the Prx−/− lenses relative to the AnkB−/+ lenses. A 36-kDa protein from the lens membrane-enriched fraction and GAPDH were used as loading controls for the lens membrane-enriched and soluble fractions, respectively. Lanes 1–3 show representative immunoblot profiles from three independent specimens. Values in B, D, G, and H are means ± SE of 7 independent specimens. *P < 0.05.
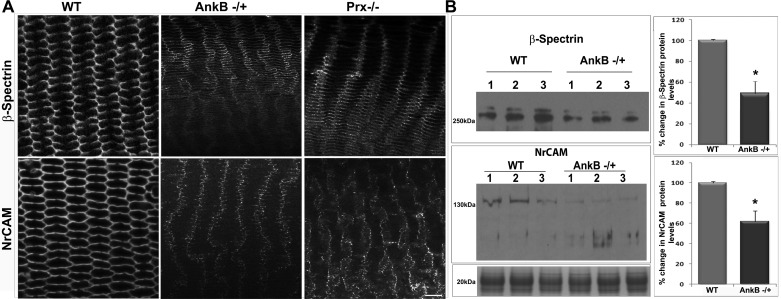
AnkB deficiency impairs membrane organization of the spectrin-actin network, NrCAM, L-type Ca2+ channel proteins, and myosin II activity in mouse lenses.
To understand the impact of changes in lens fiber cell shape, alignment, mechanics, and membrane organization of the DGC on cell adhesive interactions and membrane skeletal organization in AnkB−/+ mouse lenses, we used immunofluorescence and confocal imaging to examine membrane organization of β-spectrin and NrCAM, which colocalize with AnkB (Fig. 2) and are present in Prx immunoprecipitates from mouse lens homogenates (23). NrCAM, the transmembrane NgCAM-related L1 family of cell adhesive proteins, is involved in cell-cell adhesion and membrane organization of spectrin, ion channels, and signaling molecules (10, 24). Analysis of confocal images of lens inner cortical fibers (equatorial plane) from 5-mo-old AnkB−/+ and Prx−/− mice revealed gross disruption in the distribution pattern of β-spectrin and NrCAM (Fig. 7A), and decreased levels of β-spectrin and NrCAM in the membrane-enriched fraction of AnkB−/+ lenses, relative to WT controls (Fig. 7B). Similar to AnkB−/+ mouse lenses, Prx−/− mouse lenses have been found to exhibit decreased levels of β-spectrin in the membrane-enriched fraction (23). These results reveal the destabilization of membrane skeleton and NrCAM-based cell adhesive properties in AnkB- and Prx-deficient lenses. The bottom immunoblot in Fig. 5B shows a representative protein (20 kDa) staining profile used for normalization of loading for membrane-rich fractions.
Fig. 7.
Disruption of membrane organization of β-spectrin and NrCAM in AnkB−/+ and Prx−/− mouse lenses. A: equatorial plane sections from 5-mo-old AnkB−/+, Prx−/−, or WT lenses were immunostained for β-spectrin and NrCAM. Representative single-optical images of individual proteins are shown for comparison (in gray scale). The membrane organization of β-spectrin and NrCAM was disrupted in both AnkB−/+ and Prx−/− mouse lens fibers compared with WT lenses. B: immunoblotting-based quantification of changes in levels of β-spectrin (top) and NrCAM (bottom) proteins in membrane-enriched fractions from AnkB−/+ lenses revealed a significant decrease compared with the WT controls. Staining intensities of proteins in the range of 18–22 kDa from the lens membrane-enriched fraction were used to normalize for loading in immunoblotting analyses. Scale bar: 20 μm. Values are means ± SE of 8 independent specimens. *P < 0.05.
