Abstract
While the intestine plays an important role in digestion and absorption, the mucus lining the epithelium represents a pivotal function in mucosal protection. Goblet cells are scattered in both the crypts and among enterocytes, and they secrete an important component of mucus, mucin. We have reported that sodium/hydrogen exchanger (NHE) 8 is a novel player in mucosal protection, since loss of NHE8 function resulted in reduced mucin production and increased bacterial adhesion. While NHE8 has been shown to be expressed in enterocytes and its expression is reduced during intestinal inflammation, nothing is known about the role of NHE8 in goblet cells. This current study is designed to define the expression of NHE8 and the role of TNF-α in the regulation of NHE8 in goblet cells. Using HT29-MTX cells as an in vitro model, we detected abundant NHE8 mRNA in goblet cells. Immunohistochemical staining localized NHE8 protein on the plasma membrane and in the intracellular compartments in goblet cells. Furthermore, NHE8 expression in goblet cells is regulated by the proinflammatory cytokine TNF-α. The expression of NHE8 in HT29-MTX cells was significantly reduced at both mRNA and protein levels in the presence of TNF-α. This inhibition of NHE8 mRNA expression could be blocked by the transcriptional inhibitor actinomycin D. Promoter reporter assay showed that NHE8 promoter activity was indeed reduced by TNF-α. Mechanistically, TNF-α reduced Sp3 protein binding to the human NHE8 basal promoter region. Therefore, NHE8 is expressed in goblet cells, and the inflammatory cytokine TNF-α downregulates NHE8 expression by a transcriptional mechanism.
Keywords: sodium/hydrogen exchanger, tumor necrosis factor-α
multiple sodium/hydrogen exchanger (NHE) isoforms have been identified from mammalian cells. These NHEs display distinct tissue distribution, membrane localization, inhibitor sensitivity, and physiological regulation (17, 25, 33). They play an important part in physiological functions, such as intracellular pH homeostasis, cell volume regulation, and electroneutral NaCl absorption. Additionally, these NHEs also play roles in several diseases, including diarrheal disorders, hypertension, and cardiac ischemia. Only five of the identified NHEs are expressed in the gastrointestinal tract of mammals. NHE1 is expressed at the basolateral membrane in the intestinal epithelial cells, and it contributes to cell volume regulation and intracellular pH (pHi) regulation (4, 18). NHE2 is expressed at the brush-border membrane (BBM) in the intestinal epithelia, and is important for normal gastric function (1, 10). NHE3 is also expressed at the intestinal BBM, and it is a main player in intestinal sodium absorption (3, 10, 20). NHE4 is expressed on the basolateral membrane of gastric parietal cells and is important for acid secretion (8). NHE8 is unique compared with these NHEs. It has roles in the two major cellular phenotypes of the intestinal epithelium, enterocytes that absorb nutrients and goblet cells that make mucin. NHE8 is expressed at the apical membrane of the intestinal epithelial cells, and it mediates sodium-dependent proton exchange and plays important roles in mucosal protection (27, 29, 31).
Whereas enterocytes merely form a cellular barrier, goblet cells participate in mucosal immunity by secreting gel-forming mucins that provide an additional layer of protection. Earlier studies have shown that loss of NHE8 resulted in decreased mucin production in the colon (31), suggesting a possible role of NHE8 in the goblet cells. In this study, we identified the expression of NHE8 in goblet cells and studied the effect of TNF-α, a well-known colitis-related proinflammatory cytokine, on NHE8 expression in goblet cells.
MATERIALS AND METHODS
Cell culture.
Human intestinal goblet cells (HT29-MTX) were kindly provided by Thécla Lesuffleur (INSERM, France). Cells were cultured in DME medium (HyClone; GE Healthcare Life Sciences, Logan, UT) containing 10% fetal bovine serum (HyClone) and 1% penicillin-streptomycin (HyClone). Human intestinal epithelial cells (Caco-2) were purchased from American Type Culture Collection (Manassas, VA) and were cultured in MEM-NEAA medium (HyClone) containing 20% fetal bovine serum (HyClone) and 1% penicillin/streptomycin (HyClone). Both cell types were maintained at 37°C in a 95% air-5% CO2 atmosphere and passaged every 72 h. For TNF-α experiments, cells were exposed to different concentrations of human recombinant TNF-α (Peprotech, Rocky Hill, NJ) for 18 h before harvest. To study if the effect of TNF-α on NHE8 expression involves transcriptional regulation, cells were treated with actinomycin D (100 nM) for 18 h in the presence or absence of TNF-α.
Functional characterization of NHE8 in HT29-MTX cells.
Cells were seeded on glass cover slips and were cultured for 40 h before pHi was measured. The activity of NHE8 was monitored by measuring the rate of Na+-dependent recovery of pHi after acid load in HCO3−-free HBSS as described in previous publications (23, 25). pHi was assessed by monitoring the fluorescence emission of the pH-sensitive dye SNARF 4-AM (Invitrogen, Carlsbad, CA). The ratio of fluorescence intensity (640 nm/570 nm) was measured for individual cells, and these ratios were subsequently converted to pHi by means of an in situ-derived calibration curve.
