Abstract
Human studies use varying levels of low-dose (1-4 μg·kg−1·min−1) dopamine to examine peripheral chemosensitivity, based on its known ability to blunt carotid body responsiveness to hypoxia. However, the effect of dopamine on the ventilatory responses to hypoxia is highly variable between individuals. Thus we sought to determine 1) the dose response relationship between dopamine and peripheral chemosensitivity as assessed by the ventilatory response to hypoxia in a cohort of healthy adults, and 2) potential confounding cardiovascular responses at variable low doses of dopamine. Young, healthy adults (n = 30, age = 32 ± 1, 24 male/6 female) were given intravenous (iv) saline and a range of iv dopamine doses (1–4 μg·kg−1·min−1) prior to and throughout five hypoxic ventilatory response (HVR) tests. Subjects initially received iv saline, and after each HVR the dopamine infusion rate was increased by 1 μg·kg−1·min−1. Tidal volume, respiratory rate, heart rate, blood pressure, and oxygen saturation were continuously measured. Dopamine significantly reduced HVR at all doses (P < 0.05). When subjects were divided into high (n = 13) and low (n = 17) baseline chemosensitivity, dopamine infusion (when assessed by dose) reduced HVR in the high group only (P < 0.01), with no effect of dopamine on HVR in the low group (P > 0.05). Dopamine infusion also resulted in a reduction in blood pressure (3 μg·kg−1·min−1) and total peripheral resistance (1–4 μg·kg−1·min−1), driven primarily by subjects with low baseline chemosensitivity. In conclusion, we did not find a single dose of dopamine that elicited a nadir HVR in all subjects. Additionally, potential confounding cardiovascular responses occur with dopamine infusion, which may limit its usage.
Keywords: hypoxic ventilatory response, peripheral chemoreceptors, chemoreflex
the carotid body chemoreceptors are the primary peripheral sensors of arterial oxygen (23). Reductions in the partial pressure of oxygen will increase carotid body afferent activity, resulting in reflex increases in ventilation and sympathetic nervous system activity. Recently, there has been renewed interest in the contribution of the carotid bodies to sympathetic activity, blood pressure, and glucose regulation (11, 14, 36). Because of this, the carotid body chemoreceptors have now emerged as a potential therapeutic target for a variety of sympathoexcitatory conditions, such as hypertension, heart failure, and insulin resistance (11, 14, 36). Therefore, screening tools that can identify patients that might benefit from carotid body-focused therapies, such as resection or denervation, will be essential (25, 33). In addition, a better understanding of the integrative physiological effects of carotid body desensitization will be important in assessing the potential wide-spread physiological effects of such interventions (21).
One way to acutely manipulate carotid body activity in humans is via low doses of intravenous dopamine. On the basis of the work of Welsh and colleagues (49), many studies in humans have used low-dose dopamine to examine the influence of basal carotid body activity as well as peripheral chemosensitivity, based on its known ability to blunt the ventilatory response to hypoxia (2, 3, 7, 13, 18, 37, 42, 45–47, 49). However, the exact dosing has not been consistent between research groups and studies despite a similar goal: use a dose of dopamine to blunt carotid sinus nerve discharge without concomitant cardiovascular changes. Historically, the majority of studies have used doses of 2 μg·kg−1·min−1 (1, 16, 17, 33, 42) or 3 μg·kg−1·min−1 (7, 13, 18, 37, 44–47) to examine peripheral chemosensitivity in humans. Some groups have found that higher doses (4 μg·kg−1·min−1) elicit undesirable changes in blood pressure (42), which have been attributed to the peripheral vasodilator and cardiac effects of dopamine at higher doses. More specifically, low doses (<5 μg·kg−1·min−1) of dopamine are typically thought to target dopaminergic (D1 and D2) receptors, with D2 receptors primarily mediating the effect of dopamine on carotid sinus activity (4, 5, 10, 20, 24, 27, 38). In contrast, higher doses are thought to stimulate α- and β-adrenergic receptors within the cardiovascular system, which can affect a number of hemodynamic variables (10, 19, 28, 49). Despite this, some groups have used doses as high as 5 μg·kg−1·min−1 with minimal confounding effects on heart rate and blood pressure (2, 9, 34, 43). Thus the purpose of this study was to measure carotid body sensitivity to hypoxia during graded doses of dopamine to address the following questions: 1) what is the effect of dopamine dose on the hypoxic ventilatory response (HVR) (a measure of carotid body chemosensitivity); 2) is the effect of dopamine dose on the HVR dependent upon baseline chemosensitivity to hypoxia; and 3) what are the potential confounding cardiovascular responses that occur at varying low doses of intravenous dopamine.
MATERIALS AND METHODS
Approval.
Written informed consent was obtained from all subjects, and all experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic and conformed to the Declaration of Helsinki. Data from subjects enrolled in three separate protocols focused primarily on other topics were compiled for the present investigation.
Participants.
All participants (n = 30, 24 male/6 female) were healthy (22–45 yr), nonobese (BMI 17–30 kg/m2), and nonsmokers without chronic diseases and taking no medications known to affect cardiovascular, respiratory, or autonomic function. Women were nonpregnant (confirmed by negative pregnancy test prior to participation). Subjects refrained from alcohol, caffeine, and exercise for 24 h and fasted for 12 h prior to the study visit.
Monitoring.
Beat-by-beat heart rate was monitored with a 3-lead electrocardiogram (Cardiocap/5, Datex-Ohmeda, Louisville, CO), blood pressure by finger photoplethysmography (Model 1, Nexfin, BMEYE, Amsterdam, The Netherlands), and arterial oxygen saturation by forehead pulse oximetry (Model LNCS TF-1, Masimo, Irvine, CA) integrated within the Nexfin system (8-s average). Stroke volume, cardiac output, and total peripheral resistance were also measured noninvasively by using the Nexfin system. These hemodynamic variables were collected beat-by-beat at 1,000 Hz with a PowerLab data acquisition system (analog to digital converter, ADinstruments, Colorado Springs, CO). Breath-by-breath ventilation and inspired/expired gasses were monitored with a free-standing metabolic cart (Ultima CardiO2 gas exchange analysis system, MCG Diagnostics, Saint Paul, MN). A second forehead pulse oximeter (Model LNCS TF-1, Masimo, Irvine, CA) was fed into the metabolic cart (8-s average) and integrated into the Breeze Suite cardiorespiratory diagnostic software. Ventilatory parameters were reported breath-by-breath. Hemodynamic and ventilatory variables were time aligned for analysis.
A forearm intravenous catheter was placed in the nondominant arm for infusion of the study drug (dopamine). An additional forearm intravenous catheter was used to collect venous blood in a subset of subjects (n = 18) to measure circulating catecholamines (epinephrine, norepinephrine, and dopamine). The assays were performed by the Immunochemistry Core Laboratory of the Clinical Trial Research Unit, and the Department of Laboratory Medicine and Pathology, at the Mayo Clinic using standard procedures (48).
Assessment of peripheral chemosensitivity.
The sensitivity of the carotid body chemoreceptors was assessed by brief hypoxic challenges. Subjects breathed through a two-way nonrebreathing valve connected to a switching valve. Baseline measures were averaged from the 60-s period prior to beginning the series of hypoxic challenges. Hypoxia was achieved by randomly administering two to six breaths of 100% nitrogen followed by 2 min of room air breathing. This was repeated four times under each experimental condition. The selection of breaths was subject specific and investigator driven. That is, the initial trial consisted of three to four breaths of 100% nitrogen, and the investigator chose the following number of breaths based on the degree of desaturation achieved, with the goal of achieving oxygen saturation values ranging from 70–99% (goals: 92, 85, 78, and 70%). For example, if four breaths achieved a saturation of 85%, the next set of breaths may have been six to achieve a lower saturation. If four breaths achieved a saturation of 78%, the next set of breaths may have been two or three to achieve a higher saturation.