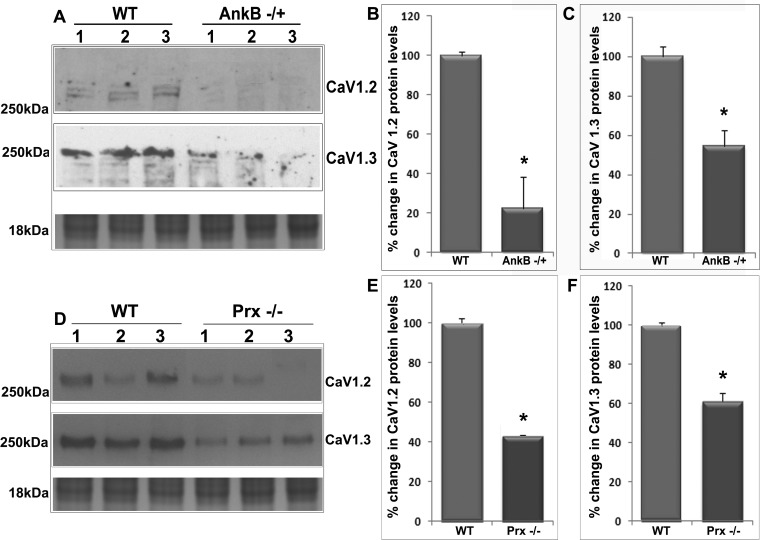
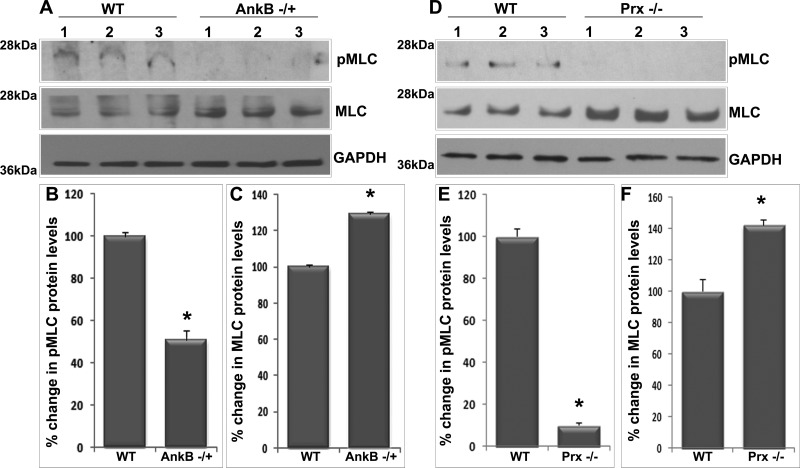
Since AnkB has been demonstrated to regulate membrane targeting of CaV1.3 L-type calcium channel in cardiac myocytes (8) and in a previous study our laboratory found that inhibition of L-type Ca2+ channels in the lens results in decreased levels of pMLC (a regulatory subunit of myosin II) (22), we asked ourselves whether AnkB or Prx deficiency might influence L-type Ca2+ channel membrane stability and MLC phosphorylation. To address this question, we determined protein content of L-type Ca2+ channel (CaV1.2 and CaV1.3) in the membrane-enriched fraction of AnkB−/+ (Fig. 8, A–C) and Prx−/− lenses (Fig. 8, D–F) by immunoblotting analysis. These analyses showed a significant decrease in the levels of CaV1.2 and CaV1.3 proteins in the membrane-enriched fraction (Fig. 8) in both AnkB−/+ and Prx−/− mouse lenses (5 mo old) compared with their respective WT controls. The bottom immunoblots in Fig. 8, A and D, represent staining profiles used for normalization of protein loading. Similarly, we also examined changes in levels of pMLC and total MLC (Fig. 9) in both AnkB−/+ and Prx−/− (5 mo old) lenses by immunoblot analysis and found that levels of pMLC (Fig. 9) were decreased significantly in these lenses compared with their WT controls. Protein levels of total MLC, in contrast, were increased significantly in both AnkB−/+ and Prx−/− (Fig. 9) compared with the WT lenses. These results indicate the importance of AnkB and Prx in regulating L-type calcium channel membrane organization and eventually influencing MLC phosphorylation in lens fibers.
