Abstract
Distal lung epithelium is maintained by proliferation of alveolar type II (AT2) cells and, for some daughter AT2 cells, transdifferentiation into alveolar type I (AT1) cells. We investigated if subpopulations of alveolar epithelial cells (AEC) exist that represent various stages in transdifferentiation from AT2 to AT1 cell phenotypes in normal adult lung and if they can be identified using combinations of cell-specific markers. Immunofluorescence microscopy showed that, in distal rat and mouse lungs, ∼20–30% of NKX2.1+ (or thyroid transcription factor 1+) cells did not colocalize with pro-surfactant protein C (pro-SP-C), a highly specific AT2 cell marker. In distal rat lung, NKX2.1+ cells coexpressed either pro-SP-C or the AT1 cell marker homeodomain only protein x (HOPX). Not all HOPX+ cells colocalize with the AT1 cell marker aquaporin 5 (AQP5), and some AQP5+ cells were NKX2.1+. HOPX was expressed earlier than AQP5 during transdifferentiation in rat AEC primary culture, with robust expression of both by day 7. We speculate that NKX2.1 and pro-SP-C colocalize in AT2 cells, NKX2.1 and HOPX or AQP5 colocalize in intermediate or transitional cells, and HOPX and AQP5 are expressed without NKX2.1 in AT1 cells. These findings suggest marked heterogeneity among cells previously identified as exclusively AT1 or AT2 cells, implying the presence of subpopulations of intermediate or transitional AEC in normal adult lung.
Keywords: intermediate cells, alveolar epithelial type II cell, alveolar epithelial type I cell, transdifferentiation, NKX2.1, homeodomain only protein x, aquaporin-5
despite progress in understanding how lung development occurs from embryo to adult, less is known about cell turnover once development is complete. Cells in the adult lung are replenished slowly at baseline but can regenerate quickly following many types of acute injury (8, 15). Recovery from acute lung injury requires distal lung epithelial cells to activate proliferation and differentiation programs to reestablish normal lung architecture. Deficiencies in normal cell turnover programs may contribute to the pathogenesis of many chronic lung diseases, including idiopathic pulmonary fibrosis (1, 15). After birth, alveolar epithelial type II (AT2) cells are the main source of renewal of distal lung epithelium and may either regenerate cuboidal surfactant-producing AT2 cells or transdifferentiate into thin elongated type I (AT1) cells (2, 9).
Although differentiated AT1 and AT2 cells differ phenotypically and express different cell-specific markers, there is evidence that both cell types exhibit considerable plasticity (6). Freshly isolated AT2 cells transdifferentiate over time in culture to AT1-like cells, recapitulating AT2 to AT1 cell transdifferentiation in vivo (3) and providing a useful model with which to investigate kinetics of the transdifferentiation process. In mouse embryonic development, lineage tracing and single-cell RNA sequencing techniques identified cells expressing markers in a pattern suggesting that they were intermediate between AT2 and AT1 cells (8, 17). The extent to which alveolar epithelial cells (AEC) exist in varying stages of differentiation in the normal adult lung, and whether such intermediate or transitional cells can be identified using combinations of putative AT2 or AT1 cell-specific markers, is as yet unknown.
One marker of the AT2 cell phenotype is NKX2.1 (also known as thyroid transcription factor 1), a transcription factor essential for lung morphogenesis and AEC differentiation. NKX2.1 is normally found in the adult in bronchial epithelium and in AT2 cells in distal lung (14). NKX2.1 regulates expression of genes that themselves serve as markers for AT2 cells, including surfactant proteins (SP-A, SP-B, SP-C) and ATP-binding cassette, subfamily A member 3 (ABCA3) (16). Several putative AT1 cell markers have been identified, including aquaporin 5 (AQP5), homeodomain only protein x (HOPX), and T1α (also known as podoplanin) (2), but when these markers become expressed during AT2 to AT1 cell transdifferentiation is unknown. In this study, we used AT1 and AT2 cell markers in various combinations to determine whether there was heterogeneity in the expression of these markers, potentially indicating differences in cells that otherwise appear phenotypically similar. We found substantial heterogeneous coexpression of AT1 and AT2 cell markers in distal epithelium, suggesting the presence of AEC subpopulations. Knowledge of how AEC are maintained under normal conditions may offer insights into how these systems are distorted in disease states.