RNA purification and PCR analysis to detect NHE8 expression.
RNA was purified from HT29-MTX cells and Caco-2 cells using Trizol reagent (Invitrogen). TaqMan technology was used to determine the expression of NHE2, NHE3, and NHE8 using human NHE2 (Hs00268166-m1), NHE3 (Hs00188200-m1), NHE8 (Hs00392302-m1), and TATA-binding protein (TBP; Hs00427620-m1) primers purchased from Applied Biosystems (Foster City, CA). Resulting data were analyzed using the comparative cycle threshold (Ct) method. The target gene cycle thresholds were adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”). TBP data were used as an endogenous reference to normalize gene expression levels.
Protein purification and Western blot analysis.
Total protein was prepared in a RIPA buffer as previously described (3). For Western blot detection, NHE8 antibody was used as a 1:3,000 dilution to detect NHE8 protein (27) while β-actin antiserum (Sigma, St. Louis, MO) was used as a 1:5,000 dilution to determine β-actin protein abundance. Western detection was performed with the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics, Indianapolis, IN). A ratio of NHE8 protein intensity over β-actin protein intensity was used to quantify protein expression.
Immunohistochemistry stain.
Mouse colon tissue sections at 8 μm thick and HT29-MTX cells were used for immunohistochemical staining. Labeling and detection of NHE8 were performed as previously described (27). Briefly, NHE8 antiserum was incubated with tissue sections and HT29-MTX cells overnight at 1:200 dilution. The tissue slides were subsequently incubated with secondary antiserum (Alexa Fluor 647 goat anti-rabbit IgG; Invitrogen) at 1:400 dilution and then visualized under ×60 objective using the EVOS FL Auto Imaging System (Invitrogen).
Transient transfection and functional promoter analysis.
The human NHE8 gene promoter constructs pGL3b/−32 and pGL3b/−671 were made as previously described (24). For transfection experiments, HT29-MTX cells grown in 24-well plates were transfected with Effectene (Qiagen, Valencia, CA) according to the manufacturer's instruction. Promoter reporter assay was performed 40 h after transfection using a dual luciferase assay kit according to the manufacturer's instructions (Promega, Madison, WI). Luciferase activities were measured with a luminometer (Femtomaster FB 12; Berthold Detection System, Pforzheim, Germany). Renilla luciferase activity driven by pRL-CMV (Promega) was used as an internal control to calculate the relative luciferase activity. To test the effect of TNF-α on human NHE8 promoter activity, transfected cells were treated with TNF-α for 18 h before conducting the promoter reporter assay. Relative change in promoter activity was calculated by the ratio of TNF-α-treated promoter activity over untreated promoter activity.
Nuclear protein extraction and gel mobility shift assay.
Nuclear protein was extracted according to a previously described method (28). Synthetic double-stranded DNA oligonucleotides that span the targeted promoter region (−18 GCCGAGGCCCCGCCTCCCGCTCTCGCC +7) were end-labeled with [γ-32P]ATP, and 4 μg of nuclear extract were incubated with 1 ng of labeled probe in gel mobility shift assay (GMSA)-binding buffer containing 10 mM HEPES (pH 7.5), 1 mM EDTA, 50 mM NaCl, 1 mM dithiothreitol, and 50 μg/ml poly[d(I-C)]. After incubation at room temperature for 20 min, the mixture was electrophoresed on a 6% polyacrylamide gel in 0.5× Tris-boric acid-EDTA buffer. Gels were dried and exposed to X-ray film. For competition experiments, a 200-fold molar excess of the unlabeled probe was added to the reaction mixture before the labeled probe was added. Reaction mixtures for supershift assays also contained 4 μg of Sp3 antibody (Santa Cruz Biotechnology).
Statistical analysis.
ANOVA post hoc tests (StatView 5.0.1; SAS Institute, Cary, NC) were used to compare values of the experimental data. P values <0.05 were considered significant.
RESULTS
Identification of NHE8 expression in HT29-MTX cells.
