Abstract
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR), an anion channel providing a major pathway for Cl− and HCO3− efflux across the apical membrane of the epithelium. In the intestine, CF manifests as obstructive syndromes, dysbiosis, inflammation, and an increased risk for gastrointestinal cancer. Cftr knockout (KO) mice recapitulate CF intestinal disease, including intestinal hyperproliferation. Previous studies using Cftr KO intestinal organoids (enteroids) indicate that crypt epithelium maintains an alkaline intracellular pH (pHi). We hypothesized that Cftr has a cell-autonomous role in downregulating pHi that is incompletely compensated by acid-base regulation in its absence. Here, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein microfluorimetry of enteroids showed that Cftr KO crypt epithelium sustains an alkaline pHi and resistance to cell acidification relative to wild-type. Quantitative real-time PCR revealed that Cftr KO enteroids exhibit downregulated transcription of base (HCO3−)-loading proteins and upregulation of the basolateral membrane HCO3−-unloader anion exchanger 2 (Ae2). Although Cftr KO crypt epithelium had increased Ae2 expression and Ae2-mediated Cl−/HCO3− exchange with maximized gradients, it also had increased intracellular Cl− concentration relative to wild-type. Pharmacological reduction of intracellular Cl− concentration in Cftr KO crypt epithelium normalized pHi, which was largely Ae2-dependent. We conclude that Cftr KO crypt epithelium maintains an alkaline pHi as a consequence of losing both Cl− and HCO3− efflux, which impairs pHi regulation by Ae2. Retention of Cl− and an alkaline pHi in crypt epithelium may alter several cellular processes in the proliferative compartment of Cftr KO intestine.
Keywords: cystic fibrosis, intestine, organoid, enteroid, anion exchanger 2
cystic fibrosis (CF) is a multisystemic disease caused by loss-of-function mutations in the anion channel CF transmembrane conductance regulator (CFTR) (90). CFTR is predominantly expressed in the epithelia of affected organs, including airways, intestine, sweat glands, salivary ducts, pancreatic ducts, and the biliary system. Recent studies also show evidence of CFTR activity in tissues not traditionally associated with manifestations of the disease, e.g., smooth muscle, macrophages, and skeletal muscle (21, 22, 35). The primary pathogenic process of CF is production of thick, viscid mucus, i.e., mucoviscidosis, which causes failure of mucociliary clearance in the lungs and plugging of ducts/glands in other organs, leading to sequelae such as respiratory infection, obstructive bowel disease, and pancreatic insufficiency (90). According to the CF Foundation Patient Registry 2013 Report (http://www.cff.org), the median predicted survival age of CF patients is 40.7 yr and approximately one-half of all CF patients living in the United States are adults. With advances in care, there is increased attention on more age-related manifestations of CF disease, including CF-related diabetes, nonclassic lung disease phenotype (e.g., disseminated bronchiectasis and diffuse panbronchiolitis), acute renal injury, metabolic bone disease, liver disease, and increased risk of gastrointestinal cancer (56).
Cftr knockout (KO) mice recapitulate the pathogenesis of CF intestinal mucoviscidosis by exhibiting a high incidence of obstructive bowel disease reminiscent of meconium ileus, distal intestinal obstructive syndrome, and chronic constipation (24, 26, 79). Other animal models of CF also prominently exhibit severe obstructive bowel disease with variable penetrance (42, 82, 85). Unlike other CF animal models, studies of CF intestinal disease in Cftr KO mice are not complicated by coincidental disease of the pancreas, liver, or lung and corresponding therapeutic measures. Low-grade inflammation of the small intestine has also been reported in both CF patients and Cftr KO mice (49, 60, 78); however, addition of a polyethylene glycol osmotic laxative to the drinking water of Cftr KO mice prevents obstructive disease and minimizes markers of inflammation (8, 12). With respect to the increased risk of gastrointestinal cancer in CF patients (41, 48), Cftr KO mice are known to exhibit a hyperproliferative small intestinal epithelium in vivo (16). The cause of intestinal hyperproliferation in Cftr KO mice is unknown, but recent reports indicate that hyperproliferation persists in intestinal organoid culture (39, 91), suggesting an epithelial-autonomous response to Cftr ablation, rather than inflammation or systemic disease.
Previous studies showed that CF epithelia often exhibit an alkaline intracellular pH (pHi) and/or a resistance to cellular acidic challenge (19, 37, 75, 92). Cftr provides a cellular HCO3− efflux pathway, both by its function as a HCO3−-permeable anion channel and through facilitation of Cl−/HCO3− exchange by recycling Cl− conductively (57, 75). Therefore, intracellular HCO3− retention is predicted in the absence of Cftr. In studies of CF airway epithelium, Willumsen and Boucher (92) showed that recovery of pHi following intracellular acidification was three times faster in CF cells than non-CF controls. Gottlieb and Dosanjh (19) reported that that pHi acidification during induction of apoptosis, which is required for DNA cleavage by endonucleases, is prevented by expression of a dysfunctional mutant of CFTR (ΔF508 CFTR) compared with normal CFTR in epithelial cells. In ex vivo studies of Cftr KO duodenum, Simpson et al. (75) found an alkaline pHi in the villous epithelium in experiments designed to isolate apical membrane Cl−/HCO3− exchange activity. More recently, investigation of WT and Cftr KO intestinal organoids (enteroids) showed that acute treatment of WT enteroids with the Cftr inhibitor Cftrinh172 will alkalize the pHi of crypt epithelium by ∼0.2 pH unit. Surprisingly, crypt epithelium from Cftr KO enteroids displayed an alkaline pHi of the same magnitude, indicating that basal Cftr activity contributes to pHi regulation, ostensibly by guarding against cell alkalinity through HCO3− efflux. Enteroids are well-differentiated, organotypic structures devoid of nonepithelial (immune, neural, and vascular) cells, systemic humoral agents, and the microbiota, which provides the opportunity to evaluate cell- or epithelial-autonomous responses and consequences of Cftr ablation. Thus the maintenance of an alkaline pHi in the isolated epithelium likely provides an intrinsic challenge against which epithelial cell and extracellular processes must operate to normalize epithelial functions in vivo. The sustained alkaline pHi in crypt epithelium of Cftr KO enteroids indicates a failure of the cell's acid-base transporters and enzymes to downregulate pHi under basal conditions, suggesting that the compensatory response is confounded in Cftr KO epithelium.
Given the number of acid-base transporters expressed in proximal intestinal epithelial cells, it is puzzling why the Cftr KO crypt epithelium would sustain an alkaline pH under relatively quiescent conditions. One reason might be that most cellular pHi regulation is directed at guarding against an acidic pHi. The upper small intestinal epithelium is exposed to luminal acid and high partial pressures of CO2 on a diurnal basis (61, 63, 67). Likewise, varying degrees of metabolic acidosis can occur in the course of normal activity and during many disease states, but metabolic alkalosis is less commonly encountered. Thus, epithelial cells may be more tolerant of an alkaline pHi. For example, Na+/H+ exchangers (NHE) 1 (NHE1), NHE2, and NHE3 and, possibly, NHE8 of the small intestinal epithelium are directed at H+ efflux, but NHE1, NHE2, and NHE3 become inactive at pH >7.2, and reverse activity is normally prevented by the extracellular-intracellular Na+ concentration gradient established by the Na+-K+-ATPase (23). Plasma membrane transporters/enzymes directed at HCO3− efflux are mainly anion exchangers. The apical membrane anion exchangers Slc26a3 [downregulated in adenoma (Dra)] and Slc26a6 [putative anion exchanger-1 (Pat-1)] support Cl−/HCO3− exchange and are expressed in the proximal intestine, although the level of their expression is less in crypt than villus epithelium (28, 89). Functional activity of these exchangers in crypt epithelium has not been directly assessed (88). On the other hand, the basolateral membrane anion exchanger 2 (Ae2) is the most likely candidate to moderate an alkaline pHi, on the basis of the inhibitory effects of intracellular protons acting on sensitive residues in the NH2-terminal cytoplasmic and transmembrane domains of the protein (2, 81). Previous studies in murine duodenum and recent studies in human airway epithelial cell lines indicate that AE2, together with the Na+-K+-2Cl− cotransporter NKCC1, provides the principal pathways of basolateral Cl− uptake for Cftr/CFTR-mediated Cl− secretion (27, 87). Interestingly, in the latter study, knockdown of AE2 by siRNA significantly enhanced CFTR expression, suggesting a reciprocal relationship between the two ion transporters. In the present study we investigate the dysregulation of pHi that yields an alkaline pHi in Cftr KO crypt epithelium. Measurements of pHi and intracellular Cl− concentration ([Cl−]i) in crypt epithelium of enteroids from wild-type (WT) and Cftr KO littermate mice are used in this study to assess the cellular impact of the loss of Cftr Cl− and HCO3− channel function.
