Abstract
Viral infections, such as respiratory syncytial virus and rhinovirus, adversely affect neonatal and pediatric populations, resulting in significant lung morbidity, including acute asthma exacerbation. Studies in adults have demonstrated that human airway smooth muscle (ASM) cells modulate inflammation through their ability to secrete inflammatory cytokines and chemokines. The role of ASM in the developing airway during infection remains undefined. In our study, we used human fetal ASM cells as an in vitro model to examine the effect of Toll-like receptor (TLR) agonists on chemokine secretion. We found that fetal ASM express multiple TLRs, including TLR3 and TLR4, which are implicated in the pathogenesis of respiratory syncytial virus and rhinovirus infection. Cells were treated with TLR agonists, polyinosinic-polycytidylic acid [poly(I:C)] (TLR3 agonist), lipopolysaccharide (TLR4 agonist), or R848 (TLR7/8 agonist), and IL-8 and chemokine (C-C motif) ligand 5 (CCL5) secretion were evaluated. Interestingly, poly(I:C), but neither lipopolysaccharide nor R848, increased IL-8 and chemokine (C-C motif) ligand 5 secretion. Examination of signaling pathways suggested that the poly(I:C) effects in fetal ASM involve TLR and ERK signaling, in addition to another major inflammatory pathway, NF-κB. Moreover, there are variations between fetal and adult ASM with respect to poly(I:C) effects on signaling pathways. Pharmacological inhibition suggested that ERK pathways mediate poly(I:C) effects. Overall, our data show that poly(I:C) initiates activation of proinflammatory pathways in developing ASM, which may contribute to immune responses to infection and exacerbation of asthma.
Keywords: airway, development, asthma, inflammation, chemokine, virus, Toll-like receptor
clinical studies in pediatric populations indicate that early-life viral respiratory infections significantly contribute to development and exacerbation of wheezing and asthma (25, 27, 54, 61). A substantial burden of health care costs is associated with hospital admissions and emergency room visits for infection-induced wheezing during the first 3 years of life (54, 66). Virus-induced wheezing during the neonatal period is associated with a heightened risk for development of airway disease in childhood (27, 32). Emerging evidence suggests that ∼80% of the causative agents of acute respiratory illness may be respiratory viruses (25, 30).
Respiratory syncytial virus (RSV), rhinovirus (RV), and influenza virus are strongly linked to the development of asthma and symptom exacerbations in young children (6, 67). RV and RSV infect the lower airways. In fact, RSV is the most common cause of lower respiratory tract illness in infants (58). Although the airway epithelial cell is the principal host cell for most respiratory viruses (36), RSV and RV also infect airway smooth muscle (ASM) (23, 44).
The neonatal time is a particularly vulnerable period, given the increased susceptibility to acquiring viral and bacterial infections (40). Certainly this susceptibility to infection and predisposition to the development of airway diseases such as asthma/wheezing is further enhanced by clinical situations, such as preterm birth or exposure to chorioamnionitis in utero (5, 21). Also, in regards to in utero infection, the maternal-fetal interface is dynamic, and viruses can traverse into the fetal lung (4, 6). Given the vulnerability to infections during the perinatal period, there is a need to understand how infections impact the developing lung, particularly with respect to the airway.
Toll-like receptors (TLRs) are a group of pathogen recognition receptors that facilitate recognition of pathogen-associated molecular patterns in bacteria, viruses, and fungi (52, 63). Activation of TLRs on airway epithelium and ASM is a pivotal event that facilitates airway inflammation during infection (14, 15, 22). For RSV and RV infection, stimulation of TLR3 and TLR4 mediates cytokine and chemokine production (31, 43, 65, 73). Prior studies have demonstrated that TLR3- and TLR4-deficient mice have altered immune response to RSV infection (35, 55, 56). Thus examination of the effects of TLR3- and TLR4-mediated pathways would improve our understanding of viral-induced airway inflammation.
Previous studies have shown that human adult ASM cells express TLRs and secrete cytokines and chemokines in response to TLR agonists (63). Recent studies in human ASM reported that, in contrast to lipopolysaccharide (LPS), polyinosinic-polycytidylic acid [poly(I:C)] has a more robust response with respect to promoting cytokine and chemokine release, including IL-6, IL-8, C-C motif chemokine 11 (CCL11; eotaxin), and CCL5 [regulated on activation, normal T expressed and secreted (RANTES)] (39). Our study focuses primarily on IL-8 and CCL5, as these inflammatory mediators have been implicated in the fetal inflammatory response. In a recent pediatric clinical study, the presence of CCL5 in nasal epithelia was linked to increased risk of childhood asthma (3), while IL-8, a chemokine that regulates neutrophil chemotaxis, is increased in young children with RSV infection (60). Furthermore, TLR3 and TLR4 ligands increase allergen sensitization and enhance allergic airway disease in mice (51). However, it is difficult to directly extrapolate these results from adult human ASM cells, other cell types, or mice to the developing human airway, given that the environmental context in which airway development or neonatal infection occur is different.
A major limitation in the study of developing human airway has been the lack of human neonatal tissues or age-appropriate human in vitro or in vivo research models. Access to postnatal (neonatal/pediatric) tissues is limited; thus we utilize human fetal ASM to model the neonatal airway. Our laboratory has previously reported a unique, nonimmortalized, noncancerous, human fetal ASM cell model (24) that offers significant advantages for mechanistic examination of signaling mechanisms in an age- and species-appropriate human tissue (albeit in vitro). Accordingly, in the present study, we investigated the effects of poly(I:C) (TLR3 agonist, synthetic double-stranded RNA) and LPS (TLR4 agonist) on TLR expression, chemokine release, and activation of TLR, mitogen-activated protein kinase (MAPK), and NF-κB pathways in human fetal ASM cells. We hypothesized that the developing airway already possesses TLRs that can mediate inflammatory responses, and that there is variation in the fetal and adult ASM response to inflammation. In this study, we found that TLR3 agonist, poly(I:C), increases chemokine expression in human fetal ASM, which represents an important means by which viral infections may contribute to pediatric airway disease.
MATERIALS AND METHODS
Materials.
Fetal bovine serum (FBS), Dulbecco's modified Eagle's medium F/12 (DMEM/F12), and additional cell culture supplies were obtained from Invitrogen (Carlsbad, CA). Remaining supplies and reagents were obtained from Sigma Aldrich (St. Louis, MO). The following ligands were utilized: LPS (E. coli 055:B5, L4524; Sigma Aldrich, St. Louis, MO), poly(I:C) (Invivogen, San Diego, CA), R848 (Invivogen, San Diego CA), and TNF-α (R&D Systems, Minneapolis, MN). Primary antibodies were obtained from Cell Signaling Technology (Danvers, MA), unless otherwise stated.
Isolation of human ASM cells.
Human fetal ASM (fetal ASM) cells were enzymatically dissociated from fetal tracheobronchial tissue as previously described under protocols approved and considered exempt by the Mayo Institutional Review Board and Ethics Committees in the UK (24, 47). Fetal ASM cells were provided by Dr. Pandya from the University of Leicester, UK and by Novogenix (Los Angeles, CA). Similarly, human adult ASM was dissociated from de-identified lung samples that became available through pneumenectomies or lobectomies. Our protocol was approved by the Mayo Clinic Institutional Review Board to collect noninfected lung specimens from surgical pathology at Saint Mary's Hospital. Cells were isolated from third to sixth generation airway branches by removing ASM layer from adventitia and epithelium. The ASM layer was placed in Hank's balanced salt solution (Invitrogen). Cells were dissociated enzymatically by collagenase and papain and then seeded in cell cultured flasks in DMEM/F12 (Invitrogen), supplemented with 10% FBS and 1% penicillin-streptomycin. Cells grown under standard conditions (95% air/5% CO2) in a humidified incubator from passages 1–5 for adult ASM and passages 2–10 for fetal ASM of subculture were used for experiments. Media was phenol red-free DMEM/F12 with 10% FBS. Cells were serum deprived in 0.5% FBS for a minimum of 24 h before experimental treatments.