MATERIALS AND METHODS
Processing of Lung Tissue
Rat or mouse lung fixed in 4% paraformaldehyde was either embedded in paraffin or prepared as cryosections. Paraffin-embedded normal human lungs were prepared from rejected lung transplant donors (13). This study was approved by the Institutional Review Board for Human Subjects Research and the Institutional Animal Care and Use Committee of the University of Southern California.
Immunofluorescence
Paraffin-embedded lung sections were deparaffinized, followed by antigen retrieval (Antigen Unmasking Solution; Vector Laboratories, Burlingame, CA). Mouse monoclonal antibodies (Abs) used included NKX2.1 (Novocastra, Buffalo Grove, IL) and ABCA3 (Seven Hills Bioreagents, Cincinnati, OH). Rabbit polyclonal Abs used included HOPX (Santa Cruz Biotechnology, Dallas, TX), pro-SP-C (Seven Hills), and AQP5 (Abcam, Cambridge, MA, or EMD Millipore, Billerica, MA). Goat polyclonal Abs used included AQP5 and pro-SP-C (Santa Cruz). Slides were incubated with the first primary Ab, followed by biotinylated secondary Abs (Vector), and then avidin-conjugated fluorescein isothiocyanate (Vector) or streptavidin-conjugated Cy3 (Jackson ImmunoResearch, West Grove, PA). Alternatively, after primary Ab incubation, slides were incubated with secondary Abs directly conjugated to a fluorochrome (either AlexaFluor 594, 633, or 488; Life Technologies, Grand Island, NY). Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector) was used for nuclear staining. Images were captured using a ZEISS LSM 510 confocal system (Carl Zeiss, Jena, Germany) or Nikon Eclipse 80i microscope (Melville, NY). To count NKX2.1+/SP-C− cells, images at ×40 magnification from three lungs from rats or mice were manually counted. Approximately 1,300 NKX2.1+ cells were counted. The number of nuclear HOPX+/NKX2.1+ cells was determined by scanning lung sections with Axio Scan.Z1 (Carl Zeiss) and counting with Imaris software (Bitplane, Concord, MA) in the Core Facility of the Broad CIRM Center (University of Southern California). A total of ∼1,350 nuclear HOPX+ cells from two rat lungs was counted. To determine if any pro-SP-C+ cells were NKX2.1−, slides were scanned, and a total of 1,454 pro-SP-C+ cells from two rat lungs was examined. Similarly, slides with NKX2.1/pro-SP-C/HOPX triple staining were scanned. A total of 1,786 NKX2.1+ cells from two rat lungs was counted to evaluate for coexpression of pro-SP-C and/or HOPX.
Rat Cell Isolation and Culture
AT2 cells were isolated from adult male Sprague-Dawley rats as previously described (3). AT2 cells were enriched by negative selection using VIIIB2 Ab with specific reactivity to rat AT1 cells (7) and positive selection with anti-ABCA3 Ab (Biolegend, San Diego, CA) using magnetic beads (Miltenyi Biotec, San Diego, CA). Cells were cultured on Transwell polycarbonate filters (Corning, Cambridge, MA). Protein from cultured cells was analyzed by Western blotting at days 0, 1, 3, 5, and 7 after plating. Cells at day 0 represent AT2 cells, whereas cells on days 5-7 have transdifferentiated toward the AT1 cell phenotype (3).
Western Analysis
Proteins were resolved by SDS-PAGE, transferred onto Immobilon-P membranes (Millipore), and then incubated with rabbit anti-HOPX, -AQP5 (Alomone Labs, Jerusalem, Israel), -T1α (Thermo Scientific, Waltham, MA), -pro-SP-C (Millipore), and mouse anti-NKX2.1 (ThermoScientific) Abs. Rabbit anti-lamin A/C (Santa Cruz) and mouse anti-β-actin (Abcam) Abs were loading controls. Proteins were visualized by enhanced chemiluminescence (ThermoScientific) with an Alpha Ease Imaging System (Alpha Innotech, San Leandro, CA).
