Abstract
Bile acids (BAs) are synthesized in the liver and secreted into the intestine. In the lumen, enteric bacteria metabolize BAs from conjugated, primary forms into more toxic unconjugated, secondary metabolites. Secondary BAs can be injurious to the intestine and may contribute to disease. The epidermal growth factor receptor (EGFR) and the nuclear farnesoid X receptor (FXR) are known to interact with BAs. In this study we examined the effects of BAs on intestinal epithelial cell proliferation and investigated the possible roles for EGFR and FXR in these effects. We report that taurine-conjugated cholic acid (TCA) induced proliferation, while its unconjugated secondary counterpart deoxycholic acid (DCA) inhibited proliferation. TCA stimulated phosphorylation of Src, EGFR, and ERK 1/2. Pharmacological blockade of any of these pathways or genetic ablation of EGFR abrogated TCA-stimulated proliferation. Interestingly, Src or EGFR inhibitors eliminated TCA-induced phosphorylation of both molecules, suggesting that their activation is interdependent. In contrast to TCA, DCA exposure diminished EGFR phosphorylation, and pharmacological or siRNA blockade of FXR abolished DCA-induced inhibition of proliferation. Taken together, these results suggest that TCA induces intestinal cell proliferation via Src, EGFR, and ERK activation. In contrast, DCA inhibits proliferation via an FXR-dependent mechanism that may include downstream inactivation of the EGFR/Src/ERK pathway. Since elevated secondary BA levels are the result of specific bacterial modification, this may provide a mechanism through which an altered microbiota contributes to normal or abnormal intestinal epithelial cell proliferation.
Keywords: bile acid, proliferation, intestine, necrotizing enterocolitis
bile acids (BAs) are important in the digestion and absorption of dietary fats (31). However, they also function as signal molecules in the intestine, liver, and other organs (17, 21, 36). The primary BAs, cholic acid (CA) and chenodeoxycholic acid (CDCA), are made in the liver and conjugated to taurine or glycine prior to excretion into the bowel lumen (30). Once in the intestine, they are subject to metabolism by enteric organisms. The classical pathway involves a two-step process of deconjugation followed by dehydroxylation to create the secondary BAs, deoxycholic (DCA) and lithocholic (LCA), from CA and CDCA, respectively. However, numerous other metabolic pathways also exist (2). Evidence suggests that secondary BAs are more toxic than their primary counterparts (59) and contribute to diseases such as neonatal necrotizing enterocolitis (NEC) (25) and inflammatory bowel disease (IBD) (39). Indeed, DCA levels are abnormally elevated in the ileum of rodents subjected to experimental NEC (26). Cholestyramine, which sequesters BAs in the gut, reduces the incidence of experimental murine NEC, suggesting that alterations in the BA pool are a cause, rather than an effect, of the disease (26, 27). In IBD, disruption of ileal BA transporters leads to an imbalance in the luminal BA pool secondary to impaired BA detoxification (39). Intestinal bacteria are responsible for modification of BAs, and an altered BA pool is the hallmark of many gastrointestinal diseases (8, 25).
In rodents, there is typically a nearly undetectable amount of secondary BAs in the liver, bile, and serum (1), although specific concentrations within various areas of the intestine have not been measured. In humans, stool contains high levels of secondary BAs being eliminated (34), which suggests that the colon may be more capable than the small intestine of tolerating secondary metabolites. While the percentage of unconjugated BAs begins to rise in the terminal ileum (54), the transition from primary to secondary metabolites typically occurs in the colon (28). It stands to reason that changes in the bacterial flora within the intestine, especially the terminal ileum, could dramatically increase the concentration of secondary BAs that is not tolerated well by the small intestine. In NEC, for example, bacteria leading to increased secondary BAs within the terminal ileum may be a mechanism through which an altered gut microbiome affects the intestinal epithelium.
The molecular mechanisms through which BAs interact with the intestine are not well understood. BAs are known to modulate signaling via the epidermal growth factor (EGF) receptor (EGFR) (3, 60, 62, 74, 75). Furthermore, they interact with the nuclear farnesoid X receptor (FXR) (48), which is best known for its regulation of BA synthesis (21, 30, 48). However, Peng et al. (55) recently reported that, in human colon cancer cells, pharmacological activation of FXR inhibited cell proliferation via Src and downstream ERK inhibition. This suggests that the EGFR and FXR pathways differentially modulate intestinal epithelial cell proliferation.
Certain BA metabolites are beneficial to the intestinal epithelium (57, 66), while others are injurious (26). In diseases such as NEC, where the intestinal microbiome is altered (9, 25), the BA pool changes, potentially contributing to injury by increasing toxic BA metabolites. Cell proliferation is required for intestinal mucosal renewal after injury in normal and disease states. Thus, in this study, we aim to understand the effects of individual BAs on the proliferation of intestinal epithelial cells and the signaling pathways involved. We hypothesized that BAs differentially control cell proliferation via preferential activation of the EGFR or FXR pathway. We found that taurine-conjugated cholic acid (TCA), a primary BA, stimulated cell proliferation, while DCA, a secondary BA, inhibited it. The pathways regulating these two opposing effects involve differential activation of EGFR vs. FXR, with the downstream pathways involved converging on Src kinase.
MATERIALS AND METHODS
Chemicals, compounds, and antibodies.
BAs, Z-guggulsterone, and GW4064 were purchased from Sigma (St. Louis, MO); PP2, PD98059, LY294002, and anti-EGFR antibody from EMD Millipore (Billerica, MA); the transarterial chemoembolization inhibitor TAPI-1, anti-FXR antibody, and Protein A/G PLUS-Agarose immunoprecipitation reagent from Santa Cruz Biotechnology (Dallas, TX); Dulbecco's modified Eagle's medium and RPMI 1640 medium from Corning (Manassas, VA); Opti-MEM and FXR (nuclear receptor subfamily 1, group H, member 4, Nr1h4, refSeq accession no. NM_021745.1; 95-bp amplicon) and fibroblast growth factor 15 (Fgf15, refSeq accession no. NM_1300753.1, 65-bp amplicon) gene primers from Life Technologies (Eugene, OR); rabbit monoclonal anti-phosphorylated (Tyr1068) EGFR, rabbit monoclonal anti-phosphorylated (Tyr416 and Tyr527) Src, monoclonal mouse and rabbit anti-Src, rabbit anti-phosphorylated (Thr202/Tyr204) ERK 1/2, mouse anti-ERK 1/2, and mouse monoclonal anti-Akt antibodies from Cell Signaling Technology (Danvers, MA); and monoclonal anti-β-actin antibody from Sigma-Aldrich.
Cell culture.
Rat small intestine (IEC-6) cells (American Type Culture Collection, Manassas, VA) were used because of their origin in the small intestine (61). Cells at passages 7–30 were used for experimentation. IEC-6 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin-streptomycin, and 0.1% insulin-transferrin-selenium (Corning). Cultures were maintained in incubators at 37°C in a 5% CO2-95% air atmosphere. Adherent cells were passaged biweekly at confluence after trypsinization. In preparation for experiments, cells were placed in FBS-deprived (0.1% FBS) medium for 24 h.
Conditionally immortalized young adult mouse colon (YAMC) cells, EGFR-null (EGFR−/−) mouse colon epithelial (MCE) cells, and mouse small intestine epithelial (MSIE) cells were obtained from Dr. Robert Whitehead (Vanderbilt Digestive Disease Research Center Novel Cell Line Core). Generation of EGFR−/− MCE cells expressing human wild-type EGFR, a kinase-dead EGFR, or a null vector is described elsewhere (72). Cells at passages 15–30 were used for experimentation. YAMC, EGFR−/− MCE, and MSIE cells were maintained in RPMI 1640 growth medium supplemented with 5% FBS, 1% penicillin-streptomycin, interferon-γ (5 IU/ml), and 0.1% insulin-transferrin-selenium. Adherent cells were passaged biweekly at confluence after trypsinization. Cultures were maintained in incubators at 33°C in a 5% CO2-95% air atmosphere. For experiments, cells were shifted to RPMI 1640 medium containing 0.5% FBS, streptomycin, and penicillin, without interferon-γ, at 37°C for 24 h prior to experimentation.
Proliferation assays.
Cells were seeded at 10,000–20,000 cells per well onto 12-well plates in complete growth medium and allowed to attach for 24 h. After 24 h of exposure to serum-deprived conditions, cells were treated with BAs and/or receptor agonists or antagonists. After 24 h, cells were fixed and stained with 0.1% crystal violet. Dye was extracted with 10% aqueous acetic acid solution. Absorbance was measured with a microplate reader (model 680, Bio-Rad, Hercules, CA) at 570 nm.
