Abstract
Since parathyroid hormone (PTH) is known to increase transient receptor potential vanilloid (TRPV)5 activity and decrease Na+-Cl− cotransporter (NCC) activity, we hypothesized that decreased NCC-mediated Na+ reabsorption contributes to the enhanced TRPV5 Ca2+ reabsorption seen with PTH. To test this, we used mDCT15 cells expressing functional TRPV5 and ruthenium red-sensitive 45Ca2+ uptake. PTH increased 45Ca2+ uptake to 8.8 ± 0.7 nmol·mg−1·min−1 (n = 4, P < 0.01) and decreased NCC activity from 75.4 ± 2.7 to 20.3 ± 1.3 nmol·mg−1·min−1 (n = 4, P < 0.01). Knockdown of Ras guanyl-releasing protein (RasGRP)1 had no baseline effect on 45Ca2+ uptake but significantly attenuated the response to PTH from a 45% increase (6.0 ± 0.2 to 8.7 ± 0.4 nmol·mg−1·min−1) in control cells to only 20% in knockdown cells (6.1 ± 0.1 to 7.3 ± 0.2 nmol·mg−1·min−1, n = 4, P < 0.01). Inhibition of PKC and PKA resulted in further attenuation of the PTH effect. RasGRP1 knockdown decreased the magnitude of the TRPV5 response to PTH (7.9 ± 0.1 nmol·mg−1·min−1 for knockdown compared with 9.1 ± 0.1 nmol·mg−1·min−1 in control), and the addition of thiazide eliminated this effect (a nearly identical 9.0 ± 0.1 nmol·mg−1·min−1). This indicates that functionally active NCC is required for RasGRP1 knockdown to impact the PTH effect on TRPV5 activity. Knockdown of with no lysine kinase (WNK)4 resulted in an attenuation of the increase in PTH-mediated TRPV5 activity. TRPV5 activity increased by 36% compared with 45% in control (n = 4, P < 0.01 between PTH-treated groups). PKC blockade further attenuated the PTH effect, whereas combined PKC and PKA blockade in WNK4KD cells abolished the effect. We conclude that modulation of NCC activity contributes to the response to PTH, implying a role for hormonal modulation of NCC activity in distal Ca2+ handling.
Keywords: parathyroid hormone, transient receptor potential vanilloid 5 channel, sodium-chloride cotransporter, thiazide, with no lysine kinase
ca2+ homeostasis is carefully maintained in the body, and renal Ca2+ handling is critical for this process. Abnormal renal Ca2+ reabsorption is associated with a compromised Ca2+ balance and accompanying pathology, such as nephrolithiasis.
Kidney tubule reabsorption of filtered Ca2+ occurs primarily in the proximal tubule (PT), thick ascending limb (TAL), distal convoluted tubule (DCT), and connecting tubule (11, 25). Ca2+ uptake in the PT and TAL occurs via a paracellular pathway, whereas DCT Ca2+ uptake occurs transcellularly (15, 30, 38). Ca2+ enters DCT cells, specifically late DCT (DCT2) and connecting tubule cells, via the epithelial Ca2+ channel transient receptor potential vanilloid (TRPV)5 (15). Once inside the cell, Ca2+ binds to calbindin-D28K to transit across the cell for eventual basolateral extrusion via ATP-dependent Ca2+-ATPase [plasma membrance Ca2+-ATPase (PMCA)1] and Na+/Ca2+ exchanger (NCX)1 (3, 20, 24, 27).
In addition to its role in Ca2+ reabsorption, the DCT is a principal site of Na+ reabsorption, taking in ∼5–10% of the filtered load of Na+ (13). DCT Na+ reabsorption occurs primarily via the Na+-Cl− cotransporter (NCC). Interestingly, blockade of NCC by thiazide diuretics has long been known to reduce urinary Ca2+ excretion (8, 23, 33). More recent studies have attributed this phenomenon primarily to increased passive proximal reabsorption of Ca2+ (31). Multiple studies have documented increased DCT Ca2+ reabsorption in response to thiazide administration (9, 14, 26, 28, 29). This suggests that in the DCT, Na+ transport does affect Ca2+ transport, and, thus, modulation of NCC activity may affect Ca2+ transport by TRPV5. Since parathyroid hormone (PTH) is known to increase TRPV5 activity and decrease NCC activity, we theorized that decreased NCC-mediated Na+ reabsorption may contribute to the enhanced TRPV5 Ca2+ reabsorption seen with PTH administration (21, 39).
One of the principle regulatory pathways for NCC involves with no lysine kinases (WNKs). In particular, WNK4 is well known for its effects on NCC, with both inhibitory and stimulatory effects (4, 5, 35, 37, 41, 42). More recently, WNK4 has been shown to affect TRPV5 (6, 17, 18). We therefore hypothesized that WNK4 may act as the link between NCC and TRPV5 activity.
Here, through the use of a cell model with native NCC and TRPV5 activity, we report that modulation of NCC activity does indeed contribute to the TRPV5 response to PTH, implying a role for hormonal modulation of NCC activity in distal Ca2+ handling. Furthermore, we demonstrate that the NCC effect on TRPV5 appears to be related to WNK4.
METHODS
Materials.
Materials were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
Cell culture and treatments.
mDCT15 or WNK4KD cells previously generated and characterized (22) were plated on cell culture dishes and grown in growth medium containing a 50:50 mix of DMEM-F-12, 5% heat-inactivated FBS, and 1% penicillin-streptomycin-neomycin at 37°C. Experiments were conducted when cells reached 90–95% confluence. For WNK4KD cells, RNA inhibitor suppression of WNK4 was reconfirmed via immunoblot analysis.
RT-PCR.
RNA was collected from mDCT15 cells grown to 95% confluence using the QIAGEN RNeasy Mini Kit. Using the SuperScript One-Step RT-PCR with Platinum Taq with a final concentration of 1 μg RNA, cDNA synthesis, PCR amplification, and final extension were carried out. The sequences were as follows: PMCA4, 5′-CTTAATGGACCTGCGAAAGC-3′ and 5′-ATCTGCAGGGTTCCCAGATA-3′; NCX1, 5′-CTCCCTTGTGC-TTGAGGAAC-3′ and 5′-CAGTGGCTGCTTGTCATCAT-3′; TRPV5, 5′-CTGGAGCTTGTGGTTTCCTC-3′ and 5′-TCCACTTCAGGCTCACCAGS-3′; and calbindin, 5′-CTAGCAGAGTACACAGACCTC-3′ and 5′-GTATCCGTTGCCATCCTGATC-3′. Samples were then analyzed using a 2% agarose gel. Loading dye was added to each sample, and 10 μl of sample were loaded onto the gel. The gel ran for 30 min at 120 V and was analyzed under ultraviolet light.