MATERIALS AND METHODS
Mice
Mice with gene-targeted disruption of the murine homolog of Cftr (Abcc7, Cftr KO) or Ae2 (Slc4a2, Ae2 KO), a gift from Lara Gawenis (University of Utah Medical Center), and sex-matched WT (+/+ or +/−) littermates were used. Mutant mice were identified using a PCR-based analysis of tail-snip DNA, as previously described (8). Mice were maintained ad libitum on standard laboratory chow (rodent chow, Formulab Diet 5008, Ralston Purina) and distilled water. For Cftr KO and WT littermates, a polyethylene glycol osmotic laxative was included in the drinking water to avoid intestinal obstruction in the Cftr KO mice (8). Mice were housed individually in a temperature (22–26°C)- and light (12:12-h light-dark cycle)-controlled room in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility at the Dalton Cardiovascular Research Center. All experiments involving animals were approved by the University of Missouri Animal Care and Use Committee.
Enteroid Culture
The enteroid culture method is described in detail elsewhere (37). Briefly, we excised proximal intestine from 6- to 8-wk-old mice (2- to 3-wk-old Ae2 KO mice) for isolation of intestinal crypts that were used for enteroid culture by a modification of the method of Sato et al. (70). Isolated crypts were plated in Matrigel (BD Bioscience) and overlaid with Ham's F-12 medium containing 5% FBS, 50 μg/ml gentamicin, 125 ng/ml R-Spondin 1, 25 ng/ml noggin, and 12.5 ng/ml epidermal growth factor. Medium was changed every 3 days, and primary enteroids were passaged on day 7 in culture. Passage 1 and 2 enteroids were used for study.
Measurement of pHi
Passage 1 or 2 enteroids were cultured for 4–5 days on glass chamber slides or glass-bottom 35-mm dishes (World Precision Instruments) for measurement of pHi using either ratiometric 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) or SNARF 5F (Life Technologies) microfluorimetry, as described previously (37, 77).
BCECF microfluorimetry.
For steady-state and acid-challenge pHi measurements, enteroids on glass-bottom dishes were loaded with BCECF-AM (16 μM) in culture medium at 37°C for 40 min. BCECF-loaded enteroids were imaged on the stage of an upright microscope (model BX50WI, Olympus) using a ×40 water-immersion objective and superfused with Krebs bicarbonate-Ringer (KBR) solution containing 10 mM N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (TES), which was gassed with 95% O2-5% CO2 (pH 7.4, 37°C). pHi was measured using dual excitation (440 and 495 nm) and imaged at 535-nm emission, which was collected using a SensiCam digital camera (COOKE, Auburn Hills, MI) and Slidebook 5.0 software (3i, Denver, CO). Data were acquired from single cells (n = 8–10) above the +4 cell position of a single enteroid crypt. The 495 nm-to-440 nm ratios were converted to pHi using the K+/nigericin technique (6, 84). For acid challenge, enteroids were exposed to a KBR solution containing 30 mM NH4Cl, which was removed after return of enteroid pHi to near-baseline levels (∼10 min). For measurement of basolateral membrane Cl−/HCO3− exchange, enteroids on glass chamber slides were punctured for insertion of a glass micropipette (30- to 50-μm-diameter tip) and retrograde microperfusion of the enteroid interior (∼1 μl/min), as recently described (38). The enteroid lumen was superfused with 0 Cl− KBR solution (Cl− substituted on an equimolar basis with isethionate−) containing 25 μM Cftrinh172 to minimize apical membrane Cl− and HCO3− transport via Slc26a exchangers and Cftr, respectively. After BCECF-AM was loaded, the enteroids were superfused on the basolateral surface with 0 Cl− KBR solution for ∼5 min and then switched to 55 mM Cl− KBR solution [both containing 1 μM 5-(N-ethyl-N-isopropyl) amiloride to inhibit Nhe1, EIPA] to initiate basolateral Cl−in/HCO3−out exchange for monitoring changes in pHi, as described elsewhere (75). The rate of pHi change during the initial 90-s period of linear ΔpH/Δt change in these experiments was converted to transmembrane flux of H+ or HCO3−, measured in mM/min, using the following equation: mM H+ or HCO3−/min = ΔpH/Δt × total buffering capacity.
SNARF 5F confocal microfluorimetry.
Enteroids on glass-bottom dishes were loaded with SNARF 5F-AM (40 μM) in culture medium for 30 min at 37°C. Enteroid cultures were mounted on the stage of a confocal-multiphoton microscope (model TCS SP5, Leica) built on an inverted platform (model DMI6000, Leica) and fitted with a temperature-controlled incubator (Life Imaging Services). Enteroids were continuously superfused with KBR solution (pH 7.4, gassed with 95% O2-5% CO2, 37°C). The excitation source for SNARF 5F was a 514-nm argon laser, and images were collected at dual-emission (580 and 640 nm) wavelengths. After z stacks (30 slices, 1 μm thick) of individual crypts were acquired, enterocytes were selected for measurement of pHi using a three-dimensional measurement sphere within the individual cells (above the +4 position) from a single enteroid crypt using Imaris software (Bitplane). The 580 nm-to-640 nm ratio was converted to pHi using methods similar to those described for BCECF microfluorimetry. pHi in Cftr KO crypt epithelia after pharmacological reduction of [Cl−]i was measured in enteroids treated with bumetanide (50 μM) for 15 min and then with bumetanide (50 μM) + carbachol (CCh, 100 μM) for 15 min.
Measurement of [Cl−]i
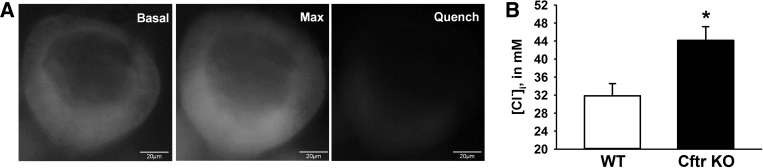
[Cl−]i of enteroid crypt epithelial cells was estimated using N-(ethoxycarboxymethyl)-6-methoxyquinolinium bromide (MQAE; Life Technologies and Enzo Life Sciences), a cell-permeant, nonratiometric indicator of Cl− concentration. Initial studies revealed that MQAE measurements were complicated by dye adherence to surrounding Matrigel. Therefore, a 200-μl pipette tip was used to scrape enteroids from the Matrigel, and the enteroids were washed twice with PBS to remove residual Matrigel and pelleted at 200 g. The pellet was resuspended in culture medium preequilibrated with 5% CO2 and containing the anoikis inhibitor Y-27632 (10 μM; R & D Systems) and MQAE (40 mM), incubated for 40 min at room temperature in a 5% CO2 atmosphere, and transferred to a coverslip for experimentation. A glass holding pipette (∼50-μm-diameter tip) was used to immobilize an enteroid at the glass interface, and cells of a single crypt were imaged using an inverted fluorescence microscope (model IX73, Olympus; 350-nm excitation and 460-nm emission) and acquired using ImageJ software (National Institutes of Health). All experimental solutions contained 10 μM Y-27632. To estimate basal [Cl−]i (Basal), bright-field and MQAE fluorescence images were acquired from WT or Cftr KO enteroids bathed in culture medium gassed with 95% air-5% CO2 at room temperature for 10 min. To determine maximal MQAE fluorescence (Max), the bath solution was changed to culture medium containing 10 μM CFTRinh172 (to minimize Cftr-dependent changes in WT cell volume during bath Cl− substitution), 20 μM nigericin, and 20 μM tributyltin for 10 min. Bright-field and maximal MQAE fluorescence images were acquired 10 min after the bath was switched to 120 mM K+/0 Cl− KBR solution containing 10 μM CFTRinh172, 20 μM nigericin, and 20 μM tributyltin. To determine minimal (Quench) MQAE fluorescence, bright-field and minimal MQAE fluorescence images were acquired 10 min after the bath solution was changed to 120 mM potassium thiocyanate-KBR solution containing 25 μM valinomycin to quench MQAE. Regions of interest were drawn postacquisition within the cell borders of 10 cells (above the +4 position); bright-field images acquired <1 min prior to MQAE fluorescence were used to ensure that the same 10 cells were analyzed at each time point. Basal MQAE fluorescence was converted to percent maximal fluorescence by the following formula: (Basal − Quench)/(Max − Quench) × 100 (see Fig. 4A). Percent maximal fluorescence was converted to mM Cl− from a calibration curve generated by the high-K+/nigericin/tributyltin method (4, 33, 34). For pharmacological reduction of [Cl−]i in Cftr KO crypt epithelium, MQAE-loaded Cftr KO enteroids were bathed in KBR solution gassed with 95% air-5% CO2 for 5 min before acquisition of bright-field and MQAE fluorescence. To reduce [Cl−]i, extracellular Cl− uptake via NKCC1 was inhibited and cellular Cl− efflux was stimulated via activation of Ca2+-dependent Cl− channels by changing the bath solution to KBR solution containing 50 μM bumetanide for 15 min followed by KBR solution containing 50 μM bumetanide + 100 μM CCh for 15 min; bright-field and MQAE fluorescence were acquired immediately before bumetanide addition, 15 min after bumetanide addition, and 15 min after bumetanide + CCh addition. Subsequently, Max and Quench fluorescence were measured, and percent maximal fluorescence at each time point was converted to Cl− concentration (mM), as described above.
Fig. 4.
Increased intracellular Cl− concentration ([Cl−]i) in Cftr KO crypt epithelium. A: representative experiment showing N-(ethoxycarboxymethyl)-6-methoxyquinolinium bromide (MQAE) fluorescence in enteroid crypts in culture medium (Basal), during maximal fluorescence in 0 Cl− KBR solution (Max), and after MQAE was quenched with 120 mM potassium thiocyanate-KBR solution (Quench). B: [Cl−]i. Values are means ± SE of 59–68 crypt epithelial cells of enteroids from 7 sex-matched WT and Cftr KO littermates. *Significantly different from WT (by unpaired t-test).