The largest three consecutive breaths within 1 min following each hypoxic challenge were averaged, and the nadir oxygen saturation within the same 1-min timeframe following each hypoxic challenge was determined and used to calculate the HVR. If a nadir was not achieved, the hypoxic episode was not included in the calculation of the HVR. A minimum change in saturation (>1%) depicting a measurable change from baseline was required. All data were inspected in real time for artifacts (i.e., coughing, movement, etc.), and any artifacts were excluded from the HVR calculations. In these instances, the hypoxic challenge was repeated. The HVR (liters/min/%) was calculated as the slope of the linear regression line obtained from baseline and the four hypoxic challenges (five total data points) (33) during each infusion condition (saline, dopamine 1–4 μg·kg−1·min−1). The average R2 for each condition was 1) saline: 0.78 ± 0.03; 2) dopamine 1 μg·kg−1·min−1: 0.69 ± 0.05; 3) dopamine 2 μg·kg−1·min−1: 0.59 ± 0.05; 4) dopamine 3 μg·kg−1·min−1: 0.71 ± 0.05; and 5) dopamine 4 μg·kg−1·min−1: 0.56 ± 0.05.
Similarly, the peak heart rate and blood pressure responses were determined following each hypoxic challenge and plotted against the nadir oxygen saturation. The hypoxic heart rate response (beats/min/%) and the hypoxic pressor response (mmHg/%) were calculated as the slope of the linear regression line obtained from baseline and the four hypoxic challenges during each infusion condition (33). This approach has been shown previously to have minimal lasting effects on subsequent HVR tests and is repeatable across time (8, 31).
Protocol.
Subjects quietly rested in a semisupine position and completed the five infusion conditions [saline, dopamine 1–4 μg·kg−1·min−1 (Hospira)] over the course of 2.5 h (see Fig. 1). Each infusion condition consisted of 15 min of normoxic breathing with steady-state measurements completed during the last 5 min. After completion of steady-state measurements, four hypoxic challenges were completed. After the fourth hypoxic challenge, the next infusion condition commenced. Infusion conditions were completed in the following order: 1) saline, 2) 1 μg·kg−1·min−1, 3) 2 μg·kg−1·min−1, 4) 3 μg·kg−1·min−1, and 5) 4 μg·kg−1·min−1.
Fig. 1.
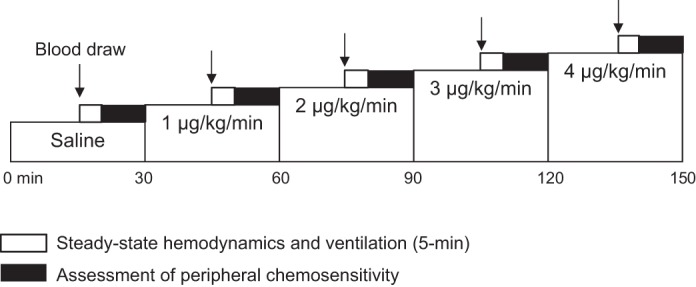
Study Timeline. Subjects completed five infusion conditions over the course of 2.5 h. Each infusion condition consisted of 15 min of normoxic breathing with steady-state measurements completed during the last 5 min. After completion of steady-state measurements, peripheral chemosensitivity was assessed via four hypoxic challenges. After completion of the final hypoxic challenge, the next infusion condition commenced.
Data analysis.
All hemodynamic measurements were collected beat-to-beat with the PowerLab data acquisition system (analog to digital converter, ADinstruments) with a sampling rate of 1,000 Hz. All respiratory variables were collected breath-by-breath during similar time points by a free-standing metabolic cart (Ultima CardiO2 gas exchange analysis system, MCG Diagnostics) and software (BreezeSuite cardiorespiratory diagnostic software). Main outcome variables were compared by a one-way repeated measures analysis of variance with the Student-Newman-Keuls method for pairwise comparisons. In all cases, distributional assumptions were assessed and nonparametric analyses (Friedman repeated measures analysis of variance on ranks) were used for the following variables: HVR, hypoxic heart rate response, hypoxic pressor response, norepinephrine, epinephrine, heart rate, stroke volume, cardiac output, total peripheral resistance, tidal volume, minute ventilation, and end-tidal carbon dioxide. To determine the influence of baseline carotid body chemosensitivity on the effect of different doses of dopamine infusion, we classified subjects into two groups based on their relative location to the mean HVR (0.35 liters·min−1·%−1). Subjects that had a HVR below the mean (0.13 to 0.27 liters·min−1·%−1, n = 17) were defined as having low baseline carotid body chemosensitivity, and subjects who had a HVR at or above the mean (0.35 to 0.93 liters·min−1·%−1, n = 13) as having high carotid body chemosensitivity. This cutoff was selected for the following reasons: 1) the group mean averages for the high HVR group were similar to those previously published by other groups (33), 2) the difference between the lowest value in the high group and the highest value in the low group was 0.07 liters·min−1·%−1 [this value is greater than the standard error of the group mean (0.04 liters·min−1·%−1)], and 3) a similar division in the data set would have been selected if we used median as the cutoff value. The dose of dopamine that elicited the greatest reduction in the HVR with less than a 10% change in mean arterial blood pressure at baseline was defined as the dose associated with the nadir HVR. This criterion was chosen to identify the dose of dopamine acting on carotid body dopamine receptors without activation of peripheral adrenergic receptors. Additionally, a change in blood pressure might influence the arterial baroreflex. This is important because of evidence indicating that activation of the arterial baroreflex is associated with a decrease in carotid body chemosensitivity (41). Data are expressed as means ± SE relative to responses observed during the saline condition (Figs. 2, 3, and 5). A P value ≤ 0.05 was considered statistically significant.
Fig. 2.
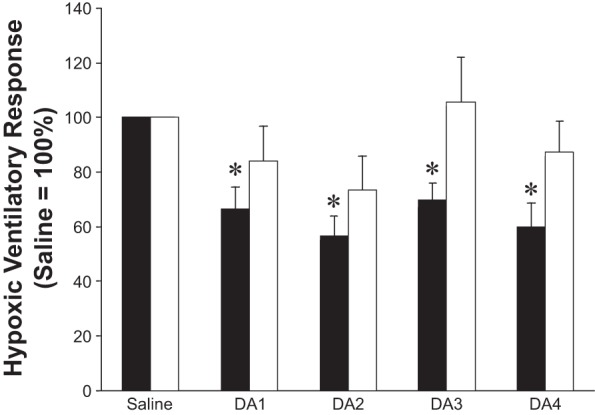
Effect of dopamine dose on the hypoxic ventilatory response (HVR), reported relative to responses during saline infusion (saline = 100%). High HVR (n = 13), low HVR (n = 17). Intravenous dopamine infusion resulted in a significant reduction in the HVR at each dopamine dose in subjects with high baseline chemosensitivity (black bars, P < 0.01). In contrast, those subjects exhibiting low baseline chemosensitivity showed no main effect of dopamine on the HVR (white bars, P = 0.20). Data are presented as means ± SE. DA, dopamine; number = dose in μg·kg−1·min−1. *P < 0.05 vs. saline.
Fig. 3.