Assessment of NCC function in cells.
mDCT15 or WNK4KD cells were seeded in 12-well plates and prepared as described above. Cells were then incubated in a serum-free growth media (Opti-Mem) for 24 h before being assayed. Cells were then treated with PTH or vehicle (DMSO) for the indicated times and concentrations. Thirty minutes before uptake, 0.1 mM metolazone (an inhibitor of NCC) or vehicle (DMSO) was added to the media to ensure NCC inhibition during the uptake period. The medium was then changed to a 22Na+-containing medium [containing 140 mM NaCl or as indicated, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES-Tris (pH 7.4), 1 μM amiloride, 0.1 mM bumetanide, 1 μM benzamil, 1 mM ouabain, and 1 μCi/ml 22Na+] with or without thiazide (0.1 mM metolazone) and incubated for 20 min. Tracer uptake was then stopped via washes with ice-cold wash buffer. Cells were subsequently lysed with 0.1% SDS. Radioactivity was measured via liquid scintillation, and protein concentrations of the lysates were determined [Bicinchoninic Acid (BCA) Protein Assay, Pierce]. Uptakes were normalized to nanomoles per milligram per minute. Thiazide-sensitive uptake was given by the difference of the uptakes with and without thiazide. For the Na+ clamping experiments, the indicated NaCl concentration was placed in the 22Na+-containing uptake medium.
Assessment of TRPV5 function in cells.
mDCT15 or WNK4KD cells were seeded in 12-well plates and prepared as described above. Cells were then incubated in serum-free growth media (Opti-Mem) for 24 h before being assayed. Cells were then treated with PTH or vehicle (DMSO) for the indicated times and concentrations. Thirty minutes before uptake, 100 nM ruthenium red (an inhibitor of TRPV5) or vehicle (DMSO) was added to the media to ensure TRPV5 inhibition during the uptake period. The medium was then changed to 45Ca2+-containing medium [containing 140 mM NaCl, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES-Tris (pH 7.4), 1 mM amiloride, 0.1 mM bumetanide, 0.1 mM benzamil, and voltage-gated Ca2+ channel inhibitors (10 μM felodipine and 10 μM verapamil), 50 μM vanadate, 1 mM ouabain, and 1 μCi/ml 45Ca2+] with or without 100 nM ruthenium red and incubated for 20 min. Tracer uptake was then stopped via washes with ice-cold wash buffer. Cells were subsequently lysed with 0.1% SDS. Radioactivity was measured via liquid scintillation, and protein concentrations of the lysates were determined (BCA Protein Assay, Pierce). Uptakes were normalized to nanomoles per milligram per minute. TRPV5 uptake was given by the difference of the uptakes with and without ruthenium red.
Electrophysiology experiments in cells.
mDCT15 cells were grown on coverglasses to confluence DMEM-F-12 (Cellgro) supplemented with 5% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 50 nM dexamethasone. The single channel activity of TRPV5-like channel on the membrane of mDCT15 cells was determined under voltage-clamp conditions in the outside-out configuration. Gap-free single channel current data from GΩ seals were acquired with an Axopatch 200B (Molecular Devices) patch-clamp amplifier interfaced via a Digidata 1440 (Molecular Devices). Currents were low-pass filtered at 1 kHz with an eight-pole Bessel filter (Warner Instruments). Events were inspected visually before acceptance. Recording pipettes had resistances of 5–8 MΩ. Bath solution contained (in mM) 140 NaCl, 10 EDTA, and 10 HEPES (pH 7.31); pipette solution containined (in mM) 150 CsCl, 10 BAPTA, and 10 HEPES (pH 7.3) Current-voltage relationships were obtained by monitoring channel activity at applied pipette voltages from −120 to +160 mV for at average of 60 s. Channel activity and open probability were assessed using Clampfit 10.5 software (Molecular Devices). To calculate open probability in paired experiments, the number of channels was fixed as the greatest number of active channels observed in control or experimental conditions. For representation, current traces were filtered at 200 Hz.
Cell surface biotinylation.
mDCT15 or WNK4KD cells were incubated as described above. Cells were washed with PBS, and cell surface proteins were labeled with Sulfo-NHS-SS-Biotin (Pierce) in PBS for 30 min at 4°C. The reaction was quenched by adding 500 μl of the quenching solution (Pierce). Cells were harvested, lysed using lysis buffer containing protease inhibitor, and homogenized by sonication on ice. Cell lysates were centrifuged briefly, and the supernatant was collected. Eighty microliters of the supernatant from each group were stored separately at −80°C. Biotinylated proteins in cell lysates were isolated by an incubation with NeutrAvidin gel (Pierce) for 60 min at room temperature. The labeled proteins were washed and eluted in SDS-PAGE sample buffer containing 50 mM DTT as per the protocol outlined in the Pierce Surface Protein Isolation Kit. Protein concentrations were determined by using a BCA Protein Assay Kit (Pierce). The eluted proteins and cell lysates were immunoblotted as detailed below.
Immunoblot analysis.
Cells were incubated as described above. Cells were harvested, lysed using lysis buffer containing protease inhibitor, and homogenized by sonication on ice. Cell lysates were centrifuged briefly, and the supernatant was collected. Proteins were resolved by SDS-PAGE and then transferred electrophoretically to polyvinylidene difluoride membranes. After being blocked with 3% BSA, membranes were probed with the following primary antibodies: NCC (1:1,000-1:5,000) (22), WNK4 (University of Dundee, 5 μg/ml), TRPV5 (AbCam, 1:35,000), and actin (Santa Cruz Biotechnology, 1:1,000) overnight at 4°C. Blots were washed in Tris-buffered saline-Tween 20. Signal detection was done via Odyssey Infrared Imaging (Li-Cor Biosciences). The secondary antibodies used were IRDye680 goat anti-mouse (Rockland Immunochemicals, dilution 1:10,000). Membranes were subsequent scanned using the Odyssey Infrared Imager. The intensity of the protein bands was analyzed using Odyssey Infrared Imaging Software (Li-Cor Biosciences).
Statistical analysis.
Statistical analysis was performed using the SigmaPlot software package (Systat, San Jose, CA). Data were analyzed for statistical significance using a paired t-test or ANOVA (Holm-Sidak). P values of <0.05 were taken as statistically significant.
RESULTS
mDCT15 cells exhibit native TRPV5 activity.