Quantitative RT-PCR Array
Passage 1 enteroids (7 days postpassage) were removed from Matrigel and processed for total RNA extraction and reverse transcription, as previously described (37). cDNA was mixed with TaqMan Gene Expression Master Mix (Applied Biosystems) according to the manufacturer's protocol and loaded onto customized mini-array 96-well plates containing TaqMan assays for the genes of interest (Table 1). A Mastercycler EP RealPlex thermocycler (Eppendorf) was used for quantitative PCR. The threshold cycle (Ct) of a gene of interest was subtracted from the geometric mean Ct of three housekeeping genes to yield ΔCt. The relative mRNA expression of Cftr KO relative to WT enteroids was calculated using the ΔΔCt method (40).
Table 1.
Gene name, symbol, and Life Technologies assay ID for custom TaqMan mini-array
| Gene Name | Symbol | Assay ID |
|---|---|---|
| Glucuronidase, β* | Gusb | Mm00446953_m1 |
| Hypoxanthine guanine phosphoribosyl transferase 1* | Hprt1 | Mm00446968_m1 |
| Mitochondrial ribosomal protein L19* | Mrpl19 | Mm00452754_m1 |
| Sodium/potassium ATPase-α1 (Na+-K+-ATPase) | Atp1a1 | Mm00523255_m1 |
| Carbonic anhydrase II (CA II) | Car2 | Mm00501572_m1 |
| Carbonic anhydrase IX (CA IX) | Car9 | Mm00519870_m1 |
| Sodium-potassium-2 chloride cotransporter 1 (Nkcc1) | Slc12a2 | Mm00436554_m1 |
| Downregulated in adenoma (Dra) | Slc26a3 | Mm00445313_m1 |
| Putative anion transporter 1 (Pat-1) | Slc26a6 | Mm00506742_m1 |
| Anion exchanger 2 (Ae2) | Slc4a2 | Mm00436617_m1 |
| Sodium-bicarbonate cotransporter e1 (Nbce1) | Slc4a4 | Mm01347935_m1 |
| Sodium/hydrogen exchanger 1 (Nhe1) | Slc9a1 | Mm00444270_m1 |
| Sodium/hydrogen exchanger 2 (Nhe2) | Slc9a2 | Mm01237129_m1 |
| Sodium/hydrogen exchanger 3 (Nhe3) | Slc9a3 | Mm01352473_m1 |
| Anoctamin 1 (Ano1) | Ano1 | Mm00724407_m1 |
Housekeeping gene. For specific assay information, see the Life Technologies product search page: https://www.lifetechnologies.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-assays/taqman-gene-expression.html?icid=fr-taqman-1.
Immunoblot Analysis
Enteroids were removed from Matrigel, suspended in ice-cold RIPA buffer containing Halt protease inhibitor (Life Technologies), and lysed at 4°C by supersonication. Total lysate protein was loaded onto 10% SDS-polyacrylamide gels for electrophoresis, membrane transfer, and immunoblotting. Anti-Ae2 (2 μg/ml; a gift from Seth Alper, Harvard Medical School) and anti-early growth response-1 (Egr-1, 1:1,000 dilution; catalog no. sc-110, Santa Cruz Biotechnology) were used as primary antibodies. Anti-β-actin (1:2,000 dilution; catalog no. sc-130656, Santa Cruz Biotechnology) was used as loading control.
Materials
BCECF-AM and SNARF 5F-AM were obtained from Life Technologies; MQAE from Life Technologies or Enzo Life Sciences; Cftrinh172 from Sigma-Aldrich; and epidermal growth factor and noggin from R & D Systems (Minneapolis, MN). Recombinant R-Spondin 1 was isolated as described previously (53). All other materials were of analytical grade and obtained from Sigma-Aldrich or Fisher Scientific.
Statistics
Values are means ± SE. Data between two groups were compared using a two-tailed Student's t-test with the assumption of equal variances between groups. ΔΔCt data were analyzed using a one-sample t-test with test mean = 0. Data from multiple treatment groups were compared using a one-way ANOVA with a post hoc Tukey's t-test. Data from sequential treatments were compared using repeated-measures ANOVA with a post hoc Tukey's t-test. P < 0.05 was considered statistically significant.
RESULTS
Cftr KO Crypt Epithelium Exhibits an Alkaline pHi and Resistance to Acidification
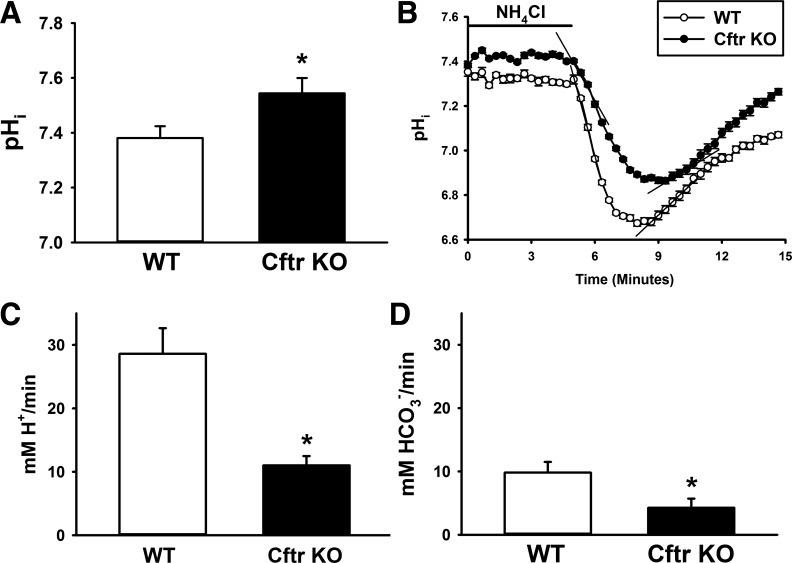
Previous studies showed that crypt epithelial cells in Cftr KO enteroids maintained a pHi that was significantly alkaline relative to WT enteroids (37). Consistent with our previous studies, pHi of cells in the Cftr KO crypt epithelium, on average, is alkaline ∼0.2 pH unit relative to WT crypt cells (Fig. 1A), indicative of Cftr-mediated loss of HCO3− efflux. To assess the effect of Cftr loss on dynamic pHi changes, WT and Cftr KO enteroids were exposed to 30 mM NH4Cl; during this time, uncharged NH3 in equilibrium enters the cell and becomes protonated. Upon removal of extracellular NH4Cl, this process is reversed, and cells are acidified as NH3 leaves the epithelial cell. The epithelial cells alkalized during exposure to NH4Cl (not shown), but pHi in WT and Cftr KO crypt epithelia spontaneously decreased to near-baseline levels (Fig. 1B). Upon removal of NH4Cl to acidify the cells, WT crypt epithelium rapidly acidified by ∼0.7 pH unit, whereas acidification of Cftr KO epithelium decreased at a slower rate by ∼0.5 pH unit. Cumulative data from several experiments showed that the initial acidification rate (30 s) upon NH4Cl removal was significantly less in Cftr KO than WT crypt epithelium (Fig. 1, B and C). These data are consistent with the hypothesis that Cftr participates in basal pHi regulation of crypt epithelium, likely through its role in regulation of cellular HCO3− efflux (10, 11). The rate of recovery from the acid load in WT and Cftr KO crypts was almost threefold slower than the acidification rate, but, interestingly, the recovery rate was significantly slower in Cftr KO than WT crypts (Fig. 1, B and D). Since Na+/H+ exchangers (Nhe1-3) play a prominent role in pHi recovery from acute cellular acidification (20, 30), the reduced rate of alkalization suggests that the absence of Cftr in crypt epithelium may result in compensatory changes in the activity of other acid-base transporters involved in pHi regulation.
Fig. 1.
Alkaline intracellular pH (pHi) and resistance to acid challenge in cystic fibrosis transmembrane conductance regulator (Cftr) knockout (KO) crypt epithelium. A: pHi of crypt epithelium in wild-type (WT) and Cftr KO enteroids as measured by 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) microfluorimetry. Values are means ± SE of 8–10 crypt epithelial cells from 4 sex-matched WT and Cftr KO littermates. *Significantly different from WT (by unpaired t-test). B: time course of pHi changes in WT and Cftr KO crypt epithelial cells during acid challenge experiment. After enteroids were exposed to 30 mM NH4Cl in Krebs bicarbonate-Ringer (KBR) solution for ∼10 min (until pHi was stable), cells were acidified by removal of NH4Cl. Each data point represents average of 8 crypt epithelial cells. Black lines depict initial acidification and alkalization rates. C: cumulative data showing initial rate of acidification following NH4Cl removal for WT and Cftr KO crypt epithelium. Values are means ± SE of enteroids from 5 WT and 4 Cftr KO sex-matched siblings. *Significantly different from WT (by unpaired t-test). D: cumulative data showing rate of pHi recovery from NH4Cl prepulse-induced acidification for WT and Cftr KO crypt epithelium. Values are means ± SE from 5 WT and 4 Cftr KO sex-matched siblings. *Significantly different from WT (by unpaired t-test).