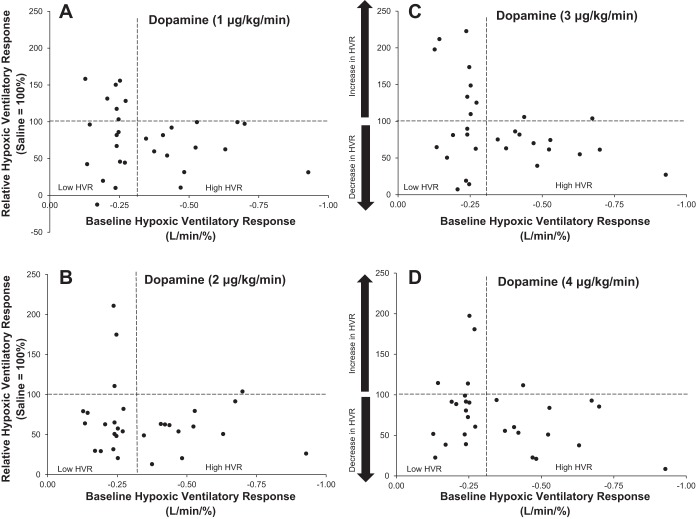
Variability in the effect of dopamine dose on the HVR. HVR data are presented relative to responses during saline infusion (saline = 100%). Values >100% indicate an increase in HVR with dopamine infusion at a given dose: 1 μg·kg−1·min−1 (A) 2 μg·kg−1·min−1 (B), 3 μg·kg−1·min−1 (C), and 4 μg·kg−1·min−1 (D). Values <100% indicate a decrease in HVR with dopamine infusion. Horizontal dashed lines represent baseline HVR (100%), and vertical dashed lines separate subjects into those with high and low baseline chemosensitivity (n = 30 total).
Fig. 5.
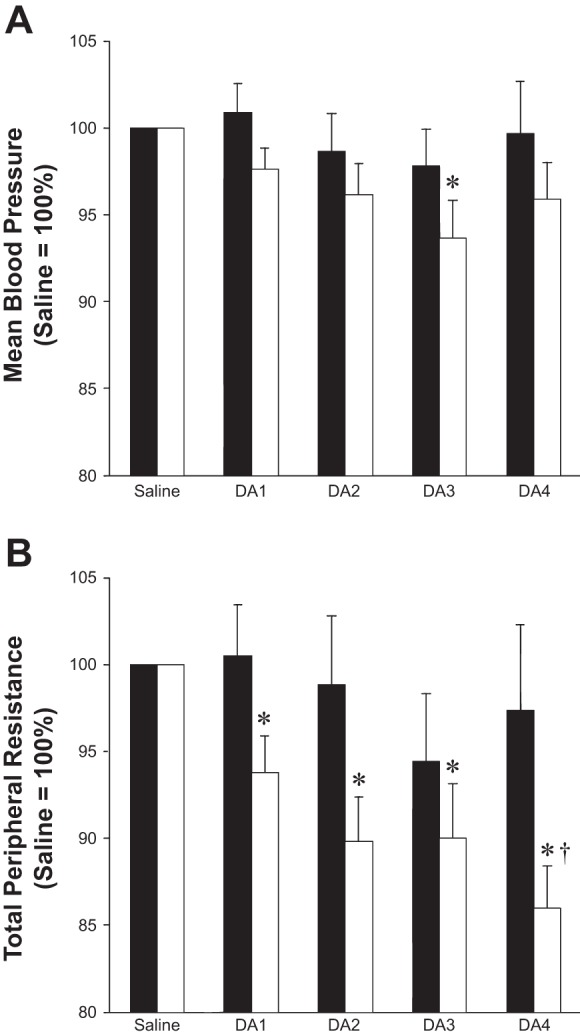
Effect of dopamine dose on steady-state hemodynamics, reported relative to responses during saline infusion (saline = 100%). High HVR (n = 13), low HVR (n = 17). A: a significant reduction in blood pressure was observed in subjects with low baseline chemosensitivity during intravenous dopamine infusion (3 μg·kg−1·min−1) (white bars, P < 0.01). No changes in blood pressure were observed in subjects with high baseline chemosensitivity (black bars, P = 0.59). B: subjects with low baseline chemosensitivity exhibited a significant decrease in total peripheral resistance at each dose of dopamine (P < 0.01) which was not observed in subjects with high baseline chemosensitivity (P = 0.51). *P < 0.05 vs. saline; †P < 0.05 vs. 1 μg·kg−1·min−1. Data are presented as means ± SE.
RESULTS
Thirty young, healthy adults participated in the current study (Table 1). Circulating dopamine concentrations increased incrementally with intravenous dopamine infusion (P < 0.01). No changes in circulating epinephrine or norepinephrine were observed (P > 0.05) (Table 2).
Table 1.
Subject demographics
| All | High | Low | Effect of Group | |
|---|---|---|---|---|
| n | 30 | 13 | 17 | |
| Sex, M/F | 14/6 | 12/1 | 12/5 | |
| Age, yr | 32 ± 1 | 32 ± 2 | 32 ± 2 | 0.765 |
| Height, cm | 178 ± 2 | 182 ± 2 | 176 ± 3 | 0.108 |
| Weight, kg | 78 ± 3 | 81 ± 4 | 75 ± 4 | 0.209 |
| BMI, kg/m2 | 24 ± 1 | 25 ± 1 | 24 ± 1 | 0.538 |
| Glucose, mg/dl | 90 ± 2 | 91 ± 3 | 90 ± 4 | 0.183 |
| Cholesterol, mg/dl | 162 ± 4 | 162 ± 6 | 163 ± 6 | 0.882 |
| Triglycerides, mg/dl | 81 ± 5 | 76 ± 8 | 85 ± 7 | 0.474 |
| HDL, mg/dl | 55 ± 2 | 52 ± 3 | 57 ± 3 | 0.282 |
| LDL, mg/dl | 91 ± 4 | 94 ± 5 | 89 ± 6 | 0.548 |
Data reported as means ± SE; n = 30 unless otherwise noted: glucose (n = 29), cholesterol (n = 27), triglycerides (n = 27), HDL (n = 27), LDL (n = 27). BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Effect of low-dose dopamine infusion on circulating plasma catecholamines
| Saline | 1 μg·kg−1·min−1 | 2 μg·kg−1·min−1 | 3 μg·kg−1·min−1 | 4 μg·kg−1·min−1 | Effect of Dose | |
|---|---|---|---|---|---|---|
| Norepinephrine, pg/ml | ||||||
| All | 230 ± 21 | 225 ± 20 | 219 ± 16 | 225 ± 17 | 220 ± 16 | 0.986 |
| High HVR | 225 ± 37 | 212 ± 29 | 227 ± 18 | 201 ± 18 | 242 ± 31 | 0.804 |
| Low HVR | 234 ± 25 | 233 ± 28 | 213 ± 24 | 242 ± 25 | 204 ± 18 | 0.413 |
| Epinephrine, pg/ml | ||||||
| All | 26 ± 6 | 31 ± 10 | 27 ± 9 | 32 ± 9 | 32 ± 12 | 0.744 |
| High HVR | 25 ± 9 | 25 ± 6 | 19 ± 2 | 30 ± 7 | 24 ± 6 | 0.608 |
| Low HVR | 28 ± 9 | 34 ± 16 | 33 ± 15 | 33 ± 15 | 38 ± 19 | 0.661 |
| Dopamine, pg/ml | ||||||
| All | 21 ± 6 | 4430 ± 500* | 12145 ± 1390*† | 19576 ± 1875*†‡ | 28956 ± 2149*†‡§ | <0.001 |
| High HVR | 18 ± 5 | 4822 ± 791* | 11098 ± 1388*† | 21171 ± 2406*†‡ | 31633 ± 2828*†‡§ | <0.001 |
| Low HVR | 23 ± 10 | 4125 ± 664 | 12960 ± 2264*† | 18336 ± 2812*†‡ | 26874 ± 3080*†‡§ | <0.001 |
Data reported as means ± SE. Norepinephrine (all n = 17; high HVR n = 7; low HVR n = 10), epinephrine (all n = 10; high HVR n = 4; low HVR n = 6), and dopamine (all n = 16; high HVR n = 7; low HVR n = 9). HVR, hypoxic ventilatory response.
P < 0.05 vs. saline,
P < 0.05 vs. 1 μg·kg−1·min−1,
P < 0.05 vs. 2 μg·kg−1·min−1,
P < 0.05 vs. 3 μg·kg−1·min−1.