To confirm crystal violet assay results, a nucleic acid incorporation assay [5-ethynyl-2′-deoxyuridine (EdU)] was also used to measure cell proliferation. Cells were plated onto 96-well dishes at 1,000 cells per well and serum-starved for 24 h and then treated. At 2 h before collection, cells were labeled with EdU, which was detected using the Click-iT assay kit (Life Technologies) following the manufacturer's instructions. After nuclear staining with Hoechst 33342 (Life Technologies), a minimum of four fields per treatment condition were photographed (Leica Microsystems, Wetzlar, Germany), and the percentage of cells in the S phase was determined using ImageJ software (National Institutes of Health, Bethesda, MD). Each data point represents the average percentage of labeled cells among the four photographs.
Immunoblotting.
Cells were seeded at 500,000 cells per well onto six-well plates in complete growth medium and allowed to attach for 24 h. After 24 h of serum starvation, cells were treated with BAs with or without receptor agonists or antagonists at specific time points. Cells were then scraped on ice into lysis buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.1% SDS, and 0.5% sodium deoxycholate) containing protease and phosphatase inhibitors. Lysates were centrifuged at 10,000 rpm for 10 min at 4°C, and the resulting supernatant was collected. Protein concentration was determined by bicinchoninic acid assay (Pierce Chemical, Rockford, IL). Protein extracts were dissolved in SDS sample buffer, and equal amounts of protein were loaded in a 10% SDS-polyacrylamide gel. Gels were transferred to nitrocellulose (Santa Cruz Biotechnology), blocked for ≥1 h at room temperature in 5% bovine serum albumin in phosphate-buffered saline (PBS) or commercially available blocking solution (Li-Cor, Lincoln, NE), and incubated overnight with appropriate primary antibodies at 4°C. On the following day, membranes were incubated with the appropriate secondary antibody (Li-Cor). Exposure and densitometry of protein expression normalized to controls of each membrane were performed using Image Studio Lite 4.0 (Li-Cor). All exposures used for densitometric analysis were within the linear range.
Immunoprecipitation.
IEC-6 cells were grown to confluence, serum-starved for 24 h, and then treated with TCA at 5 and 60 min. Cells were washed with PBS, lysed, and centrifuged at 10,000 g at 4°C. The supernatant was precleared with resuspended Protein A/G PLUS-Agarose beads. After incubation overnight with mouse anti-Src antibody at 4°C, immunocomplexes were incubated with Protein A/G PLUS-Agarose beads for 1 h. Immunoprecipitates were collected by centrifugation, washed, and resuspended in SDS sample buffer. Samples were boiled and analyzed by gel electrophoresis and immunoblotting using rabbit anti-Src and rabbit anti-EGFR antibodies and the rabbit TrueBlot Western blot kit (Rockland Immunochemicals, Limerick, PA) according to the manufacturer's instructions.
siRNA transfections.
IEC-6 cells were seeded at 1,000 cells per well onto 96-well plates and allowed to attach for 24 h. Cells were transfected with 10 nM nontargeting siRNA or siRNA specific to FXR using Lipofectamine (Life Technologies) diluted in Opti-MEM. All siRNA was obtained from GE Dharmacon's ON-TARGETplus SMARTpool (Lafayette, CO). After transfection, cells were incubated for 48 h in serum-starved conditions prior to treatment with DCA to assess cell proliferation via nucleic acid incorporation (see above). Parallel immunoblots were done on protein extracts from IEC-6 cells plated to 70% confluence onto six-well plates and treated with siRNA to FXR diluted in Lipofectamine for 48 h. These lysates were probed for total FXR to show percent reduction in protein levels.
Real-time RT-PCR.
IEC-6 cells were treated with DCA or TCA and lysed after 30 min and 24 h. RNA was extracted from cells using TRI Reagent solution (Life Technologies). After cDNA synthesis, FXR or Fgf15 expression was determined using TaqMan gene expression assay (Life Technologies) and a multicolor real-time RT-PCR detection system (model IQ5, Bio-Rad) according to the manufacturers' protocols. Relative FXR or Fgf15 levels were calculated using the cycle threshold (ΔΔCt) method; hypoxanthine-guanine phosphoribosyltransferase was used as a reference gene.
Intracellular BA uptake measurements.
Confluent IEC-6 cells were treated with TCA or DCA. Medium was collected at 30 min and 24 h after treatment and compared with medium collected at time 0 (control). Cells were washed with PBS and treated with 100 μl of 0.2% Triton X-100 in PBS prior to cell scraping and further disruption by 10 passes through a 21-gauge needle. BAs were extracted with methanol using a previously published protocol (34) and measured using a high-performance liquid chromatography-mass spectroscopy system (4000 Q Trap, Applied Biosystems, Waltham, MA).
Formula feeding-hypoxia rat model of intestinal injury.
A well-defined model of intestinal injury in neonatal rats was used (35, 52). Briefly, newborn Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were gavage-fed 200 μl of formula (Similac 60/40, Abbott Nutritional, Columbus, OH) in 75 ml of Esbilac canine milk replacement (PetAG, Hampshire, IL) or 200 μl of formula plus 5 mmol/l TCA or DCA and exposed to hypoxia (95% N2-5% O2) three times daily. After 4 days, the animals were euthanized, and the terminal ileum was harvested. Rats received an intraperitoneal injection of EdU (25 mg/kg in PBS) 6 h before harvesting of the terminal ileum. Intestinal injury was graded histologically, on the basis of the presence of epithelial sloughing, loss of villous architecture, submucosal edema, or intestinal perforation, on a scale of 0–4. A score of ≥2 was confirmation of intestinal injury. EdU-positive cells were detected using the Click-iT assay kit (Life Technologies) according to the manufacturer's instructions, with 4′,6-diamidino-2-phenylindole (Life Technologies) used as a nuclear stain. A minimum of four fields per specimen containing at least four villi were photographed (Leica Microsystems). The percentage of cells in the S phase was then determined using ImageJ software. Each data point represents the average percentage of labeled cells in ≥10 villi per specimen. These experiments were approved and monitored by the Animal Care and Use and Biosafety Committees of Children's Hospital Los Angeles (Protocol No. 364-14).
Statistical analysis.
Values are means ± SE of at least three independent experiments. Statistical calculations were performed using ANOVA and Student's unpaired t-test with Bonferroni's modification to correct for multiple comparisons (Prism 6.05, GraphPad, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
BA metabolites exert a differential effect on intestinal epithelial cell proliferation.
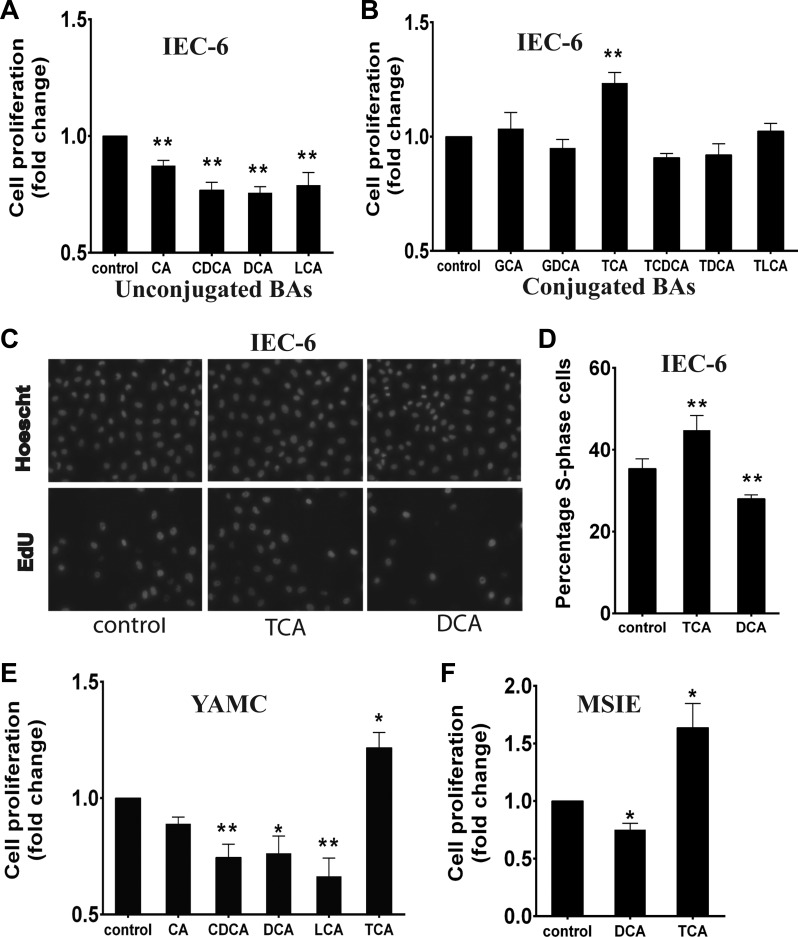
To determine the effects of BAs on cell proliferation, IEC-6 cells were initially treated with BAs at 200 μM, a concentration within the normal physiological range for individual BAs (54). Administration of the unconjugated primary BA cholic acid (CA) resulted in a modest, but statistically significant, decrease in cell proliferation (12.7 ± 3.6%; Fig. 1A). Because initial treatment with the other unconjugated BAs was toxic at 200 μM as assessed by visual inspection and confirmed using a modified MTS-based assay (not shown), doses were lowered until no toxicity was seen. Proliferation was inhibited more by treatment with unconjugated primary (50 μM CDCA) and unconjugated secondary (50 μM DCA and 25 μM LCA) BAs than by treatment with CA (23.2 ± 4.0%, 24.3 ± 3.4%, and 21.1 ± 4.1% respectively; Fig. 1A). In contrast, glycine-conjugated BA and TCA did not kill cells or inhibit cell proliferation at 200 μM; in fact, TCA stimulated intestinal cell proliferation (23.5 ± 4.4% above baseline; Fig. 1B). The effects of TCA and DCA on proliferation were confirmed by EdU incorporation assays in IEC-6 cells (Fig. 1, C and D). Similar results were seen in YAMC and MSIE cells, suggesting that these effects are not specific to the IEC-6 cell line (Fig. 1, E and F). Because DCA is the bacterial metabolic product of TCA, we narrowed our focus of investigation to these two molecules in the reminder of our studies.