For both fetal and adult human ASM cells, phenotype was confirmed from cell passages 1–5 for adult and passages 2–10 for fetal ASM, with expression of smooth muscle markers as described in previous studies (7, 24). In our laboratory's prior investigations, we identified that the expression pattern of smooth muscle actin, calponin, and acetylcholine receptor in fetal ASM cells was similar to that of adult human ASM cells (7, 24). This previous work supported the use of this in vitro ASM cell model for the remainder of this study.
Cell treatments.
Fetal ASM cells were treated with control (sterile PBS), 10 ng/ml TNF-α, 1 μg/ml LPS, or 1 μg/ml poly(I:C) for 24 h, unless otherwise specified. Dose responses were performed to determine the optimum concentration of poly(I:C) and R848 for all cell treatments. In some experiments, fetal ASM cells were pretreated with 10 μM of extracellular signal-regulated kinase (ERK) activation inhibitor peptide I (MEK1 inhibitor; Calbiochem, San Diego, CA), 10 μM p38 MAPK inhibitor (Calbiochem), 20 μM NF-κB activation inhibitor III (SM-7368; EMD Millipore, Billerica, MA), or 20 μM TLR3 antagonist, N-[(3-chloro-6-fluorobenzo[b]thien-2-yl)carbonyl]-d-phenylalanine (CU CPT 4a; Tocris Bioscience, Minneapolis, MN) for 1 h before treatment with control (sterile PBS), 1 μg/ml poly(I:C), LPS, or R848 for 24 h. Nuclear extraction was performed after 2 h of cell treatment using the NE-PER nuclear and cytoplasmic extraction kit per the manufacturer's protocol (ThermoScientific, Rockford, IL).
Western blot analysis.
After 24 h of treatment, cells were harvested using standard techniques with cell lysis buffer containing protease inhibitors. Duration of cell treatment for examination of the MAPK pathway ranged from 5 min to 1 h. Lysate protein concentration was determined with a DC protein assay (Bio-Rad, Hercules, CA), and ∼30 μg total protein was loaded onto either 10% or 4–15% gradient gels (Criterion Gel System, Bio-Rad, Hercules, CA). A Trans-Blot Turbo system was used to transfer proteins to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked at room temperature with Odyssey Blocking buffer (Li-Cor Biosciences, Lincoln, NE) for 1 h. Membranes were incubated overnight at 4°C in 1 μg/ml of primary antibody of interest. Primary antibodies included the following: TLR2, TLR3, TLR4, TLR7, TLR9, phosphorylated and total ERK (Santa Cruz Biotechnology, Dallas, TX), total ERK-1/2 (Santa Cruz Biotechnology), phosphorylated and total c-Jun NH2-terminal kinase (JNK) (Santa Cruz Biotechnology), phosphorylated and total p38, p50 (Santa Cruz Biotechnology), and p65 (Santa Cruz Biotechnology). GAPDH or TATA-box binding protein (Abcam) were used to control for protein loading. Membranes were washed with Tris-buffered saline before incubation with infrared dye-conjugated secondary antibodies (LiCor Biosciences) for 60 min at room temperature. Membranes were imaged on a Li-Cor OdysseyXL system, and densitometry quantified with Image Studio software. All blots were normalized to GAPDH, unless otherwise specified.
Real-time PCR.
Following the manufacturer's protocol, total RNA was extracted from cells 6 h following cell treatments using an RNeasy Mini Kit (Qiagen). Standard techniques were used to synthesize and amplify cDNA. Real-time PCR was performed in triplicates per cDNA template using a Roche Light Cycler 480. mRNA expression was calculated by normalization of cycle threshold (Ct) values of target gene to reference gene (GAPDH). The relative fold change was calculated by ΔΔCt method. Human primers used include the following: CCL5, CD14, GAPDH, IL-8, myeloid differentiation 2 (MD2), myeloid differentiation factor 88 (MyD88), TLR3, TLR4, and TIR domain containing adapter-inducing interferon-β (TRIF) (Table 1).
Table 1.
Human primers used for quantitative real-time PCR
| Forward Primer | Reverse Primer | |
|---|---|---|
| CCL5 | AGTGTGTGCCAACCCAGAGAAGAA | TGTGGTAGAATCTGGGCCCTTCAA |
| CD14 | ACGCCAGAACCTTGTGAGC | GCATGGATCTCCACCTCTACTG |
| GAPDH | AAGGTGAAGGTCGGAGTCAACGGATT | CCATGGAATTTGCCATGGGAGGAATC |
| IL-8 | CCTGATTCTGCAGCTCTGTGTGA | AATTTCTGTGTTGGCGCAGTGTGG |
| MD2 | AACTGCACGGTCCCAATGG | GGGCAAAGGACTGGAACACAA |
| MyD88 | GGCTGCTCTCAACATGCGA | CTGTGTCCGCACGTTCAAGA |
| TLR3 | CCTGGTTTGTTAATTGGATTAACG | TGAGGTGGAGTGTTGCAAAGG |
| TLR4 | CCTCGGCGGCAACTTCATAA | AGAGCGGATCTGGTTGTACTG |
| TRIF | ATATGCCGCAATTTTACAGGGT | ACCTGCAATAAAGCAGCCTGG |
CCL5, C-C motif chemokine 5; IL-8, interleukin-8; MD2, myeloid differentiation 2; MyD88, myeloid differentiation factor 88; TLR, Toll-like receptor; TRIF, TIR domain containing adapter-inducing interferon-β.
Enzyme-linked immunosorbent assay.
Concentrated cell culture supernatants from cells treated with control (sterile PBS), 10 ng/ml TNF-α, 1 μg/ml LPS, or increasing concentrations of either poly(I:C) or R848, ranging from 0.01 to 1 μg/ml over 24 h, were assayed for levels of CCL5 (RANTES) (range: 31.2-2,000 pg/ml), and IL-8/chemokine (C-X-C motif) ligand (CXCL) 8 (31.2-2,000 pg/ml) using enzyme-linked immunosorbent assay (ELISA) plates from R&D Systems (Minneapolis, MN). IL-8/CXCL8 and CCL5 ELISA plates were performed according to the manufacturer's instructions.
MAPK inhibitor, NF-κB inhibitor, and TLR3 antagonist cell treatments.
Concentrated cell culture supernatants from cells pretreated for 1 h with ERK inhibitor, p38 MAPK inhibitor, NF-κB inhibitor, or TLR3 antagonist before exposure with poly(I:C) over 24 h were assayed for levels of CCL5 and IL-8/CXCL8 as per above.
Statistical analysis.
Experiments were performed using cells from at least three different fetal ASM samples, with at least three repetitions per sample. Data were analyzed using Student's t-test and one-way ANOVA or the nonparametric Kruskal-Wallis test, when appropriate. Comparisons between groups were made using Tukey post hoc analysis. Values are means ± SE, and statistical significance was established at P < 0.05.
RESULTS
Expression of TLRs and adaptor molecules in human fetal ASM.