Cell Proliferation
Thymidine analog 5-ethynyl-2′-deoxyuridine (EdU) was administered intraperitoneally 24 h before mice were killed. HOPX staining was performed as described above, followed by EdU incorporation detection with the Click-iT Plus EdU Alexa Fluor 488 Imaging Kit (Life Technologies). Lung sections were then scanned (Axio Scan.Z1). A total of ∼6,000 nuclear HOPX+ cells from three mouse lungs was counted (Imaris software) to determine the percentage of nuclear HOPX+/EdU+ cells.
RESULTS
Coexpression of NKX2.1 with AT1 and AT2 Cell-Specific Markers
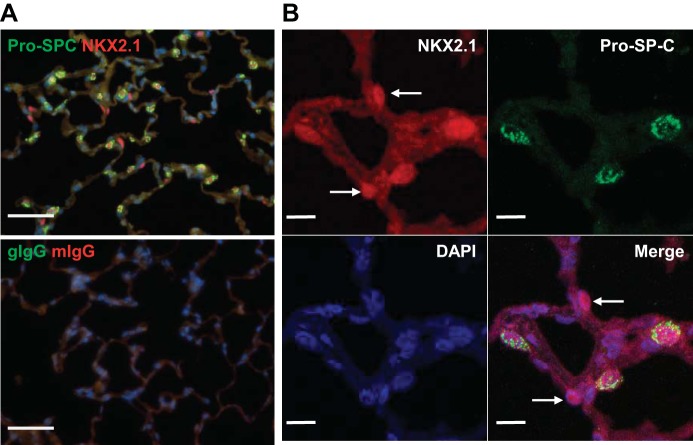
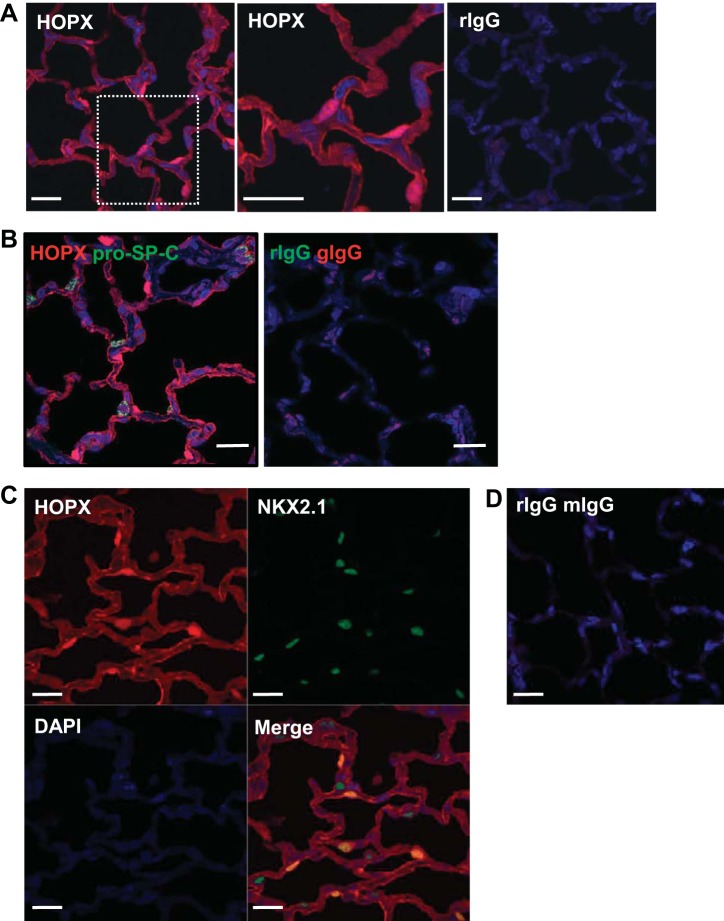
Lung cells were readily found in both rat (Fig. 1) and mouse (data not shown) that expressed nuclear NKX2.1 and cytoplasmic pro-SP-C. In distal lung, 21.8 ± 0.5% of NKX2.1+ cells in mouse and 32.4 ± 1.8% in rat were pro-SP-C−. We examined 1,454 pro-SP-C+ cells from two different rats, and all cells coexpressed NKX2.1. Figure 2A demonstrates nuclear and membrane/cytoplasmic localization in normal rat lung of a recently identified AT1 cell marker, HOPX (2). With the use of a double-labeling technique, pro-SP-C did not appear to be expressed in HOPX+ cells (Fig. 2B), consistent with a previous report (2). However, NKX2.1 was expressed in most nuclear HOPX+ cells (only 1 of 1,355 nuclear HOPX+ cells did not express NKX2.1) (Fig. 2, C and D). Using a triple-label immunofluorescence technique (Fig. 2E), we found that among 1,786 NKX2.1+ cells viewed, cells either expressed pro-SP-C or HOPX in normal rat lung, but not both, or were indeterminate (0.2% of total).