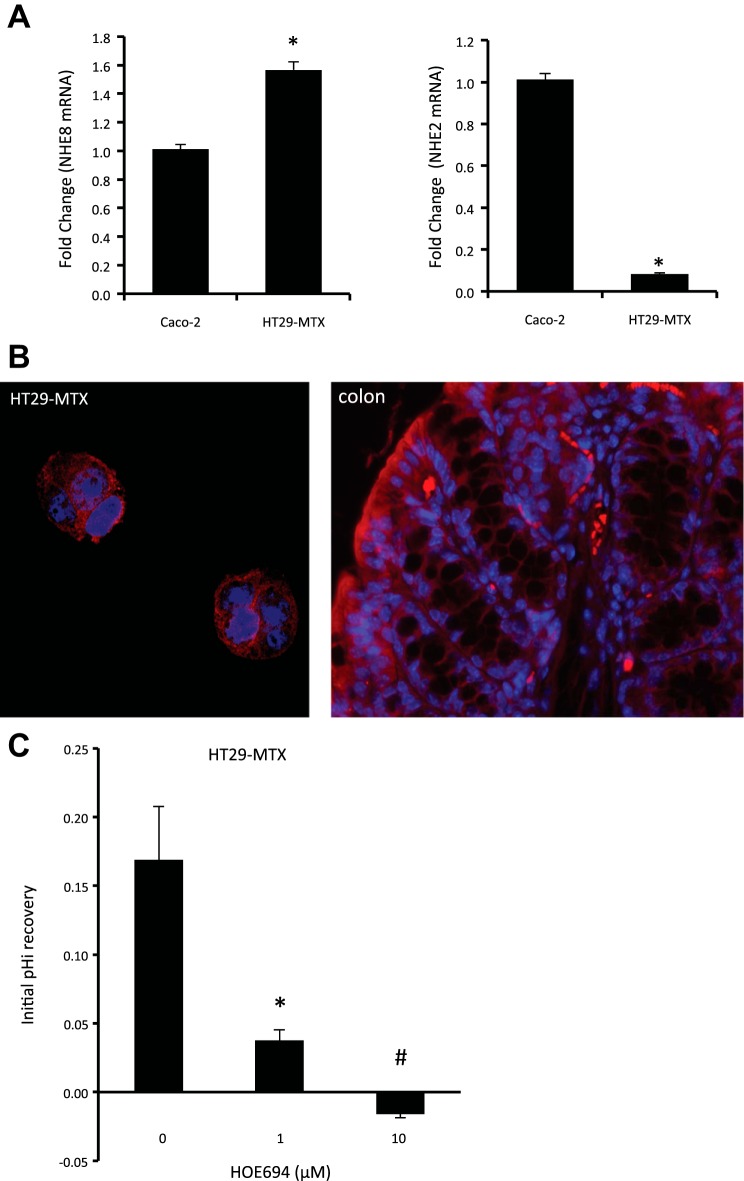
Real-time PCR was performed on RNA isolated from cultured HT29-MTX and Caco-2 cells to compare NHE2, NHE3, and NHE8 gene expression. As shown in Fig. 1A, only NHE2 and NHE8 were detected by Real-time PCR in HT29-MTX cells. NHE8 expression in HT29-MTX cells was higher than in Caco-2 cells (1.57 ± 0.03 in HT29-MTX cells compared with 1.01 ± 0.03 in Caco-2 cells; n = 4, P = 0.00001), and NHE2 expression in HT29-MTX cells was lower than in Caco-2 cells (0.08 ± 0.01 in HT29-MTX cells compared with 1.01 ± 0.03 in Caco-2 cells; n = 4, P < 0.00001). Immunohistochemical stain using NHE8 antibody also detected plasma membrane and intracellular compartment localization of NHE8 in HT29-MTX cells as well as in mouse colonic tissue (Fig. 1B).
Fig. 1.
Identification of sodium/hydrogen exchanger (NHE) 8 expression in goblet cells. A: PCR detection of NHE isoform expression in HT29-MTX cells. Total RNA was isolated from cells and used for 1st-strand cDNA synthesis. Real-time PCR was performed using human NHE2, NHE3, NHE8, and TATA-binding protein (TBP) primers. Data are presented as means ± SE from 6 separate experiments. *P < 0.05 for Caco-2 cells vs. HT29-MTX cells. B: NHE8 protein localization in goblet cells. Mouse colonic tissue section and HT29-MTX cells were reacted with NHE8 antibody following the procedure described in materials and methods. NHE8 is labeled with red, and nuclei are labeled with blue. C: characterization of functional NHE8 in HT29-MTX cells. Cells were loaded with 40 mM NH4Cl for 5 min and then washed with sodium-free HBSS before addition of sodium-containing HBSS. The rate of intracellular pH (pHi) recovery was analyzed during the initial 60 s following addition of sodium-containing HBSS in the presence or absence of NHE inhibitor HOE-694 (0, 1, and 10 μM). HOE-694 was included both during the NH4Cl washout phase and the recovery phase. Data were collected from 19 to 29 cells for each group. *P < 0.00001 for 1 μM HOE-694 vs. others; #P < 0.00001 for 10 μM HOE-694 vs. others.
NHE8 functions as NHE in HT29-MTX cells.
Using a NHE specific inhibitor, we identified that only NHE1 and NHE8 activity was detected at functional levels in HT29-MTX cells. The rate of pHi recovery in HT29-MTX cells was 0.169 ± 0.039 pH/min in the absence of HOE-694 and 0.038 ± 0.008 pH/min in the presence of 1 μM HOE-694. HT29-MTX cells failed to recover from acid load in the presence of 10 μM HOE-694 (Fig. 1C).