Numbers in bold represent statistical significance.
Peripheral chemosensitivity.
Intravenous dopamine infusion resulted in a significant reduction in the HVR (P < 0.01). Compared with the saline infusion condition, the reduction was observed at all doses (1–4 μg·kg−1·min−1) of intravenous dopamine infusion (Table 3). When subjects were divided into those with high baseline chemosensitivity (n = 13) and low baseline chemosensitivity (n = 17, P < 0.01), intravenous dopamine infusion resulted in a significant reduction in the HVR in only the high group (P < 0.01). In those subjects, a reduction in the HVR from saline levels was observed during each dose of intravenous dopamine infusion (Table 3 and Figs. 2 and 3). Subjects that exhibited low baseline chemosensitivity showed no main effect of dopamine on the HVR (P = 0.32) (Table 3 and Figs. 2 and 3).
Table 3.
Effect of low-dose dopamine infusion on the hypoxic and cardiovascular response to hypoxia
| Saline | 1 μg·kg−1·min−1 | 2 μg·kg−1·min−1 | 3 μg·kg−1·min−1 | 4 μg·kg−1·min−1 | Effect of Dose | |
|---|---|---|---|---|---|---|
| Hypoxic ventilatory response [(liters/min)/%] | ||||||
| All | 0.35 ± 0.04 | 0.26 ± 0.03* | 0.23 ± 0.03*† | 0.28 ± 0.03*†‡ | 0.24 ± 0.03*†§ | <0.001 |
| High HVR | 0.53 ± 0.05 | 0.35 ± 0.05* | 0.31 ± 0.05* | 0.35 ± 0.04* | 0.30 ± 0.05* | <0.001 |
| Low HVR | 0.22 ± 0.01 | 0.19 ± 0.03 | 0.16 ± 0.03 | 0.23 ± 0.04 | 0.20 ± 0.03 | 0.319 |
| Hypoxic heart rate response [(beat/min)/%] | ||||||
| All | 1.08 ± 0.11 | 0.95 ± 0.09 | 0.89 ± 0.07 | 0.84 ± 0.08 | 1.01 ± 0.09 | 0.238 |
| High HVR | 1.35 ± 0.22 | 0.98 ± 0.16* | 0.85 ± 0.08* | 0.79 ± 0.10* | 1.11 ± 0.16* | 0.023 |
| Low HVR | 0.88 ± 0.08 | 0.93 ± 0.11 | 0.92 ± 0.11 | 0.88 ± 0.12 | 0.94 ± 0.10 | 0.948 |
| Hypoxic blood pressure response (mmHg/%) | ||||||
| All | 0.52 ± 0.08 | 0.36 ± 0.04* | 0.40 ± 0.06† | 0.49 ± 0.06† | 0.47 ± 0.05† | 0.047 |
| High HVR | 0.93 ± 0.19 | 0.56 ± 0.10* | 0.63 ± 0.15 | 0.62 ± 0.14 | 0.62 ± 0.10 | 0.034 |
| Low HVR | 0.36 ± 0.05 | 0.33 ± 0.06 | 0.37 ± 0.06 | 0.51 ± 0.08 | 0.47 ± 0.08 | 0.050 |
Data reported as means ± SE, n = 30 unless otherwise noted (high n = 13, low n = 17).
P < 0.05 vs. saline,
P < 0.05 vs. 1 μg·kg−1·min−1,
P < 0.05 vs. 2 μg·kg−1·min−1,
P < 0.05 vs. 3 μg·kg−1·min−1.
Numbers in bold represent statistical significance.
To take into account interindividual variability within groups, we further examined the individualized dose of dopamine that elicited a nadir HVR for each subject (with a <10% change in blood pressure). Compared with baseline, the average of individual nadirs (independent of dose) resulted in an ∼50% reduction in the HVR in both groups (low group: 0.22 ± 0.01 to 0.11 ± 0.02 liters·min−1·%−1, P < 0.01; high group: 0.53 ± 0.05 to 0.25 ± 0.05 liters·min−1·%−1, P < 0.01). These results illustrate that the individualized dose of dopamine that elicits the nadir HVR is quite variable (low group: 2.1 ± 0.4 μg·kg−1·min−1; high group: 2.6 ± 0.3 μg·kg−1·min−1), and there is no uniform dose that causes a maximum reduction in the HVR in all subjects (Fig. 4A); however, a consistent reduction (∼50%) can be achieved if the selected dose is allowed to vary (Fig. 4B).
Fig. 4.
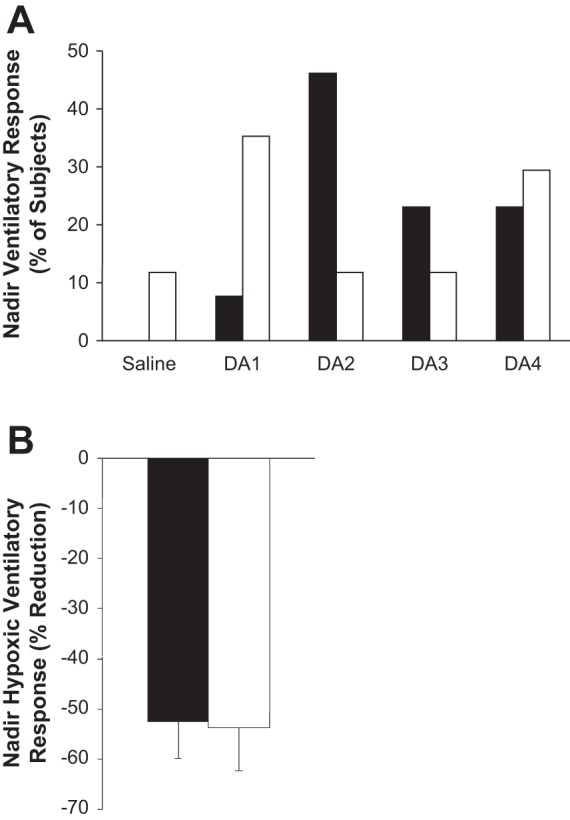
Distribution of dopamine doses that elicited a nadir HVR, by percent of subjects studied. High HVR (n = 13), low HVR (n = 17). The dose of dopamine (1–4 μg·kg−1·min−1) that elicited a nadir HVR (with a <10% change in blood pressure) was determined for each subject. A: these results illustrate there is no uniform dose that is sufficient to cause a maximum reduction in the HVR in all subjects. Further, subjects with low baseline chemosensitivity (white bars) appear to require a relatively lower dose than those subjects with high baseline chemosensitivity (black bars). B: a consistent reduction (∼50%) in the HVR can be achieved with low-dose dopamine if the selected dose is allowed to vary. Data are presented as means ± SE.
Intravenous dopamine infusion had no effect on the hypoxic heart rate response (P = 0.24). However, when subjects were divided into groups with high or low baseline chemosensitivity, intravenous dopamine infusion resulted in a significant reduction in the heart rate response to hypoxia in only the high group (P = 0.02 vs. P = 0.95). In the high group, a reduction in hypoxic heart rate response from saline levels was observed during each dose (1–4 μg·kg−1·min−1) of intravenous dopamine infusion (Table 3).
Intravenous dopamine infusion resulted in a reduction in the hypoxic blood pressure response (P = 0.05). Compared with the saline infusion condition, the reduction was observed only during the 1 μg·kg−1·min−1 infusion (Table 3). When subjects were divided into high or low baseline chemosensitivity, this reduction was seen in both groups (P = 0.03 and P = 0.05, respectively). In those subjects with high baseline chemosensitivity, a reduction in the hypoxic blood pressure response from saline levels was observed during 1 μg·kg−1·min−1 (P = 0.04). In those subjects with low baseline chemosensitivity, only a main effect was observed (Table 3).