Fig. 1.
Immunofluorescence of NKX2.1 and pro-surfactant protein (SP)-C in normal rat lung. A: double immunofluorescence for pro-SP-C (green) and NKX2.1 (red) shows that not all NKX2.1+ cells are pro-SP-C+. Negative controls are goat IgG (gIgG) and mouse IgG (mIgG). 4′,6-Diamidino-2-phenylindole (DAPI, blue) is the nuclear counterstain. Scale bar = 50 μm. B: confocal z-stack images demonstrate that some NKX2.1+ cells are pro-SP-C− (arrows). Scale bar = 10 μm.
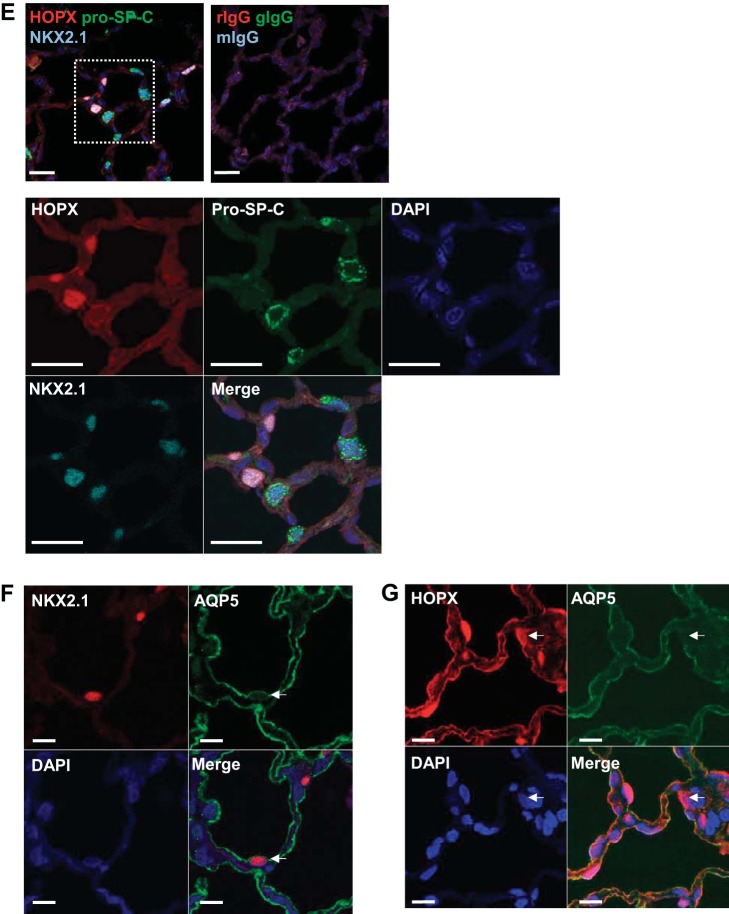
Fig. 2.