Transcriptional Responses by Cftr KO Enteroids Are Consistent With Compensation of an Alkaline pHi
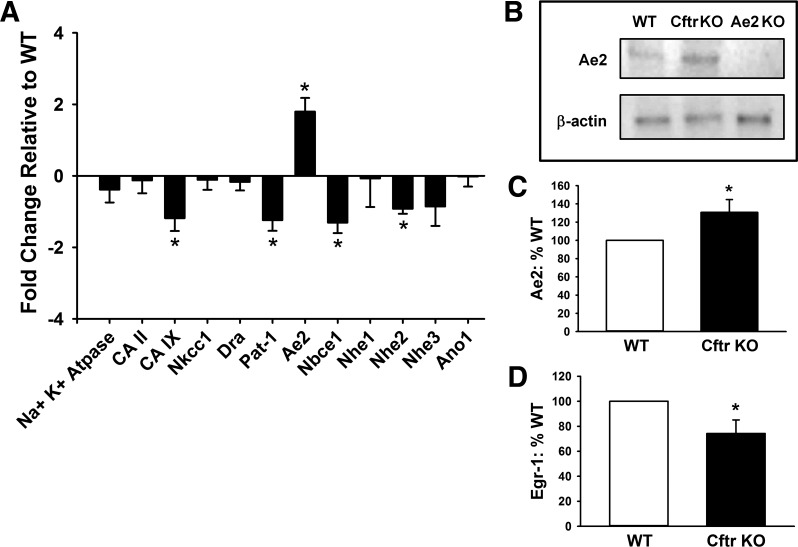
To investigate why pHi compensation does not occur in crypt epithelium of Cftr KO enteroids, quantitative RT-PCR was used to examine the expression level of several acid-base transport proteins that regulate pHi in intestinal epithelium. As shown in Fig. 2A, the transcriptional profile of proteins that are involved in pHi regulation/acid-base transport is different between Cftr KO and WT enteroids. mRNA expression in the Cftr KO enteroids was less for proteins associated with base (HCO3−) loading of the epithelial cell. The changes included carbonic anhydrase IX (CA IX, Car9), a transmembrane enzyme with an extracellular catalytic site that facilitates H+ efflux from or HCO3− import into epithelial cells (83); Pat-1 (Slc26a6), an anion exchanger that can provide HCO3− loading in murine intestinal epithelium (76); the electrogenic sodium bicarbonate cotransporter (Nbce1, Slc4a4), a major pathway for epithelial HCO3− uptake (66); and Na+/H+ exchanger 2 (Nhe2, Slc9a2), which provides H+ efflux from the epithelium during cellular acidification and may preferentially localize to the crypt epithelium (18, 20). In contrast to downregulation of base-loading transporters, mRNA expression of one major HCO3−-unloading transporter, Ae2, was significantly increased. Ae2 is located at the basolateral membrane and serves a housekeeping function of pHi regulation in intestinal epithelium (1). These differences in expression are consistent with changes in transcription to decrease base (HCO3−) loading and increase HCO3− efflux from Cftr KO epithelium.
Fig. 2.
Changes in expression of pHi regulators in Cftr KO crypt enteroids. A: quantitative real-time PCR of mRNA from 8 sex-matched WT and Cftr KO littermate pairs. Genes represented are the Na+-K+-ATPase, carbonic anhydrases (CA II and CA IX), Na+-K+-2Cl− cotransporter (Nkcc1, Slc12Aa2), downregulated in adenoma (Dra, Slc26a3), putative anion transporter-1 (Pat-1, Slc26a6), anion exchanger 2 (Ae2, Slc4a2), electrogenic NaHCO3 cotransporter 1 (Nbce1, Slc4a4), Na+/H+ exchangers 1, 2, and 3 (Nhe1-3, Slc9a1-3), and the anion channel anoctamin 1 (Ano1, Tmem16a). *Significantly different from 0 (by 1-sample t-test). B: representative immunoblot for Ae2 and β-actin of protein lysate from enteroids cultured from a sex-matched WT and Cftr KO littermate pair and a sex-matched Ae2 KO mouse. C: cumulative data for Ae2 immunoblot densitometry of enteroids from sex-matched WT and Cftr KO littermate pairs. Values are means ± SE (n = 11). *Significantly different from WT (by unpaired t-test). D: cumulative data for early growth response-1 (Egr-1) immunoblot densitometry of enteroids from sex-matched WT and Cftr KO littermate pairs. Values are means ± SE (n = 8). *Significantly different from WT (by unpaired t-test).
Cftr KO Enteroids Exhibit Increased Ae2 Protein Expression and Downregulation of the Acid-Sensitive Transcription Factor Egr-1
To determine whether increased transcriptional activation of Ae2 translated to increased Ae2 protein expression, protein lysates of enteroids from sex-matched WT and Cftr KO littermates were examined by immunoblotting. As shown in Fig. 2, B and C, Ae2 expression was significantly greater in Cftr KO than WT enteroids, although the fold change was less than that measured by quantitative RT-PCR. Recent studies demonstrated that cellular acidification of intestinal (C2BBe1) cells increases expression of the acid-sensitive transcription factor Egr-1, resulting in increased transcription of Nhe2 through increased promoter activity at an Egr-1/Sp-1/Egr-1 (ESE) response element (47). Since Nhe2 expression was downregulated in the Cftr KO enteroids (Fig. 2A), we measured protein expression of Egr-1 in WT and Cftr KO enteroids. As shown in Fig. 2D, Egr-1 was significantly decreased in Cftr KO relative to WT enteroids. ESE response elements are found in each of the significantly downregulated transporters in Fig. 2A, consistent with transcriptional regulation by acid-sensitive immediate early gene transcription factors such as Egr-1 (94).
Functional Activity of Ae2 Cl−/HCO3− Exchange Is Increased in Cftr KO Crypt Epithelium
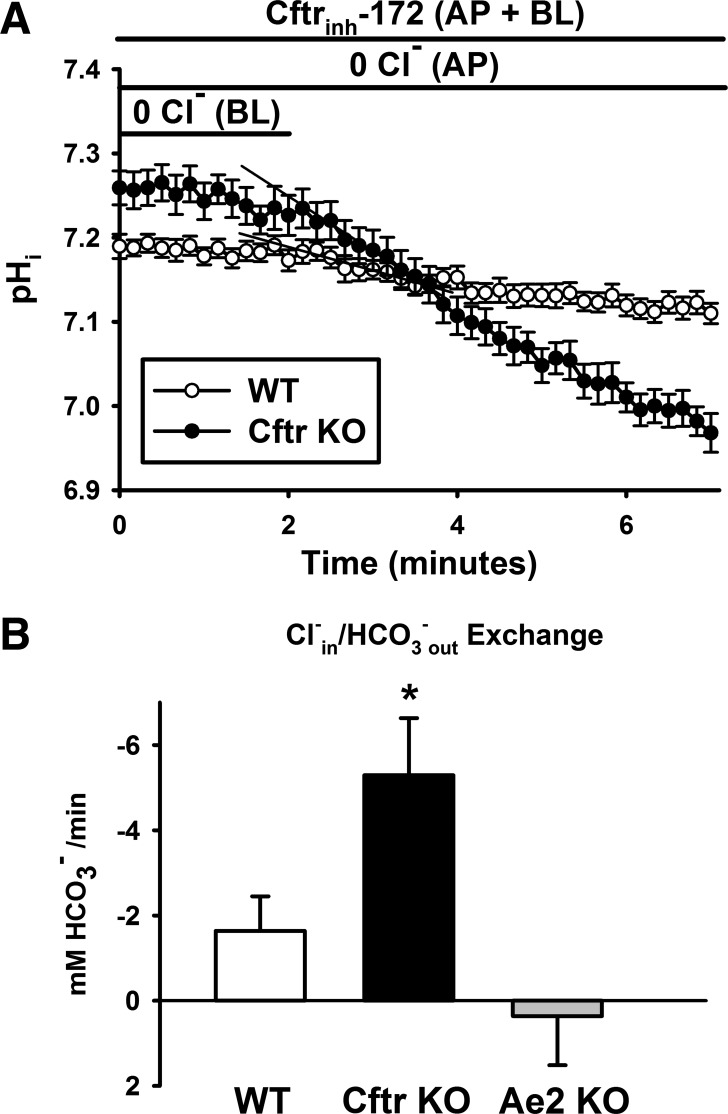
On the basis of our finding of increased Ae2 expression, we asked whether the functional activity of the transporter was impaired and, therefore, whether the transporter was unable to downregulate pHi in Cftr KO crypt epithelium. To isolate basolateral membrane Cl−/HCO3− exchange, the luminal interior of WT and Cftr KO littermate mouse enteroids was microperfused, as previously described (38), with a Cl−-free KBR solution containing 25 μM Cftrinh172 to minimize apical membrane Cl− and HCO3− transport by Slc26a Cl−/HCO3− exchangers and WT Cftr. The basolateral KBR bath solution also contained 25 μM Cftrinh172 and 1 μM EIPA. Using BCECF microfluorimetry to measure pHi, we determined the functional activity of basolateral Cl−/HCO3− exchange by exposing the basolateral membrane to a 0 Cl− KBR solution to lower [Cl−]i and increase intracellular HCO3− uptake by forcing the basolateral membrane Cl−/HCO3− exchanger to run in “reverse” mode (Cl−out/HCO3−in exchange). Subsequently, the basolateral bath solution was switched to KBR solution containing 55 mM Cl− to enable the exchanger to run in “forward” mode (Cl−in/HCO3−out exchange), and the initial (∼30 s) rate of cell acidification by HCO3− efflux was measured. As shown in Fig. 3A, the rate of HCO3− efflux was greater in Cftr KO than WT crypt epithelium. The cumulative data in Fig. 3B indicate that the rate of Cl−in/HCO3−out exchange in Cftr KO epithelium was approximately double that in WT epithelium. The absolute rates of Cl−/HCO3− exchange are lower than reported for recombinant AE2 (80), which is likely due to a slow change in bath Cl− concentration because of interference with diffusion in the Matrigel surrounding the enteroid crypt. To determine whether the measured basolateral Cl−/HCO3− exchange was due to Ae2 activity, the experimental paradigm was repeated using enteroids from Ae2 KO mice. As shown in Fig. 3B, basolateral Cl−/HCO3− exchange was essentially eliminated in Ae2 KO crypt epithelium. These studies indicate that increased expression of Ae2 and a corresponding increase in Ae2 transport function are exhibited by Cftr KO crypt epithelium.