Fig. 1.
Bile acids (BAs) differentially affect intestinal epithelial cell proliferation. A: IEC-6 cells were serum-starved for 24 h and then treated with unconjugated BAs at nontoxic doses: 200 μM cholic acid (CA), 50 μM chenodeoxycholic acid (CDCA), 50 μM deoxycholic acid (DCA), and 25 μM lithocholic acid (LCA). After 24 h, cells were stained with crystal violet and counted. B: IEC-6 cells were exposed to conjugated BAs [200 μM glycocholic acid (GCA), glycodeoxycholic acid (GDCA), taurine-conjugated CA (TCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), and taurolithocholic acid (TLCA)] for 24 h, stained with crystal violet, and counted. C and D: IEC-6 cells were labeled with 5-ethynyl-2′-deoxyuridine (EdU) after 24 h of BA exposure, and the percentage of EdU-positive nuclei was determined. E and F: young adult mouse colon (YAMC) and mouse small intestine epithelial (MSIE) cells were exposed to BAs for 24 h after serum starvation, stained with crystal violet, and counted. Values are means ± SE; n > 10 for each (A), n ≥ 4 for each (B), n > 10 for each (D), and n ≥ 3 (E and F). *P < 0.05; **P < 0.01.
TCA and DCA differentially modulate cell proliferation in a dose-dependent manner.
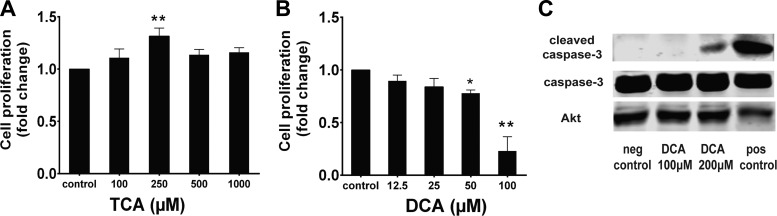
TCA, an abundant component of normal bile (64), promoted cell proliferation. DCA, which inhibited cell proliferation (Fig. 1), is the product of bacterial modification of TCA by deconjugation followed by dehydroxylation (2). To determine if the effects shown in Fig. 1 were dose-dependent, IEC-6 cells were treated with varying concentrations of TCA or DCA. TCA-induced cell proliferation was maximized at 250 μM, while DCA-induced inhibition of proliferation was maximal at 50 μM (Fig. 2, A and B). At >50 μM, DCA induced significant cell death, identified by visual inspection of cells and comparison of cell counts with a parallel baseline plate, fixed at the time of BA treatment. DCA is known to stimulate cell apoptosis (24). To confirm that apoptosis was not involved in the inhibitory effect of <100 μM DCA on cell proliferation, cells were treated with varying doses of DCA, lysed, and probed by Western blotting for caspase-3 cleavage. DCA inhibited proliferation at concentrations much lower than those required to stimulate caspase-3 cleavage (Fig. 2C). These data, in addition to the reduced proportion of EdU incorporation in DCA-treated cells (Fig. 1), strongly argue for an inhibitory effect on cell proliferation. Thus, 250 μM TCA and 50 μM DCA were used for the remainder of our experiments.
Fig. 2.
Effects of TCA and DCA on proliferation are dose-dependent. A and B: IEC-6 cells were treated with varying doses of TCA and DCA, stained with crystal violet, and counted. Maximal effects were seen at 250 μM TCA (31.6 ± 9.5%) and 50 μM DCA (22.1 ± 8.0%). Values are means ± SE; n > 5. DCA at 100 μM was toxic as determined by visual inspection and by cell counts. C: lysates from cells treated with 100 or 200 μM DCA for 24 h were assessed by immunoblotting for caspase-3 cleavage. Akt was used as a loading control; 500 μM LCA was used as a positive control. Because of abundant cell death, there was less protein in the positive control condition. *P < 0.05; **P < 0.01.
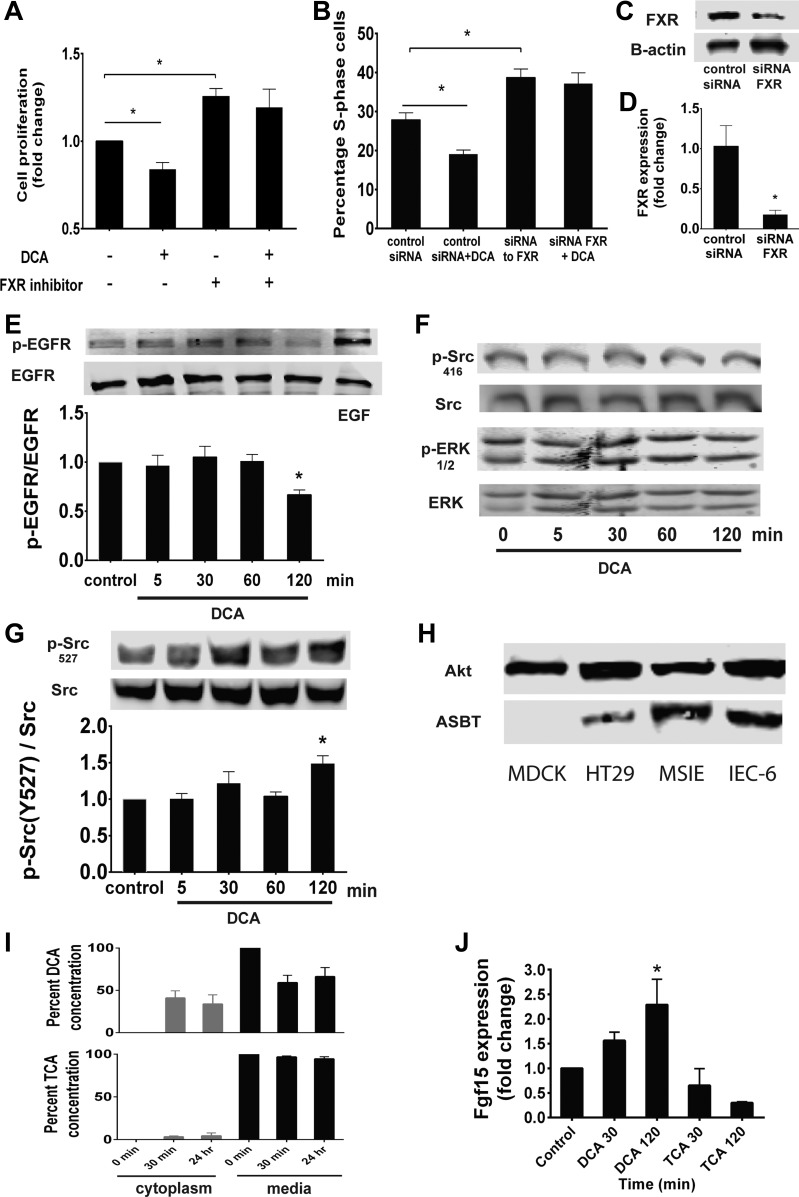
TCA stimulates Src, EGFR, and ERK phosphorylation.