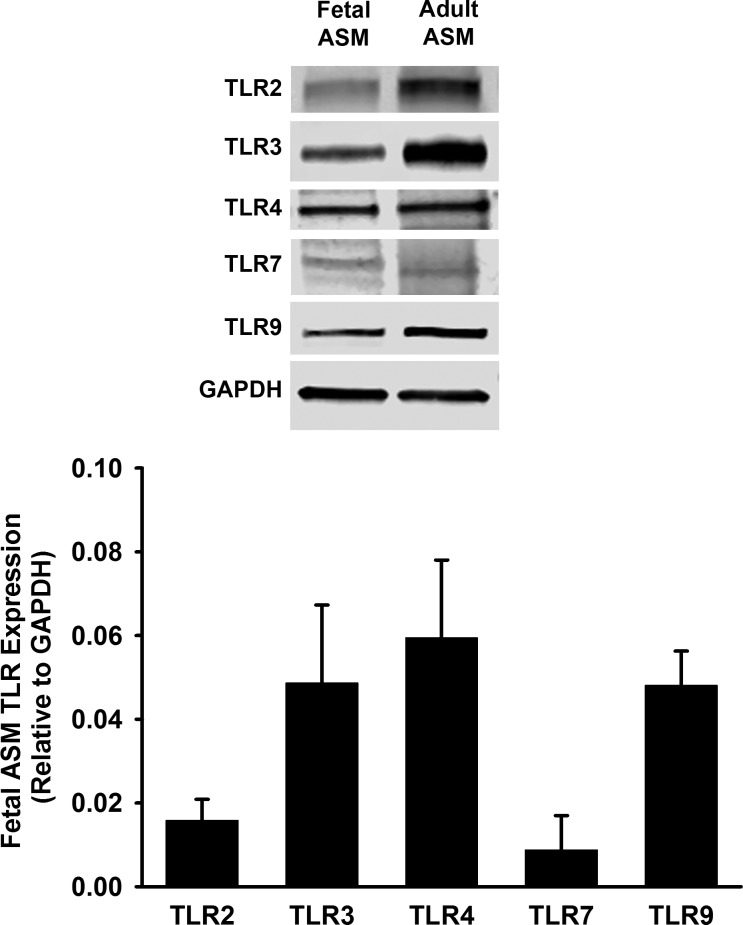
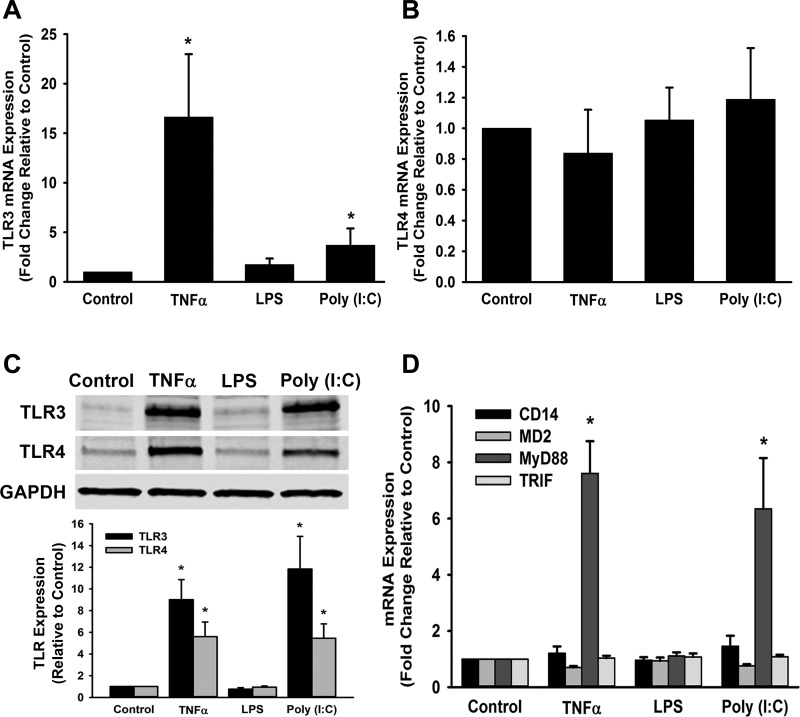
Similar to studies performed in human adult ASM (39), we found that human fetal ASM cells express TLR2, TLR3, TLR4, TLR7, and TLR9, as demonstrated via Western blot using human adult ASM as a positive control (Fig. 1). Based on these findings and the relevance of TLR3 and TLR4 to pediatric lung infection, we investigated the effects of TLR3 and TLR4 agonists on fetal ASM. We performed real-time PCR for mRNA expression of TLR3 and TLR4. We found that poly(I:C) and TNF-α, but not LPS, significantly increased TLR3 mRNA expression 6 h following treatment (Fig. 2A). TLR4 mRNA expression was not significantly altered by poly(I:C), TNF-α, or LPS (Fig. 2B). However, protein expression of TLR3 and TLR4 was significantly increased by TNF-α and poly(I:C), compared with control cells (Fig. 2C), suggesting differential regulation of these TLRs by inflammatory stimuli. TLR3 and TLR4 have associated adaptor molecules, which are important for their signaling (38). We found that cell treatments with TNF-α and poly(I:C) resulted in a significant increase of MyD88 mRNA expression, but no effect was seen on MD2, TRIF, or CD14 (Fig. 2D).
Fig. 1.
Expression of Toll-like receptors (TLRs) in fetal airway smooth muscle (ASM). Top: expression of TLR2, 3, 4, 7, and 9 was detected in fetal ASM by Western blot. Bottom: fetal ASM were treated with media only (control) for 24 h, and TLR2, 3, 4, 7, and 9 expression relative to GAPDH was evaluated. Adult ASM treated with media only was used as a positive control. Values are means ± SE; n = 3–4 samples.
Fig. 2.
Effect of TLR agonists on TLR3 and TLR4 and adaptor molecule expression. Cells were treated with media only (control), TNF-α, lipopolysaccharide (LPS), or polyinosinic-polycytidylic acid [poly(I:C)] for 6 or 24 h. A: TNF-α and poly(I:C) increased TLR3 mRNA expression after 6 h. B: no differences were observed in TLR4 mRNA expression after 6 h when cells were exposed to either TNF-α or poly(I:C). C: TNF-α and poly(I:C) increased TLR3 and TLR4 protein expression after 24 h. D: there was a notable increase in myeloid differentiation factor 88 (MyD88) TLR adaptor molecule mRNA expression with cells exposed to TNF-α and poly(I:C) treatments, but no changes in myeloid differentiation 2 (MD2), TIR domain containing adapter-inducing interferon-β (TRIF), or CD14. Values are means ± SE; n = 3–6 samples. *Significant difference from control, P < 0.05.
Effects of TLR3 and TLR4 agonists on chemokine secretion and expression.
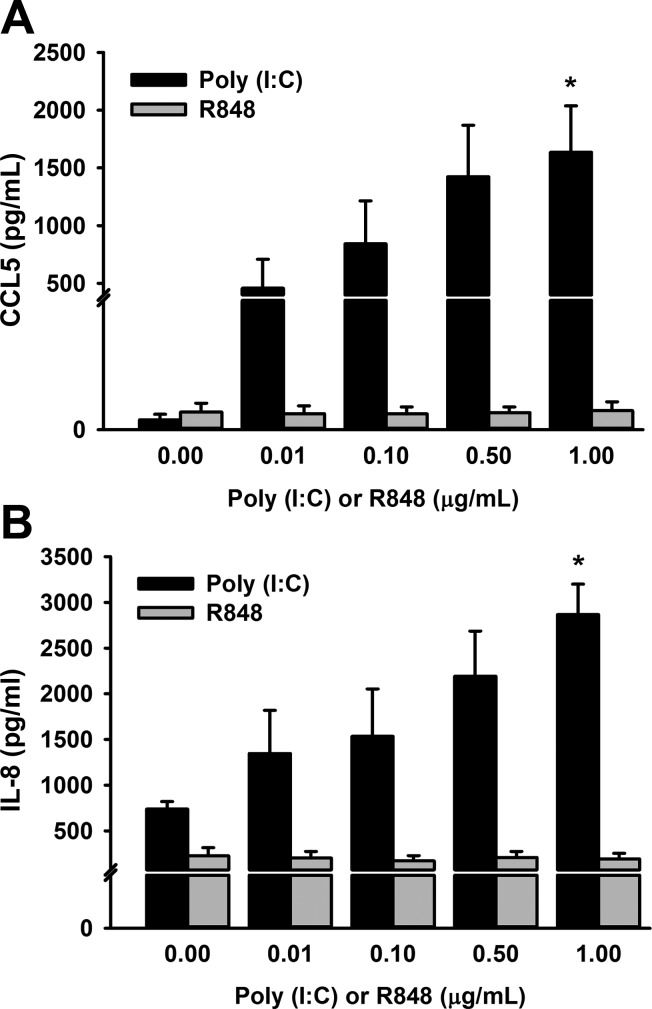
To determine optimal poly(I:C) treatment concentration, human fetal ASM were exposed to control and increasing concentrations of poly(I:C), ranging from 0.01 to 1 μg/ml over a 24-h period, to assess CCL5 and IL-8 secretion (Fig. 3, A and B). We found that the highest concentration of 1 μg/ml induced a significant increase in both CCL5 and IL-8 secretion compared with the other exposures. We assessed the effects of increasing concentrations of TLR7/8 ligand, R848 (0.01–1 μg/ml), on CCL5 and IL-8 secretion, respectively, and found no differences compared with control at all concentrations with R848 cell treatments (Fig. 3, A and B).
