Abstract
We tested the efficacy of attractive toxic sugar bait (ATSB) with garlic oil microencapsulated in beta-cyclodextrin as active ingredient against Aedes albopictus in suburban Haifa, Israel. Two three-acre gardens with high numbers of Ae. albopictus were selected for perimeter spray treatment with ATSB and ASB (bait containing no active ingredient). Baits were colored with food dye to verify feeding of the mosquitoes. The mosquito population was monitored by human landing catches and sweep net catches in the surrounding vegetation. Experiments lasted for 44 days. Treatment occurred on day 13. The mosquito population collapsed about 4 days after treatment and continued to drop steadily for 27 days until the end of the study. At the experimental site the average pre-treatment landing rate was 17.2 per 5 mins. Two days post-treatment, the landing rate dropped to 11.4, and continued to drop to an average of 2.6 during the following 26 days. During the same period, the control population was stable. Few sugar fed females (8–10%) approached a human bait and anthrone tests showed relatively small amounts of sugar within their crop/gut. Around 60–70 % of males caught near our human bait were sugar positive which may indicate that the males were feeding on sugar for mating related behavior. From the vegetation treated with the toxic bait, we recovered significantly fewer (about 10–14%) males and females stained by ATSB than at the ASB-treated control. This may indicate that the toxic baits alter the resting behavior of the poisoned mosquitoes within the vegetation. Almost no Ae. albopictus females (5.2 ± 1.4) approached human bait after treatment with ATSB. It therefore appears that microencapsulated garlic oil is an effective pesticide against Ae. albopictus when used in an ATSB system.
Keywords: Culicidae, Aedes albopictus, Sugar feeding, ATSB, Mosquito control, Israel
1. Introduction
A novel method for controlling mosquitoes, attractive toxic sugar baits (ATSB), was developed and extensively tested in Israel (Müller and Schlein, 2006, 2008; Müller at al., 2008, 2010c). This method is based on the requirement for male and female mosquitoes to consume plant-derived sugars for survival (Yuval, 1992; Foster, 1995). A “attract and kill” approach has been developed that uses fruit or flower scent as an attractant, a sugar solution that acts as a feeding stimulant, and an oral toxin to kill the targeted insects. A solution of ATSB can either be sprayed on vegetation or suspended in portable bait stations where the insects ingest the toxic solutions and are killed.
Since its inception, ATSB has continually been improved and optimized through testing with various oral toxins (Xue et al., 2008, 2011; Khallaayoune et al., 2013; Qualls et al., 2014) and attractants (Müller et al., 2010a,c). Initially, toxic sugar baits consisted strictly of an oral insecticide and sugar with the addition of food dye for marking feeding mosquitoes. Field studies in Israel by Müller and Schlein (2006) and Schlein and Müller (2008) reported that ATSB's sprayed on highly attractive plant blossoms in the field virtually eliminated local mosquito populations. Later formulations included fruit-based attractants (such as wine with overripe/fermented nectarine, honey melon, or guava juice) for spray application on non-attractive vegetation (Müller et al., 2010a,c) and use in portable bait stations (Müller and Schlein, 2008; Müller et al., 2008, 2010b). Additional laboratory studies have shown that several insecticides from different classes of chemistry have potential for use in the ATSB system (Allan, 2011). Low-risk compounds such as spinosad, boric acid, and the neonicotinoid dinotefuran were found to be highly effective against several anopheline and culicine as well as Aedes species (Müller and Schlein, 2008; Schlein and Müller, 2008; Beier et al., 2012; Revay et al., 2014; Qualls et al., 2014).
Ideally, commercialized ATSB for widespread use should be a safe alternative to traditional insecticides by using compounds with the lowest risk to mammals as active ingredients, yet must be effective and economical. Many essential oils from various plant species have been reported to possess ovicidal, larvicidal, and repellent properties against numerous insect species and are regarded as “environmentally friendly pesticides” (Isman, 2000; Cetin et al., 2004). Of these, essential oil of garlic has been shown to possess strong insecticidal properties against Chinese pear pests, nasal bot-flies of camels and mosquito larvae (Zhao et al., 2013; Khater, 2014; Kimbaris et al., 2009).
In this study, we test the efficacy of garlic oil microencapsulated in beta-cyclodextrin, a material exempt from registration with the United States Environmental Protection Agency due to its low toxicity to mammals (U.S. EPA, 2015), as the insecticide in a new commercial formulation of ATSB against pest populations of Aedes albopictus in Haifa, Israel. Ae. albopictus is an invasive species native to Southeast Asia that spread to Europe, Africa, the Middle East, and the Americas during the 20th century and continues to spread today (Benedict et al., 2007). It is the vector for many arboviruses such as dengue, chikungunya, West Nile, and eastern equine encephalitis (Gratz, 2004) and transmits Dirofilaria immitis (heartworm) in dogs (Pietrobelli, 2008). Ae. albopictus is difficult to control because of the widespread availability of suitable natural and artificial sites for larval development (Hawley, 1988). Larvae are capable of development in almost any water-holding receptacle in rural, suburban and urban environments (Hawley, 1988).