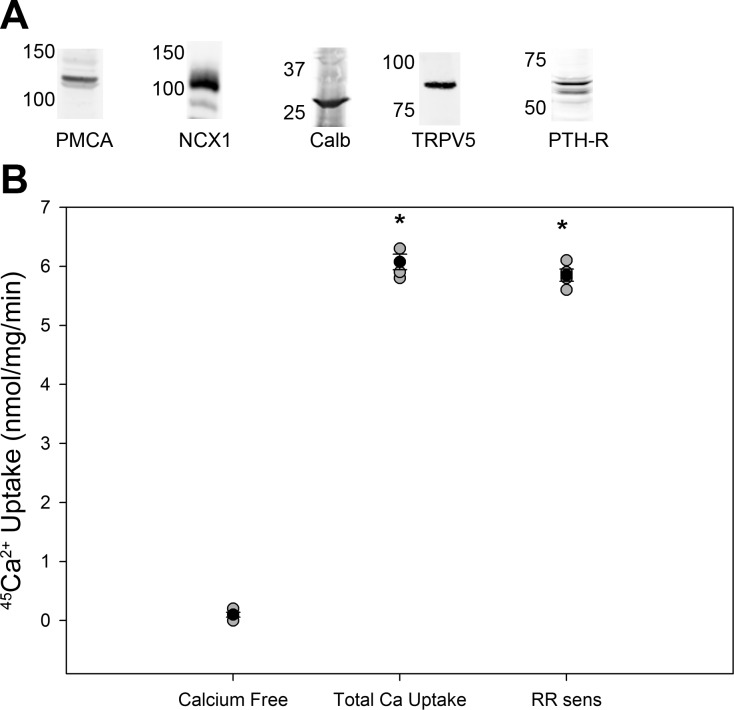
To study the effects of NCC activity on TRVP5 activity, mDCT15 cells were used. mDCT15 cells are known to exhibit native NCC activity (22). To assess for native Ca2+ transport, the presence of tubular proteins needed for Ca2+ transport was assessed by immunoblot analysis, confirming the presence of TRPV5, PMCA1, NCX, PTH receptor, and calbindin (Fig. 1A). Functional Ca2+ uptake was then confirmed via 45Ca2+ radiotracer uptake as described above in methods. mDCT15 cells exhibited native Ca2+ transport activity, with an uptake of 6.1 ± 0.3 nmol·mg−1·min−1 (Fig. 1B). Virtually all of this uptake, 5.9 ± 0.2 nmol·mg−1·min−1, was sensitive to ruthenium red, indicating that this native Ca2+ uptake was due to TRPV5 activity.
Fig. 1.
Demonstration of functional Ca2+ transport in mDCT15 cells. A: representative Western blots demonstrating expression of plasma membrane Ca2+-ATPase (PMCA)1, Na+/Ca2+ exchanger (NCX)1, calbindin (Calb), transient receptor potential vanilliod (TRPV)5, and parathyroid hormone (PTH) receptor (PTH-R). B: 45Ca2+ uptake in mDCT15 cells under Ca2+-free conditions and with Ca2+ and Ca2+ and 200 nM ruthenium red (RR sens; n = 4). *P < 0.01 compared with Ca2+-free conditions. Solid circles indicate means, shaded circles indicate individual data points, and bars represent SEs.
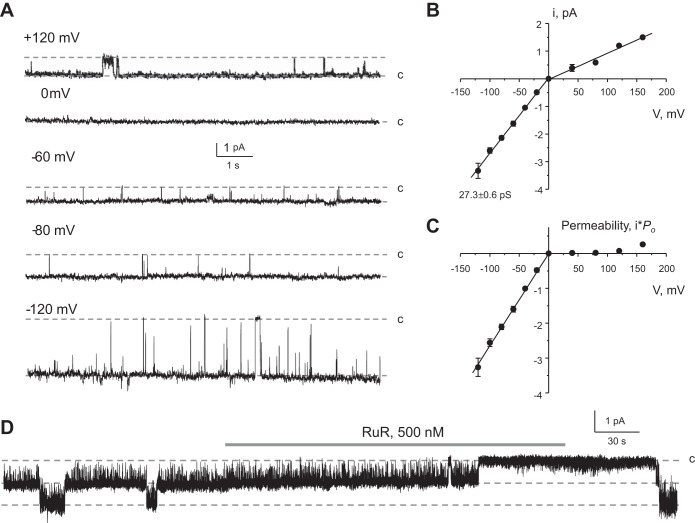
To confirm the presence of active TRPV5 channels, we next characterized TRPV5 activity at the single channel level in outside-out patches from mDCT15 cells. Since single channel conductance becomes negligibly small in the presence of Ca2+ (34), TRPV5 activity was monitored in divalent-free solutions. Typical patch-clamp recordings at different pipette potentials and the respective current-voltage relationships are shown in Fig. 2, A and B, respectively. Channel activity was high at negative voltages with an estimated single channel conductance of 27.3 ± 0.6 pS. This value is similar to that reported for single channel TRPV5 activity (30–40 pS) in excised patches (10, 36, 40). In contrast, channel activity and open probability were very low at positive voltages (Fig. 2A), which are also characteristics of TRPV5 (32). Overall, the recorded channel demonstrated remarkable inward rectification (Fig. 2C). Furthermore, channel activity can be reversibly inhibited by submicromolar concentrations of ruthenium red (500 nM), as shown in a representative continuous experiment in Fig. 2D. This is consistent with the reported high sensitivity of TRPV5 (ECaC1) to this blocker [IC50: ∼125 nM (see Ref. 16)]. In contrast, TRPV6 (ECaC2) is less sensitive to ruthenium red and can be inhibited by much higher concentrations of ruthenium red (IC50: ∼9 μM). Overall, the single channel conductance, strong inward rectification, and high sensitivity to ruthenium red strongly suggest that the detected channel in mDCT15 cells has properties attributable to TRPV5.
Fig. 2.
Functional properties of TRPV5-like channel in mDCT15 cells. A: representative current traces of single channel activity recorded from the same outside-out patch at different pipette potentials as indicated. c denotes the closed nonconductant state. B: average current-voltage (i-V) relationship of the unitary current amplitude for channels similar to that shown in A. Each value presented as the mean ± SE from an average of 4 individual experiments. The single channel conductance at negative voltages (27.3 ± 0.6 pS) was estimated as a slope of the linear fit. C: summary graph of channel permeability calculated as single channel amplitude multiplied by the value of open probability (Po) at any given voltage. D: representative continuous current trace from an outside-out patch monitoring activity of TRPV5-like channel in the control, upon application of 500 nM RuR (shown as the shaded bar at the top), and after washout with control media. The patch was clamped to −40 mV.
PTH and thiazides enhance TRPV5 Ca2+ uptake.