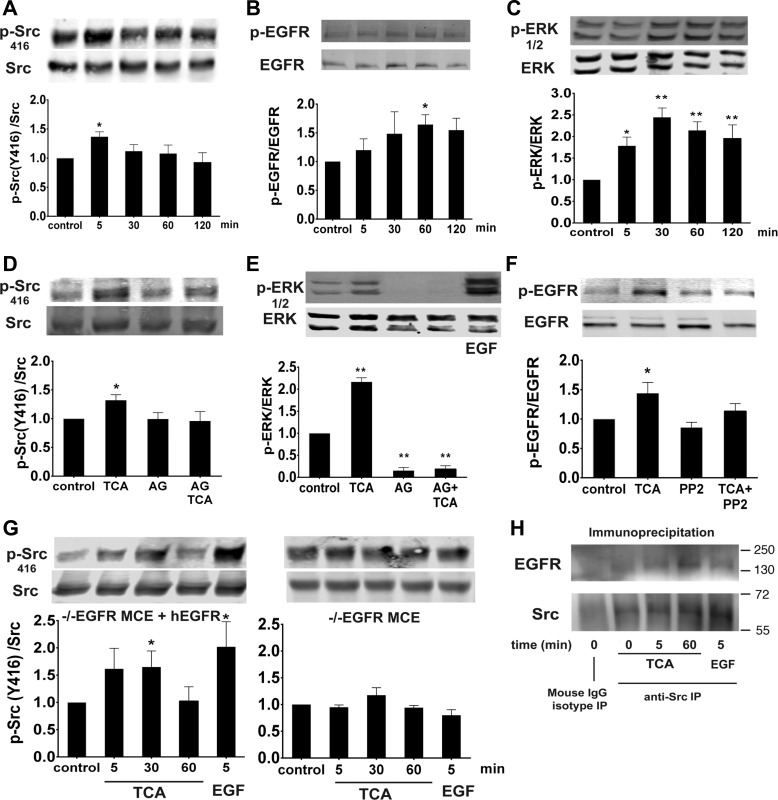
To assess the role of TCA in activation of the EGFR/Src/ERK pathway, IEC-6 cells were exposed to TCA, lysed, and probed via immunoblotting using phospho-specific antibodies. TCA stimulated Src (Tyr416), EGFR (Tyr1068), and ERK (Thr202/Tyr204) phosphorylation in a time-dependent manner, with maximal effect at 5, 60, and 30 min, respectively (Fig. 3, A–C). Pretreatment with the EGFR inhibitor AG1478 abolished TCA-induced Src (Tyr416) and ERK activation at 5 and 30 min, respectively (Fig. 3, D and E). Interestingly, inhibition of Src kinase with PP2 also abolished TCA-induced EGFR phosphorylation at 60 min (Fig. 3F). The role of EGFR in TCA-induced Src activation was further assessed using EGFR−/− MCE cells (19, 72) transfected with an empty vector (EGFR knockout) or wild-type human EGFR. TCA treatment of EGFR−/− MCE cells stably expressing wild-type human EGFR resulted in Src (Tyr416) activation 1.6 ± 0.36 fold above baseline, as expected. However, EGFR−/− MCE cells expressing an empty vector did not respond to TCA administration (Fig. 3G). Taken together, these results suggest that TCA induces intestinal ERK activation that requires EGFR and Src coactivation. To determine if EGFR and Src are colocalized in this process, immunoprecipitation of Src was followed by analysis of total Src and EGFR by immunoblotting. However, administration of TCA did not appreciably increase baseline levels of EGFR-Src association (Fig. 3H), suggesting that driving this complex is not a key part of the mechanism by which these kinases are activated.
Fig. 3.
TCA stimulates phosphorylation of Src at Tyr416, epidermal growth factor (EGF) receptor (EGFR), and ERK. IEC-6 cells were exposed to TCA at 5–120 min, and lysates were immunostained by Western blotting. A–C: phosphorylation of Src at Tyr416, EGFR, and ERK was increased, with maximal phosphorylation at 5, 60, and 30 min, respectively. D and E: cells were pretreated with the EGFR inhibitor AG1478 (AG, 150 nM) prior to TCA administration, and phosphorylation of Src at Tyr416 was assessed at 5 min and ERK phosphorylation at 30 min. F: Src inhibition with PP2 (10 μM) eliminated TCA-induced EGFR phosphorylation at 1 h. G: EGFR−/− mouse colon epithelial (MCE) cells expressing wild-type human EGFR (hEGFR) or EGFR−/− MCE cells lacking EGFR were treated with TCA, and Src activation was assessed. H: cells were treated with TCA at 5 or 60 min and immunoprecipitated (IP) with Src, and total EGFR was analyzed. Values are means ± SE; n ≥ 4 (A–G) and n = 3 (H). *P < 0.05; **P < 0.01.
TCA-induced cell proliferation is dependent on EGFR, Src, and ERK activation.
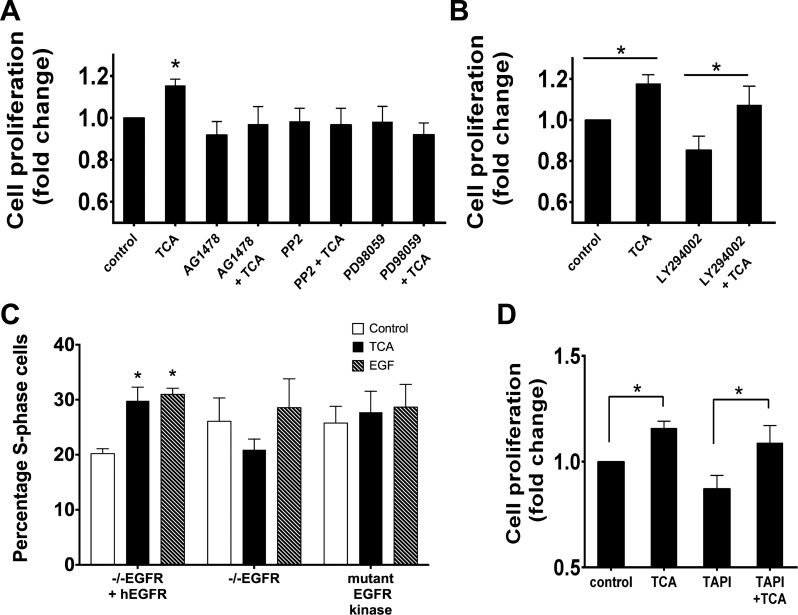
Activation of the Src/EGFR/ERK pathway has been implicated in promotion of cell proliferation in the intestine (40, 55). To test if this pathway is required for TCA-induced responses, IEC-6 cells were pretreated with the Src kinase inhibitor PP2 (10 μM), the EGFR inhibitor AG1478 (150 nM), or the MEK/ERK inhibitor PD98059 (10 μM) with or without TCA. Src, EGFR, or ERK inhibition abolished TCA-induced cell proliferation (Fig. 4A) as measured by crystal violet assay. In contrast, blockade of phosphoinositide 3-kinase with LY294002 (10 μM) had no effect on TCA-induced intestinal epithelial cell proliferation (Fig. 4B). The role of EGFR in TCA-induced cell proliferation was further assessed by nucleic acid incorporation using EGFR−/− MCE cells (19, 72) transfected with an empty vector (EGFR knockout), wild-type human EGFR, or a kinase-inactive EGFR. TCA treatment of EGFR−/− MCE cells expressing wild-type human EGFR resulted in increased proliferation (29.8 ± 2.5%), while EGFR−/− MCE cells expressing an empty vector or kinase-inactive EGFR did not respond to TCA administration (Fig. 4C). Since metalloproteinases (MMPs) have been implicated in the EGFR-Src interaction (42), we used the transarterial chemoembolization inhibitor TAPI-1 to test their role in this signaling cascade. Inhibition of MMP did not alter the stimulatory effect of TCA on IEC-6 cell proliferation (Fig. 4D).
Fig. 4.
Effect of TCA on cell proliferation is mediated by EGFR, Src, and ERK. A: after 24 h of serum starvation, cells were pretreated for 1 h with EGFR, Src, or ERK inhibitors (150 nM AG1478, 10 μM PP2, or 10 μM PD98059, respectively). Proliferation was assessed by crystal violet counting and confirmed by EdU incorporation. B: cells were pretreated with the phosphoinositide 3-kinase inhibitor LY294002 (10 μM). C: proliferation was assessed after TCA administration with EdU incorporation in EGFR knockout cells transfected with a plasmid containing human EGFR (−−EGFR +hEGFR), a kinase-dead EGFR (mutant EGFR kinase), or an empty vector (−−EGFR). EGFR was required for TCA-induced proliferation. D: cells were pretreated with the matrix metalloproteinase inhibitor transarterial chemoembolization inhibitor TAPI-1 for 1 h prior to exposure to TCA and assessment of cell proliferation. Values are means ± SE; n > 6 for each (A), n = 8 (B), n = 5 (C), and n = 4 (D). *P < 0.05.