Steady-state hemodynamics.
When examining the effect of intravenous dopamine on cardiovascular variables under normoxic conditions, we found no effect of dopamine on resting, steady-state heart rate (P > 0.05). However, at higher doses of dopamine, a significant reduction in blood pressure (3 μg·kg−1·min−1) was observed (P < 0.05). These results were primarily driven by subjects with a low baseline chemosensitivity (Table 4). Subjects with low baseline chemosensitivity also exhibited a significant decrease in total peripheral resistance and increase in cardiac output at each dose of dopamine (1–4 μg·kg−1·min−1), and these results were not observed in subjects with a high baseline chemosensitivity (Table 4 and Fig. 5). No changes in stroke volume were observed.
Table 4.
Effect of low-dose dopamine infusion on normoxic, steady-state, systemic hemodynamics
| Saline | 1 μg·kg−1·min−1 | 2 μg·kg−1·min−1 | 3 μg·kg−1·min−1 | 4 μg·kg−1·min−1 | Effect of Dose | |
|---|---|---|---|---|---|---|
| Heart rate (beat/min) | ||||||
| All | 62 ± 2 | 62 ± 2 | 63 ± 2 | 64 ± 2 | 64 ± 2 | 0.150 |
| High HVR | 63 ± 3 | 63 ± 3 | 64 ± 3 | 65 ± 3 | 65 ± 3 | 0.505 |
| Low HVR | 61 ± 2 | 62 ± 2 | 62 ± 2 | 63 ± 2 | 64 ± 2 | 0.197 |
| Mean blood pressure (mmHg) | ||||||
| All | 96 ± 2 | 95 ± 2 | 93 ± 2 | 91 ± 1*† | 93 ± 1 | 0.005 |
| High HVR | 94 ± 3 | 95 ± 2 | 93 ± 2 | 92 ± 2 | 93 ± 2 | 0.550 |
| Low HVR | 97 ± 2 | 94 ± 2 | 93 ± 2 | 90 ± 2* | 92 ± 1 | 0.009 |
| Total peripheral resistance [mmHg/(liters/min)] | ||||||
| All | 15.3 ± 0.7 | 14.6 ± 0.6* | 14.0 ± 0.5*† | 13.8 ± 0.5*† | 13.6 ± 0.7*†‡§ | <0.001 |
| High HVR | 14.3 ± 1.1 | 14.2 ± 1.0 | 13.8 ± 1.8 | 13.2 ± 0.9 | 13.7 ± 1.0 | 0.376 |
| Low HVR | 16.0 ± 0.8 | 15.0 ± 0.7* | 14.2 ± 0.6* | 14.2 ± 0.5* | 13.5 ± 0.4*†‡§ | <0.001 |
| Cardiac output (liters/min) | ||||||
| All | 6.6 ± 0.2 | 6.7 ± 0.2 | 6.8 ± 0.2 | 6.8 ± 0.2 | 7.1 ± 0.2*† | 0.006 |
| High HVR | 7.0 ± 0.4 | 7.0 ± 0.4 | 7.0 ± 0.4 | 7.3 ± 0.4 | 7.2 ± 0.4 | 0.459 |
| Low HVR | 6.3 ± 0.2 | 6.5 ± 0.2* | 6.7 ± 0.1* | 6.5 ± 0.2* | 7.0 ± 0.2*†‡§ | 0.003 |
| Stroke volume (ml) | ||||||
| All | 113 ± 5 | 114 ± 4 | 112 ± 3 | 110 ± 3 | 114 ± 4 | 0.135 |
| High HVR | 122 ± 8 | 122 ± 7 | 116 ± 6 | 117 ± 5 | 120 ± 6 | 0.123 |
| Low HVR | 106 ± 5 | 107 ± 4 | 109 ± 4 | 105 ± 4 | 110 ± 4 | 0.072 |
Data reported as means ± SE, n = 30 unless otherwise noted (high n = 13, low n = 17).
P < 0.05 vs. saline,
P < 0.05 vs. 1 μg·kg−1·min−1,
P < 0.05 vs. 2 μg·kg−1·min−1,
P < 0.05 vs. 3 μg·kg−1·min−1.
Numbers in bold represent statistical significance.
Steady-state ventilation.
In regard to ventilation under normoxic conditions (Table 5), intravenous dopamine infusion resulted in 1) reductions in tidal volume, 2) increases in respiratory rate, 3) reductions in minute ventilation, and 4) increases in end-tidal carbon dioxide levels (all P < 0.01). These effects were observed primarily in those subjects with low baseline chemosensitivity (Table 5). Compared with the saline infusion condition, the reduction in tidal volume was observed at each dose of intravenous dopamine infusion, and the increase in respiratory rate was primarily observed at the higher (3–4 μg·kg−1·min−1) doses (P < 0.01). The increase in end-tidal carbon dioxide, while slight (∼2 mmHg), was observed in all groups across the majority of doses (Table 5).
Table 5.
Effect of low-dose dopamine infusion on normoxic, steady-state ventilation
| Saline | 1 μg·kg−1·min−1 | 2 μg·kg−1·min−1 | 3 μg·kg−1·min−1 | 4 μg·kg−1·min−1 | Effect of Dose | |
|---|---|---|---|---|---|---|
| Tidal volume (ml/breath) | ||||||
| All | 764 ± 50 | 700 ± 45* | 662 ± 36* | 651 ± 31* | 664 ± 32* | <0.001 |
| High HVR | 708 ± 54 | 648 ± 37 | 617 ± 28 | 613 ± 23 | 636 ± 27 | 0.109 |
| Low HVR | 807 ± 77 | 740 ± 75* | 685 ± 60* | 680 ± 52* | 685 ± 53* | 0.002 |
| Respiratory rate (breath/min) | ||||||
| All | 13 ± 0.8 | 13 ± 0.7 | 14 ± 0.7* | 14 ± 0.6* | 14 ± 0.6*† | 0.001 |
| High HVR | 15 ± 1.4 | 15 ± 1.1 | 16 ± 1.2 | 15 ± 0.7 | 16 ± 0.6 | 0.238 |
| Low HVR | 12 ± 0.8 | 12 ± 0.8 | 12 ± 0.8 | 13 ± 0.8* | 13 ± 0.8*† | 0.003 |
| Minute ventilation (liters/min) | ||||||
| All | 8.8 ± 0.3 | 8.2 ± 0.3* | 8.4 ± 0.3* | 8.3 ± 0.3* | 8.6 ± 0.3†‡§ | 0.004 |
| High HVR | 9.3 ± 0.5 | 8.6 ± 0.5 | 9.1 ± 0.5 | 9.0 ± 0.5 | 9.3 ± 0.3 | 0.151 |
| Low HVR | 8.3 ± 0.3 | 7.9 ± 0.4 | 7.8 ± 0.3 | 7.8 ± 0.2 | 8.1 ± 0.4 | 0.126 |
| End tidal carbon dioxide (mmHg) | ||||||
| All | 41.5 ± 0.7 | 43.4 ± 0.7* | 43.5 ± 0.8* | 44.1 ± 0.7*†‡ | 44.2 ± 0.6*†‡ | <0.001 |
| High HVR | 41.2 ± 0.9 | 43.8 ± 0.9* | 44.0 ± 1.0* | 44.5 ± 1.0* | 44.4 ± 0.9* | <0.001 |
| Low HVR | 41.7 ± 1.1 | 43.1 ± 1.1* | 43.2 ± 1.1* | 43.9 ± 1.1*† | 43.9 ± 0.9*† | <0.001 |
Data reported as means ± SE, n = 30 unless otherwise noted (high n = 13, low n = 17).
P < 0.05 vs. saline,
P < 0.05 vs. 1 μg·kg−1·min−1,
P < 0.05 vs. 2 μg·kg−1·min−1,
P < 0.05 vs. 3 μg·kg−1·min−1.