Fig. 3.
Effects of increasing concentrations of poly(I:C) and R848 on C-C motif chemokine 5 (CCL5; A) and interleukin (IL)-8 (B) secretion. Fetal ASM were treated with media only (control), poly(I:C), or R848 at 0.01, 0.1, 0.5, and 1 μg/ml, respectively, for 24 h. Poly(I:C), but not R848, increased CCL5 and IL-8 secretion in a dose-response manner, and treatment of 1 μg/ml resulted in the most significant increase in CCL5 (A) and IL-8 (B) secretion. Values are means ± SE; n = 3–4 samples. *Significant difference from control, P < 0.05.
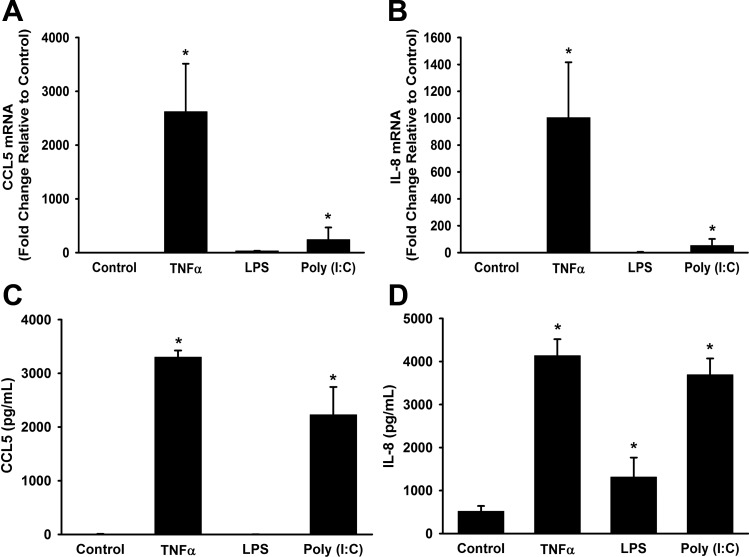
Next, we examined the effects of poly(I:C) and LPS on IL-8 and CCL5 mRNA expression and secretion. CCL5 and IL-8 mRNA levels were increased by poly(I:C) and TNF-α (Fig. 4, A and B). Similarly, poly(I:C) and TNF-α both significantly increased CCL5 and IL-8 secretion (Fig. 4, C and D). Conversely, LPS did not increase IL-8 or CCL5 mRNA expression levels (Fig. 4, A and B). LPS did increase IL-8 secretion, but to a lesser extent than poly(I:C) or TNF-α (Fig. 4D).
Fig. 4.
Effects of poly(I:C) on CCL5 and IL-8 expression and secretion. Fetal ASM were treated with media only (control), TNF-α, LPS, or poly(I:C) for 6 or 24 h. TNF-α and poly(I:C), but not LPS, increased CCL5 (A) and IL-8 mRNA (B) expression. C: TNF-α and poly(I:C), but not LPS, increased CCL5 secretion. D: TNF-α, poly(I:C), and LPS all significantly increased IL-8 secretion. Values are means ± SE; n = 3–5 samples. *Significant difference from control, P < 0.05.
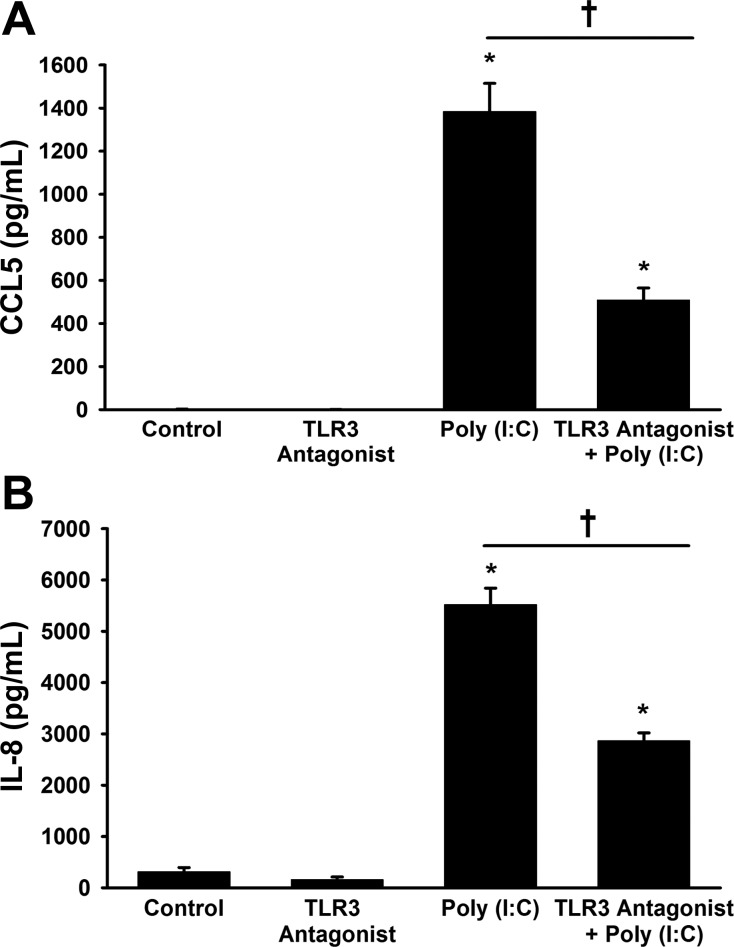
To determine if the effects of poly(I:C) were mediated through TLR3, we utilized TLR3 antagonist, CU CPT 4a. TLR3 antagonist significantly reduced the ability of poly(I:C) to increase both CCL5 and IL-8 secretion, as measured by ELISA (Fig. 5, A and B).
Fig. 5.
Effect of a TLR3 antagonist on poly(I:C)-induced CCL5 (A) and IL-8 (B) expression. Fetal ASMs were pretreated with a TLR3 antagonist, N-[(3-chloro-6-fluorobenzo[b]thien-2-yl)carbonyl]-d-phenylalanine (CU CPT 4a), for 1 h and subsequently treated with media only (control) or poly(I:C) for 24 h. Pretreatment with TLR3 antagonist significantly decreased CCL5 (A) and IL-8 (B) secretion induced by poly(I:C). Values are means ± SE; n = 4 samples. *Significant difference from control, P < 0.05. †Significant difference between poly(I:C) and TLR3 antagonist + poly(I:C) treatments, P < 0.05.