Effect of TNF-α treatment on NHE8 protein expression in HT29-MTX cells.
NHE8 protein expression in HT29-MTX cells after exposure to standard or TNF-α-containing medium was assessed by Western blot. As shown in Fig. 2, TNF-α treatment reduced NHE8 protein abundance in HT29-MTX cells, and this inhibition is dose-dependent. TNF-α concentration at <10 ng/ml had no effect on NHE8 protein abundance in HT29-MTX cells (0.175 ± 0.004 in TNF-α-treated cells compared with 0.185 ± 0.004 in control cells). When the concentration of TNF-α was 30 ng/ml, NHE8 protein expression was reduced from 0.185 ± 0.004 in control cells to 0.12 ± 0.07 in TNF-α-treated cells. At 100 ng/ml TNF-α, NHE8 protein expression was also reduced, from 0.185 ± 0.004 in untreated cells to 0.115 ± 0.011 in treated cells.
Fig. 2.
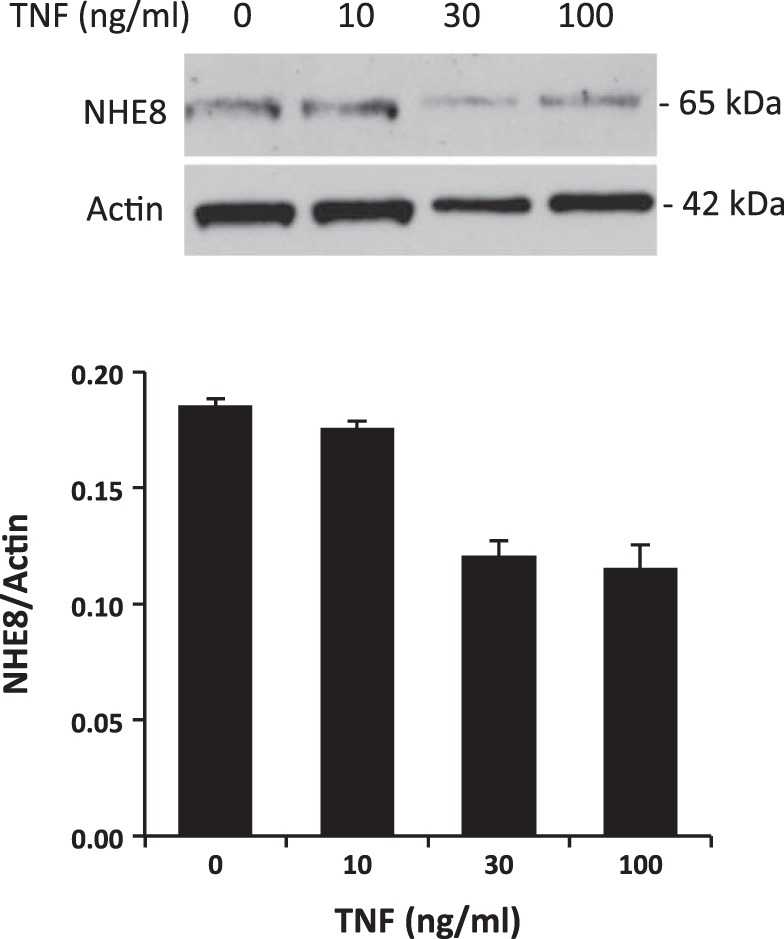
Dose-dependent effect of TNF-α on NHE8 protein expression in goblet cells. HT29-MTX cells were treated with different concentrations of TNF-α for 18 h. Total protein was then isolated and used for Western blot detection. Top, representative Western blot image. Bottom, summary data (means ± SE) from 2 separate experiments.
Effect of TNF-α treatment on NHE8 mRNA expression in HT29-MTX cells.
RNA was purified from cells exposed to standard or TNF-α-containing medium (30 ng/ml TNF-α) and was used for Real-time PCR analysis. As shown in Fig. 3A, NHE8 mRNA expression in HT29-MTX cells was significantly decreased by TNF-α treatment (1.00 ± 0.03 in control cells compared with 0.46 ± 0.09 in TNF-α-treated cells; n = 6, P = 0.009). In the presence of 100 nM actinomycin D (Act D), TNF-α-induced NHE8 mRNA expression inhibition was completely blocked (0.45 ± 0.03 in Act D-treated cells compared with 0.47 ± 0.01 in Act D- and TNF-α-treated cells; n = 3) (Fig. 3B).
Fig. 3.