Numbers in bold represent statistical significance.
DISCUSSION
The major observations from this study are the following: 1) a range of low doses of intravenous dopamine reduce the ventilatory response to hypoxia, with large intersubject variability (Figs. 2 and 3); 2) the effect of dopamine on the HVR appears to be driven by subjects with high baseline chemosensitivity (Fig. 2); 3) with an individualized dose of dopamine, the HVR can be attenuated similarly in subjects with both high and low baseline chemosensitivity (Fig. 4); and 4) potential confounding cardiovascular responses occur at increasing doses of intravenous dopamine which may limit their usage, especially in adults with low baseline chemosensitivity (Fig. 5).
Hypoxic ventilatory response.
On the basis of the work of Welsh and colleagues (49), many studies in humans have exogenously administered low-dose (1–4 μg·kg−1·min−1) dopamine to examine peripheral chemosensitivity. This methodology is based on dopamine's well-established ability to blunt the ventilatory response to hypoxia (2, 3, 7, 13, 15, 16, 18, 37, 42–47, 49) and additional evidence suggesting it can also reduce resting, normoxic ventilation (34, 37, 43, 46, 49). The depressant effect of exogenous dopamine is thought to be through activation of D2-like receptors on Type 1 glomus cells, which block calcium currents, leading to membrane hyperpolarization, reduced neurotransmitter release, and decreased carotid sinus nerve discharge (4, 5, 10, 20, 24, 27, 38). However, the exact dosing has not been consistent between research groups and studies. In the present investigation, we observed a reduction in the HVR during intravenous dopamine infusions of 1, 2, 3, and 4 μg·kg−1·min−1 in healthy adults (Table 3). These findings are consistent with other investigators who have used intravenous infusions of dopamine to blunt carotid body chemosensitivity (referenced above).
Dopamine attenuated minute ventilation and increased end-tidal carbon dioxide values during normoxic conditions (Table 5), thus producing a mild hypercapnic stimulus to both the carotid body chemoreceptors and central chemoreceptors. Recent evidence indicates that activation of the carotid body chemoreceptors via hypoxia is associated with a hyperadditive ventilatory response when the central chemoreceptors are exposed to hypercapnia (6, 40). Consequently, the mild hypercapnia that was induced by the dopamine infusions during normoxia might have masked an even further attenuation of the ventilatory response to hypoxia. Along these lines, a reduction in basal carotid body activity might have subsequently altered the chemosensitivity to hypoxia. Although our data provide some evidence that basal carotid body activity was attenuated (e.g., lower ventilation and elevated end-tidal carbon dioxide), it is difficult to accurately determine the degree to which tonic carotid body activity is lowered in healthy humans.
Some investigators suggest the magnitude of any dopamine-induced reduction in chemosensitivity is related to baseline chemosensitivity or endogenous dopamine (44), although others disagree (7). To determine if the effect of dopamine on the HVR was dependent upon baseline chemosensitivity to hypoxia, we divided subjects into groups with high baseline chemosensitivity (a HVR greater than the mean) and low baseline chemosensitivity (below the mean). Interestingly, subjects with high baseline chemosensitivity exhibited a reduction in the HVR during intravenous dopamine infusions; meanwhile the HVR was not influenced by dopamine infusion (at any dose) in subjects with low chemosensitivity (Table 3 and Fig. 2). To our knowledge, this is the first study to systematically examine the effect of baseline chemosensitivity on responses to several doses of intravenous dopamine. Only one previous study examined the relationship between peripheral chemosensitivity and changes induced by a single dopamine dose (7). Although Boetger and Ward (7) reported no significant correlation between baseline chemosensitivity and the response to dopamine, data were not presented to directly support this conclusion. Therefore, we analyzed the raw data presented in their manuscript [Table 1, (7)] and found a trend for a relationship (R = −0.76, P = 0.13, n = 5) between the baseline HVRs and the fall in this response with dopamine infusion. That is, subjects with the highest baseline chemosensitivity exhibited the greatest reduction in the ventilatory response to hypoxia during a given dose of dopamine infusion. Although this correlation was not statistically significant, it was likely underpowered because of the small sample size. In this way, the results of Boetger and Ward (7) are in agreement with our findings, and support the relationship between baseline chemosensitivity and the ability of a single low dose of dopamine to attenuate the HVR.
On the basis of our results, there is also no uniform dose of dopamine that is sufficient to elicit the nadir HVR in all subjects and, rather, we found large interindividual variability in the dose of dopamine necessary to elicit a nadir HVR (Fig. 3). Notably, the distribution of doses was different between subjects with high and low baseline chemosensitivity (Fig. 4). Furthermore, in 7% (n = 2) of the subjects studied, a nadir was not observed at any dose used (1–4 μg·kg−1·min−1). We speculate that in these instances a dose <1 μg·kg−1·min−1 may be necessary to elicit a nadir HVR independent of changes in cardiovascular variables (discussed in further detail below). Thus it is likely inappropriate to compare the influence of intravenous dopamine infusion at similar doses between subjects with high carotid body chemosensitivity to those with low carotid body chemosensitivity. Importantly, when the individualized nadir HVR during dopamine infusion was selected, we found that the HVR was attenuated by ∼50% in both groups (high group: 53 ± 7%; low group: 54 ± 9%; P = 0.93; Fig. 4B). This is a very critical finding because it indicates that as long as an individualized dose of dopamine is selected, carotid body chemosensitivity can be consistently and significantly reduced, regardless of baseline chemosensitivity.
Hypoxic cardiovascular response.
Emerging evidence indicates that the carotid body chemoreceptors are important modulators of cardiovascular function in both healthy and diseased populations (29–33, 36). Additionally, the potential exists for distinct connections between the peripheral chemoreceptors and central nervous system, with possibly different glomus cells targeting different reflex pathways (e.g., ventilatory vs. sympathetic/hemodynamic control) (35). Therefore, both the heart rate and blood pressure responses to hypoxia have recently been used as additional indicators of carotid body chemosensitivity (33). In the healthy adults studied presently, we observed a reduction in the hypoxic heart rate response only in those adults with high baseline chemosensitivity (Table 3). In contrast to this finding, our group and others have shown that the heart rate response to hypoxia is preserved after carotid body resection (26, 32). In these instances, it has been postulated that the aortic bodies may be responsible for the heart rate response to hypoxia. However, higher doses of exogenous dopamine (7–14 μg·kg−1·min−1) have been shown to reduce aortic body afferent activity in response to hypoxia in anesthetized cats (39), suggesting dopamine infusion may attenuate not only the carotid, but also the aortic body response. It is also important to consider tachycardia as a secondary response to chemoreceptor-mediated hyperventilation [Hering-Breuer reflex (22)]. In considering this possibility, we observed a significant relationship between the fall in the HVR and the fall in the heart rate response to hypoxia during dopamine infusion (all subjects and doses pooled; R = 0.65, P < 0.01). Considering that a reduction in the HVR was only observed in those adults with high baseline chemosensitivity, it may not be surprising that those adults also exhibited a reduction in the hypoxic heart rate response (Table 3).
Similar to the heart rate response, the hypoxic pressor responses obtained during dopamine infusion (2–4 μg·kg−1·min−1) were not statistically distinguishable from the saline condition. These findings are not consistent with those obtained by Niewinski and colleagues (33) who found a reduction in the hypoxic pressor response in healthy subjects during 2 μg·kg−1·min−1 intravenous dopamine infusion. However, the healthy subjects studied by Niewinski and colleagues exhibited higher baseline HVRs than our cohort (Table 3). In this context, when we examined the hypoxic pressor response in the group of individuals with high chemosensitivity, our results were consistent with Niewinski and colleagues such that the hypoxic pressor response during low-dose (1 μg·kg−1·min−1) dopamine infusions is attenuated (Table 3). These data again support the idea that there is no uniform dose of dopamine that is sufficient to elicit a fall in peripheral chemosensitivity (e.g., ventilatory, heart rate, and pressor response to hypoxia) in all individuals, and thus it may be inappropriate to compare physiological responses to the same intravenous dose of dopamine between individuals. This may be especially important when comparing responses in patient populations to that of healthy controls (36).