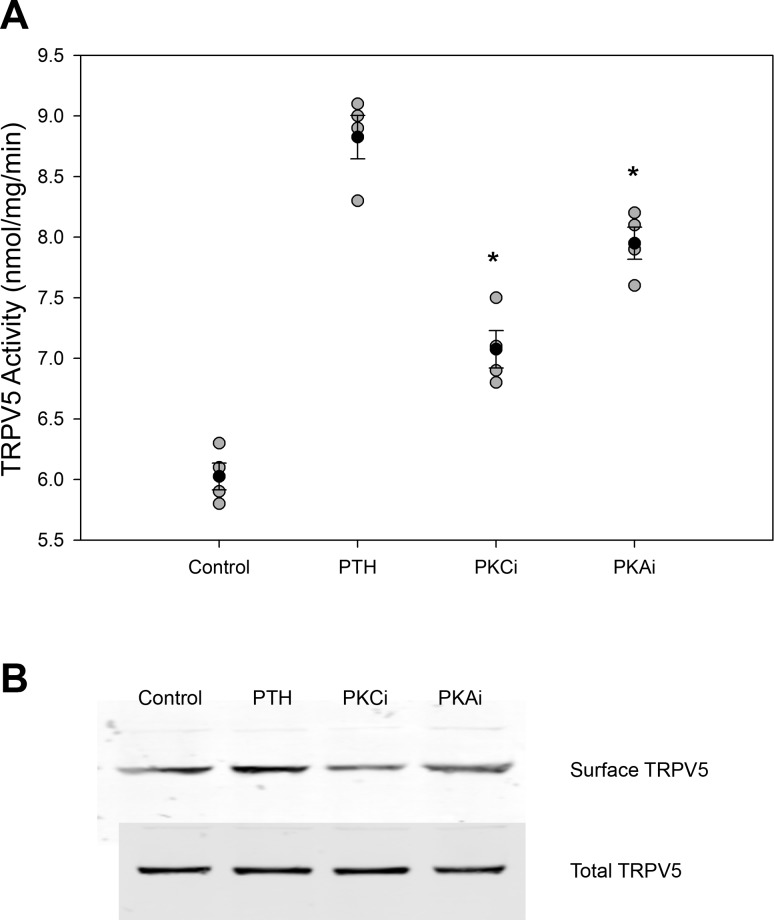
Having demonstrated that mDCT15 cells exhibit TRPV5 protein expression and ruthenium red-inhibitable Ca2+ transport consistent with TRPV5 activity, we examined the effect of PTH on TRPV5 function in our system. As expected, treatment of mDCT15 cells with 100 nM PTH for 15 min increased TRPV5 activity. 45Ca2+ uptakes significantly increased by 49% from 5.9 ± 0.3 to 8.8 ± 0.7 nmol·mg−1·min−1 (n = 4, P < 0.01 compared with control; Fig. 3A). This increase is similar to that reported in the literature (12).
Fig. 3.
Effect of PTH on TRPV5 activity. A: TRPV5 activity with administration of 100nM PTH for 15 min and either vehicle (control), 500 nM Gö-6976 [a PKC inhibitor (PKCi)], or 1 μM H89 [a PKA inhibitor (PKAi); n = 4]. *P < 0.01 compared with PTH. Solid circles indicate means, shaded circles indicate individual data points, and bars represent SEs. B: representative blot showing TRPV5 surface and total expression for the above groups (n = 4).
The effect of PTH on TRPV5 is known to be due to increased channel activity mediated by PKC and PKA in addition to an effect on plasma membrane surface expression (7, 10, 12). To confirm that PTH was acting on TRPV5 in a similar manner in mDCT15 cells, plasma membrane surface expression, as measured by cell surface biotinylation, of TRPV5 was measured and increased significantly to response to PTH (Fig. 3A). As shown in Fig. 3, inhibition of PKC by 500 nM Gö-6976 and PKA by 1 μM H89 sharply attenuated the effects of PTH on TRPV5 activity and surface expression, consistent with prior reports.
PTH decreases NCC Na+ uptake.
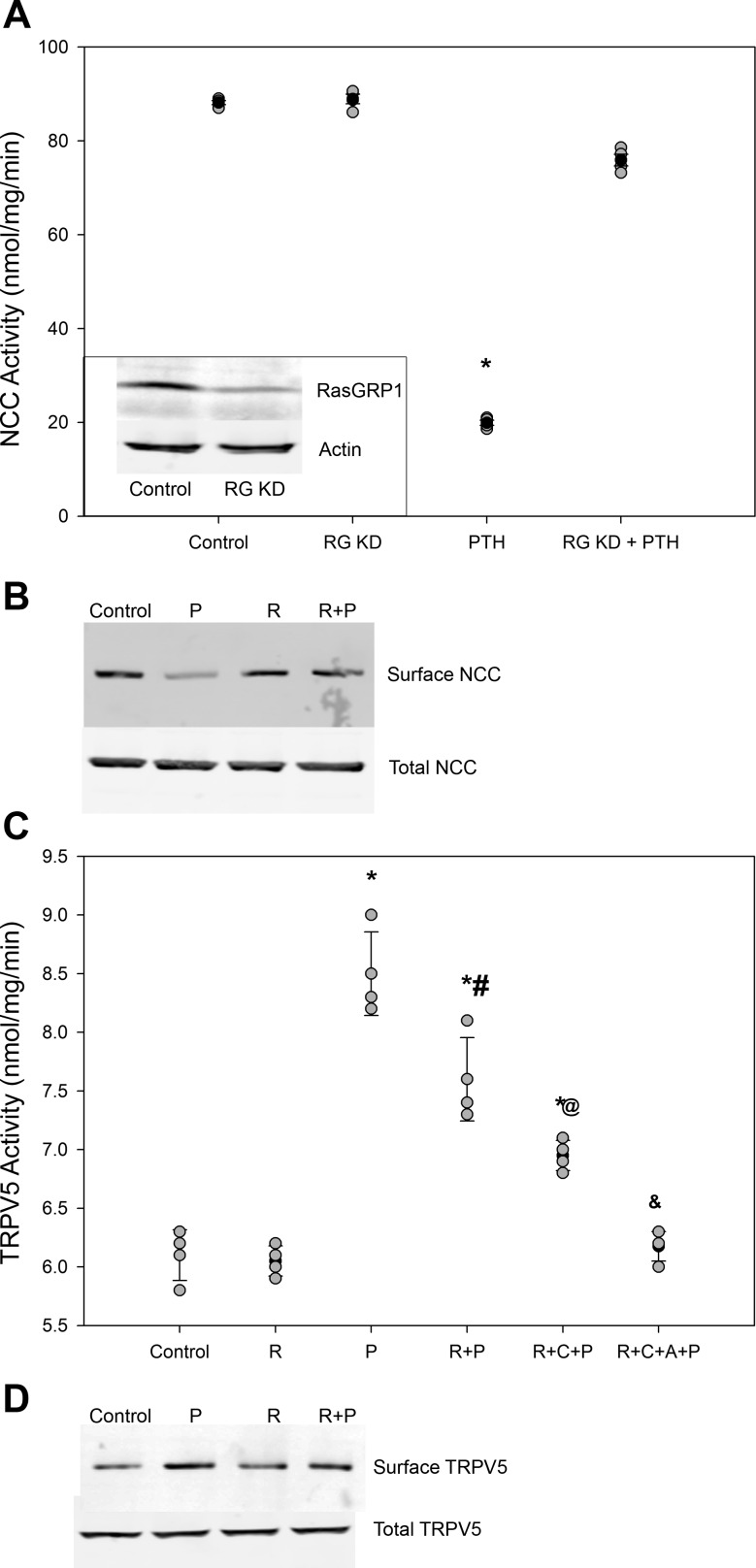
Having established that mDCT15 cells exhibit native TRPV5 activity that is PTH responsive, the effect of PTH on NCC activity was assessed. Consistent with our previous report (21) in mDCT cells, 22Na+ uptake experiments demonstrated a sharp inhibition of NCC activity with 15-min 100 nM PTH treatment. NCC activity significantly decreased by 73% from 75.4 ± 2.7 to 20.3 ± 1.3 nmol·mg−1·min−1 (n = 4, P < 0.01 compared with control; Fig. 4A). To verify that this effect was dependent upon Ras guanyl-releasing protein (RasGRP)1, as has been previously described, mDCT15 cells were treated with small interfering RNA specific for RasGRP1 (or nontargeting control). Under conditions of 70% RasGRP1 knockdown, NCC activity was not significantly affected by PTH, confirming the RasGRP1 dependence of PTH action. Similarly, NCC surface expression was sharply reduced by PTH, an effect inhibited by RasGRP1 knockdown (Fig. 4B). This established that the mDCT15 cell line exhibis native TRPV5 and NCC activity responsive to PTH with mechanisms consistent with previously published reports.