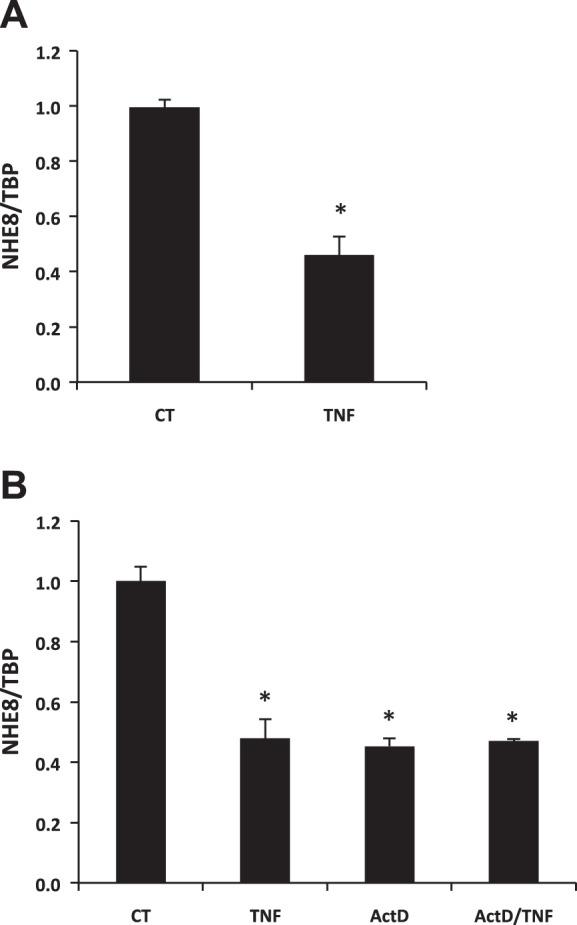
Effect of TNF-α on NHE8 mRNA expression in goblet cells. A: real-time PCR data from HT29-MTX cells treated with TNF-α (30 ng/ml). Cells were treated with control medium or TNF-α-containing medium for 18 h. Total RNA was isolated from cells and used for Real-time PCR. Data are presented as means ± SE from 6 separate experiments. *P < 0.05 for control cells vs. TNF-α-treated cells. B: real-time PCR data from HT29-MTX cells treated with TNF-α (30 ng/ml) in the presence of actinomycin D. Cells were treated with control medium or TNF-α-containing medium in the presence or absence of actinomycin D for 18 h. Total RNA was isolated from cells and used for Real-time PCR. Results are means ± SE from 3 separate experiments. *P < 0.01 for control cells (CT) vs. TNF-α treated cells (TNF), actinomycin D-treated cells (ActD), TNF-α- and actinomycin D-treated cells (ActD/TNF).
Effect of TNF-α treatment on NHE8 gene promoter activity.
To explore if the human NHE8 gene promoter activity is affected by TNF-α, HT29-MTX cells were first transfected with NHE8 gene promoter constructs and then treated with TNF-α (30 ng/ml) for 18 h before promoter activity was studied. The activity of human NHE8 promoter in HT29-MTX cells was decreased in the presence of TNF-α. TNF-α treatment reduced pGL3b/−671 promoter activity from 1.02 ± 0.02 in control cells to 0.61 ± 0.06 in treated cells (n = 6, P = 0.0004). TNF-α treatment also reduced pGL3b/−32 promoter activity from 1.00 ± 0.02 in control cells to 0.58 ± 0.11 in treated cells (n = 6, P = 0.008) (Fig. 4).
Fig. 4.
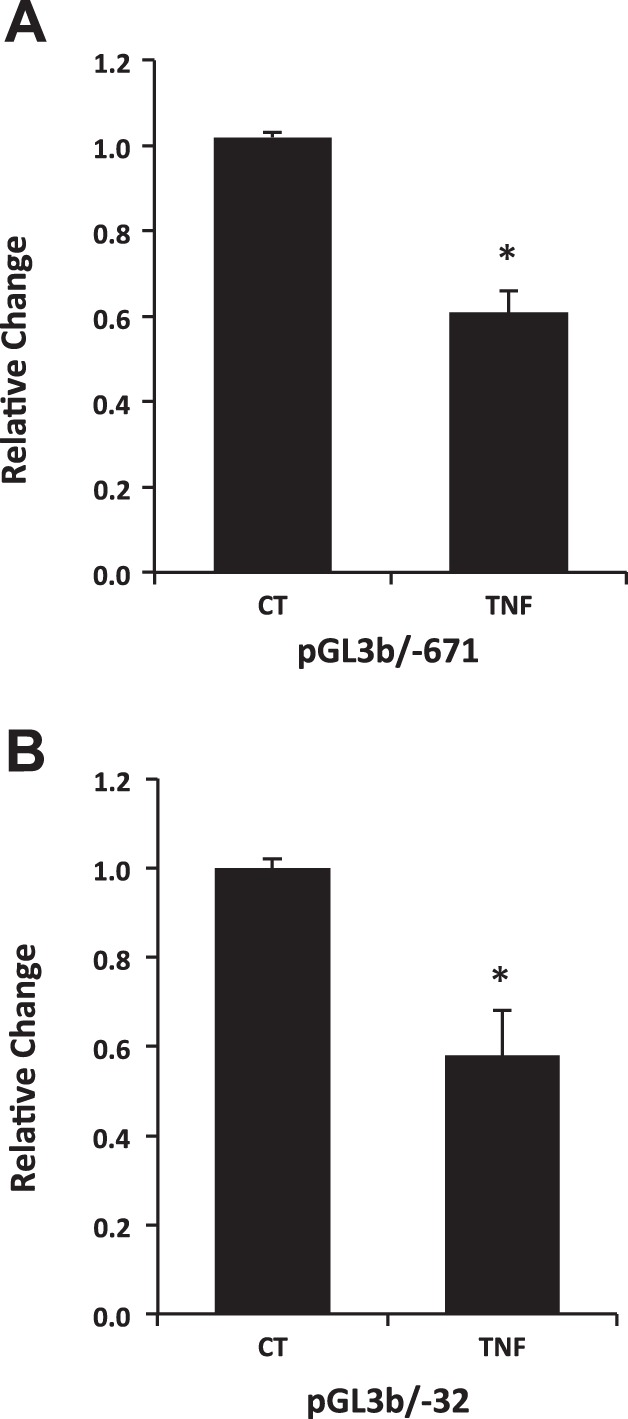
Localization of the TNF-α response region on human NHE8 gene promoter in goblet cells. HT29-MTX cells were transfected with human NHE8 promoter constructs pGL3B/671 (A) and pGL3B/32 (B). TNF-α were then applied 18 h before measuring promoter activities. The degree of inhibition is shown as the ratio of luciferase activity in TNF-α-treated cells over luciferase activity in nontreated cells. Results are means ± SE from 4 separate experiments. *P < 0.01 for control cells vs. TNF-α treated cells.