Fig. 8.
Decreased levels of L-type Ca2+ channel proteins in AnkB−/+ and Prx−/− mouse lenses. A–C: significantly decreased voltage-gated calcium channels CaV1.2 (A and B) and CaV1.3 (A and C) protein content in AnkB−/+ lenses (membrane-enriched fraction from 5-mo-old mice) compared with WT lenses based on immunoblot quantification. D–F: significantly decreased CaV1.2 (D and E) and CaV1.3 (D and F) channel protein content in Prx−/− lenses (5 mo old) compared with WT lenses. In A and D, lanes 1–3 show representative immunoblot profiles from three independent specimens. Staining of proteins from membrane-enriched fraction (in the range of 16–18 kDa) was used as a loading control. Values in B, C, E, and F are means ± SE of 4–6 independent observations. *P < 0.05.
Fig. 9.
Deficiency of AnkB and Prx impairs myosin light chain (MLC) phosphorylation (pMLC) in the mouse lens. A–C: significantly decreased pMLC (A and B) and increased MLC (A and C) in AnkB−/+ lenses based on immunoblot quantification. GAPDH serves as a loading control. D–F: significantly decreased pMLC (D and E) and increased MLC (D and F) in Prx−/− lenses. Values in B, C, E, and F are means ± SE of 4 independent observations. *P < 0.05.
DISCUSSION
The main goal of this study was to determine whether AnkB regulates membrane association of Prx, and whether the interaction between AnkB and Prx is required for establishment and maintenance of lens fiber cell hexagonal geometry, membrane organization, and biomechanical properties. The intrinsic influence of AnkB on membrane tethering of Prx and its colocalization with Prx in the fiber cell, together with the parallel and progressive changes observed in fiber cell phenotype and stiffness in the AnkB haploinsufficient and Prx null mouse lenses in this study, reveal that the membrane scaffolding role played by Prx in lens fiber cells is regulated in part by interaction of Prx with AnkB. The results of this study also demonstrate that AnkB plays a crucial role in maintaining fiber cell hexagonal shape, membrane organization, and biomechanical characteristics, which are key determinants of lens cytoarchitecture, optical clarity, and tensile properties via Prx-based, as well as Prx-independent, mechanisms.
Differentiated fiber cells, which constitute the bulk of the lens, are derived from epithelial cells that exit the cell cycle at the lens equator. Subsequently, they embark on a differentiation process that induces extensive cell elongation, membrane changes, reorganization of the cortical cytoskeleton, and cell adhesive complexes, and expression of various fiber cell abundant and specific proteins (20, 32). Postmitotic lens fiber cells progressively attain a perfect hexagonal shape as they differentiate, undergo compaction and denucleation, and are moved from the outer cortical area into the inner cortical region (19). Fiber cells are closely packed and arranged in perfect radial rows in the cortical region (25). In contrast, fiber cells in the core (center or nucleus) region of the lens, while transparent, do not possess a hexagonal shape and symmetric alignment (19, 29). This spatial program of structural specializations of lens fiber cells poses several interesting questions, including the following. What are the molecular determinants of fiber cell hexagonal geometry and membrane organization in lens? Why do cortical fiber cells need to maintain a hexagonal shape? Does a definitive functional correlate drive the formation of the unique prismoidal shape assumed by the inner cortical fibers of the lens? It is also not clear whether the hexagonal geometry of cortical lens fibers is ideally suited for withstanding the frequent changes in mechanical load that these cells encounter during accommodation-associated lens deformability. The observations documented in this study offer several novel insights into the role(s) of AnkB and Prx in these less than well-understood aspects of lens fiber geometry and function.