DCA-induced inhibition of cell proliferation is mediated by the nuclear receptor FXR.
FXR is a nuclear receptor that interacts with BAs and regulates BA transport and synthesis in the intestine and liver (21, 48). DCA is a strong FXR agonist, while TCA is thought to have little FXR activity (70). We sought to assess if DCA-induced inhibition of intestinal epithelial cell proliferation is functioning through an FXR-dependent mechanism. Since IEC-6 cells express FXR (13), we blocked FXR in IEC-6 cells pharmacologically with 10 μM Z-guggulsterone (Fig. 5A), a previously described FXR antagonist (15), or with FXR-specific siRNA knockdown (Fig. 5B) prior to treatment with DCA. The antiproliferative effects of DCA were abolished when FXR activity was blocked using both methods. We achieved an 88% reduction of FXR protein using siRNA (Fig. 5C) and an 82% reduction in FXR gene expression (Fig. 5D).
Fig. 5.
DCA inhibits proliferation through the farnesoid X receptor (FXR). A and B: after 24 h of serum starvation, proliferation was assessed using crystal violet and confirmed by EdU incorporation on cells after 1 h of pretreatment with the FXR inhibitor Z-guggulsterone (10 μM) or transfection with the FXR-specific siRNA (10 nM) or nontargeting control for 48 h prior to assessment of proliferation. C: FXR protein knockdown was assessed by immunoblotting of cell lysates exposed to FXR-specific siRNA. D: reduction of FXR gene expression was assessed by real-time RT-PCR. E–G: IEC-6 cells were treated with DCA for 5–120 min and assessed for EGFR phosphorylation, Src (Tyr416) and ERK phosphorylation, and Src (Tyr527) phosphorylation. H: Madin-Darby canine kidney (MDCK), HT29, MSIE, and IEC-6 cell lysates were probed for apical sodium-dependent BA transporter (ASBT) by immunoblotting. I: IEC-6 cells were treated with DCA or TCA for 30 min or 24 h, media and cell lysates were collected, and BA levels were measured using high-performance liquid chromatography-mass spectroscopy. J: IEC-6 cells were treated for 30 or 120 min with DCA or TCA, and fibroblast growth factor 15 (Fgf15) levels were measured using real-time RT-PCR. Values are means ± SE; n ≥ 5 each (A and B), n = 4 (D and J), n ≥ 3 (E–G), and n = 3 (I). *P < 0.05.
FXR has recently been shown to inhibit Src phosphorylation and may regulate the EGFR pathway (55). Indeed, in cells treated with DCA, EGFR phosphorylation was decreased to below baseline levels at 2 h (Fig. 5E). Src (Tyr416) and ERK phosphorylation did not change with DCA administration up to 2 h (Fig. 5F). Since DCA treatment did not decrease Src phosphorylation at Tyr416 below baseline, we sought to better understand the interaction between DCA and Src by investigating phosphorylation at Tyr527, the inhibitory Src phosphorylation site (49). We found that DCA treatment stimulated Src Tyr527 phosphorylation, which was maximized at 2 h (Fig. 5G). This suggests that DCA may inhibit the EGFR pathway, possibly through its activation of FXR, resulting in Src inactivation.
In vivo, BAs are taken up into the cell via passive and active methods. The apical sodium-dependent BA transporter (ASBT) is the best described and has the highest affinity for conjugated metabolites (17). Since FXR is an intracellular receptor, we tested cellular entry of DCA. Madin-Darby canine kidney cells did not express ASBT, while HT29, MSIE, and IEC-6 cells expressed ASBT at some level (Fig. 5H). However, DCA is much more hydrophobic than TCA and can diffuse passively through the cell membrane without the need for a transporter (65). To test this hypothesis in our system, we treated cells with DCA or TCA for 30 min and 24 h. BAs were extracted from media or cell lysates and measured using high-performance liquid chromatography-mass spectroscopy. DCA was detected within IEC-6 cytoplasm after 30 min. TCA, in contrast, remained largely in the surrounding medium (Fig. 5I). To further support the model that DCA is gaining entry into the cell and acting via an FXR-dependent mechanism, we measured mRNA levels of Fgf15, a known transcription product of FXR activation by BAs (22). We extracted mRNA from IEC-6 cells exposed to TCA and DCA. Fgf15 expression increased at 30 min and 2 h after DCA exposure, while there was no increase in Fgf15 expression in TCA-treated cells (Fig. 5J).
TCA and DCA function through convergent pathways.
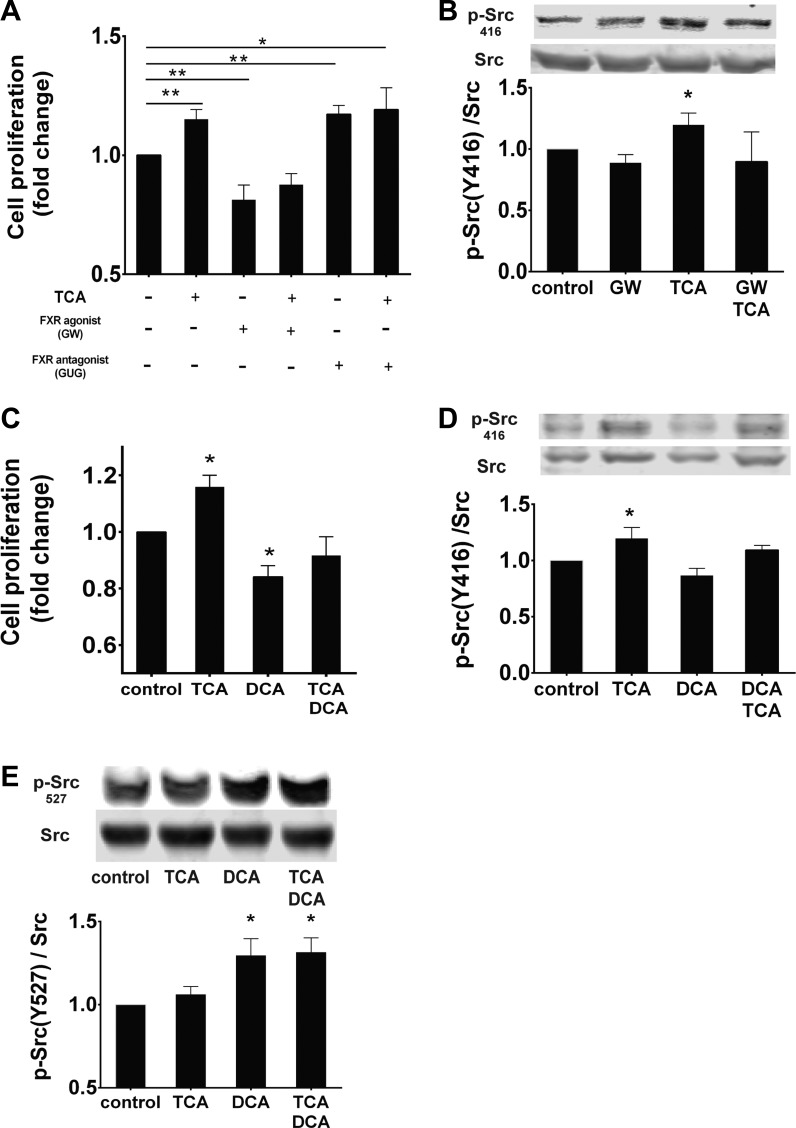
Our data suggest that the Src/EGFR/ERK and FXR pathways regulate intestinal epithelial cell proliferation. To assess how the FXR pathway interacts with TCA-induced cell proliferation, we determined the effect of a pharmacological agonist and antagonist of FXR on IEC-6 cell proliferation. TCA-induced proliferation was blocked by the FXR agonist GW4064. FXR antagonism with Z-guggulsterone, which can stimulate proliferation (55), or TCA alone increased intestinal cell proliferation (Fig. 6A). This effect did not seem to be additive, possibly suggesting maximal stimulation. GW4064 blocked TCA-induced Src phosphorylation (Fig. 6B). TCA and DCA appear to offset each other's effects on proliferation, resulting in no significant changes from baseline treatment when these two BAs are combined (Fig. 6C). Cells treated with TCA, DCA, or both were probed for Src phosphorylation at Tyr416 and at the inhibitory site, Tyr527. TCA-induced phosphorylation at Tyr416 was partially blocked by DCA treatment (Fig. 6D). DCA treatment increased Tyr527 phosphorylation that was not ablated by TCA treatment (Fig. 6E). Taken together, these data suggest that TCA and DCA function through two competing pathways that converge at the level of Src kinase.