Identification of trans-factor and cis-element involved in TNF-α response of the human NHE8 promoter in HT29-MTX cells.
GMSA was used to study the DNA/protein interaction involved in TNF-α-mediated NHE8 regulation. Promoter assay indicated that the shortest human NHE8 gene promoter construct, pGL3b/−32, was responsive to TNF-α treatment. Thus we focused on determining the DNA/protein interaction at this promoter region. Previous studies showed that pGL3b/−32 contains a GC-box and that this region recruits Sp3 protein to activate basal NHE8 gene transcription (24). We tested whether TNF-α-mediated NHE8 transcription inhibition in HT29-MTX cells was due to an altered interaction in nuclear protein binding at this DNA region. As shown in Fig. 5A, TNF-α treatment reduced DNA-protein interaction at the basal promoter region of the human NHE8 gene, and this interaction could be blocked by GC-box consensus DNA oligos. Supershift experiments indicated that this DNA/protein complex could be further shifted by Sp3 antibody in nuclear protein isolated from control and TNF-α-treated HT29-MTX cells (Fig. 5B).
Fig. 5.
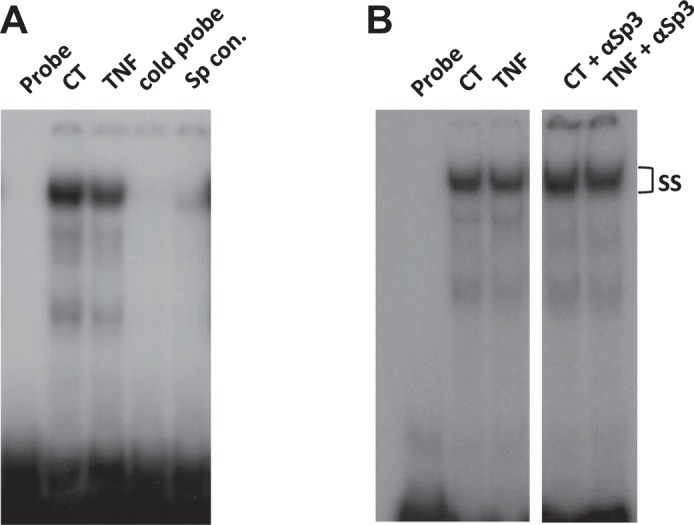
Identification of DNA/protein interaction involved in TNF-α regulation at the human NHE8 gene promoter by gel mobility shift assay (GMSAs). Oligo DNA was end labeled with [γ-32P]ATP and used as a probe in GMSAs. Nuclear protein was isolated from control cells or TNF-α-treated cells. GMSAs (A) and supershift (B) were performed as indicated in materials and methods. Unlabeled probe (cold probe) and GC-box consensus oligo (Sp Cons.) were used to compete Sp protein family binding. Sp3 antibodies (αSp3) were used for supershift experiments in the presence of labeled probe. ss, Supershift complex.
DISCUSSION
The diverse secretory and absorptive function of the intestinal epithelium is constructed by a mixed population of predominantly absorptive cells displaying a brush-border membrane and mucin-producing goblet cells (22). Goblet cells form a significant component of the gastrointestinal tract, comprising ∼10% of the small intestinal epithelium and 24% of the total epithelial cell population in the distal colon (7). The main role of goblet cells is to secrete mucin to protect the mucous membranes where they are found. Mucins are large glycoproteins formed mostly by carbohydrates. Their glycans (bound carbohydrates) attract relatively large quantities of water to produce gel-like properties of mucins (12). On the inner surface of the intestine, hydrated mucin forms a thick layer that lubricates and protects the wall of the organ (11). Distinct forms of mucin are produced in different organs. While Muc2 is prevalent in the intestine, Muc5AC and Muc5B are the main forms found in the airway and conjunctiva in the eye (9, 19). Mucins are stored in granules inside the goblet cells before being released to the lumen of the organ (12). Their secretion may be stimulated by irritants such as microbes, dust, and smoke (9).