Immunofluorescence for alveolar type 1 (AT1) and 2 (AT2) cell markers in normal rat lung. A: left, immunofluorescence for homeodomain only protein x (HOPX, red) shows both nuclear and cytoplasm/membrane staining. Middle, magnified views of the rectangles shown on left. Right, negative control is rIgG. DAPI (blue) is the nuclear counterstain. Bar = 20 μm. B: double immunofluorescence shows HOPX (red) does not colocalize with pro-SP-C (green). Bar = 20 μm. C: double immunofluorescence shows nuclear HOPX (red) colocalizing with NKX2.1 (green). Bar = 20 μm. D: negative controls for C are rIgG and mIgG. E: triple immunofluorescence shows that NKX2.1 (light blue) colocalizes with either nuclear HOPX (red) or pro-SP-C (green), but not both. High magnification of rectangle is shown on bottom with individual channels. Bar = 20 μm. F: immunofluorescent localization of NKX2.1 (red) and AQP5 (green). Bar = 20 μm. Some cells are seen that colocalize both markers (arrows). G: immunofluorescent localization of HOPX (red) and AQP5 (green). Bar = 20 μm. Although most cells coexpress both markers, some do not (arrows).
We next determined if coexpression of NKX2.1 with HOPX was unique to HOPX, or if NKX2.1 can be coexpressed with another AT1 cell marker as well, such as AQP5. Although AT1 cells are technically difficult to count accurately using fluorescence microscopy due to their thin elongated shape, we were able to determine that some AQP5+ cells also express NKX2.1 (Fig. 2F), consistent with the existence of cells displaying features of both AT1 and AT2 cells. We observed that cytoplasmic/membrane HOPX colocalizes with the AT1 cell marker AQP5, but some nuclear HOPX+ cells are AQP5− (Fig. 2G), also suggesting heterogeneity within the AT1 cell population. Although nearly all nuclear HOPX+ cells are NKX2.1+, only 0.12% of nuclear HOPX+ cells in three normal mice examined incorporated EdU into newly synthesized DNA, suggesting that proliferation is a rare event under normal conditions.
Changing Expression of AT2 and AT1 Cell Markers Over Time in Culture