Fig. 4.
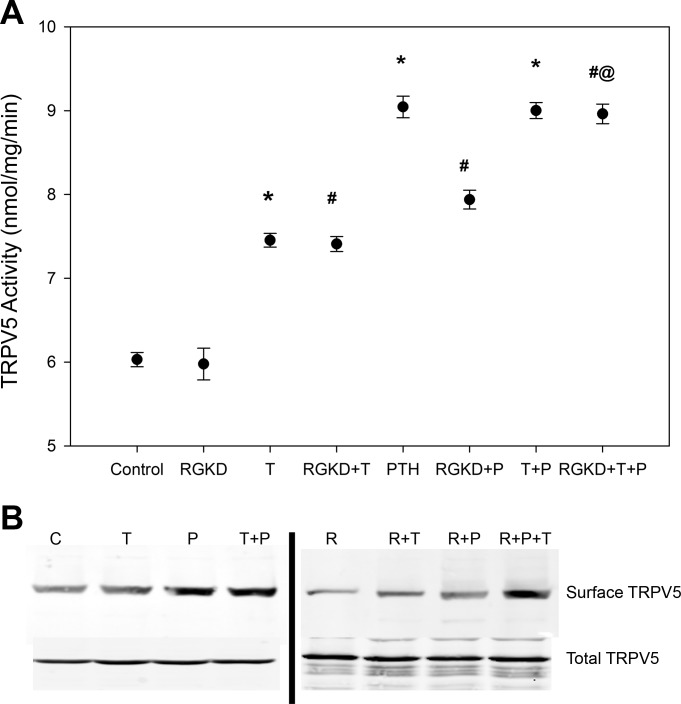
Role of Ras guanyl-releasing protein (RasGRP1) on PTH stimulation of TRPV5 activity. A: Na+-Cl− cotransporter (NCC) activity with administration of 100 nM PTH for 15 min in control cells or in cells with 70% reduced RasGRP1 expression (RG KD; n = 4). *P < 0.01 compared with PTH. Solid circles indicate means, shaded circles indicate individual data points, and bars represent SEs. Inset, representative blot of RasGRP1 expression in RG KD cells. B: representative blot showing NCC surface and total expression for the above groups (n = 4). C: TRPV5 activity with administration of 100 nM PTH for 15 min in control cells and mDCT15 cells with reduced RasGRP1 expression (R). Cells were treated with 100 nM PTH (P) for 15 min with and without inhibitors of PKC (C) and PKA (A). n = 4. *P < 0.01 compared with R; #P < 0.01 compared with P; @P < 0.01 compared with R + P; &P < 0.01 compared with R + C + P. Solid circles indicate means, shaded circles indicate individual data points, and bars represent SE. D: representative blot showing TRPV5 surface and total expression for control, PTH-treated, RasGRP1 knockdown, and RasGRP1 knockdown cells treated with PTH (n = 4).
Inhibition of the PTH effect on NCC attenuates TRPV5 Ca2+ uptake.
To assess whether the effect of PTH on Na+ transport contributed to the enhanced Ca2+ uptake, TRPV5 activity was studied in mDCT15 cells demonstrating 70% knockdown of RasGRP1. As shown in Fig. 4C, RasGRP1 knockdown had no effect on baseline TRPV5 activity. However, PTH administration in these cells resulted in an attenuated 45Ca2+ uptake compared with nontargeting controls. TRPV5 activity increased by 45% from 6.0 ± 0.2 to 8.7 ± 0.4 nmol·mg−1·min−1 in nontargeting controls (n = 4, P < 0.01 compared with control; Fig. 5). However, inhibition of the PTH effect by RasGRP1 knockdown significantly reduced the increase from 6.1 ± 0.1 to 7.5 ± 0.2 nmol·mg−1·min−1, only a 22% increase (n = 4, P < 0.01 between PTH-treated groups; Fig. 5). The increase in TRPV5 surface expression with PTH was attenuated with RasGRP1 knockdown (Fig. 4D).
Fig. 5.
Effect of thiazide on RasGRP1-mediated inhibition of the PTH effect. A: TRPV5 activity with administration of 100 nM PTH for 15 min in control cells and mDCT15 cells with reduced RasGRP1 expression (RG1). Cells were treated with 100 nM PTH for 15 min with and without thiazide diuretic (T). n = 4. *P < 0.01 compared with control; #P < 0.01 compared with RG; @P < 0.01 compared with RG + P. Solid circles indicate means, shaded circles indicate individual data points, and bars represent SEs. B: representation blot showing TRPV5 surface and total expression for control (C), thiazide-treated (T), PTH-treated (P), thiazide and PTH-treated (T + P), RasGRP1 knockdown (R), RasGRP1 knockdown treated with thiazide (R + T), RasGRP1 knockdown treated with PTH (R + P), and RasGRP1 knockdown treated with thiazide and PTH (R + P + T) cells (n = 4).
Inhibition of PKC and PKA resulted in a further attenuation of the PTH effect, with PKC inhibition decreasing uptake to 14% (6.1 ± 0.1 to 6.9 ± 0.1 nmol·mg−1·min−1, n = 4, P < 0.01 compared with RasGRP1 knockdown + PTH-treated groups; Fig. 4C). The addition of a PKA inhibitor to the PKC inhibitor completely eliminated any response to PTH.
The effect of RasGrp1 on PTH is mediated by its actions on NCC.