Fig. 6.
Proliferative effects of TCA and DCA converge on Src kinase. Proliferation was assessed by counting after crystal violet staining and confirmed by EdU incorporation. A: cells were treated with TCA, the FXR agonist GW4064 (GW, 10 μM), and the FXR antagonist Z-guggulsterone (GUG, 10 μM) alone or in combination. B: Western blots on cell lysates were exposed to GW4064 for Src (Tyr416) phosphorylation. C–E: cells were treated with TCA and DCA, alone or in combination, and assessed for proliferation, phosphorylation of Src (Tyr416), and phosphorylation of Src (Tyr527). Values are means ± SE; n ≥ 4 for each (A), n = 5 (B), and n ≥ 3 (C–E). *P < 0.05; **P < 0.01.
TCA and DCA have differential proliferative effects in vivo.
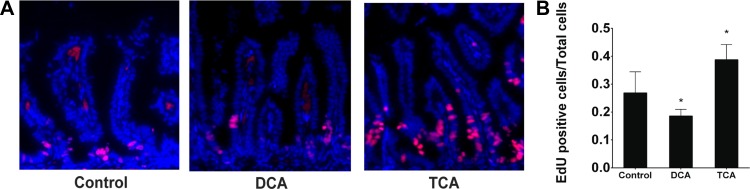
To test whether TCA and DCA affect enterocyte proliferation in vivo, newborn rat pups were given BA in the context of a well-established neonatal intestinal injury model (35, 52). Pups were gavage-fed formula three times daily with or without 5 mM TCA or DCA and exposed to hypoxia. On day 4 of life, the animals were euthanized 6 h after intraperitoneal EdU injection. The terminal 1 cm of small intestine was harvested and stained for EdU. Animals treated with TCA had a reduced incidence of intestinal injury (Table 1) and a greater proportion of EdU-positive cells (Fig. 7). Animals treated with DCA had reduced labeling and appeared sicker at euthanization. DCA levels in the intestine are increased in this model, and oral gavage of DCA alone without formula feeding leads to similar intestinal injury (26). This, along with a short experimental time course, may explain why DCA administration with formula feeding did not increase the incidence of intestinal injury but decreased EdU labeling.
Table 1.
Injury incidence in treatment group
| Treatment | Incidence |
|---|---|
| Formula feeding | 14 of 33 (42%) |
| TCA | 7 of 21 (33%) |
| DCA | 8 of 18 (44%) |
Injury is a score of ≥2 on a scale of 0–4, as measured by histology.
TCA, taurine-conjugated cholic acid; DCA, deoxycholic acid.
Fig. 7.
Newborn rats were fed formula with or without TCA or DCA 3 times daily and subjected to hypoxia for 4 days. EdU was injected 6 h prior to euthanasia, and the terminal ileum was harvested. A and B: slides were stained for EdU-positive cells, and proportion of EdU-positive cells was determined. Values are means ± SE; n = 5.
DISCUSSION
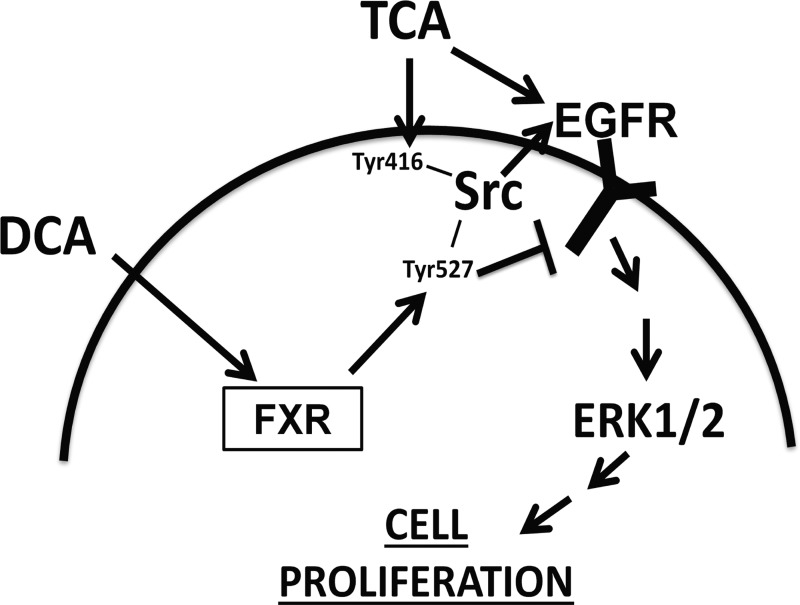
All BA metabolites have similar chemical structure due to their common parent molecule, yet they have vastly different physiological properties (17, 21, 31, 36). Our present data show that the conjugated, primary BA, TCA, is mitogenic to the intestinal epithelium. In contrast, DCA, formed by bacterial metabolism of TCA in the gut, inhibits intestinal cell proliferation. This appears to be due to TCA and DCA acting on distinct, competing pathways that converge on Src kinase. TCA promoted enterocyte proliferation via activation of Src kinase, EGFR, and ERK. While ERK is downstream, it appears that upstream Src and EGFR are independently required for proliferation and may transactivate one another. The antiproliferative effect seen with DCA administration appears to involve FXR activation, which results in Src phosphorylation at its inhibitory site (Tyr527). Activation of Tyr527 may be the mechanism through which DCA inhibits EGFR activation. This proposed mechanism is summarized in Fig. 8 and describes how BA metabolites may have differential effects on the intestinal epithelium.
Fig. 8.
Proposed mechanism of differential effects of TCA and DCA on intestinal epithelial cell proliferation.
It is interesting that TCA, but not other conjugated BAs, stimulated cell proliferation as early as 24 h. TCA has been shown to stimulate liver cell proliferation in a cancer model (47). In the intestine, cell proliferation is stimulated by taurodeoxycholate (68) or glycochenodeoxycholic acid (38), although as many as 6 days were required to see these effects. Here, using both an increase in cell number and EdU incorporation, we show that only TCA stimulates intestinal epithelial cell proliferation in 24 h. It is not known how, whether through direct binding or another mechanism, TCA specifically activates Src or EGFR. One possible mechanism is via another cell surface receptor, such as TGR5, which is also known to be involved in BA signaling (58). This pathway presents an avenue for future study.
Interestingly, reports that the microbiome dictates the BA pool (2, 20, 31) and that bacterial dysbiosis is seen in intestinal diseases such as NEC (25, 26) and IBD (39) may point to a mechanism by which the microbiome alters the host intestine. Indeed, in the formula-feeding-hypoxia neonatal rat model of NEC, DCA levels are elevated (vs. dam-fed littermates) in the lumen of the terminal ileum by 24–48 h (26, 27), while histological damage is not seen until 72 h. DCA levels are much lower in human infants that are breast-fed (29), and breast feeding has long been considered protective from NEC (45).
Several investigators have reported roles for BAs in intestinal cell proliferation (38, 43, 76). However, the literature is not always in agreement. Similar to our results, DCA has been shown to inhibit IEC-6 cell proliferation (38), but it has also been shown to stimulate proliferation in colon cancer cells (50) and mouse colon in vivo (33). The causes of these types of discrepancies are not clear. One possible explanation is that BAs have different effects on normal bowel compared with colon cancer models, as we show inhibition of proliferation in a rodent injury model. Clearly, BAs differentially contribute to tumor progression (11). The variable doses used in different studies could also explain the discrepancies. In our experiments we use nontoxic doses of BAs that are within the physiological range, since total postprandial BA concentrations within the terminal ileum are ∼1 mM (54).
Levels of BAs will vary with fed and fasted states. In the proximal bowel, total maximum BA concentration, including all metabolites, is 5–10 mM, while the concentration in the ileum is ∼1 mM (54, 56). Data identifying the concentration of specific BA metabolites in various areas of the bowel are scarce. In human cecal samples from healthy individuals, BA metabolite concentration has been measured at ∼0.4 mM, ∼25% of which is DCA (28); therefore, the doses we use in this study are physiologically relevant.
While BAs have been shown to interact with the EGFR pathway in numerous tissues, including the colon (10, 44), liver (63), bile ducts (74, 75), stomach (73), and esophagus (3), discrepancies in the literature exist here as well. For example, in Caco-2 cells, DCA was found to inhibit barrier function via EGFR phosphorylation (62), although EGF administration is typically associated with improved barrier function (6, 7). DCA-induced phosphorylation of EGFR has been shown in T84 colon cells as well (41). This suggests a differential role of BAs within the colon specifically. Indeed, in gastroesophageal junction cells, DCA has been shown to inhibit EGFR phosphorylation (4). Our results clearly show that DCA inhibits EGFR and intestinal epithelial cell proliferation. DCA is a strong FXR agonist, and FXR activation inhibits intestinal cell proliferation. TCA, in contrast, stimulates EGFR and proliferation in small intestine epithelial cells.