When evaluated in the context of absence of developmental defects in lenses of AnkB null (24) and haploinsufficient mice and Prx null mice, these data indicate that the primary role(s) of AnkB and Prx are relevant to lens cytoarchitecture and biomechanical properties, which are dependent on cell shape and adhesive interactions. Identical to what was seen in the Prx−/− mouse lenses(23), AnkB−/+ lens fibers revealed obvious abnormalities, especially in the inner cortical region, with fiber cells losing their characteristic hexagonal shape and radial packing pattern and exhibiting instead a collapsible cell shape with widely varying widths (Fig. 3). Importantly, Prx organization was disrupted dramatically in the fiber cells of both younger (<P30) and aged (>4 mo old) AnkB−/+ lenses. This disruption in membrane organization of Prx was found to be due to impaired membrane tethering, as evidenced by the accumulation of Prx in the soluble fraction, in contrast to the predominantly membrane-bound distribution in WT lenses (Fig. 1). Moreover, AnkB and Prx appear to colocalize at vertices, long and short arms and membrane subdomains of lens fiber cells (Fig. 2), indicating their role in maintaining fiber cell hexagonal geometry and other characteristics.
Although, the structural requirements for colocalization between AnkB and Prx are not clear at present, AnkB appears to intrinsically regulate Prx membrane organization in lens fibers. Interestingly, at the fiber cell vertices, we also found AnkB exhibiting relatively intense colocalization with β-spectrin and NrCAM. Similarly, Prx also exhibits colocalization with β-spectrin and NrCAM at the fiber cell vertices. These observations infer that, at fiber cell vertices, Prx coexist as a multiprotein complex with AnkB, β-spectrin, and NrCAM. Moreover, in the absence of AnkB or Prx, there was a dramatic and parallel disruption in fiber cell hexagonal shape and spectrin-actin network, indicating that AnkB and Prx regulate fiber cell hexagonal shape through mutual interactions with β-spectrin and NrCAM. While the direct interactions of AnkB with β-spectrin and NrCAM have been well established (6, 9, 10), further studies focused on specific protein-protein interaction analyses are required to confirm the existence of direct interaction of Prx with AnkB, β-spectrin, and NrCAM. Additionally, AnkB extensive colocalization with NrCAM at the lateral membrane subdomains of long arms of fiber cells, which is not found with Prx, along with the noted decrease of NrCAM in the membrane-enriched fraction derived from AnkB−/+ lenses (data not shown), indicate the significance of AnkB in maintaining lens fiber cell shape and mechanics, independent of Prx through NrCAM. AnkB has been demonstrated to interact directly with NrCAM and together regulate membrane domain organization of several proteins in neurons (9, 10). The absence of NrCAM has been reported to develop cataract and disruption of fiber cell arrangement in mice (24). These observations collectively support the importance of AnkB in lens architecture, wherein AnkB regulates the membrane organization of Prx, NrCAM, β-spectrin, and other proteins. In contrast to the influence of AnkB on Prx membrane organization discussed above, a complete absence of Prx had very little to no effect on the membrane localization of AnkB in young and clear lenses. Although there was a reduction of AnkB in the membrane-enriched fraction in 5-mo-old Prx null lenses, there was no concomitant accumulation of this protein in the soluble fraction. It is possible that the observed decrease in AnkB levels in Prx null lenses is partly related to transcriptional alterations.