Mechanisms of poly (I:C)-induced effects in chemokine expression.
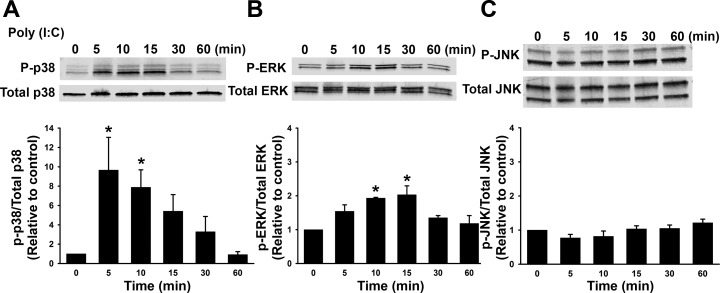
The production of proinflammatory chemokines can be regulated by NF-κB, in addition to MAPKs p38, JNK, and ERK-mediated pathways (37, 49, 53). To evaluate potential mechanisms by which poly(I:C) may be functioning, we examined phosphorylation of MAPKs. We first performed a timed treatment course, where cells were treated with control or poly(I:C) for 5, 10, 15, 30, or 60 min and assessed phosphorylation of ERK, p38, and JNK by Western blot (Fig. 6, A–C). Poly(I:C) significantly increased phosphorylation of ERK (Fig. 6B) ∼15 min following poly(I:C) stimulation, and p38 phosphorylation was increased at 5 min following poly(I:C) stimulation (Fig. 6A). Treatment with poly(I:C) did not alter phosphorylation of JNK during the time course (Fig. 6C).
Fig. 6.
Activation of extracellular signal-regulated kinase (ERK) and p38 phosphorylation by poly(I:C). Fetal ASM were treated with media only (control) or poly(I:C) for 0–60 min. Poly(I:C) increased phosphorylation of p38 (A) and ERK (B), but not c-Jun NH2-terminal kinase (JNK) (C). Values are means ± SE; n = 4 samples. *Significant difference from control, P < 0.05.
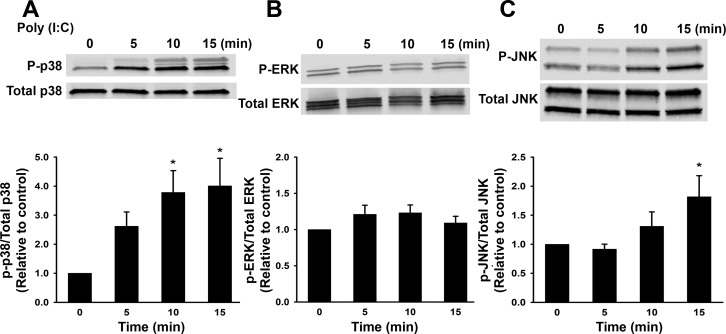
We sought to compare and contrast mechanisms of poly(I:C)-induced effects in chemokine expression between human fetal and adult ASM. Similar to experiments conducted in fetal ASM, we examined NF-κB, as well as phosphorylation of MAPK p38, JNK, and ERK, in adult ASM. We performed a timed treatment course where adult ASM cells were treated with control or poly(I:C) for 5, 10, and 15 min (Fig. 7, A–C). Poly(I:C) did not have an effect on ERK (Fig. 7B), but significantly increased phosphorylation of p38 and JNK ∼10–15 min following poly(I:C) stimulation (Fig. 7, A and C).
Fig. 7.
Activation of ERK and p38 phosphorylation (p) by poly(I:C). Adult ASM were treated with media only (control) or poly(I:C) for 0–15 min. Poly(I:C) increased phosphorylation of p38 (A), but not ERK (B), and also increased phosphorylation of JNK (C). Values are means ± SE; n = 5 samples. *Significant difference from control, P < 0.05.
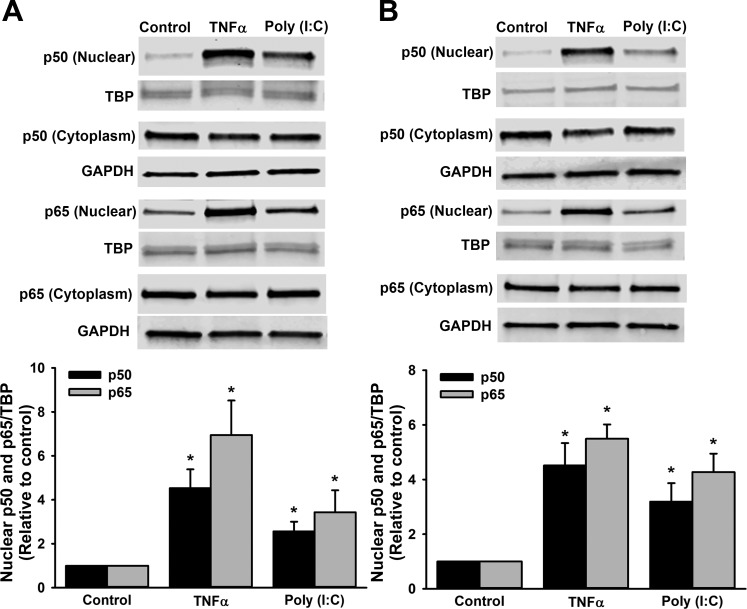
After nuclear extraction, we also assessed the effect of the various treatments for a 2-h duration on nuclear translocation of the NF-κB subunit p50 and p65 in human fetal ASM. Treatment with TNF-α and poly(I:C) increased both p50 and p65 nuclear levels (Fig. 8A). Likewise, in nuclear fractions, we assessed the effect of the TNF-α and poly(I:C) treatments over a 2-h duration on nuclear translocation of the NF-κB subunits p50 and p65 in human adult ASM. Similar to findings in the human fetal ASM, treatment with both TNF-α and poly(I:C) in human adult ASM induced p50 and p65 nuclear translocation (Fig. 8B).
Fig. 8.
Nuclear translocation of the p50 and p65 NF-κB subunits. In human fetal ASM (A) and human adult ASM (B), p50, p65, and TATA-box binding protein (TBP) in nuclear extracts, in addition to p50, p65, and GAPDH in cytoplasm extracts, were, respectively, measured by Western blot showing that TNF-α and poly(I:C) promoted nuclear translocation of the p50 and p65 NF-κB subunits in both human fetal and adult ASM. There was no global increase in either p50 or p65 expression in the cytoplasm fraction. Values are means ± SE; n = 4–7 samples. *Significant difference from control, P < 0.05.
MAPK and NF-κB inhibitor treatments and effects in chemokine expression.