BAs have also been implicated in activation of the EGFR-Src complex (3, 60, 62, 74). However, the exact mechanism by which BAs modulate the complex interaction between these two molecules has yet to be elucidated. Src activation by BAs has been reported to be upstream of EGFR (51) and can involve MMP (42) and shedding of the ectodomain of the EGFR ligand (53). While we investigated one EGFR phosphorylation site, other sites, such as Tyr845, may be involved in the EGFR-Src interaction (16). Interestingly, the interaction of mutated EGFR in cancer with Src is different from the interaction of normal EGFR with Src (14), which may account for the longer time required for Src activation in our EGFR-mutant cell line (Fig. 3). Others have shown EGFR and Src colocalization to lipid rafts, where inhibition of both EGFR and Src leads to effects not seen with inhibition of either molecule separately (37). Here, we show that both Src and EGFR are required for TCA-induced proliferation, and inhibition of either blocks phosphorylation of the other. Interestingly, unless EGFR is rapidly being phosphorylated selectively on a tyrosine residue we did not study, it appears that Src phosphorylation occurs first. A growing literature showing asymmetric dimerization of EGFR family members (71) may suggest a mechanism for interdependent EGFR and Src activation, despite differing apparent time courses of phosphorylation, if another ErbB family member is functioning as a heterodimer partner for EGFR. Alternatively, recruitment of another necessary molecule to an EGFR-Src complex may be required. EGFR-Src association does not seem to be substantially changed by TCA administration, and the TCA effect does not appear to be MMP-dependent in our system. Alternatively, TCA may also be stimulating EGFR from outside the cell and Src via another mechanism from within the cell. Further understanding of the complex EGFR-Src interaction in TCA-treated cells is the subject of ongoing studies.
While FXR is best known for its regulation of BA synthesis (21, 30, 48), it is also involved in regulation of proliferation in many cell types (18, 46, 55) and acts as an intestinal tumor suppressor decreasing colon cancer proliferation (55). In fact, FXR is often silenced in colon tumors in humans (5). Interestingly, FXR activation also plays an anti-inflammatory role in experimental models of IBD (69). Our findings demonstrate that FXR activation, either pharmacologically or via DCA, decreases intestinal epithelial cell proliferation, but this response is negated by adequate levels of TCA. While this may be beneficial in cancer models, proliferation is desirable for injury repair. Furthermore, FXR may be overactive when levels of DCA increase in the terminal ileum (26, 27), where, typically, the presence of DCA is minimal. In a disease such as NEC, where DCA levels are increased (25, 26), activation of FXR by DCA could contribute to intestinal injury. The specific genes transcribed by FXR to control cell proliferation are unknown and beyond the scope of this study. Further understanding of this pathway is crucial, however, to development of therapeutic strategies targeting this receptor.
While the proliferative effects shown in vitro are modest, they are statistically significant, similar in magnitude to those seen in other settings (12, 23, 32, 67), and likely biologically significant. Our in vivo model of intestinal injury provides further evidence that BAs can alter intestinal cell proliferation (Fig. 7). During times of injury, even modest changes in cell proliferation can be the difference between gut barrier integrity and failure.
We have shown the opposing effects on intestinal cell proliferation of two distinct BA metabolites: TCA and its product of bacterial modification, the secondary BA DCA. Understanding the pathways that control these differential effects will allow us to design therapeutic strategies in diseases where the intestinal bacterial population and BA pool are altered.
GRANTS
This research was funded by a Saban Research Institute Career Developmental Award (to C. P. Gayer) and National Institutes of Health Grants R01 DK-095004 (to M. R. Frey) and R01 AI-01403233 (to H. R. Ford).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.Y.D., M.R.F., H.R.F., and C.P.G. developed the concept and designed the research; A.Y.D., O.E., J.G., and C.P.G. performed the experiments; A.Y.D., O.E., J.G., H.R.F., and C.P.G. analyzed the data; A.Y.D., M.R.F., and C.P.G. interpreted the results of the experiments; A.Y.D. prepared the figures; A.Y.D. drafted the manuscript; A.Y.D., O.E., J.G., M.R.F., H.R.F., and C.P.G. edited and revised the manuscript; A.Y.D., O.E., J.G., M.R.F., H.R.F., and C.P.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the following members of the Saban Research Institute at Children's Hospital Los Angeles for technical help, gifts of reagents, and/or loan of equipment: Stephanie Papillon and Anne Roberts (Gayer Laboratory), Anatoly Grishin and Jordan Bowling (Ford Laboratory), and Marie Nguyen and Jessica Zagory (Wang Laboratory).
REFERENCES
- 1.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 873: 209–217, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aries V, Crowther JS, Drasar BS, Hill MJ. Degradation of bile salts by human intestinal bacteria. Gut 10: 575–576, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avissar NE, Toia L, Hu Y, Watson TJ, Jones C, Raymond DP, Matousek A, Peters JH. Bile acid alone, or in combination with acid, induces CDX2 expression through activation of the epidermal growth factor receptor (EGFR). J Gastrointest Surg 13: 212–222, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bae KB, Jeong YJ, Won HJ, Hong KH, Choi IW, Seo SK, Park SG. A human monoclonal antibody scFv to urokinase plasminogen activator. Hybridoma 29: 147–152, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bailey AM, Zhan L, Maru D, Shureiqi I, Pickering CR, Kiriakova G, Izzo J, He N, Wei C, Baladandayuthapani V, Liang H, Kopetz S, Powis G, Guo GL. FXR silencing in human colon cancer by DNA methylation and KRAS signaling. Am J Physiol Gastrointest Liver Physiol 306: G48–G58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banan A, Fields JZ, Zhang Y, Keshavarzian A. Key role of PKC and Ca2+ in EGF protection of microtubules and intestinal barrier against oxidants. Am J Physiol Gastrointest Liver Physiol 280: G828–G843, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Banan A, Fields JZ, Zhang Y, Keshavarzian A. Phospholipase Cγ inhibition prevents EGF protection of intestinal cytoskeleton and barrier against oxidants. Am J Physiol Gastrointest Liver Physiol 281: G412–G423, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Berr F, Kullak-Ublick GA, Paumgartner G, Münzing W, Hylemon PB. 7α-Dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology 111: 1611–1620, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Brower-Sinning R, Zhong D, Good M, Firek B, Baker R, Sodhi CP, Hackam DJ, Morowitz MJ. Mucosa-associated bacterial diversity in necrotizing enterocolitis. PLos One 9: e105046, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H, Luo S, Xu M, Zhang Y, Song S, Wang S, Kong X, He N, Cao X, Yan F, Wang B. The secondary bile acid deoxycholate accelerates intestinal adenoma-adenocarcinoma sequence in Apc (min/+) mice through enhancing Wnt signaling. Fam Cancer 13: 563–571, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Centuori SM, Martinez JD. Differential regulation of EGFR-MAPK signaling by deoxycholic acid (DCA) and ursodeoxycholic acid (UDCA) in colon cancer. Dig Dis Sci 59: 2367–2380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi LS, Basson MD. Glucagonlike peptide 2 analogue teduglutide: stimulation of proliferation but reduction of differentiation in human Caco-2 intestinal epithelial cells. JAMA Surg 148: 1037–1042, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem 278: 19909–19916, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Chung BM, Raja SM, Clubb RJ, Tu C, George M, Band V, Band H. Aberrant trafficking of NSCLC-associated EGFR mutants through the endocytic recycling pathway promotes interaction with Src. BMC Cell Biol 10: 84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J, Huang L, Zhao A, Lew JL, Yu J, Sahoo S, Meinke PT, Royo I, Pelaez F, Wright SD. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem 278: 10214–10220, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta S, Menezes ME, Das SK, Emdad L, Janjic A, Bhatia S, Mukhopadhyay ND, Shao C, Sarkar D, Fisher PB. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res 19: 4621–4633, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res 56: 1085–1099, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan JH, Fang L. MicroRNA-92 promotes gastric cancer cell proliferation and invasion through targeting FXR. Tumour Biol 35: 11013–11019, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J 25: 5683–5692, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fromm H, Hofmann AF. The importance of bile acids in human diseases. Ergeb Inn Med Kinderheilkd 37: 143–192, 1975. [DOI] [PubMed] [Google Scholar]