Cardiovascular responses during steady-state normoxia.
Important strengths of using low-dose dopamine to examine carotid body chemosensitivity are threefold: 1) dopamine has a short half-life and thus can be used acutely; 2) dopamine can be combined with hypoxia (in contrast to using hyperoxia to blunt carotid body activation) to examine peripheral chemosensitivity to a common carotid body stimuli; and 3) dopamine in low doses has been shown to have minimal independent cardiovascular effects, allowing for the study of the independent contribution of the carotid chemoreceptors to cardiovascular adaptations with disease (e.g., heart failure). However, these strengths require that the dopamine dose used has minimal confounding cardiovascular effects. It is known that dopamine at higher doses can independently activate adrenergic receptors, resulting in increased heart rate and contractility, decreased neurotransmitter uptake, peripheral vasoconstriction via α-receptor activation, and vasodilation through β-receptor activation (10). In this way, some studies suggest a dose greater than 2 μg·kg−1·min−1, given under resting normoxic conditions, will increase heart rate (43), blood pressure (9), and cardiac output (33) and decrease systemic vascular resistance (33). In contrast, we found no effect of dopamine on resting, steady-state heart rate (Table 4). At higher doses of dopamine (3 μg·kg−1·min−1) we observed a significant reduction in blood pressure (Fig. 5), which was driven primarily by those subjects with a low baseline chemosensitivity. Throughout the dopamine infusion, subjects with a low baseline chemosensitivity also exhibited a significant fall in systemic vascular resistance and increase in cardiac output which was not observed in subjects with a high baseline chemosensitivity (Fig. 5 and Table 4). Our data are thus consistent with independent effects of dopamine on the peripheral vasculature. These potential confounding cardiovascular responses to increasing doses of intravenous dopamine may limit its use, especially in subjects with low baseline chemosensitivity. For example, changes in cardiovascular variables might alter baroreflex-mediated control of autonomic outflow and obscure the independent effects of reduced chemoreflex function. In this way, we speculate that a dose <1 μg·kg−1·min−1 may be necessary in some individuals with low baseline chemosensitivity to observe a nadir in HVR independent of changes in cardiovascular variables.
Experimental considerations.
There are some important limitations that should be considered when interpreting the present data. First, the dose of dopamine administration was not randomized. Therefore, the combination of increasing dopamine dose and protocol time (∼2.5 h) might have influenced the HVR, as well as resting cardiovascular and ventilatory variables. Importantly, our experimental approach to assessing the HVR has been shown previously to have limiting lasting effects of subsequent tests and is repeatable across time (8, 31). Second, we studied only healthy individuals and did not include examination of a clinical population known to have elevated carotid body chemosensitivity (36). Hence, we cannot comment on any possible dopamine-dose relationship that might exist in these patient groups. Third, subjects were split into groups of high and low baseline chemosensitivity based on their ventilatory response to acute hypoxia. It is possible that whereas subjects who have a low ventilatory response to hypoxia, tonic carotid body afferent activity may be high. In this context, a reduction of the ventilatory response to hypoxia during dopamine does not necessarily indicate that basal activity of the carotid body chemoreceptors is also reduced. The idea that dopamine infusions could alter basal activity with or without a change in sensitivity could explain the variability in some of our results (16). Furthermore, as a result of hyperventilation achieved while breathing 100% nitrogen, some subjects experienced acute, mild hypocapnia. If isocapnia had been maintained, it is possible the HVR observed during initial saline infusion would be higher and any reduction in the HVR elicited by dopamine would potentially be greater. Along these lines, dopamine also reduces the carotid body response to carbon dioxide (45); thus the lack of isocapnia may have impacted differences between the low and high carotid body chemosensitivity groups. For this reason, future studies should consider including a background of mild hypercapnia during hypoxic exposures (12). Fourth, we observed a decrease in total peripheral resistance during the dopamine infusions. This physiological change might have influenced stroke volume calculations, as our methodology requires stable impedance values for accurate measurements. Last, in contrast to a decrease in the HVR, we observed an increase in the HVR in 30% of individuals at the 3 μg·kg−1·min−1 dose of dopamine (n = 10) and 17% of individuals at the 4 μg·kg−1·min−1 dose (n = 5). In addition to cardiovascular effects, higher doses of dopamine may override D2-like receptor activation (which have higher affinity than D1 receptors) and, via postsynaptic D1-like receptors, increase afferent activity from the carotid body. These data further support the idea that although the majority of individuals will exhibit a dopamine-mediated reduction in carotid body chemosensitivity to hypoxia, this effect may not be seen at the same dose for each individual.
CONCLUSION
Intravenous low-dose dopamine infusions are a common experimental method for examining the independent contribution of the carotid chemoreceptors to a variety of physiological responses. Surprisingly, this is the first study to systematically examine both the direct effects of dopamine on carotid body chemosensitivity and the potential confounding cardiovascular changes throughout a range of doses commonly used. We found that there is no uniform dose that is sufficient to elicit a reduction in the HVR in all individuals. However, when the dose of dopamine is individualized for each subject, peripheral chemosensitivity can be reduced by ∼50%. Therefore, a careful selection of the dose of dopamine should be performed when comparing subjects with high and low baseline carotid body chemosensitivity. Along these lines, it appears inappropriate to compare the influence of a given intravenous dopamine infusion dose between subjects with high carotid body chemosensitivity to those with low carotid body chemosensitivity. For example, if a fall in carotid body chemosensitivity is not achieved with low-dose dopamine, it would suggest the carotid chemoreceptors remain active and thus their contribution is not adequately being examined in subsequent tests. This is especially important in light of the large systemic cardiovascular effects observed in subjects with low baseline chemosensitivity at even relatively low (1 μg·kg−1·min−1) doses of intravenous dopamine. Such potential confounding, dopamine-mediated changes in cardiovascular variables limit the ability to understand the independent integrative physiological effects (e.g., glucoregulation and blood pressure regulation) of carotid body desensitization. These data highlight the importance of determining an individualized dose of dopamine when designing experiments focused on the independent contribution of the carotid body chemoreceptors.
GRANTS
This work was supported by National Institutes of Health Grants DK-090531 (to M. Joyner), NS-32352 (to M. Joyner), HL-119337 (to M. Joyner), F32 HL-120570 (to J. Limberg), UL1 TR-000135 (Mayo Clinic Center for Translational Science Activities, to M. Joyner), and American Heart Association Grant 13POST14380027 (to B. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.K.L., B.D.J., and M.J.J. conception and design of research; J.K.L., B.D.J., W.W.H., S.M.R., and M.T.M. performed experiments; J.K.L., B.D.J., W.W.H., and M.T.M. analyzed data; J.K.L., B.D.J., W.W.H., S.M.R., M.T.M., and M.J.J. interpreted results of experiments; J.K.L. prepared figures; J.K.L. and B.D.J. drafted manuscript; J.K.L., B.D.J., W.W.H., S.M.R., M.T.M., and M.J.J. edited and revised manuscript; J.K.L., B.D.J., W.W.H., S.M.R., M.T.M., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
Our deepest appreciation and thanks to our research participants. The authors also thank Dr. Timothy Curry, Sarah Wolhart, Shelly Roberts, Pam Engrav, Nancy Meyer, Jaime Long, Maja Johnson, Kate Malterer, Christopher Johnson, and Andrew Miller at the Mayo Clinic. Additionally, we thank the Clinical Research Unit staff and the Immunochemical Core Laboratory at the Mayo Clinic.