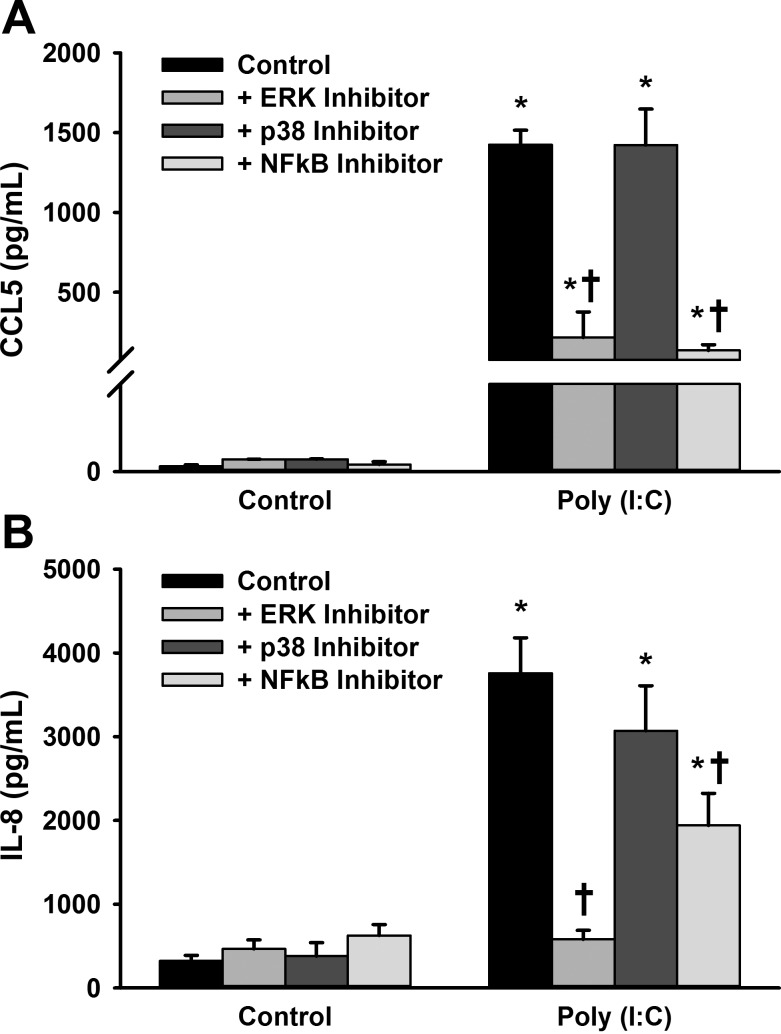
In the fetal ASM cells, we pharmacologically inhibited ERK activation, p38 kinase activity, and NF-κB to determine whether these pathways contribute to CCL5 and IL-8 secretion upon treatment with poly(I:C). Fetal ASM cells were pretreated with MEK1 inhibitor (inhibits ERK activation), p38 inhibitor, or NF-κB inhibitor for 1 h, followed by treatment with control (sterile PBS) or poly(I:C) for 24 h. Treatment with the MEK1 inhibitor and NF-κB inhibitor attenuated poly(I:C)-induced CCL5 and IL-8 secretion, while the p38 inhibitor did not have a significant effect on either chemokine (Fig. 9, A and B).
Fig. 9.
Effect of ERK, p38, and NF-κB inhibition on poly(I:C)-induced CCL5 and IL-8 expression. Fetal ASM were pretreated with ERK, p38, or NF-κB inhibitor for 1 h and subsequently treated with media only (control) or poly(I:C) for 24 h. A: pretreatment with ERK and NF-κB inhibitors, but not p38 inhibitor, decreased CCL5 secretion induced by poly(I:C). B: pretreatment with ERK and NF-κB inhibitors, but not p38 inhibitor, attenuated IL-8 secretion induced by poly(I:C). Values are means ± SE; n = 5–6 samples. *Significant difference from control, P < 0.05. †Significant difference of ERK inhibitor or NF-κB inhibitor compared with poly(I:C) only, P < 0.05.
DISCUSSION
Airway inflammation triggered by viral pathogens is an important contributor to the development of wheezing and asthma in the pediatric population. Recent epidemiologic studies reveal that severe viral lower respiratory tract infection during the neonatal period is associated with increased risk of developing asthma in children (26, 59). Premature infants are even more susceptible to acquiring viral infections. RSV, a RNA virus, is frequently detected in preterm neonates, making it relevant to study. A recent prospective study indicated that RSV infections in premature infants resulted in increased health care utilization and costs in the first 2 years of life (18). The presence of airway inflammation induced by viral infections could result in deleterious long-term effects on airway structure and function, including bronchoconstriction, enhanced airway contractility, increased cell proliferation, and remodeling (6, 48). These changes may translate clinically into the development of asthma and wheezing disorders (6, 48).
Using human fetal ASM cells as a model for the neonatal airway, we evaluated the role of poly(I:C) and its receptor, TLR3, in addition to other proinflammatory mediators, LPS and TNF-α, on chemokine secretion. We compared mechanisms by which poly(I:C) induced effects in chemokine expression between human fetal and adult ASM through examination of the MAPK and NF-κB pathways. We acknowledge that there are limitations in the use of fetal vs. neonatal ASM cells. Prior studies in neonatal ASM by Fayon et al. (20) showed greater cell proliferation in neonatal ASM than adult ASM. Our group observed similar findings in fetal ASM, suggesting there may be similarities between fetal and neonatal ASM (20, 24).
Viral infections instigate asthma exacerbations through activation of TLRs, which promote airway inflammation (14). ASM contributes to proinflammatory responses due to its ability to augment expression of inflammatory mediators, such as cytokines and chemokines (14). TLR mRNA expression has been characterized in a prior study showing that human adult ASM expressed TLR1 through TLR10 (63). Human ASM exposure to RV or RSV results in production of a variety of cytokines, including IL-1β, IL-6, IL-8, IL-11, IFN-γ, and TNF-α, and enhances expression of TLRs (8, 14, 15, 42, 46). However, there is a paucity of data on the expression of TLRs and chemokine secretion in the developing ASM. Analogous to human adult ASM, we found that fetal ASM express TLRs, which are known to mediate pulmonary inflammatory responses. With respect to TLR3 activation, poly(I:C) stimulates the production of multiple chemokines, including CCL11 (45). Similarly, our studies demonstrate that poly(I:C) stimulates IL-8 and CCL5 production in human fetal ASM. Importantly, the effects of poly(I:C) were significantly reduced by a TLR3 antagonist.
CCL5 and IL-8 are important chemokines for airway inflammation during infection and asthma (33, 69). Chemokines not only mediate cell recruitment to areas of inflammation, but also function to mediate the production of cytokines (13, 62). IL-8 acts primarily as a mediator for neutrophil chemotaxis, whereas, CCL5 (RANTES) functions as a potent chemoattractant for eosinophils (13, 62). In a study examining mature asthmatic human ASM cells, exposure to RV induced IL-8 release (46). Additionally, in primary culture of adult human bronchial smooth muscle cells, poly(I:C) enhanced CCL5 production (45). Consistent with previous studies, our data in developing ASM demonstrate that exposure to poly(I:C) results in increased secretion of IL-8 and CCL5. These data suggest that activation of TLRs in the developing ASM may contribute to inflammatory cell recruitment during infection.
Although we found that fetal ASM express TLR4, the effects of LPS on chemokine expression were less pronounced than poly(I:C). LPS did not instigate significant effects on IL-8 and CCL5 mRNA expression, and IL-8 secretion following LPS stimulation was significantly less than with poly(I:C). Our data are consistent with a prior investigation in human ASM demonstrating that poly(I:C) stimulates chemokine secretion, while LPS had minimal effects on chemokine production (39). We speculated that limited responses to LPS in ASM could be attributed to lack of expression of key TLR4 adaptor molecules, MD2, CD14, and MyD88. Our data show that fetal ASM express MD2, CD14, and MyD88. Interestingly, poly(I:C) and TNF-α increased MyD88 expression, suggesting potential modulation of TLR4 by TLR3- and TNFR-mediated signaling. We speculate that there may be intrinsic differences in the TLR4 pathway that limit the effects of LPS in ASM in regard to chemokine production. Certainly, additional studies will be needed to further interrogate TLR signaling pathway in developing ASM.
TNF-α is a potent proinflammatory cytokine that has multiple effects during airway inflammation (11). Similar to TLR3 and TLR4 signaling, TNF-α activates multiple signaling pathways, including MAPK and NF-κB (16). Treatment with TNF-α increased TLR3 and TLR4 mRNA and protein expression in fetal ASM and also increased IL-8 and CCL5 production. The data suggest that TNF-α may potentiate the effects of TLR ligands through enhancement of TLR expression (10, 38).