- 21.Gadaleta RM, Cariello M, Sabbà C, Moschetta A. Tissue-specific actions of FXR in metabolism and cancer. Biochim Biophys Acta 1851: 30–39, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801: 683–692, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem 284: 2001–2011, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glinghammar B, Inoue H, Rafter JJ. Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-κB and AP-1. Carcinogenesis 23: 839–845, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Halpern MD, Dvorak B. Does abnormal bile acid metabolism contribute to NEC? Semin Perinatol 32: 114–121, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpern MD, Weitkamp JH, Mount Patrick SK, Dobrenen HJ, Khailova L, Correa H, Dvorak B. Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G623–G631, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton JP, Xie G, Raufman JP, Hogan S, Griffin TL, Packard CA, Chatfield DA, Hagey LR, Steinbach JH, Hofmann AF. Human cecal bile acids: concentration and spectrum. Am J Physiol Gastrointest Liver Physiol 293: G256–G263, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Hammons JL, Jordan WE, Stewart RL, Taulbee JD, Berg RW. Age and diet effects on fecal bile acids in infants. J Pediatr Gastroenterol Nutr 7: 30–38, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol 6: 15–27, 2007. [PubMed] [Google Scholar]
- 31.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159: 2647–2658, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Holscher HD, Davis SR, Tappenden KA. Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J Nutr 144: 586–591, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Hori T, Matsumoto K, Sakaitani Y, Sato M, Morotomi M. Effect of dietary deoxycholic acid and cholesterol on fecal steroid concentration and its impact on the colonic crypt cell proliferation in azoxymethane-treated rats. Cancer Lett 124: 79–84, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Humbert L, Maubert MA, Wolf C, Duboc H, Mahé M, Farabos D, Seksik P, Mallet JM, Trugnan G, Masliah J, Rainteau D. Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J Chromatogr B Analyt Technol Biomed Life Sci 899: 135–145, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Hunter CJ, Williams M, Petrosyan M, Guner Y, Mittal R, Mock D, Upperman JS, Ford HR, Prasadarao NV. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect Immun 77: 1031–1043, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res 50: 1509–1520, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin ME, Bohin N, Boerner JL. Src family kinases mediate epidermal growth factor receptor signaling from lipid rafts in breast cancer cells. Cancer Biol Ther 12: 718–726, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishizuka S, Shiwaku M, Hagio M, Suzuki T, Hira T, Hara H. Glycochenodeoxycholic acid promotes proliferation of intestinal epithelia via reduction of cyclic AMP and increase in H2AX phosphorylation after exposure to γ-rays. Biomed Res (Tokyo) 33: 159–165, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Jahnel J, Fickert P, Hauer AC, Högenauer C, Avian A, Trauner M. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos 42: 1423–1431, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Jijon HB, Buret A, Hirota CL, Hollenberg MD, Beck PL. The EGF receptor and HER2 participate in TNF-α-dependent MAPK activation and IL-8 secretion in intestinal epithelial cells. Mediators Inflamm 2012: 207398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keating N, Mroz MS, Scharl MM, Marsh C, Ferguson G, Hofmann AF, Keely SJ. Physiological concentrations of bile acids down-regulate agonist induced secretion in colonic epithelial cells. J Cell Mol Med 13: 2293–2303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitamura T, Srivastava J, DiGiovanni J, Kiguchi K. Bile acid accelerates erbB2-induced pro-tumorigenic activities in biliary tract cancer. Mol Carcinog 54: 459–472, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Krishna-Subramanian S, Hanski ML, Loddenkemper C, Choudhary B, Pagès G, Zeitz M, Hanski C. UDCA slows down intestinal cell proliferation by inducing high and sustained ERK phosphorylation. Int J Cancer 130: 2771–2782, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Lee HY, Crawley S, Hokari R, Kwon S, Kim YS. Bile acid regulates MUC2 transcription in colon cancer cells via positive EGFR/PKC/Ras/ERK/CREB, PI3K/Akt/IκB/NF-κB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway. Int J Oncol 36: 941–953, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Lim JC, Golden JM, Ford HR. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int 31: 509–518, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Tong SJ, Wang X, Qu LX. Farnesoid X receptor inhibits LNcaP cell proliferation via the upregulation of PTEN. Exp Ther Med 8: 1209–1212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, Zhang L, Shi R, Wang G, Pandak WM, Sirica AE, Hylemon PB, Zhou H. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 60: 908–918, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Michels S, Trautmann M, Sievers E, Kindler D, Huss S, Renner M, Friedrichs N, Kirfel J, Steiner S, Endl E, Wurst P, Heukamp L, Penzel R, Larsson O, Kawai A, Tanaka S, Sonobe H, Schirmacher P, Mechtersheimer G, Wardelmann E, Büttner R, Hartmann W. SRC signaling is crucial in the growth of synovial sarcoma cells. Cancer Res 73: 2518–2528, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Milovic V, Teller IC, Faust D, Caspary WF, Stein J. Effects of deoxycholate on human colon cancer cells: apoptosis or proliferation. Eur J Clin Invest 32: 29–34, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Monaghan-Benson E, McKeown-Longo PJ. Urokinase-type plasminogen activator receptor regulates a novel pathway of fibronectin matrix assembly requiring Src-dependent transactivation of epidermal growth factor receptor. J Biol Chem 281: 9450–9459, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Nagathihalli NS, Beesetty Y, Lee W, Washington MK, Chen X, Lockhart AC, Merchant NB. Novel mechanistic insights into ectodomain shedding of EGFR ligands amphiregulin and TGF-α: impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res 74: 2062–2072, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Northfield TC, McColl I. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut 14: 513–518, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Z, Raufman JP, Xie G. Src-mediated cross-talk between farnesoid X and epidermal growth factor receptors inhibits human intestinal cell proliferation and tumorigenesis. PLos One 7: e48461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J, Augustijns P. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J Pharm Pharmacol 58: 1079–1089, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Perrone EE, Liu L, Turner DJ, Strauch ED. Bile salts increase epithelial cell proliferation through HuR-induced c-Myc expression. J Surg Res 178: 155–164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis 29: 37–44, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powell AA, LaRue JM, Batta AK, Martinez JD. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem J 356: 481–486, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W, Molkentin J, Schmidt-Ullrich R, Fisher PB, Grant S, Hylemon PB, Dent P. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell 12: 2629–2645, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol 294: G906–G913, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Rao YP, Studer EJ, Stravitz RT, Gupta S, Qiao L, Dent P, Hylemon PB. Activation of the Raf-1/MEK/ERK cascade by bile acids occurs via the epidermal growth factor receptor in primary rat hepatocytes. Hepatology 35: 307–314, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Schiff ER, Dietschy JM. Current concepts of bile acid absorption. Am J Clin Nutr 22: 273–278, 1969. [DOI] [PubMed] [Google Scholar]
- 66.Strauch ED, Bass BL, Rao JN, Vann JA, Wang JY. NF-κB regulates intestinal epithelial cell and bile salt-induced migration after injury. Ann Surg 237: 494–501, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sueyoshi R, Woods Ignatoski KM, Okawada M, Hartmann B, Holst J, Teitelbaum DH. Stimulation of intestinal growth and function with DPP4 inhibition in a mouse short bowel syndrome model. Am J Physiol Gastrointest Liver Physiol 307: G410–G419, 2014. [DOI] [PubMed] [Google Scholar]
- 68.Toledo A, Yamaguchi J, Wang JY, Bass BL, Turner DJ, Strauch ED. Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-κB. Dig Dis Sci 49: 1664–1671, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 183: 6251–6261, 2009. [DOI] [PubMed] [Google Scholar]
- 70.Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res 18: 1087–1095, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Ward MD, Leahy DJ. Kinase activator-receiver preference in ErbB heterodimers is determined by intracellular regions and is not coupled to extracellular asymmetry. J Biol Chem 290: 1570–1579, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaoka T, Frey MR, Dise RS, Bernard JK, Polk DB. Specific epidermal growth factor receptor autophosphorylation sites promote mouse colon epithelial cell chemotaxis and restitution. Am J Physiol Gastrointest Liver Physiol 301: G368–G376, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun 354: 154–159, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology 122: 985–993, 2002. [DOI] [PubMed] [Google Scholar]
- 75.Yoon JH, Werneburg NW, Higuchi H, Canbay AE, Kaufmann SH, Akgul C, Edwards SW, Gores GJ. Bile acids inhibit Mcl-1 protein turnover via an epidermal growth factor receptor/Raf-1-dependent mechanism. Cancer Res 62: 6500–6505, 2002. [PubMed] [Google Scholar]
- 76.Zahiri HR, Perrone EE, Strauch ED. Bile salt supplementation acts via the farnesoid X receptor to alleviate lipopolysaccharide-induced intestinal injury. Surgery 150: 480–489, 2011. [DOI] [PubMed] [Google Scholar]