Fig. 3.
Increased basolateral Ae2 activity in Cftr KO crypt epithelium. A: time course of induced Cl−in/HCO3−out exchange at the basolateral (BL) membrane of WT and Cftr KO crypt epithelial cells. Enteroids were microperfused at the apical (AP) membrane with 0 Cl− KBR solution containing 25 μM Cftrinh172 to limit changes in pHi contributed by apical membrane Cftr and Cl−/HCO3− exchange activities. Basolateral bath was composed of 0 Cl− KBR (superfused ∼10 min to achieve a stable pHi) that was switched to KBR containing 55 mM Cl−. Basolateral bath also contained 25 μM Cftrinh172 and 1 μM EIPA. Each data point represents average pHi of 7–10 crypt epithelial cells. Black lines depict initial acidification rates. B: cumulative data showing initial acidification rates after basolateral bath was switched to 55 mM Cl− KBR for WT, Cftr KO, and Ae2 KO crypt epithelium. Values are means ± SE from 6 WT and Cftr KO sex-matched littermates and 4 Ae2 KO mice. *Significantly different from WT and Ae2 KO (by 1-way ANOVA and post hoc Tukey's test).
Cftr KO Crypt Epithelium Maintains an Increased [Cl−]i
The finding of increased Ae2 activity raised the following question: Why does basolateral Ae2 Cl−/HCO3− exchange fail to normalize pHi in Cftr KO crypt epithelium? Previous electron microprobe analysis of human CF intestinal sections showed that both villus and crypt epithelium have a higher Cl− content (50), suggesting that loss of CFTR Cl− channel activity causes cellular Cl− retention in intestinal epithelium. To determine whether a higher [Cl−]i is sustained in live Cftr KO crypt epithelial cells, the Cl−-sensitive fluorescent dye MQAE was used to estimate [Cl−]i in WT and Cftr KO enteroid crypt epithelium. Because our original observation of an alkaline pHi in Cftr KO crypt epithelium was in a study using enteroids maintained in culture medium (37), [Cl−]i was estimated in enteroids maintained in culture medium (Fig. 4A). Under these conditions, as shown in Fig. 4B, [Cl−]i was significantly increased in Cftr KO relative to WT crypt epithelium. A higher [Cl−]i in Cftr KO epithelium would be predicted to provide a less favorable [Cl−]out-[Cl−]in gradient for Cl−/HCO3− exchange by basolateral Ae2.
Acute Pharmacological Correction of [Cl−]i in Cftr KO Crypt Epithelium Normalizes pHi
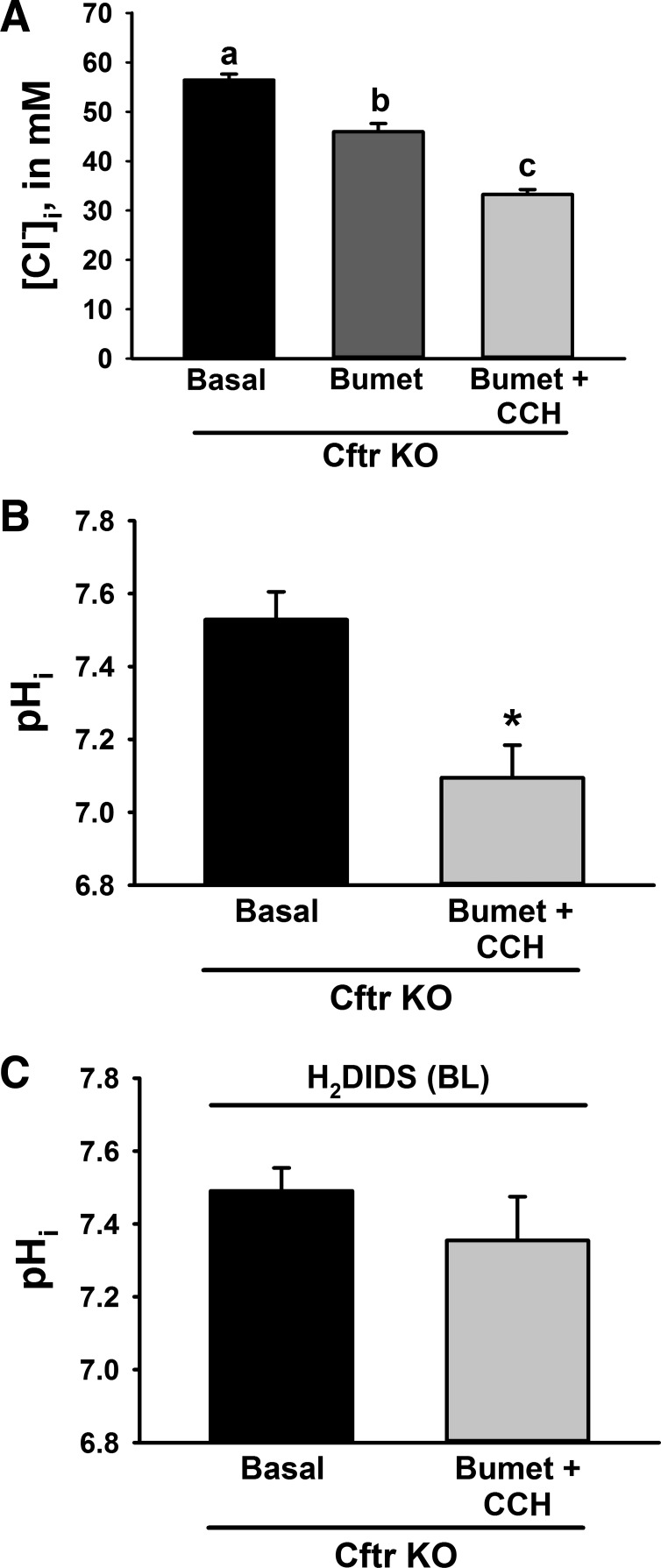
To investigate whether it is the combined effect of Cl− and HCO3− retention that prevents autoregulation of pHi in Cftr KO crypt epithelial cells, we hypothesized that acute reduction of [Cl−]i in Cftr KO crypt epithelium by pharmacological means would reestablish a favorable [Cl−]out-[Cl−]in gradient, thereby enabling Ae2 downregulation of pHi. Reduction of [Cl−]i in Cftr KO crypt epithelium was accomplished by dual treatment with bumetanide (50 μM), an inhibitor of Cl− uptake by NKCC1, and CCh (100 μM), a cholinomimetic that stimulates anion secretion via intracellular Ca2+-sensitive Cl− channels in crypt epithelium, e.g., anoctamin 1. As shown by the MQAE study in Fig. 5A, the measured [Cl−]i in Cftr KO crypt epithelium superfused with oxygenated KBR solution was somewhat higher compared with culture medium maintained with an air-CO2 atmosphere (compare with Cftr KO in Fig. 4B). Treatment of Cftr KO crypt epithelium with bumetanide alone significantly reduced [Cl−]i from basal conditions within 15 min, and addition of CCh to the basolateral bath further reduced [Cl−]i. A similar effect has been reported for CF, but not normal, human airway epithelium measured using double-barreled Cl−-sensing microelectrodes (93). To determine the effect of reduced [Cl−]i on pHi in Cftr KO crypt epithelial cells, pHi was measured before and after sequential treatment with bumetanide (50 μM for 15 min) and CCh (100 μM for 15 min). As shown in Fig. 5B, the combined pharmacological treatment (bumetanide + CCh) to acutely lower [Cl−]i was associated with a significant reduction of pHi in Cftr KO crypt epithelium. To assess whether the acidifying effect of lowering [Cl−]i could be blocked by inhibition of Ae2, the enteroids were treated with the distilbene H2DIDS in the basolateral solution during treatment with bumetanide + CCh. Treatment with H2DIDS would be expected to inhibit both Ae2 and Nbce1 at the basolateral membrane of the enteroids (27, 65) and, therefore, induce opposing effects on pHi regulation. However, as shown in Fig. 5C, H2DIDS prevented the acidifying effect of bumetanide + CCh in Cftr KO crypt epithelial cells, suggesting that Ae2 is dominant in downregulation of pHi when [Cl−]i is normalized. Additional studies verified that H2DIDS exposure did not independently prevent the reduction of [Cl−]i during treatment with bumetanide + CCh in Cftr KO crypt epithelium (in mM): Δ[Cl−]i = −23.15 ± 1.39 without H2DIDS (from Fig. 5A) and −22.04 ± 1.26 with H2DIDS (not significant, n = 18 cells from 2 cultures/2 Cftr KO mice). These findings are consistent with the hypothesis that Cl− retention is responsible for failure of Ae2 to properly downregulate pHi in Cftr KO crypt epithelium.