Proinflammatory cytokines and TLR ligands activate the MAPK and NF-κB pathways, and through these pathways regulate key processes during airway inflammation, such as airway remodeling, resulting in cell proliferation and extracellular matrix deposition (17). We found that poly(I:C) stimulated phosphorylation of ERK and p38 and activated NF-κB (p50 and p65) in human fetal ASM, similar to recent findings in human bronchial epithelial cells showing increased phosphorylation of ERK with poly(I:C) exposure (64). Another study in murine ASM found that TLR3 stimulation with poly(I:C) increased NF-κB and JNK signaling pathways (2). We speculate that possible differences between species (human and murine) ASM could explain variations between observations with respect to how we did not observe activation of JNK by poly(I:C) in human fetal ASM. In our studies, pretreatment with an ERK inhibitor, but not p38 inhibitor, blunted IL-8 and CCL5 secretion stimulated by poly(I:C). A previous study similarly found that utilization of an ERK inhibitor resulted in a reduction in IL-1β-induced CCL11 (eotaxin) production in adult human ASM (71). Our experiments in human fetal ASM with specific inhibitors of MAPKs suggest that the poly(I:C)-induced expression of chemokines in human fetal ASM is mediated, at least in part, through the ERK pathway.
Our data suggest variations in mechanisms between human adult and fetal ASM. We observe that the phosphorylation of ERK is upregulated by poly(I:C) in fetal ASM, but this is not seen in the adult ASM. Moreover, phosphorylation of JNK is not increased by poly(I:C) in fetal ASM, but this is observed in adult ASM. NF-κB-mediated signaling is integral to asthmatic inflammation (9, 19). Infection with RSV and RV, RNA type viruses, have been shown to induce activation of NF-κB in adult human nasal epithelial cells, adult human airway epithelium, and adult human ASM; however, there is a paucity of data specifically focused on the developing airway (12, 41, 46). Consistent with prior studies, in the human adult ASM we observed a significant increase in the nuclear translocation of NF-κB subunits p50 and p65, with inflammation triggered by TLR3 agonist, poly(I:C), and TNF-α (1, 7, 28, 71, 72). Secretion of CCL5 and IL-8 was reduced after pretreatment with NF-κB inhibitor in the presence of poly(I:C). NF-κB has been shown to play an important role in proinflammatory cytokine production and hypercontractility in human ASM (50, 57).
In our study, we found that fetal ASM express not only TLR3 and TLR4, which were the focus of this paper, but other TLRs, including TLR2, TLR7, and TLR9. TLR2 prompts immune response through recognition of bacteria, TLR7 is activated by viral single-stranded RNA, and TLR9 responds to unmethylated CpG DNA present in bacterial and viral DNA (29, 43). In addition to TLRs, other receptors, such as retinoic acid-inducible gene 1 (RIG-1) and melanoma differentiation-associated protein 5 (MDA5), are also involved in responses to viral infection (70). Recent studies demonstrated a role for RIG-1 and MDA5 in interferon production by ASM upon RV infection (8). Future experiments examining the roles of other TLRs and RNA virus sensing receptors in the developing airway would improve our understanding of the significance of these receptors in the developing ASM.
Inflammation is a central feature of infection, which can promote asthmatic exacerbations and contribute to altered airway structure and function. Pulmonary infection, triggered by viral pathogens, is prevalent in the neonatal population and represents an important contributor to the development of asthma and wheezing. The present studies suggest that poly(I:C), TLR3 agonist, can enhance the inflammatory response in the developing airway through upregulation of CCL5 and IL-8 through involvement of mechanisms related to the TLR3 receptor, in addition to the MAPK and NF-κB signaling pathways. Although there are limitations in using TLR agonists as opposed to a live virus, it does offer the opportunity to study mechanisms, as there is less concern about variation in virulence or viral load. Prior in vitro work suggests that RV epithelial infection decreases β2-adrenoceptor function on ASM (68). In addition, another study shows that RV infection induces airway remodeling through increased deposition of extracellular matrix proteins (34). Future studies will be needed to examine the effects of live RSV or RV on fetal ASM.
Our investigations demonstrate that fetal ASM cells respond to stimulation with poly(I:C). Clinically, these findings suggest that fetal ASM contributes to inflammatory responses in the fetal lung during antenatal infection, which is relevant to postnatal lung development in preterm infants and possibly more long-term in childhood. Mechanistically, it appears that there are variations demonstrated between human fetal and adult ASM that may have important implications in the development of potential treatments to offer more specific, targeted therapy for the developing airway. Further studies aiming to identify therapeutic measures to target TLR signaling and to examine ways to downregulate inflammation in the developing airway will be important for clinical advances in the treatment of neonatal airway disease.
GRANTS
This study was supported by grants from the National Heart, Lung, and Blood Institute [T32 HL-105355 (R. D. Britt, E. R. Vogel), F32 HL-123075 (R. D. Britt), and R01 HL-056470 (Y. S. Prakash, R. J. Martin)], and by collaborative grants from the Mayo Clinic Center for Biomedical Discovery and the Center for Clinical and Translational Sciences and the Children's Research Center (C. M. Pabelick). This study was also supported by the Departments of Obstetrics and Gynecology (A. Faksh) and Anesthesiology (Y. S. Prakash, C. M. Pabelick, E. R. Vogel) of Mayo Clinic, Rochester.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. conception and design of research; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. performed experiments; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. analyzed data; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. interpreted results of experiments; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. prepared figures; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. drafted manuscript; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. edited and revised manuscript; A.F., R.D.B.J., E.R.V., M.A.T., H.C.P., R.J.M., C.M.P., and Y.S.P. approved final version of manuscript.