Because loss of NHE8 expression results in reduced mucin production in the intestine and in the conjunctiva (31, 32), we wondered if NHE8 is expressed in the goblet cells and, if NHE8 is expressed there, where it is located in the goblet cells. To address this question, we chose HT29-MTX cells as an in vitro model. HT29-MTX cells are mucin-secreting cells (13) and are widely used to study mucin secretion and bacterial adhesion (6, 14, 21). We first identified what kind of NHE isoforms are expressed in goblet cells using Real-time PCR. Our data showed that mRNA of NHE2 and NHE8 but not NHE3 could be detected in HT29-MTX cells. The expression of NHE8 in the mucin-secreting cells is 1.6-fold higher than in Caco-2 cells. Although PCR detected NHE2 mRNA signals, the expression level of NHE2 in HT29-MTX cells was very low (<10% of Caco-2 cells). Using the pHi measurement method, we were able to identify the functional NHEs in HT29-MTX cells. In the presence of 1 μM HOE-694, a concentration inhibiting NHE1 activity, HT29-MTX cells retained ∼19% of NHE activity, which indicates functional NHE8 expression. Because pHi recovery after acid load was completely blocked by 10 μM HOE-694, the NHE activity detected in the presence of 1 μM HOE-694 was indeed NHE8. These observations suggest that the only functional NHEs in HT29-MTX cells are NHE1 and NHE8.
Furthermore, immunohistochemical stain detected NHE8 protein in HT29-MTX cells as well as in the epithelial cells and goblet cells of mouse colonic tissue sections. Interestingly, NHE8 has different localization in goblet cells compared with enterocytes and Leydig cells. In enterocytes, NHE8 is located at the plasma membrane in the enterocytes (23, 27). In Leydig cells, NHE8 is located only in the intracellular compartments (26). However, in goblet cells, NHE8 was detected in the plasma membrane and intracellular compartments. The varied NHE8 protein localization suggests different roles of NHE8 in different cells. Whereas NHE8 mediates NHE in enterocytes, NHE8 has a role in luteinizing hormone receptor trafficking in Leydig cells (26). Because NHE8 protein is also detected inside the goblet cells, the role of NHE8 in goblet cells is likely more than mediating the sodium/hydrogen exchange.
The effect of NHE8 deficiency on mucin expression strongly supports an important role of NHE8 in intestinal mucosal protection (15, 29, 31, 32). Loss of NHE8 expression in the intestine resulted in decreased Muc2 expression, increased bacterial adhesion, and elevated inflammatory cytokine expression. The elevated proinflammatory cytokine expression creates a never-ending cycle. TNF-α is a potent proinflammatory cytokine, and it affects many functions of the epithelial cells in the intestine, such as inhibiting NHE3 and NHE8 expression (2, 24), modulating mucin production (5, 21), and damaging tight junctions (2, 16). Because we previously showed that intestinal inflammation and TNF-α exposure reduced NHE8 expression in the intestine and in Caco-2 cells, we wondered if NHE8 expression in the goblet cells is also subject to regulation by the proinflammatory cytokine TNF-α.
Our data showed that NHE8 protein expression was inhibited by TNF-α in HT29-MTX cells and that the inhibition was concentration-dependent. Although a higher concentration of TNF-α was required to inhibit NHE8 expression in goblet cells compared with Caco-2 cells (30 vs. 10 ng/ml) (24), these results suggest that TNF-α impairs NHE8 function by inhibiting NHE8 protein expression in the goblet cells. Further study confirmed that the NHE8 mRNA level was also decreased in HT29-MTX cells after exposure to TNF-α. This inhibition could be blocked by Act D, which suggests that TNF-α-mediated reduction of NHE8 protein was the result of reduced NHE8 mRNA expression in goblet cells. Promoter reporter assay indicated that TNF-α treatment resulted in significant decrease in human NHE8 gene promoter reporter activity in HT29-MTX cells, so TNF-α indeed regulated NHE8 gene expression by inhibiting NHE8 gene promoter activity. Because the pGL3/−32 promoter construct contains the minimal promoter sequence required for basal NHE8 gene expression activation and TNF-α treatment suppressed this promoter construct activity in Caco-2 cells (24), the effect of TNF-α on NHE8 gene expression in HT29-MTX cells was most likely mediated by decreasing basal NHE8 gene transcription.