Interestingly, in addition to spectrin-actin membrane skeleton, the membrane organization of β-dystroglycan, Dp71, and Dp40 was also found to be disrupted dramatically in 5-mo-old AnkB−/+ and Prx null lenses. Dp71, the predominant isoform of dystrophin in lens, was noted to accumulate in the soluble fraction, concomitant with a significant decrease in the membrane-enriched fraction of both AnkB−/+ and Prx−/− lenses, indicating that reduced levels of AnkB or the absence of Prx result in impaired membrane association of Dp71. Although both AnkB and Prx are known to interact directly with dystrophin and Drp-2 in skeletal muscle and Schwann cells, respectively, serving to connect the actin cytoskeleton to the extracellular matrix through a transmembrane component (β-dystroglycan) and an extracellular component of dystroglycan (α-dystroglycan), Dp71, and Drp-2 (1, 27), no appreciable colocalization was noted between AnkB and β-dystroglycan in lens fibers. Moreover, the disruption of membrane organization of β-dystroglycan in lens fibers was found to be dramatic in aged compared with young AnkB−/+ and Prx−/− mice, indicating the changes in DGC and dystrophin-associated protein complex appear to be via acquired rather than an intrinsic activity of AnkB or Prx in lens. Collectively, it is conceivable that the acquired and coordinated responses of Prx and AnkB on the membrane organization of DGC and Dp71 suggest their critical role for maintaining lens fiber cell shape and mechanical properties. Additionally, disruption of the coordinated scaffolding activity of AnkB and Prx also appears to destabilize organization of other membrane proteins, including L-type Ca2+ channels in lens fibers. Further studies are needed to determine whether AnkB and Prx directly influence membrane organization of L-type Ca2+ channels in lens fibers.
Our data also revealed decreased compressive stiffness of both AnkB−/+ and Prx−/− lenses, consistent with the disruption of membrane organization of DGC, and the spectrin-actin network in fiber cells from these lenses. In addition to optical properties, the focusing ability of the lens depends on its finely tuned tensile properties. In accommodating species, the lens changes its shape to accommodate the near and far focusing of an object (3, 31). The mechanical properties of the lens fibers must, therefore, be permissive to rapid shape changes that occur during accommodation (13, 16). Disruption of the fiber cell membrane skeleton (spectrin-actin) in the absence of tropomodulin-1 has been reported to result in reduction of lens mechanical stiffness (16). Although we have not examined the status of tropomodulin-1 in either AnkB−/+ or Prx−/− lenses, we speculate that the organization of this protein is likely disrupted in the absence of AnkB and Prx based on the altered spectrin-actin cytoskeleton noted in these lenses. Our observations imply that the cell shape changes resulting from the primary deficiency of AnkB and Prx, in conjunction with secondary deficiency of proteins, which interact with AnkB, such as dystrophin/dystroglycan, β-spectrin, NrCAM, and L-type Ca2+ channels, along with impaired myosin II activity noted in this study, directly influence the lens stiffness. Changes in lens stiffness imply disruptions in mechanical load-bearing characteristics of the fiber cell and likely the mechanotransduction of lens fibers, which are relevant to lens deformability and accommodation. Decreased MLC phosphorylation noted in the AnkB−/+ and Prx−/− lenses could be partly related to calcium signaling regulated by L-type Ca2+ channels (21, 22). In contrast to pMLC, total MLC levels were increased under deficiency of AnkB and Prx. This effect on MLC is consistent with our earlier observation with inhibition of L-type Ca2+ channels in mouse lens (22) and suggestive of an adaptive response toward impaired MLC phosphorylation in the AnkB- and Prx-deficient lenses. From these observations, we can also conclude that, unlike in rodent lenses, the influence of AnkB and Prx on lens mechanical stiffness is expected to be a key factor of physiological significance for vision in accommodative species, including humans.
GRANTS
This work is supported by National Eye Institute Grants to P. V. Rao (R01-EY-025096 and R01-EY-018590) and the National Eye Institute Vision Core Grant P30-EY-005722. V. Bennett is an investigator of the Howard Hughes Medical Institute. P. J. Brophy is supported by the Wellcome Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M., V.B., and P.V.R. conception and design of research; R.M., M.W., and P.V.R. performed experiments; R.M., M.W., P.J.B., V.B., and P.V.R. analyzed data; R.M., M.W., P.J.B., V.B., and P.V.R. interpreted results of experiments; R.M. prepared figures; R.M. and P.V.R. drafted manuscript; R.M., M.W., P.J.B., V.B., and P.V.R. edited and revised manuscript; R.M., M.W., P.J.B., V.B., and P.V.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Janell Hostettler for arranging the AnkB−/+ mice.