REFERENCES
- 1.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bachar O, Adner M, Uddman R, Cardell LO. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, an effect mediated by JNK and NF-kappa B signaling pathways. Eur J Immunol 34: 1196–1207, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, Schechtman KB, Strunk RC, Castro M. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 130: 91–100 e103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastek JA, Gomez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol 38: 385–406, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 11: e1001596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt RD Jr, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Expert Rev Respir Med 7: 515–531, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britt RD Jr, Faksh A, Vogel ER, Thompson MA, Chu V, Pandya HC, Amrani Y, Martin RJ, Pabelick CM, Prakash YS. Vitamin d attenuates cytokine-induced remodeling in human fetal airway smooth muscle cells. J Cell Physiol 230: 1189–1198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calven J, Yudina Y, Uller L. Rhinovirus and dsRNA induce RIG-I-like receptors and expression of interferon beta and lambda1 in human bronchial smooth muscle cells. PLoS One 8: e62718, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang PJ, Michaeloudes C, Zhu J, Shaikh N, Baker J, Chung KF, Bhavsar PK. Impaired nuclear translocation of the glucocorticoid receptor in corticosteroid-insensitive airway smooth muscle in severe asthma. Am J Respir Crit Care Med 191: 54–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiou YL, Lin CY. Der p2 activates airway smooth muscle cells in a TLR2/MyD88-dependent manner to induce an inflammatory response. J Cell Physiol 220: 311–318, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Cho JY, Pham A, Rosenthal P, Miller M, Doherty T, Broide DH. Chronic OVA allergen challenged TNF p55/p75 receptor deficient mice have reduced airway remodeling. Int Immunopharmacol 11: 1038–1044, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary S, Boldogh S, Garofalo R, Jamaluddin M, Brasier AR. Respiratory syncytial virus influences NF-kappaB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-kappaB2. J Virol 79: 8948–8959, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, Hussell T, Openshaw PJ. Role of CCL5 (RANTES) in viral lung disease. J Virol 80: 8151–8157, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damera G, Panettieri RA Jr. Does airway smooth muscle express an inflammatory phenotype in asthma? Br J Pharmacol 163: 68–80, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damera G, Tliba O, Panettieri RA Jr. Airway smooth muscle as an immunomodulatory cell. Pulm Pharmacol Ther 22: 353–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev 14: 193–209, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol 19: 676–680, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Drysdale SB, Alcazar-Paris M, Wilson T, Smith M, Zuckerman M, Peacock JL, Johnston SL, Greenough A. Viral lower respiratory tract infections and preterm infants' healthcare utilisation. Eur J Pediatr 174: 209–215, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther 121: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayon M, Andrieux A, Bara I, Rebola M, Labbe A, Marthan R, Berger P. An age-wise comparison of human airway smooth muscle proliferative capacity. PLoS One 10: e0122446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, Jacobsen SJ. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med 164: 187–192, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Greene CM, McElvaney NG. Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz) 53: 418–427, 2005. [PubMed] [Google Scholar]
- 23.Hakonarson H, Maskeri N, Carter C, Hodinka RL, Campbell D, Grunstein MM. Mechanism of rhinovirus-induced changes in airway smooth muscle responsiveness. J Clin Invest 102: 1732–1741, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L711–L719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, Hayden FG, Hatley TK, Chamberlain R. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 114: 239–247, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illi S, von Mutius E, Lau S, Bergmann R, Niggemann B, Sommerfeld C, Wahn U. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 322: 390–395, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF Jr. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 178: 667–672, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of CD38 expression by tumor necrosis factor-alpha in human airway smooth muscle cells: role of NF-kappaB and sensitivity to glucocorticoids. FASEB J 20: 1000–1002, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31: 873–884, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Kimura H, Yoshizumi M, Ishii H, Oishi K, Ryo A. Cytokine production and signaling pathways in respiratory virus infection. Front Microbiol 4: 276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolli D, Velayutham TS, Casola A. Host-viral interactions: role of pattern recognition receptors (PRRs) in human pneumovirus infections. Pathogens 2: 232–263, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy–the first sign of childhood asthma? J Allergy Clin Immunol 111: 66–71, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, Wenzel SE. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med 163: 1338–1343, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Kuo C, Lim S, King NJ, Johnston SL, Burgess JK, Black JL, Oliver BG. Rhinovirus infection induces extracellular matrix protein deposition in asthmatic and nonasthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L951–L957, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1: 398–401, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 18: 684–692, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Mansson Kvarnhammar A, Tengroth L, Adner M, Cardell LO. Innate immune receptors in human airway smooth muscle cells: activation by TLR1/2, TLR3, TLR4, TLR7 and NOD1 agonists. PLoS One 8: e68701, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marodi L. Neonatal innate immunity to infectious agents. Infect Immun 74: 1999–2006, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masaki T, Kojima T, Okabayashi T, Ogasawara N, Ohkuni T, Obata K, Takasawa A, Murata M, Tanaka S, Hirakawa S, Fuchimoto J, Ninomiya T, Fujii N, Tsutsumi H, Himi T, Sawada N. A nuclear factor-kappaB signaling pathway via protein kinase C delta regulates replication of respiratory syncytial virus in polarized normal human nasal epithelial cells. Mol Biol Cell 22: 2144–2156, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Message SD, Johnston SL. The immunology of virus infection in asthma. Eur Respir J 18: 1013–1025, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22: 240–273, Table of Contents, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore PE, Cunningham G, Calder MM, DeMatteo AD Jr, Peeples ME, Summar ML, Peebles RS Jr. Respiratory syncytial virus infection reduces beta2-adrenergic responses in human airway smooth muscle. Am J Respir Cell Mol Biol 35: 559–564, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niimi K, Asano K, Shiraishi Y, Nakajima T, Wakaki M, Kagyo J, Takihara T, Suzuki Y, Fukunaga K, Shiomi T, Oguma T, Sayama K, Yamaguchi K, Natori Y, Matsumoto M, Seya T, Yamaya M, Ishizaka A. TLR3-mediated synthesis and release of eotaxin-1/CCL11 from human bronchial smooth muscle cells stimulated with double-stranded RNA. J Immunol 178: 489–495, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Oliver BG, Johnston SL, Baraket M, Burgess JK, King NJ, Roth M, Lim S, Black JL. Increased proinflammatory responses from asthmatic human airway smooth muscle cells in response to rhinovirus infection. Respir Res 7: 71, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandya HC, Snetkov VA, Twort CH, Ward JP, Hirst SJ. Oxygen regulates mitogen-stimulated proliferation of fetal human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L1220–L1230, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Prakash YS, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacol Ther 143: 74–86, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puddicombe SM, Davies DE. The role of MAP kinases in intracellular signal transduction in bronchial epithelium. Clin Exp Allergy 30: 7–11, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Redhu NS, Saleh A, Halayko AJ, Ali AS, Gounni AS. Essential role of NF-kappaB and AP-1 transcription factors in TNF-alpha-induced TSLP expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L479–L485, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Reuter S, Dehzad N, Martin H, Bohm L, Becker M, Buhl R, Stassen M, Taube C. TLR3 but not TLR7/8 ligand induces allergic sensitization to inhaled allergen. J Immunol 188: 5123–5131, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Reuven EM, Fink A, Shai Y. Regulation of innate immune responses by transmembrane interactions: Lessons from the TLR family. Biochimica Biophys Acta 1838: 1586–1593, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9: 180–186, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Rosenthal LA, Avila PC, Heymann PW, Martin RJ, Miller EK, Papadopoulos NG, Peebles RS Jr, Gern JE. Viral respiratory tract infections and asthma: the course ahead. J Allergy Clin Immunol 125: 1212–1217, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol 79: 3350–3357, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol 176: 1937–1942, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Sathish V, Abcejo AJ, VanOosten SK, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 in cytokine-induced enhancement of intracellular Ca2+ in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L607–L614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282: 1440–1446, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 171: 137–141, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Smyth RL, Mobbs KJ, O'Hea U, Ashby D, Hart CA. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatr Pulmonol 33: 339–346, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354: 541–545, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Strieter RM. Interleukin-8: a very important chemokine of the human airway epithelium. Am J Physiol Lung Cell Mol Physiol 283: L688–L689, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, Chung KF. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol 118: 641–648, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Tadaki H, Arakawa H, Mizuno T, Suzuki T, Takeyama K, Mochizuki H, Tokuyama K, Yokota S, Morikawa A. Double-stranded RNA and TGF-alpha promote MUC5AC induction in respiratory cells. J Immunol 182: 293–300, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 140: 805–820, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Thomas AO, Lemanske RF Jr, Jackson DJ. Infections and their role in childhood asthma inception. Pediatr Allergy Immunol 25: 122–128, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 23: 74–98, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trian T, Moir LM, Ge Q, Burgess JK, Kuo C, King NJ, Reddel HK, Black JL, Oliver BG, McParland BE. Rhinovirus-induced exacerbations of asthma: how is the β2-adrenoceptor implicated? Am J Respir Cell Mol Biol 43: 227–233, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Wenzel S. Mechanisms of severe asthma. Clin Exp Allergy 33: 1622–1628, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Wornle M, Sauter M, Kastenmuller K, Ribeiro A, Mussack T, Ladurner R, Sitter T. Role of toll-like receptor 3, RIG-I, and MDA5 in the expression of mesothelial IL-8 induced by viral RNA. Appl Biochem Biotechnol 160: 1179–1187, 2010. [DOI] [PubMed] [Google Scholar]
- 71.Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. Involvement of p38 MAPK, JNK, p42/p44 ERK and NF-kappaB in IL-1beta-induced chemokine release in human airway smooth muscle cells. Respir Med 97: 811–817, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J Virol 84: 7267–7277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng R, Cui Y, Hai Y, Liu Y. Pattern recognition receptors for respiratory syncytial virus infection and design of vaccines. Virus Res 167: 138–145, 2012. [DOI] [PubMed] [Google Scholar]