REFERENCES
- 1.Bain AR, Dujic Z, Hoiland RL, Barak OF, Madden D, Drvis I, Stembridge M, Macleod DB, MacLeod DM, Ainslie PN. Peripheral chemoreflex inhibition with low-dose dopamine; new insight into mechanisms of extreme apnea. Am J Physiol Regul Integr Comp Physiol 309: R1162–R1171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainbridge CW, Heistad DD. Effect of haloperidol on ventilatory responses to dopamine in man. J Pharmacol Exp Ther 213: 13–17, 1980. [PubMed] [Google Scholar]
- 3.Bascom DA, Clement ID, Dorrington KL, Robbins PA. Effects of dopamine and domperidone on ventilation during isocapnic hypoxia in humans. Respir Physiol 85: 319–328, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Bisgard GE, Forster HV, Klein JP, Manohar M, Bullard VA. Depression of ventilation by dopamine in goats—effects of carotid body excision. Respir Physiol 40: 379–392, 1980. [DOI] [PubMed] [Google Scholar]
- 5.Black AM, Comroe JH Jr, Jacobs L. Species difference in carotid body response of cat and dog to dopamine and serotonin. Am J Physiol 223: 1097–1102, 1972. [DOI] [PubMed] [Google Scholar]
- 6.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2). J Physiol 588: 2455–2471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boetger CL, Ward DS. Effect of dopamine on transient ventilatory response to exercise. J Appl Physiol 61: 2102–2107, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Chua TP, Coats AJ. The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur J Clin Invest 25: 887–892, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Ciarka A, Rimacchi R, Vincent JL, Velez-Roa S, Dumonceaux M, Leeman M, van de Borne P. Effects of low-dose dopamine on ventilation in patients with chronic obstructive pulmonary disease. Eur J Clin Invest 34: 508–512, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Ciarka A, Vincent JL, van de Borne P. The effects of dopamine on the respiratory system: friend or foe? Pulm Pharmacol Ther 20: 607–615, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC, Ribeiro MJ. Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol 5: 418, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahan A, van den Elsen MJ, Berkenbosch A, DeGoede J, Olievier IC, Burm AG, van Kleef JW. Influence of a subanesthetic concentration of halothane on the ventilatory response to step changes into and out of sustained isocapnic hypoxia in healthy volunteers. Anesthesiology 81: 850–859, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Dahan A, Ward D, van den Elsen M, Temp J, Berkenbosch A. Influence of reduced carotid body drive during sustained hypoxia on hypoxic depression of ventilation in humans. J Appl Physiol 81: 565–572, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Del Rio R, Andrade DC, Marcus NJ, Schultz HD. Selective carotid body ablation in experimental heart failure: a new therapeutic tool to improve cardiorespiratory control. Exp Physiol 100: 136–142, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docherty RJ, McQueen DS. Inhibitory action of dopamine on cat carotid chemoreceptors. J Physiol 279: 425–436, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgell H, McMurtry MS, Haykowsky MJ, Paterson I, Ezekowitz JA, Dyck JR, Stickland MK. Peripheral chemoreceptor control of cardiovascular function at rest and during exercise in heart failure patients. J Appl Physiol 118: 839–848, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg LI. The role of dopamine receptors in the treatment of congestive heart failure. J Cardiovasc Pharmacol 14 Suppl 5: S19–S27, 1989. [PubMed] [Google Scholar]
- 18.Henson LC, Ward DS, Whipp BJ. Effect of dopamine on ventilatory response to incremental exercise in man. Respir Physiol 89: 209–224, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz D, Fox Sm D, Goldberg LI. Effects of Dopamine in man. Circ Res 10: 237–243, 1962. [DOI] [PubMed] [Google Scholar]
- 20.Ide T, Shirahata M, Chou CL, Fitzgerald RS. Effects of a continuous infusion of dopamine on the ventilatory and carotid body responses to hypoxia in cats. Clin Exp Pharmacol Physiol 22: 658–664, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BD, Joyner MJ. Carotid body denervation: too soon to get breathless about heart failure? J Am Coll Cardiol 62: 2431–2432, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P. Systemic effects resulting from carotid body stimulation-invited article. Adv Exp Med Biol 648: 223–233, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahiri S, Nishino T. Inhibitory and excitatory effects of dopamine on carotid chemoreceptors. Neurosci Lett 20: 313–318, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Limberg JK, Joyner MJ. Should we be ‘doping’ the peripheral chemoreceptors? J Physiol 592: 1177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, Rizza RA, Curry TB, Joyner MJ, Wehrwein EA. Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension 65: 1365–1371, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llados F, Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol 274: 487–499, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lollgen H, Drexler H. Use of inotropes in the critical care setting. Crit Care Med 18: S56–60, 1990. [PubMed] [Google Scholar]
- 29.Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592: 391–408, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus NJ, Del Rio R, Schultz HD. Central role of carotid body chemoreceptors in disordered breathing and cardiorenal dysfunction in chronic heart failure. Front Physiol 5: 438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niewinski P, Engelman ZJ, Fudim M, Tubek S, Paleczny B, Jankowska EA, Banasiak W, Sobotka PA, Ponikowski P. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J Card Fail 19: 408–415, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Jazwiec P, Banasiak W, Sobotka PA, Hart EC, Paton JF, Ponikowski P. Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp Physiol 99: 552–561, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Niewinski P, Tubek S, Banasiak W, Paton JF, Ponikowski P. Consequences of peripheral chemoreflex inhibition with low-dose dopamine in humans. J Physiol 592: 1295–1308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson LG, Hensley MJ, Saunders NA. Ventilatory responsiveness to hypercapnic hypoxia during dopamine infusion in humans. Am Rev Respir Dis 126: 783–787, 1982. [DOI] [PubMed] [Google Scholar]
- 35.Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA, Narkiewicz K. Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep 15: 273–280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61: 5–13, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Sabol SJ, Ward DS. Effect of dopamine on hypoxic-hypercapnic interaction in humans. Anesth Analg 66: 619–624, 1987. [PubMed] [Google Scholar]
- 38.Sampson SR. Mechanism of efferent inhibition of carotid body chemoreceptors in the cat. Brain Res 45: 266–270, 1972. [DOI] [PubMed] [Google Scholar]
- 39.Smatresk NJ, Lahiri S. Aortic body chemoreceptor responses to dopamine, haloperidol, and pargyline. J Appl Physiol Respir Environ Exerc Physiol 53: 596–602, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Smith CA, Blain GM, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2: role of carotid body CO2. J Physiol 593: 4225–4243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87: 1953–1957, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stickland MK, Fuhr DP, Haykowsky MJ, Jones KE, Paterson DI, Ezekowitz JA, McMurtry MS. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol 589: 6219–6230, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Borne P, Oren R, Somers VK. Dopamine depresses minute ventilation in patients with heart failure. Circulation 98: 126–131, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Ward DS. Stimulation of hypoxic ventilatory drive by droperidol. Anesth Analg 63: 106–110, 1984. [PubMed] [Google Scholar]
- 45.Ward DS, Bellville JW. Effect of intravenous dopamine on hypercapnic ventilatory response in humans. J Appl Physiol Respir Environ Exerc Physiol 55: 1418–1425, 1983. [DOI] [PubMed] [Google Scholar]
- 46.Ward DS, Bellville JW. Reduction of hypoxic ventilatory drive by dopamine. Anesth Analg 61: 333–337, 1982. [PubMed] [Google Scholar]
- 47.Ward DS, Voter WA, Karan S. The role of the carotid bodies in the counter-regulatory response to hypoglycemia. Adv Exp Med Biol 648: 273–280, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol 588: 4593–4601, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh MJ, Heistad DD, Abboud FM. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex J Clin Invest 61: 708–713, 1978.641149 [Google Scholar]