To further understand what types of AEC subsets might be identified temporally by combinations of cell markers, we isolated rat AT2 cells, allowed them to transdifferentiate on Transwell filters, and harvested protein for Western analysis (Fig. 3A). Expression of pro-SP-C protein drops off quickly and is not seen after day 1 in culture. Total protein for HOPX and other AT1 cell markers (T1α and AQP5) increases over time. HOPX levels rise earlier than T1α or AQP5, suggesting that cells in situ, which express both NKX2.1 and HOPX, may represent late AT2 cells/early AT1 cells, whereas NKX2.1+/AQP5+ or NKX2.1+/T1α+ cells may represent those that are in later stages of AT1 transdifferentiation. A proposed time course of these in vitro changes is illustrated in Fig. 3B.
Fig. 3.
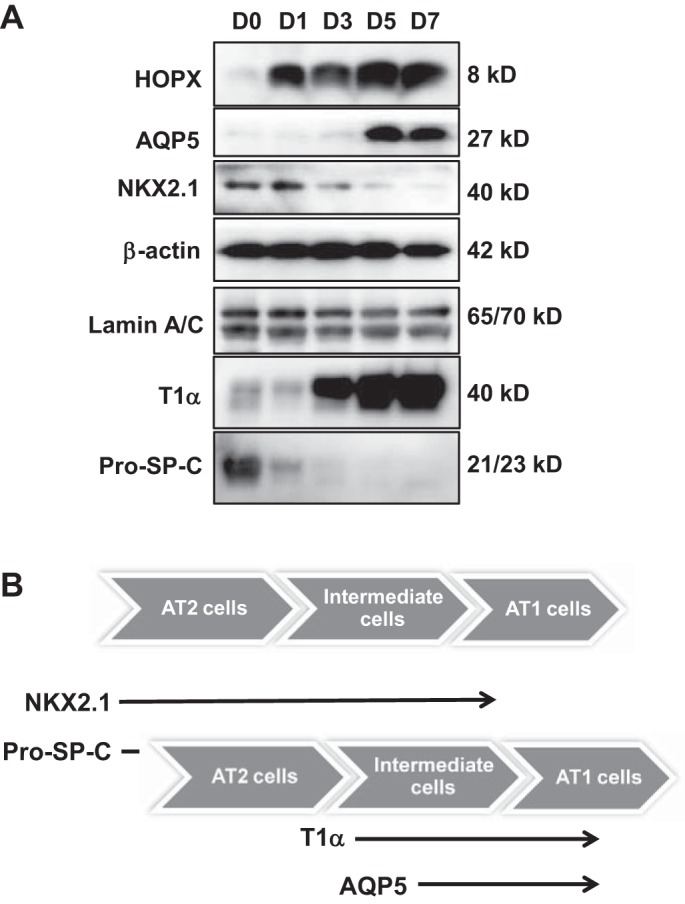
Expression of AT1 and AT2 cell markers changes over time in culture during in vitro transdifferentiation. A: immunoblot with AT1 and AT2 cell markers shows HOPX is highly expressed from day 1 and increases only minimally in culture. AQP5 is expressed at very low levels on day 1 and increases over time. NKX2.1 decreases as cells transdifferentiate to the AT1-like phenotype; n = 3. B: diagram of proposed sequence of temporal changes in AT1 and AT2 cell markers as AT2 cells transdifferentiate toward AT1-like cells. Pro-SP-C expression drops abruptly as cells begin to transdifferentiate while NKX2.1 expression declines more slowly as cells progress though the intermediate (transitional) cell time period.
Expression of AT1 Cell Markers in Human Lung Tissue
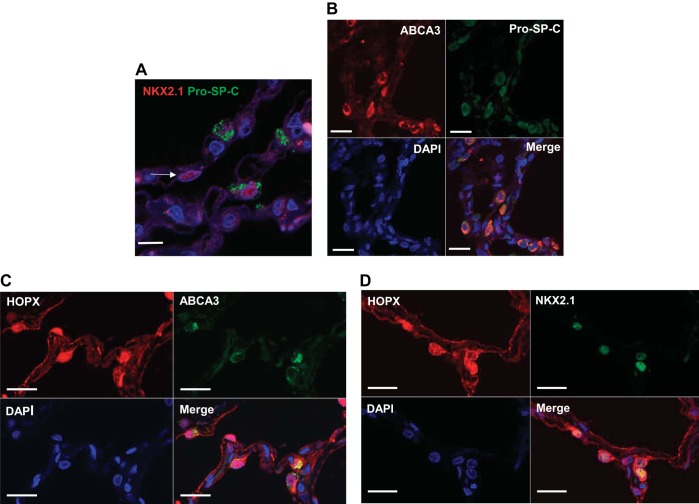
Similar to rat and mouse studies, NKX2.1+ cells were readily found in normal adult human lung tissue that did not colocalize pro-SP-C (Fig. 4A). Because of Ab incompatibility in human lung, we double-labeled HOPX with another AT2 cell marker that colocalizes with pro-SP-C, ABCA3 (16). We found excellent (>95%) colocalization of ABCA3 and pro-SP-C, as seen in Fig. 4B. Surprisingly, HOPX+ cells, which did not colocalize with pro-SP-C in mouse or rat lung, did colocalize ABCA3 (Fig. 4C). In addition, most HOPX+ cells colocalized with NKX2.1, similar to findings in rat and mouse (Fig. 4D).
Fig. 4.
AT1 and AT2 cell markers in human lung tissue. A: not all NKX2.1+ cells colocalize with pro-SP-C in normal human lung (arrow, confocal z-stack image). DAPI (blue) was used to identify nuclei. Bar = 20 μm. B: ABCA3+ cells (>95%) coexpress pro-SP-C. Bar = 20 μm. C: many cells that express HOPX (red) also express the AT2 cell marker ABCA3 (green). Bar = 20 μm. D: most cells that express nuclear HOPX are also NKX2.1+. Bar = 20 μm.