Having shown that RasGRP1 knockdown attenuates the TRPV5 response to PTH, we sought to establish the site of action of RasGRP1. The administration of thiazide diuretics resulted in an increase in TRPV5 activity from 6.0 ± 0.1 to 7.5 ± 0.1 nmol·mg−1·min−1 (n = 4, P < 0.01 compared with control; Fig. 5A). As shown in Fig. 5B, thiazides had no apparent effect on TRPV5 surface or total abundance. The addition of thiazide to RasGRP1 knockdown caused a 23% increase in TRPV5 activity (6.0 ± 0.2 to 7.4 ± 0.1 nmol·mg−1·min−1), similar to that of thiazide administration in control cells. While RasGRP1 knockdown, as previously shown, decreases the magnitude of the TRPV5 response to PTH (7.9 ± 0.1 nmol·mg−1·min−1 for knockdown compared with 9.1 ± 0.1 nmol·mg−1·min−1 in control), the addition of thiazide eliminates this effect (a nearly identical 9.0 ± 0.1 nmol·mg−1·min−1). This indicates that functionally active NCC is required for RasGRP1 knockdown to impact the PTH effect on TRPV5 activity.
The effect of PTH on TRPV5 is partially WNK4 dependent.
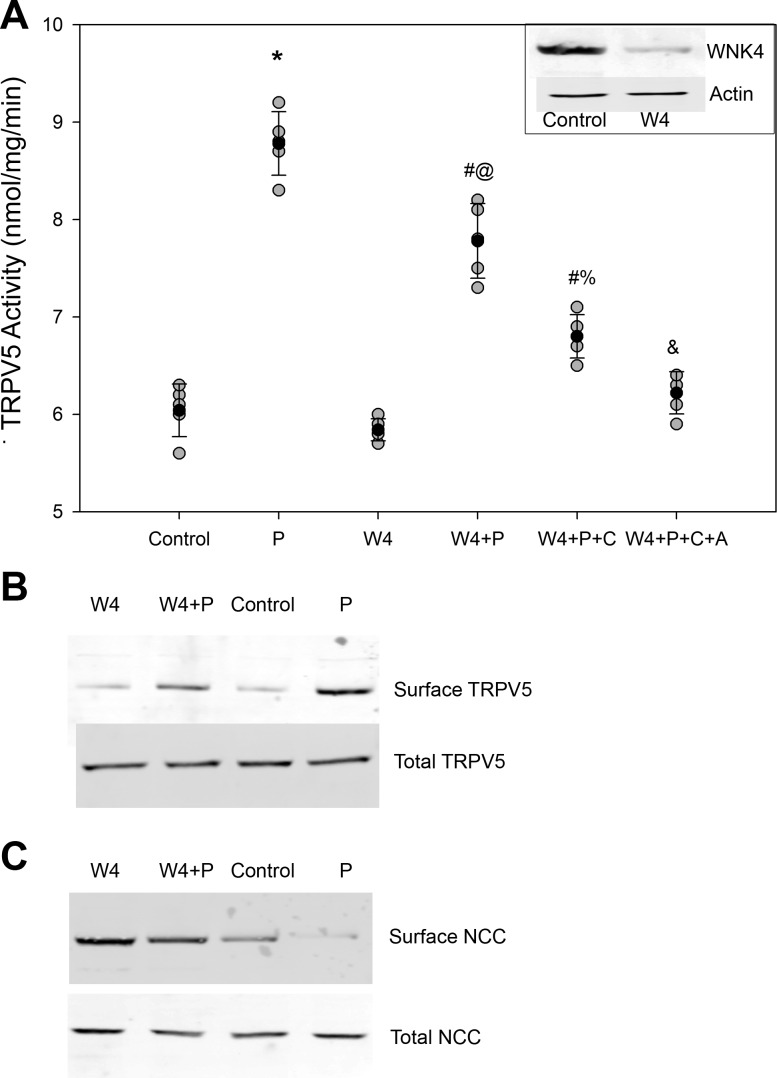
WNK4 has long been known to regulate NCC activity and is sensitive to intracellular Cl−1 concentrations (4, 5, 35, 37, 41, 42). More recently, WNK4 has been shown to regulate TRPV5 activity, suggesting that WNK4 may act as a potential mediator for the effect of NCC on TRPV5 (6, 17, 18). To assess this potential mechanism, WNK4KD cells, which are mDCT15 cells stably displaying a 75% knockdown for WNK4, were used (22). As shown in Fig. 6, TRPV5 activity does not significantly change in WNK4KD cells compared with control. The addition of PTH resulted in an attenuated increase in TRPV5 activity. The TRPV5 activity of WNK4KD cells increased from 5.8 ± 0.1 to 7.9 ± 0.4 nmol·mg−1·min−1, a 36% increase compared with 45% in control cells (n = 4, P < 0.01 between PTH-treated groups; Fig. 6). Again, PKC blockade resulted in a further attenuation of the PTH effect (5.8 ± 0.1 to 6.8 ± 0.4 nmol·mg−1·min−1, n = 4, P < 0.01 vs. the WN4KD + PTH-treated group), whereas combined PKC and PKA blockade in these WNK4KD cells completely abolished the effect.
Fig. 6.
Effect of WNK4 knockdown on PTH stimulation of TRPV5 activity. A: TRPV5 activity with administration of 100 nM PTH for 15 min in control cells and mDCT15 cells with reduced WNK4 expression (W4). Inset, representative blot of WNK4 expression. Cells were treated with 100 nM PTH (P) for 15 min with and without inhibitors of PKC (C) and PKA (A). n = 4. *P < 0.01 compared with control; #P < 0.01 compared with W4; @P < 0.01 compared with P; %P < 0.01 compared with W4 + P + C; &P < 0.01 compared with W4 + P + C. Solid circles indicate means, shaded circles indicate individual data points, and bars represent SEs. B: representative blot showing TRPV5 surface and total expression for the above groups (n = 4). C: representative blot showing NCC surface and total expression for the above groups (n = 4).
Taken together, these experiments indicate that TRPV5 Ca2+ uptake is modulated by decreases in NCC activity. Furthermore, a portion of the increased TRPV5 activity seen with PTH is due to alterations in NCC activity and may be mediated by WNK4.
DISCUSSION
Thiazide diuretics have long been associated with altered renal tubule handling of Ca2+. Urinary Ca2+ excretion falls ∼50% with thiazide treatment, reflecting increased Ca2+ reabsorption (19). The site of increased Ca2+ reabsorption has been the topic of considerable debate. Over time, a considerable number of studies have demonstrated changes in renal Ca2+ handling, with some favoring a PT mechanism and others pointing distally. The volume contraction seen with thiazides has been shown to play a major role in determining the degree of urinary Ca2+ excretion, indicating a proximal response. In studies of distal transport, microperfusion work has shown increased distal Ca2+ reabsorption in response to thiazides, and animal studies in which thiazide were selectively given to only one kidney showed hypocalciuria only in the treated kidney administration (9, 14, 26, 28, 29). A study (26) in animals has shown increased TRPV5 expression compared with thiazide administration.