Fig. 5.
Pharmacological reduction of [Cl−]i normalizes pHi in Cftr KO crypt epithelium. A: [Cl−]i of crypt epithelium from Cftr KO enteroids under basal conditions (Basal) and after addition of 50 μM bumetanide (Bumet) for 15 min followed by 100 μM carbachol (Bumet + CCh) for 15 min. Values are means ± SE of 30 crypt epithelial cells in enteroids from 3 Cftr KO mice. a,b,cSignificantly different (by repeated-measures ANOVA with post hoc Tukey's test). B: pHi of crypt epithelium from Cftr KO enteroids measured before (Basal) and after sequential treatment with bumetanide (50 μM) for 15 min and CCh (100 μM) for 15 min. Values are means ± SE of 10 crypt epithelial cells in enteroids from 3 Cftr KO mice. *Significantly different from Basal (by unpaired t-test). C: effect of H2DIDS on pHi of crypt epithelium from Cftr KO enteroids measured before (Basal) and after sequential treatment with bumetanide (50 μM) for 15 min and CCh (100 μM) for 15 min. Enteroids were treated with 200 μM H2DIDS [in the basolateral (BL) solution] throughout the experiment. Values are means ± SE of 17 crypt epithelial cells in enteroids from 3 Cftr KO mice.
DISCUSSION
Through the development of culture methods to generate primary intestinal epithelial organoids, the opportunity is presented to examine cell- or epithelial-autonomous effects of protein dysfunction in a well-differentiated epithelium for genetic diseases such as CF. These primary cultures avoid many of the interpretational limitations presented by immortalized intestinal cell lines. In the present investigation the enteroid system enabled measurements of the intracellular milieu in live crypt enterocytes in an anatomic setting without the influence of neurotransmission, circulating hormonal or humoral factors, submucosal cells with associated products such as eicosanoids, immune cell interactions, or the luminal microbiome. As previously observed in murine enteroids (37), Cftr KO crypt enterocytes maintained an alkaline pHi and resisted pHi change during an acidic challenge compared with WT. Previous studies of pHi regulation suggested a propensity for cellular alkalinity and/or increased recovery from an acid challenge in Cftr KO duodenal and CF airway epithelia (75, 92), as well as in cell lines expressing mutant CFTR (19). Beyond this, cellular alkalinity is not generally recognized as a property of CF epithelia; consequently, few studies have examined this aspect when investigating abnormal cellular processes in CF disease.
Cftr plays a dual role in the efflux of HCO3− across the apical membrane of proximal small intestine, which, depending on the ionic content of the extracellular fluid, would be predicted to downregulate pHi (10). CFTR permeability to HCO3− (PHCO3−) under basal conditions is ∼0.20–0.25 relative to Cl− (36, 51, 57), but PHCO3− can be altered by changes in extracellular Cl− concentration (73), which is likely in response to the activity of the Cl−-sensitive with-no-lysine (WNK) kinase cascade (55). The other role of Cftr is facilitation of apical membrane Cl−/HCO3− exchange activity by direct intermolecular actions and providing a Cl− leak pathway that avoids subapical membrane Cl− accumulation and an unfavorable [Cl−]out-[Cl−]in gradient (32, 75). In the absence of these Cftr functions, crypt epithelium is predisposed to an alkaline pHi, if it is assumed that Cftr is basally active in WT enteroids. Evidence for basal Cftr activity in the absence of nonepithelial stimuli was shown by a greater crypt cell shrinkage response to CCh in WT than Cftr KO enteroids (37), despite no differences in expression of the intracellular Ca2+-activated Cl− channel anoctamin 1 (Fig. 2A). Therefore, it is possible that the cellular/paracrine environment of rapidly dividing progenitor cells of the crypt provides sufficient Cftr phosphorylation for basal activity, perhaps by the HCO3−-sensitive soluble adenylate cyclase (5) or changes in cAMP related to the cell cycle (7, 64).
Changes in expression of pHi regulatory proteins to compensate for an alkaline pHi were exhibited by the isolated Cftr KO crypt epithelium. Decreased expression of Ncbe1 (Slc4a4), a major epithelial HCO3− uptake pathway, would be predicted to be an appropriate response. Previous microelectrode analyses did not find differences in baseline membrane potential of crypt epithelial cells between WT and Cftr KO enteroids (37), indicative of both a rheological balance between Ncbe1 and Cftr activities in WT epithelium and a compensatory rheological response to Cftr loss in Cftr KO epithelium, perhaps by changes in basolateral K+ diffusion potential (9). The anion exchanger Pat-1 (Slc26a6) transports Cl−, HCO3−, sulfate, or oxalate (46), and gene-targeted deletion results in acidic pHi in intestinal epithelium, thereby indicating that Pat-1 can function under physiological conditions as a HCO3− uptake pathway (76, 77, 88). The putative stoichiometry of Pat-1 (1 Cl−:2 HCO3−) (52) would be conducive to HCO3− loading, although a favorable electrical gradient would not be present because of the similarity in membrane potential between WT and Cftr KO crypt epithelium. Pat-1 is functional in the lower villus epithelium (88), but definitive studies of Pat-1 activity in crypt epithelium have not been performed. Decreased expression of CA IX in Cftr KO enteroids is also consonant with reduced HCO3− loading of the epithelium. CA IX catalytic site has an extracellular locale and provides cellular H+ efflux/HCO3− influx (83). Although known for its role in protection of cancer stem cells from hypoxia (13, 83), CA IX demonstrates physiological function (54) and normally is expressed at significant levels in crypt epithelium (69). Nhe2 is expressed in intestinal epithelia and may preferentially function in crypt epithelium (20, 25). Together with a trend for decreased Nhe3 expression, downregulation of Nhe2, which provides epithelial proton efflux, is also predicted to be an appropriate response by the epithelial cells to offset an alkaline pHi. The reduced pHi recovery from NH4Cl prepulse cell acidification in Cftr KO crypts (Fig. 1, B and D) may reflect diminished Nhe2 and, possibly, Nhe3 activity corresponding to decreased expression of these transporters (Fig. 2A). Notably, recent studies of NHE2 gene regulation have shown that upregulation of NHE2 expression in response to extracellular acidification is dependent on increased expression of the transcription factor Egr-1, which interacts with an ESE motif in the upstream elements of NHE2 (47). Our finding that Egr-1 protein expression is downregulated in Cftr KO enteroids is consistent with a regulatory arrangement that is responsive to sustained changes in pHi. Egr-1 binding sites are found in the promoter regions of Nbce1, Pat-1, CA IX, and Nhe2 (Genomatix search not shown), suggesting that compensatory downregulation of base (HCO3−)-loading transporters/enzymes in response to an alkaline pHi in Cftr KO crypt epithelium is coordinated by pH-sensitive transcription factors/kinases of the growth-related immediate early response genes (94).
In addition to reduced transcription of HCO3−-loading transporters/enzymes, mRNA and protein expression of Ae2, a definitive HCO3−-unloading transporter, was upregulated in Cftr KO enteroids. Ae2 at the basolateral membrane is a major participant in pHi regulation in intestinal epithelium and provides a Cl− uptake pathway for Cftr-mediated Cl− secretion in parallel with NKCC1 (3, 17, 72, 87). Ae2 is a distilbene-sensitive 1 Cl−:1 HCO3− exchanger that increases in activity in response to increases in pHi (81). Thus, despite a relatively modest increase in protein expression, the functional activity of Ae2 was two- to threefold greater in Cftr KO than WT crypt epithelium as determined by initial rates of pHi change during experimental removal and replacement of extracellular Cl−. The transport rates for basolateral Cl−/HCO3− exchange, which are largely a measure of Ae2 activity (Fig. 3B), were somewhat less than typically found in recombinant protein expression systems (31), but this likely reflects diminished diffusion through the Matrigel surrounding the enteroids during solution changes. Since baseline pHi tends alkaline in Cftr KO crypt epithelium, the central question of this investigation was as follows: Why doesn't increased activity of Ae2 in Cftr KO epithelium normalize pHi? A previous electron microanalysis of snap-frozen sections of jejunum from normal subjects and CF patients showed an increase of 18–41% in the Cl− content of both villous and crypt epithelium in the CF intestine (50). Therefore, it was reasoned that the major permeant anions of Cftr, i.e., HCO3− and Cl−, might be retained in isolated, viable intestinal epithelium. In the case of Cl−, retention would establish a less favorable [Cl−]out-[Cl−]in gradient for Cl−/HCO3− exchange by Ae2.