DISCUSSION
Cell renewal in the normal adult lung is a slow process, occurring in only ∼7% of alveoli per year (8). In the absence of injury, resident AT2 cells function as progenitors for other AT2 cells or transdifferentiate into AT1 cells to maintain lung homeostasis (2). AT1 and AT2 cells are morphologically distinct, with AT2 cells demonstrating a cuboidal shape, apical microvilli, and lamellar bodies while AT1 cells are thin and elongated without villus structures or lamellar bodies. A variety of cell-specific markers may assist in the identification of AT1 and AT2 cells beyond relying on morphological characteristics alone. However, when AT1 and AT2 cell markers were combined using immunolocalization in fixed lung tissue, AEC could be identified that coexpressed both AT1 and AT2 cell markers. This suggests considerable heterogeneity among cells previously identified as exclusively AT1 or AT2 cells.
A subpopulation of AEC in adult and neonatal lungs that expresses features of both AT1 and AT2 cells has previously been described during repair from lung injury in experimental animal models (10, 18). These intermediate cells are less well described in normal lung tissue. For example, using markers of mature AT1 and AT2 cells, Clegg et al. (5) saw numerous AEC that coexpressed markers of both cell types during recovery from experimental lung injury, but not in control lungs. Using a recently developed technique of single-cell RNA sequencing, embryonic mouse distal lung epithelial cells were able to be divided into transcriptional patterns that suggested separate groups comprised of AEC progenitors, early and late AT2 cells, and early and late AT1 cells (17). These clusters of RNA expression are likely associated with differences in protein expression, suggesting that subpopulations of intermediate cells (potentially similar to cells in embryonic development) may be identified even in normal adult lung tissue.
NKX2.1 plays a key role in lung development, emerging as the first sign of specification of the lung field (14). NKX2.1 is required for lung morphogenesis and expression of surfactant protein and ABCA3 (associated with AT2 cell lamellar body membranes) genes (16). Previously, NKX2.1 was thought to be associated exclusively with AT2 cells in distal lung (11). Our data suggest that NKX2.1 is expressed by cells other than those that produce surfactant proteins, and its expression may persist into early AT1 cell transdifferentiation.
By comparison, HOPX is also expressed in developing lung and can function downstream of NKX2.1 to inhibit surfactant proteins (19). HOPX was recently identified as a marker for AT1 cells in mouse lung, with expression found in scattered nuclei, cytoplasm, and cell membranes (2). HOPX was also shown to be involved in regulation of AT2 cell transdifferentiation together with another transcription factor, GATA6 (4). We observed that nearly all nuclear HOPX+ cells were also NKX2.1+, suggesting nuclear HOPX may play a role in determination of phenotype for this intermediate or transitional cell population via coordination with NKX2.1. Whether and how nuclear and cytoplasmic/membrane HOPX function differently during AEC transdifferentiation will require further investigation. Using lineage tracing, Jain et al. (12) showed that some HOPX-labeled cells were capable of proliferation after partial pneumonectomy in the mouse. Our data suggest that nuclear HOPX+ cells have very low proliferation activity under basal conditions. However, this subpopulation of nuclear HOPX+/NKX2.1+ cells may retain the transcriptional capacity to respond like AT2 cells when a stimulus such as pneumonectomy is present.
Our findings have implications for researchers working with AEC isolation or identification. Most investigators use SP-C expression to identify or lineage trace AT2 cells, and SP-C does appear to identify a population of mature AT2 cells that do not coexpress AT1 cell markers. However, use of AT1 cell markers such as HOPX or AQP5 to isolate AT1 cells will likely also capture some intermediate cell types that coexpress NKX2.1. Whether these intermediate cells behave differently from exclusively HOPX+ or AQP5+ cells that are NKX2.1− is as yet unknown.
Species differences may exist between rodent (rat and mouse) and human lung tissue in expression of AT1 and AT2 cell markers. In human lung, we found many cells that coexpressed HOPX and ABCA3, an AT2 cell marker of surfactant production. We did not see HOPX in cells that were pro-SP-C+ in mouse or rat lungs, suggesting that HOPX expression may occur earlier in the transdifferentiation of AT2 to AT1 cells in humans than is the case in rodents.