More recently, a study (31) in knockout animals clearly indicated a proximal effect in the absence of NCC or even TRPV5. A human study (2) has also shown an increase in PT Ca2+ reabsorption with thiazide administration. The importance of a PT effect is not surprising, as driving down distal delivery by increased PT transport will tend to minimize even a large distal effect. Nevertheless, a wealth of studies has clearly demonstrated a relationship between distal NCC function and Ca2+ transport. Here, using the mDCT15 cell line, we demonstrated that the response of TRPV5 to PTH is mediated in part by NCC activity. Furthermore, this attenuation of TRPV5 activity is dependent on RasGRP1, possibly mediated by WNK4.
The mDCT15 cells used in this study have been well characterized, especially with regard to Na+. These cells exhibit native NCC activity and expression of common regulators of NCC, such as SPS1-related proline/alanine-rich kinase/oxidative stress-responsive kinase 1 and WNK kinases (22). Here, we demonstrate that mDCT15 cells express not only TRPV5 but also PMCA1, NCX1, PTH receptor, and calbindin. These cells exhibit single channel conductance, strong inward rectification, and high sensitivity to ruthenium red indicative of TRPV5. Furthermore, 45Ca2+ radiotracer uptake studies have shown significant ruthenium red-sensitive Ca2+ uptake. Taken together, these data represent clear evidence for native TRPV5 activity in mDCT15 cells. Unlike many other models, mDCT15 cells are not only PTH responsive but also exhibit native NCC activity in addition to native TRPV5 activity, making them an ideal model to examine a role for Na+ uptake in the regulation of TRPV5 activity by PTH.
As we have seen in other models, PTH suppresses NCC function, dependent on RasGRP1 (21). PTH is well known to activate TRPV5, an effect primarily mediated by PKC- and PKA-dependent open probability, as described in the literature (7, 10, 12). In our study, inhibition of the PTH mediated-decrease in NCC activity by RasGRP1 knockdown attenuated the TRPV5 response to PTH. RasGRP1 knockdown had no baseline effect on TRPV5 activity.
These RasGRP1-mediated effects are additive to those triggered by the established PKC and PKA pathways that act on TRPV5. Furthermore, these effects are not seen in the presence of total NCC inhibition with thiazide treatment. These results strongly imply a role for NCC activity in modulating TRPV5 Ca2+ transport by PTH. Secreted by the parathyroid glands, PTH is a 9.4-kDa hormone that, among its functions, is a powerful regulator of serum Ca2+ levels. In the DCT, PTH is known to enhance Ca2+ reabsorption by enhancing the activity of TRPV5. Traditionally, PTH effects on TRPV5 have been ascribed to activation of PKC and PKA pathways, which act directly on the Ca2+ channel (7, 10, 12). Here, in addition to these known pathways, we describe an indirect regulation of TRPV5 activity via RasGRP1-mediated effects on NCC activity.
Traditionally, distal effects of thiazide administration on Ca2+ reabsorption were attributed to one of two theories. The first theory involves a relatively direct effect on TRPV5. NCC inhibition by thiazide would decrease intracellular Na+, thereby facilitating basolateral Ca2+ extrusion by NCX1 and allowing for increased TRPV5 Ca2+ transport. In addition, the intracellular Cl− depletion from NCC inhibition could cause membrane potential differences facilitating TRPV5 activity. In the second theory, hypomagnesemia resulting from thiazide use stimulates TRPM6/TRPV5 cross-talk, leading to increased TRPV5 activity.
While we cannot rule out an effect of intracellular Cl− depletion and the resultant hyperpolarization affecting our model, our experiments of TRPV5 activity involve Ca2+ uptake only and are in the setting of NCX1 blockade. Therefore, the mechanism of intracellular Na+ depletion cannot explain the phenomenon observed in mDCT15 cells. Similarly, hypomagnesemic conditions were not present in our uptake media, and thus the latter mechanism cannot explain the observed effect on TRPV5.
Instead, we found that the PTH effect on TRPV5 is attenuated by WNK4KD. WNK4 is best known for its effects on NCC but also is known to stimulate TRPV5 (6, 17, 18). Depending on the situation, WNK4 can stimulate or inhibit NCC activity (1, 4, 5, 35, 37, 41, 42). WNK4 is known to be Cl− responsive; interestingly, recent work by Bazúa-Valenti et al. (1) has shown that intracellular Cl− depletion may determine whether WNK4 acts in an inhibitory or stimulatory role. We therefore propose that PTH-mediated decreases in NCC activity trigger intracellular Cl− depletion, which, in turn, may modulate WNK4 activity.
This modulation could take two possible forms. Previous studies into the TRPV5 interaction with WNK4 have shown that WNK4 increases TRPV5 activity via increasing TRPV5 plasma membrane expression (17, 18). Therefore, Cl− depletion by PTH-mediated decreases in NCC activity could activate WNK4, which would, in turn, further increase TRPV5 activity. This increase would only be evident in experimental models expressing functional TRVP5, NCC, and WNK4.
Alternatively, data from Cha et al. (6) have shown that WNK4 stimulates the process of constitutive TRPV5 endocytosis. These data are in direct opposition to the previous findings, and the reasons for this difference are not entirely clear. Since the effects of WNK4 on NCC were recently shown to be dependent on intracellular Cl− concentration, perhaps a dual effect of WNK4 on TRPV5 is also present. Either mechanism, however, could plausibly explain the phenomenon demonstrated in our study. In this case, Cl− depletion would signal WNK4 in an inhibitory fashion, leading to increased TRPV5 expression and activity. An investigation into this potential mechanism is warranted but is beyond the scope of the present study. Furthermore, since WNK4 is known to increase baseline NCC activity in mDCT15 cells (22), the lack of a baseline change of TRPV5 activity in WNK4KD cells suggests that WNK4 itself might be the mediator of these effects.
While the contributions of WNK4, PKC, and PKA pathways are additive, similar to those of RasGRP1, PKC, and PKA, WNK4KD alone did not suppress the PTH effect by the same magnitude as RasGRP1KD. While the knockdown levels were broadly similar, this could simply be due to differences critical expression levels in signaling pathways, with 30% expression of WNK4 being sufficient for some degree of interaction with TRPV5 but 30% expression of RasGRP1 being too low for a significant interaction with NCC. WNK4 may also be directly regulated by PTH and our WNK4 findings are unrelated to NCC, but as the combination of WNK4KD and PKC/PKA inhibition eliminated the PTH effect of Ca2+ transport without need for blockade of RasGRP1, it seems more likely that the WNK4 phenomenon is related to NCC.