REFERENCES
- 1.Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135: 1189–1200, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Ayalon G, Hostettler JD, Hoffman J, Kizhatil K, Davis JQ, Bennett V. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem 286: 7370–7378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banh A, Bantseev V, Choh V, Moran KL, Sivak JG. The lens of the eye as a focusing device and its response to stress. Prog Retin Eye Res 25: 189–206, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 119: 199–207, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81: 1353–1392, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Bennett V, Lorenzo DN. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Top Membr 72: 1–37, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, Brophy PJ. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature 431: 191–195, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cunha SR, Hund TJ, Hashemi S, Voigt N, Li N, Wright P, Koval O, Li J, Gudmundsson H, Gumina RJ, Karck M, Schott JJ, Probst V, Le Marec H, Anderson ME, Dobrev D, Wehrens XH, Mohler PJ. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation 124: 1212–1222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem 269: 27163–27166, 1994. [PubMed] [Google Scholar]
- 10.Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J Cell Biol 135: 1355–1367, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dytrych L, Sherman DL, Gillespie CS, Brophy PJ. Two PDZ domain proteins encoded by the murine periaxin gene are the result of alternative intron retention and are differentially targeted in Schwann cells. J Biol Chem 273: 5794–5800, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Fort PE, Darche M, Sahel JA, Rendon A, Tadayoni R. Lack of dystrophin protein Dp71 results in progressive cataract formation due to loss of fiber cell organization. Mol Vis 20: 1480–1490, 2014. [PMC free article] [PubMed] [Google Scholar]
- 13.Fudge DS, McCuaig JV, Van Stralen S, Hess JF, Wang H, Mathias RT, FitzGerald PG. Intermediate filaments regulate tissue size and stiffness in the murine lens. Invest Ophthalmol Vis Sci 52: 3860–3867, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie CS, Sherman DL, Blair GE, Brophy PJ. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron 12: 497–508, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie CS, Sherman DL, Fleetwood-Walker SM, Cottrell DF, Tait S, Garry EM, Wallace VC, Ure J, Griffiths IR, Smith A, Brophy PJ. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron 26: 523–531, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Gokhin DS, Nowak RB, Kim NE, Arnett EE, Chen AC, Sah RL, Clark JI, Fowler VM. Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens. PLoS One 7: e48734, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355: 696–702, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155: 739–746, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res 78: 673–687, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol 280: 1–14, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Mace OJ, Morgan EL, Affleck JA, Lister N, Kellett GL. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol 580: 605–616, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddala R, Nagendran T, de Ridder GG, Schey KL, Rao PV. L-type calcium channels play a critical role in maintaining lens transparency by regulating phosphorylation of aquaporin-0 and myosin light chain and expression of connexins. PLoS One 8: e64676, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddala R, Skiba NP, Lalane R 3rd, Sherman DL, Brophy PJ, Rao PV. Periaxin is required for hexagonal geometry and membrane organization of mature lens fibers. Dev Biol 357: 179–190, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.More MI, Kirsch FP, Rathjen FG. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J Cell Biol 154: 187–196, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak RB, Fischer RS, Zoltoski RK, Kuszak JR, Fowler VM. Tropomodulin1 is required for membrane skeleton organization and hexagonal geometry of fiber cells in the mouse lens. J Cell Biol 186: 915–928, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salim C, Boxberg YV, Alterio J, Fereol S, Nothias F. The giant protein AHNAK involved in morphogenesis and laminin substrate adhesion of myelinating Schwann cells. Glia 57: 535–549, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Sherman DL, Fabrizi C, Gillespie CS, Brophy PJ. Specific disruption of a schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron 30: 677–687, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci 116: 4985–4995, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci 37: 1396–1410, 1996. [PubMed] [Google Scholar]
- 30.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol 147: 995–1008, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeber HA, van der Heijde RG. On the relationship between lens stiffness and accommodative amplitude. Exp Eye Res 85: 602–607, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem 57: 479–504, 1988. [DOI] [PubMed] [Google Scholar]