We recognize that there are limitations to using immunolocalization techniques alone for identification of potential AEC subpopulations. Results are dependent on the quality, availability, and compatibility of the antibodies employed, sensitivity of the staining methods, and clarity of imaging. Additional morphologic, molecular, and functional analyses would be useful in confirming these findings, in conjunction with validation in isolated cells.
In summary, we found substantial heterogeneous coexpression of AT1 and AT2 cell markers in distal lung epithelium, suggesting the presence of subpopulations of AEC at varying stages of transdifferentiation in normal adult lung tissue. These data suggest that NKX2.1 and pro-SP-C colocalize in AT2 cells, NKX2.1 and HOPX or AQP5 colocalize in intermediate or transitional cells, and HOPX and AQP5 are expressed without NKX2.1 in AT1 cells. Use of combinations of cell-specific markers should lead to better characterization of AT1 and AT2 cell subsets and help provide insights into discrete functional properties and differentiation/regenerative potential of these subpopulations. However, there may be species variation in expression of these markers, and findings from rat and mouse studies should be confirmed in human tissue when possible.
GRANTS
This work was supported by the Hastings Foundation, Whittier Foundation, and Research Grants HL-114959 (B. Zhou) and HL-114094 (C. N. Marconett and I. A. Laird-Offringa) from the National Heart, Lung, and Blood Institute (NHLBI). Microscopy services were provided by the Cell and Tissue Imaging Core of the University of Southern California Research Center for Liver Diseases (NHLBI Grant P30-DK-048522).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.L. and B.Z. conception and design of research; J.M.L., C.N.M., N.J., H.W., and Y.L. performed experiments; J.M.L., C.N.M., N.J., H.W., Y.L., P.F., I.A.L.-O., P.M., and B.Z. analyzed data; J.M.L., C.N.M., P.F., I.A.L.-O., P.M., and B.Z. interpreted results of experiments; J.M.L., C.N.M., and B.Z. prepared figures; J.M.L. drafted manuscript; J.M.L., C.N.M., P.F., I.A.L.-O., P.M., and B.Z. edited and revised manuscript; J.M.L. and B.Z. approved final version of manuscript.
REFERENCES
- 1.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BLM, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA 112: 5099–5104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BLM. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borok Z, Danto SI, Zabski SM, Crandall ED. Defined medium for primary culture de novo of adult rat alveolar epithelial cells. In Vitro Cell Dev Biol Anim 30A: 99–104, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Cheung W, Zhao M, Liu Z, Stevens L, Cao P, Fang J, Westbrook T, Nguyen D. Control of alveolar differentiation by the lineage transcription factors GATA6 and HOPX inhibits lung adenocarcinoma metastasis. Cancer Cell 23: 725–738, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC. Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol 288: L382–L390, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 12: 497–502, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Danto SI, Zabski SM, Crandall ED. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol 6: 296–306, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507: 190–194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975. [DOI] [PubMed] [Google Scholar]
- 10.Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S, Xue X. Hyperoxia stimulates the transdifferentiation of type II alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell Mol Physiol 308: L861–L872, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem 270: 8108–8114, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, Padmanabhan A, Manderfield LJ, Gupta M, Li D, Li L, Trivedi CM, Hogan BLM, Epstein JA. Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat Commun 6: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconett CN, Zhou B, Rieger ME, Selamat SA, Dubourd M, Fang X, Lynch SK, Stueve TR, Siegmund KD, Berman BP, Borok Z, Laird-Offringa IA. Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation. PLoS Genet 9: e1003513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1. Mouse Embryos Dev Biol 209: 60–71, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Rock JR, Hogan BLM. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27: 493–512, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Stahlman MT, Besnard Vr Wert SE, Weaver TE, Dingle S, Xu Y, Zychlin Kv Olson SJ, Whitsett JA. Expression of ABCA3 in developing lung and other tissues. J Histochem Cytochem 55: 71–83, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509: 371–375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhal BD. Cell cycle kinetics in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 272: L1031–L1045, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM, Kimura S, Ballard PL, Beers MF, Epstein JA, Morrisey EE. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am J Physiol Lung Cell Mol Physiol 291: L191–L199, 2006. [DOI] [PubMed] [Google Scholar]