An effect of NCC modulating TRPV5 activity could provide additional acute regulation of DCT Ca2+ reabsorption but might not be apparent chronically. Under conditions of prolonged NCC inhibition (such as by thiazide), proximal Na+ and Ca2+ reabsorption are undeniably increased, resulting in such reduced distal deliveries of Na+ and Ca2+ that the DCT contribution becomes relatively negligible. However, in states of submaximal or not prolonged DCT inhibition, proximal reabsorption would not be increased and distal delivery would be preserved. In this setting, NCC inhibition may represent a powerful means of regulating DCT Ca2+ transport.
Importantly, the present study cannot exclude low intracellular Cl−-mediated cellular hyperpolarization nor assess the role of enhanced NCX1 activity as additional contributors to this phenomenon. These mechanisms would only serve to enhance DCT Ca2+ uptake further, and, therefore, the role of NCC activity in regulating TRPV5 activity may be in fact greater than observed here. Further studies are needed to assess the full impact of NCC on TRPV5 activity, but here we provide clear evidence that PTH mediates DCT Ca2+ reabsorption in part via regulation of NCC.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants P01-DK-56788 (to B. Ko), R01-DK-085097 (to R. S. Hoover), and R01-DK-095029 (to O. Pochynyuk), American Heart Association Grant-In-Aid 13GRNT16220002 (to O. Pochynyuk), the Research Service, the Atlanta Veterans Affairs Medical Center (to R. S. Hoover), and Russian Foundation for Basic Research Grant 15-04-00938.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.S.H., V.T., O.P., and B.K. interpreted results of experiments; R.S.H., O.P., and B.K. edited and revised manuscript; R.S.H., V.T., L.N.H., O.P., and B.K. approved final version of manuscript; V.T., L.N.H., O.P., and B.K. performed experiments; V.T., L.N.H., O.P., and B.K. analyzed data; O.P. and B.K. prepared figures; O.P. and B.K. drafted manuscript; B.K. conception and design of research.
REFERENCES
- 1.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, García-Valdés J, Hadchouel J, Gamba G. The effect of WNK4 on the Na+-Cl− cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26: 1781–1786, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsland KJ, Worcester EM, Coe FL. Role of proximal tubule in the hypocalciuric response to thiazide of patients with idiopathic hypercalciuria. Am J Physiol Renal Physiol 305: F592–F599, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brini M, Carafoli E. The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 3: a004168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha SK, Huang CL. WNK4 kinase stimulates caveola-mediated endocytosis of TRPV5 amplifying the dynamic range of regulation of the channel by protein kinase C. J Biol Chem 285: 6604–6611, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha SK, Wu T, Huang CL. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294: F1212–F1221, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med 327: 1141–1152, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo LS, Windhager EE. Calcium and sodium transport by the distal convoluted tubule of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 235: F492–F506, 1978. [DOI] [PubMed] [Google Scholar]
- 10.de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20: 1693–1704, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte CG, Watson JF. Calcium reabsorption in proximal tubule of the dog nephron. Am J Physiol 212: 1355–1360, 1967. [DOI] [PubMed] [Google Scholar]
- 12.Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA. Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving protein kinase A and protein kinase C. Endocrinology 137: 13–20, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994. [PubMed] [Google Scholar]
- 14.Gesek FA, Friedman PA. Mechanism of calcium transport stimulated by chlorothiazide in mouse distal convoluted tubule cells. J Clin Invest 90: 429–438, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274: 8375–8378, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol 537: 747–761, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Ferguson WB, Peng JB. WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol 292: F545–F554, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Jing H, Na T, Zhang W, Wu G, Liu C, Peng JB. Concerted actions of NHERF2 and WNK4 in regulating TRPV5. Biochem Biophys Res Commun 404: 979–984, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen FS. Effect of thiazide diuretics upon calcium metabolism. Dan Med Bull 23: 223–230, 1976. [PubMed] [Google Scholar]
- 20.Kip SN, Strehler EE. Characterization of PMCA isoforms and their contribution to transcellular Ca2+ flux in MDCK cells. Am J Physiol Renal Physiol 284: F122–F132, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Ko B, Cooke LL, Hoover RS. Parathyroid hormone (PTH) regulates the sodium chloride cotransporter via Ras guanyl releasing protein 1 (Ras-GRP1) and extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) pathway. Transl Res 158: 282–289, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko B, Mistry AC, Hanson L, Mallick R, Cooke LL, Hack BK, Cunningham P, Hoover RS. A new model of the distal convoluted tubule. Am J Physiol Renal Physiol 303: F700–F710, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laerum E, Larsen S. Thiazide prophylaxis of urolithiasis. A double-blind study in general practice Acta medica. Scandinavica 215: 383–389, 1984. [PubMed] [Google Scholar]
- 24.Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ. Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J 25: 2978–2988, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of renal tubular reabsorption of calcium in normal rodents. Am J Physiol 204: 771–775, 1963. [Google Scholar]
- 26.Lee CT, Shang S, Lai LW, Yong KC, Lien YH. Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am J Physiol Renal Physiol 287: F1164–F1170, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Morsing P, Velazquez H, Wright FS, Ellison DH. Adaptation of distal convoluted tubule of rats. II. Effects of chronic thiazide infusion. Am J Physiol Renal Fluid Electrolyte Physiol 261: F137–F143, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Na KY, Oh YK, Han JS, Joo KW, Lee JS, Earm JH, Knepper MA, Kim GH. Upregulation of Na+ transporter abundances in response to chronic thiazide or loop diuretic treatment in rats. Am J Physiol Renal Physiol 284: F133–F143, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Ng RC, Rouse D, Suki WN. Calcium transport in the rabbit superficial proximal convoluted tubule. J Clin Invest 74: 834–842, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nijenhuis T, Vallon V, van der Kemp AWCM, Loffing J, Hoenderop JGJ, Bindels RJM. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol 527: 239–248, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohkawa M, Tokunaga S, Nakashima T, Orito M, Hisazumi H. Thiazide treatment for calcium urolithiasis in patients with idiopathic hypercalciuria. Br J Urol 69: 571–576, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Peng JB. TRPV5 and TRPV6 in transcellular Ca2+ transport: regulation, gene duplication, and polymorphisms in African populations. Adv Exp Med Biol 704: 239–275, 2011. [DOI] [PubMed] [Google Scholar]
- 35.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenova SB, Vassilieva IO, Fomina AF, Runov AL, Negulyaev YA. Endogenous expression of TRPV5 and TRPV6 calcium channels in human leukemia K562 cells. Am J Physiol Cell Physiol 296: C1098–C1104, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suki WN, Rouse D, Ng RC, Kokko JP. Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest 66: 1004–1009, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca2+ transport proteins by parathyroid hormone. Kidney Int 68: 1708–1721, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Vassilieva IO, Tomilin VN, Marakhova II, Shatrova AN, Negulyaev YA, Semenova SB. Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in human blood lymphocytes and Jurkat leukemia T cells. J Membr Biol 246: 131–140, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]