Our measurements revealed an increase in [Cl−]i in Cftr KO crypt epithelium that would be predicted to impede Ae2 Cl−/HCO3− exchange activity by ∼30%. The apparent remaining activity of Ae2 (∼70%), based on the unfavorable [Cl−]out-[Cl−]in gradient, is likely an overestimation of Ae2 transport function in Cftr KO crypt because of two additional factors. 1) CA IX is known to form a membrane HCO3− transport metabolon with Ae2 that increases its activity in gastric epithelia (45); this metabolon may form in intestinal epithelial cells as well. If so, the reduced expression of CA IX in Cftr KO crypt epithelial cells (Fig. 2A) may diminish metabolon formation and, thus, further impair Ae2 function. 2) It was found that the Cftr KO crypt epithelium exhibits a significant, although puzzling, level of NKCC1 activity under unstimulated conditions, as shown by the reduction of [Cl−]i upon bumetanide treatment (Fig. 5A). Whether this represents a requirement to sustain cell volume in rapidly proliferating crypt cells, perhaps in response to local Wnt signals, or a pathophysiological response to the absence of Cftr in these cells requires further investigation. Nonetheless, it is likely that increased NKCC1 activity not only contributes to the increased [Cl−]i in Cftr crypt epithelium but also, given the cellular location of NKCC1, could sustain a basolateral submembrane [Cl−]i that amplifies an unfavorable [Cl−]out-[Cl−]in gradient for Ae2 activity.
Reducing [Cl−]i by the combined pharmacological actions of NKCC1 Cl− uptake inhibition and CCh-induced activation of Ca2+-activated Cl− channels resulted in normalization of pHi within 30 min in Cftr KO crypt epithelium. The reduction of pHi was prevented by basolateral treatment with the distilbene H2DIDS. Although H2DIDS treatment inhibits both Ae2 and Nbce1 (58), which have opposite effects on intracellular [HCO3−], preventing normalization of pHi suggests that Ae2 has a dominant role in pHi regulation in the presence of a favorable [Cl−]out-to-[Cl−]in gradient. It is unclear why cellular transport processes do not normalize [Cl−]i in Cftr KO crypt epithelium. Loss of Cftr-mediated anion secretion by crypt epithelium likely places demands on cellular electrolyte homeostasis that are apparently met by Cl− retention. Since NKCC1 is a Na+ gradient-driven transporter, normal or increased NKCC1 activity in the absence of Cftr continues to provide intracellular Na+, which contributes to the activity of the Na+-K+-ATPase as well as the volume status of the epithelial cell. Similarly, cellular K+ retention (50) to sustain normal membrane potential may be another feature that contributes to increased [Cl−]i in Cftr KO crypt epithelial cells.
The tendency of isolated Cftr KO intestinal epithelium to sustain an alkaline pHi in the absence of extracellular regulatory influences likely has subtle, but broad-ranging, effects on cellular processes. One potential area of influence would be cell proliferation, where alkaline pHi in the proliferative compartment of intestinal epithelium may foster hyperproliferation by a process that can be directly tied to loss of Cftr function. An alkaline pHi is known to favor cell cycle transitions (59), optimize DNA replication (71), enhance membrane biogenesis (95), and alter Wnt/β-catenin signaling (74). Previous in vivo studies of Cftr KO intestinal epithelia indicate a status of hyperproliferation (16), which we have reported to be recapitulated in Cftr KO enteroids, consistent with an epithelial-autonomous effect of Cftr loss (39, 91). Hyperproliferation provides a platform for neoplasia (14, 86), and it is known that CF patients have a sixfold-increased risk for gastrointestinal cancer (48), an alarming statistic for a relatively young population. A variety of other disturbances in cellular processes in CF epithelium, including ATP release (29), mucus release (38, 44), cholesterol metabolism (15), microtubule acetylation (68), and Ca2+ signaling (43, 62), have been identified. Few of these studies have evaluated the possibility of altered pHi or [Cl−]i, which would be expected to impact the activity of proteins/enzymes involved in the cellular process. Since pHi regulation is largely directed to prevent cell acidification, which can be encountered frequently in normal physiological states, it is unclear whether the resistance of CF epithelium to acidification would have a detrimental effect on cellular function. On the other hand, alkalosis is infrequently encountered in physiological or pathological states, so it may not be surprising that sustained cellular alkalinity is tolerated in CF intestinal epithelium, especially since it is conducive to cell growth and proliferation. Together, the present findings provide evidence that isolated Cftr KO crypt epithelium maintains an altered intracellular milieu against which other intracellular and extracellular regulatory processes must operate in vivo and, in WT crypt epithelium, identifies Cftr as providing a cellular Cl− and HCO3− efflux pathway that establishes an upper limit to cellular alkalization.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-48816 (to L. L. Clarke) and Cystic Fibrosis Foundation Grants Clarke11G0 and Clarke15G0 (to L. L. Clarke) and LIU13F0 (to J. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.M.W., J.L., and L.L.C. developed the concept and designed the research; N.M.W., J.L., S.R.S., C.D.S., and A.M.S. performed the experiments; N.M.W., J.L., S.R.S., C.D.S., and L.L.C. analyzed the data; N.M.W. and L.L.C. interpreted the results of the experiments; N.M.W., J.L., C.D.S., and L.L.C. prepared the figures; N.M.W., J.L., C.D.S., A.M.S., and L.L.C. edited and revised the manuscript; N.M.W., J.L., S.R.S., C.D.S., A.M.S., and L.L.C. approved the final version of the manuscript; L.L.C. drafted the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the expertise and assistance of Dr. Luis Martinez-Lemus and the Dalton Live Cell Imaging Core.
REFERENCES
- 1.Alper SL, Darman RB, Chernova MN, Dahl NK. The AE gene family of Cl−/HCO3− exchangers. J Nephrol 15: S41–S53, 2002. [PubMed] [Google Scholar]
- 2.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212: 1672–1683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G332, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Andersson C, Servetnyk Z, Roomans GM. Activation of CFTR by genistein in human airway epithelial cell lines. Biochem Biophys Res Commun 308: 518–522, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Baudouin-Legros M, Hamdaoui N, Borot F, Fritsch J, Ollero M, Planelles G, Edelman A. Control of basal CFTR gene expression by bicarbonate-sensitive adenylyl cyclase in human pulmonary cells. Cell Physiol Biochem 21: 75–86, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl−/HCO3− exchangers. Am J Physiol Cell Physiol 255: C857–C869, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Burger MM, Bombik BM, Breckenridge BM, Sheppard JR. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol 239: 161–163, 1972. [DOI] [PubMed] [Google Scholar]
- 8.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice using an oral osmotic laxative. Lab Anim Sci 46: 612–618, 1996. [PubMed] [Google Scholar]
- 9.Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol Gastrointest Liver Physiol 270: G259–G267, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Clarke LL, Stien X, Walker NM. Intestinal bicarbonate secretion in cystic fibrosis mice. J Pancreas 2 Suppl 4: 263–267, 2001. [PubMed] [Google Scholar]
- 12.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293: G577–G584, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J, Supuran CT, Pastorekova S, Pastorek J. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res 71: 7558–7567, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 411: 342–348, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, West RH, Manson ME, Ruddy J, Jiang D, Previs SF, Sonawane ND, Burgess JD, Kelley TJ. Increased plasma membrane cholesterol in cystic fibrosis cells correlates with CFTR genotype and depends on de novo cholesterol synthesis. Respir Res 11: 61, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher AM, Gottlieb RA. Proliferation, not apoptosis, alters epithelial cell migration in small intestine of CFTR null mice. Am J Physiol Gastrointest Liver Physiol 281: G681–G687, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE. AE2 Cl−/HCO3− exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol 298: G493–G503, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gens JS, Du H, Tackett L, Kong SS, Chu S, Montrose MH. Different ionic conditions prompt NHE2 and NHE3 translocation to the plasma membrane. Biochim Biophys Acta 1768: 1023–1035, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb RA, Dosanjh A. Mutant cystic fibrosis transmembrane conductance regulator inhibits acidification and apoptosis in C127 cells: possible relevance to cystic fibrosis. Proc Natl Acad Sci USA 93: 3587–3591, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan Y, Dong J, Tackett L, Meyer JW, Shull GE, Montrose MH. NHE2 is the main apical NHE in mouse colonic crypts but an alternative Na+-dependent acid extrusion mechanism is upregulated in NHE2-null mice. Am J Physiol Gastrointest Liver Physiol 291: G689–G699, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Guo JJ, Stoltz DA, Zhu V, Volk KA, Segar JL, McCray PB Jr, Roghair RD. Genotype-specific alterations in vascular smooth muscle cell function in cystic fibrosis piglets. J Cyst Fibros 13: 251–259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem 282: 31422–31428, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi H, Szaszi K, Coady-Osberg N, Orlowski J, Kinsella JL, Grinstein S. A slow pH-dependent conformational transition underlies a novel mode of activation of the epithelial Na+/H+ exchange-3 isoform. J Biol Chem 277: 11090–11096, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hodges CA, Grady BR, Mishra K, Cotton CU, Drumm ML. Cystic fibrosis growth retardation is not correlated with loss of Cftr in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 301: G528–G536, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Houwen RH, van der Doef HP, Sermet I, Munck A, Hauser B, Walkowiak J, Robberecht E, Colombo C, Sinaasappel M, Wilschanski M. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J Pediatr Gastroenterol Nutr 50: 38–42, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Shan J, Kim D, Liao J, Evagelidis A, Alper SL, Hanrahan JW. Basolateral chloride loading by AE2: role in fluid secretion by the human airway epithelial cell line Calu-3. J Physiol 590: 5299–5316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Mak D, Devidas S, Schwiebert EM, Bragin A, Zhang Y, Skach W, Guggino WB, Foskett JK, Engelhardt JF. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J Cell Biol 143: 645–657, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity and role in cell proliferation. J Biol Chem 269: 23544–23552, 1994. [PubMed] [Google Scholar]