Sp3 has been shown to be an essential transcriptional factor to activate the basal NHE8 gene promoter in Caco-2 cells (24). Therefore, we focused on Sp3 binding as a possible transcriptional factor involved in NHE8 regulation in goblet cells. Because TNF-α also inhibited NHE8 basal promoter activity in HT29-MTX cells, we suspected that the Sp3-binding region might also be involved. GMSA results showed decreased DNA-protein interaction at the human NHE8 proximal promoter region (−18 bp/+7 bp) in HT29-MTX cells treated with TNF-α. Supershift studies confirmed that Sp3 protein was bound on this DNA sequence in the human NHE8 proximal promoter. These observations suggested that Sp3 protein is the key transcription factor that regulates NHE8 gene expression in HT29-MTX cells in response to TNF-α. Our results on Sp3 strongly support the conclusion that TNF-α is a potent regulator for NHE8 expression during inflammation through its effect on both enterocytes and goblet cells in the intestine. Because Sp3 protein is required for basal NHE8 transcriptional activation in the epithelial cells (24), and increased Sp3 nuclear translocation is observed in butyrate-stimulated NHE8 expression (30), it is possible that TNF-α could affect Sp3 nuclear translocation in the goblet cells.
In summary, we showed for the first time that NHE8 is highly expressed in intestinal goblet cells. We also demonstrated that TNF-α could inhibit NHE8 expression by reducing NHE8 gene promoter activation in HT29-MTX cells. Specifically, TNF-α inhibits Sp3 binding on the NHE8 gene promoter. This work adds new insight on understanding the role of TNF-α on NHE8 expression in the intestinal mucosal protection.
GRANTS
This investigation was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-073638.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.X. and F.K.G. conception and design of research; H.X., Q.L., Y.Z., and J.L. performed experiments; H.X., Q.L., Y.Z., and J.L. analyzed data; H.X., Q.L., and J.L. interpreted results of experiments; H.X., Q.L., and Y.Z. prepared figures; H.X. drafted manuscript; H.X. and F.K.G. edited and revised manuscript; H.X. and F.K.G. approved final version of manuscript.
REFERENCES
- 1.Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med 50: 511–515, 2000. [PubMed] [Google Scholar]
- 2.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Ontogeny of basolateral membrane sodium-hydrogen exchange (NHE) activity and mRNA expression of NHE-1 and NHE-4 in rat kidney and jejunum. Biochim Biophys Acta 1369: 247–258, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Dharmani P, Leung P, Chadee K. Tumor necrosis factor-alpha and Muc2 mucin play major roles in disease onset and progression in dextran sodium sulphate-induced colitis. PLoS One 6: e25058, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fergie N, Guo L, Pearson JP, Birchall JP. The influence of prednisolone on the secretion of MUC5AC from TH29-MTX cell culture. Clin Otolaryngol Allied Sci 25: 570–576, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Forstner JFF. Gastrointestinal Mucus. New York, NY: Raven, 1994, p. 1255–1283. [Google Scholar]
- 8.Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem 280: 12781–12789, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Guzman-Aranguez A, Argueso P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul Surf 8: 8–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Johansson ME, Hansson GC. Mucus and the goblet cell. Dig Dis 31: 305–309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10: 352–361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res 50: 6334–6343, 1990. [PubMed] [Google Scholar]
- 14.Lievin-Le Moal V, Huet G, Aubert JP, Bara J, Forgue-Lafitte ME, Servin AL, Coconnier MH. Activation of mucin exocytosis and upregulation of MUC genes in polarized human intestinal mucin-secreting cells by the thiol-activated exotoxin listeriolysin O. Cell Microbiol 4: 515–529, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol 305: C121–C128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 286: G367–G376, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447: 549–565, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331–9339, 1992. [PubMed] [Google Scholar]
- 19.Rubin BK. Secretion properties, clearance, and therapy in airway disease (Abstract). Transl Respir Med 2: 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Smirnova MG, Birchall JP, Pearson JP. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine 12: 1732–1736, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol Cell Physiol 260: C183–C193, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Xu H, Chen H, Li J, Zhang B, Tang C, Ghishan FK. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am J Physiol Cell Physiol 300: C375–C382, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Chen H, Li J, Zhao Y, Ghishan FK. Disruption of NHE8 expression impairs Leydig cell function in the testes. Am J Physiol Cell Physiol 308: C330–C338, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Inouye M, Hines ER, Collins JF, Ghishan FK. Transcriptional regulation of the human NaPi-IIb cotransporter by EGF in Caco-2 cells involves c-myb. Am J Physiol Cell Physiol 284: C1262–C1271, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol 304: G257–G261, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, McCoy A, Li J, Zhao Y, Ghishan FK. Sodium butyrate stimulates NHE8 expression via its role on activating NHE8 basal promoter activity. Am J Physiol Gastrointest Liver Physiol 309: G500–G505, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Zhao Y, Li J, Wang M, Lian F, Gao M, Ghishan FK. Loss of NHE8 expression impairs ocular surface function in mice. Am J Physiol Cell Physiol 308: C79–C87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]