- 31.Karumanchi SA, Jiang L, Knebelmann B, Stuart-Tilley AK, Alper SL, Sukhatme VP. VHL tumor suppressor regulates Cl−/HCO3− exchange and Na+/H+ exchange activities in renal carcinoma cells. Physiol Genomics 5: 119–128, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koncz C, Daugirdas JT. Use of MQAE for measurement of intracellular [Cl−] in cultured aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 267: H2114–H2123, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Krapf RB, Berry CA, Verkman AS. Estimation of intracellular chloride activity in isolated perfused rabbit proximal convoluted tubules using a fluorescent indicator. Biophys J 53: 955–962, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol 67: 802–808, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Linsdell P, Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol 110: 355–364, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Walker NM, Cook MT, Ootani A, Clarke LL. Functional Cftr in crypt epithelium of organotypic enteroid cultures from murine small intestine. Am J Physiol Cell Physiol 302: C1492–C1503, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Walker NM, Ootani A, Strubberg AM, Clarke LL. Defective goblet cell exocytosis contributes to murine cystic fibrosis-associated intestinal disease. J Clin Invest 125: 1056–1068, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Walker NM, Clarke LL. Hyperproliferation in the CF mouse intestine is associated with increased Wnt/β-catenin signaling (Abstract). Pediatr Pulmonol 34 Suppl: 255–256, 2011. [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods (Duluth) 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Maisonneuve P, Fitzsimmons SC, Neglia JP, Campbell PW, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst 95: 381–387, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol 176: 1377–1389, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miele L, Cordella-Miele E, Xing M, Frizzell R, Mukherjee AB. Cystic fibrosis gene mutation (ΔF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol 16: 749–759, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Montserrat C, Merten M, Figarella C. Defective ATP-dependent mucin secretion by cystic fibrosis pancreatic epithelial cells. FEBS Lett 393: 264–268, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR. Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am J Physiol Cell Physiol 293: C738–C748, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch 447: 710–721, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Muthusamy S, Cheng M, Jeong JJ, Kumar A, Dudeja PK, Malakooti J. Extracellular acidosis stimulates NHE2 expression through activation of transcription factor Egr-1 in the intestinal epithelial cells. PLos One 8: e82023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neglia JP, Fitzsimmons SC, Maisonneuve P, Schoni MH, Schoni-Affolter F, Corey M, Lowenfels AB. The risk of cancer among patients with cystic fibrosis. N Engl J Med 332: 494–499, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 286: G1032–G1041, 2004. [DOI] [PubMed] [Google Scholar]
- 50.O'Loughlin EV, Hunt DM, Bostrom TE, Hunter D, Gaskin KJ, Gyory A, Cockayne DJ. X-ray microanalysis of cell elements in normal and cystic fibrosis jejunum: evidence for chloride secretion in villi. Gastroenterology 110: 411–418, 1996. [DOI] [PubMed] [Google Scholar]
- 51.O'Reilly CW, Winpenny JP, Argent BE, Gray MA. Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: inhibition by bicarbonate ions. Gastroenterology 118: 1187–1196, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587: 2179–2185, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orlowski A, de Giusti VC, Morgan PE, Aiello EA, Alvarez BV. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/HCO3− cotransporter enhances NBCe1-mediated HCO3− influx in the rat heart. Am J Physiol Cell Physiol 303: C69–C80, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139: 620–631, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Plant BJ, Goss CH, Plant WD, Bell SC. Management of comorbidities in older patients with cystic fibrosis. Lancet Respir Med 1: 164–174, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91: 5340–5344, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol 290: F580–F599, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Putney LK, Barber DL. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem 278: 44645–44649, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Raia V, Maiuri L, De Ritis G, De Vizia B, Vacca L, Conte R, Auricchio S, Londei M. Evidence of chronic inflammation in morphologically normal small intestine of cystic fibrosis patients. Pediatr Res 47: 344–350, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Rhodes J, Apsimon HT, Lawrie JH. pH of the contents of the duodenal bulb in relation to duodenal ulcer. Gut 7: 502–508, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro CM. Measurements of intracellular calcium signals in polarized primary cultures of normal and cystic fibrosis human airway epithelia. Methods Mol Biol 742: 113–126, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Robinson PJ, Smith AL, Sly PD. Duodenal pH in cystic fibrosis and its relationship to fat malabsorption. Dig Dis Sci 35: 1299–1304, 1990. [DOI] [PubMed] [Google Scholar]
- 64.Rocha AS, Paternot S, Coulonval K, Dumont JE, Soares P, Roger PP. Cyclic AMP inhibits the proliferation of thyroid carcinoma cell lines through regulation of CDK4 phosphorylation. Mol Biol Cell 19: 4814–4825, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romero MF, Boron WF. Electrogenic Na+/HCO3− cotransporters: cloning and physiology. Annu Rev Physiol 61: 699–723, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch 447: 495–509, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Rune SJ, Henriksen FW. Carbon dioxide tensions in the proximal part of the canine gastrointestinal tract. Gastroenterology 56: 758–762, 1969. [PubMed] [Google Scholar]
- 68.Rymut SM, Harker A, Corey DA, Burgess JD, Sun H, Clancy JP, Kelley TJ. Reduced microtubule acetylation in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 305: L419–L431, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastorekova S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem 46: 497–504, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. [DOI] [PubMed] [Google Scholar]
- 71.Schreiber R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol 205: 129–137, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Schultheiss G, Horger S, Diener M. The bumetanide-resistant part of forskolin-induced anion secretion in rat colon. Acta Physiol Scand 164: 219–228, 1998. [PubMed] [Google Scholar]
- 73.Shcheynikov N, Kim KH, Kim K, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J Biol Chem 279: 21857–21865, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol 11: 286–294, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates basal Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Simpson JE, Walker NM, Supuran CT, Soleimani M, Clarke LL. Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in the duodenal villous epithelium. Am J Physiol Gastrointest Liver Physiol 298: G683–G691, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smyth RL, Croft NM, O'Hea U, Marshall TG, Ferguson A. Intestinal inflammation in cystic fibrosis. Arch Dis Child 82: 394–399, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. [DOI] [PubMed] [Google Scholar]
- 80.Sterling D, Casey JR. Transport activity of AE3 chloride/bicarbonate anion-exchange proteins and their regulation by intracellular pH. Biochem J 344: 221–229, 1999. [PMC free article] [PubMed] [Google Scholar]
- 81.Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl− transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J Gen Physiol 120: 707–722, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 120: 3149–3160, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 29: 6509–6521, 2010. [DOI] [PubMed] [Google Scholar]
- 84.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979. [DOI] [PubMed] [Google Scholar]
- 85.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, Chambers A, Ji D, Gamber K, Chu KK, Tearney G, Tang LP, Fortenberry JA, Du M, Cadillac JM, Bedwell DM, Rowe SM, Sorscher EJ, Fanucchi MV. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLos One 9: e91253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36: 131–149, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker NM, Flagella M, Gawenis LR, Shull GE, Clarke LL. An alternate pathway of cAMP-stimulated Cl− secretion across the NKCC1-null murine duodenum. Gastroenterology 123: 531–541, 2002. [DOI] [PubMed] [Google Scholar]
- 88.Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL. Functional activity of Pat-1 (Slc26a6) Cl−/HCO3− exchange in the lower villus epithelium of murine duodenum. Acta Physiol (Oxf) 201: 21–31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002. [DOI] [PubMed] [Google Scholar]
- 90.Welsh MJ, Tsui LC, Boat TF, Beaudet AL. Cystic fibrosis. In: Metabolic and Molecular Basis of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Valle D, Fredrickson DS. New York: McGraw Hill, 1995, p. 3799–3863. [Google Scholar]
- 91.Williams AM, Liu J, Walker NM, Clarke LL. Loss of CFTR results in intestinal stem cell hyperproliferation (Abstract). Gastroenterology 146: S517, 2014. [Google Scholar]
- 92.Willumsen NJ, Boucher RC. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J Physiol 455: 247–269, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Willumsen NJ, Davis CW, Boucher RC. Intracellular Cl− activity and cellular pathways in cultured human airway epithelium. Am J Physiol Cell Physiol 256: C1033–C1044, 1989. [DOI] [PubMed] [Google Scholar]
- 94.Yamaji Y, Moe OW, Miller RT, Alpern RJ. Acid activation of immediate early genes in renal epithelial cells. J Clin Invest 94: 1297–1303, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Young BP, Shin JJ, Orij R, Chao JT, Li SC, Guan XL, Khong A, Jan E, Wenk MR, Prinz WA, Smits GJ, Loewen CJ. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science 329: 1085–1088, 2010. [DOI] [PubMed] [Google Scholar]