2. Materials and methods
2.1. Study site and conditions
Experiments were conducted in urban Haifa from September 22 to November 02, 2013. The local climate is temperate Mediterranean and most of the annual rainfall of 500–700 mm occurs in winter between November and February. The dry season is from June to August. The highest average temperatures (30 °C) are reached in July/August and the lowest (9 °C) are reached January/February (Ashbel, 1951; Orni and Efrat, 1980). During the study period, light rain (<0.25 cm) occurred on October 05, 18, 19, 30, 31 and November 01.
The experimental and control sites each consisted of three-acre plots of garden vegetation containing high numbers of Ae. albopictus as determined by initial sampling using human landing rates (methods mentioned in a later section of this paper) prior to application. The control site was located 800 m from the experimental site. Both sites were surrounded by similar non-irrigated gardens and parkland. At the time of the study, only about 10% of the vegetation was flowering. The majority of the vegetation consisted of herbaceous plants with green leaves.
2.2. ASB and ATSB formulation and application
At the experimental site we applied ATSB while at the control site ASB (attractive sugar bait) was applied. The ATSB formulation (Westham Innovations LTD, Tel Aviv, Israel) consists of 0.4% beta-cyclodextrin microencapsulated garlic oil and 99.6% mixture of date syrup, citrus juice, sucrose and water (US Patent No. 8420070). The food dye used in the ATSB solution was Tartrazine 19140 “special green” (Stern, Netanya, Israel). The ASB solution contained 100% of the same mixture of date syrup, citrus juice, sucrose and water used in the ATSB solution but no active ingredient was added. The food dye used in the ASB solution was E132, Indigotine “Food Blue No. 1” (Stern, Netanya, Israel). Mixtures were applied with a 16 l back-pack sprayer (Killaspray, Model 4526, Hozelock, Birmingham UK) as a perimeter treatment which is the application of ASB or ATSB solution to vegetation in a continuous band approximately 1.5 feet (45.7 cm) wide between 1.0 and 5.0 feet (30.5 and 152.4 cm) above the ground. The mixture was applied according to the manufacturer's (Westham Innovations LTD, Tel Aviv, Israel) instructions at a rate of 15–20 oz (443.6–591.5 ml) per 100 linear feet (30.5 M), to the point of runoff.
2.3. Monitoring the mosquito population
The effect of ATSB and ASB on the mosquito population was monitored by human landing catches conducted every second day for the first 11 days of the experimental period and every third day after bait application (from day 15 to day 44 of the trial). Mosquitoes attempting to land on the legs of human baits were collected with a Power Vac Back-Pack unit (John W. Hock, Gainesville, FL, USA) before they could settle down and probe/bite, in 5 min intervals from 15:00 to 16:30. The number of mosquitoes collected while landing during these intervals was used to define the “landing rate” at the sites. At both sites there were 9 repetitions of the landing catch experiments on each monitoring day. Mosquitoes within collecting containers were anesthetized with ethyl acetate-soaked cotton swabs and kept in a cooling bag before they were processed immediately or frozen at −70 °C.
Four authors of this study, 2 males and 2 females served as the volunteer subjects and were therefore fully informed of the nature and purposes of the test and of any physical and mental health consequences resulting from mosquito bites which were reasonably foreseeable.
Human landing catch trials were conducted according to U.S. EPA guidelines (U.S. EPA, 2000) as follows: exposed legs (from knee to ankle) of each volunteer were used as a test area. The skin outside the test area was covered with regular clothes to protect from mosquito bites. Volunteers wore short trousers and long-sleeved shirts. Immediately before each trial, the exposed skin on each volunteer was cleaned with 70% isopropyl alcohol. Volunteers were advised to avoid alcohol, caffeine, and fragrance products (e.g., perfume, cologne, hair spray, lotion, etc.) during the entire experimental period.
Mosquitoes were also collected from the vegetation surrounding the treatment and control areas, between 7:00 and 9:00 AM, with a Power Vac and a hand sweep net consisting of strong gauze net, fixed on a heavy iron frame, 50 cm in diameter, with a 1.5 m long handle. Collection from vegetation occurred on 4 pre-treatment days and 5 post-treatment days and was carried out on the day following a human landing catch collection. Using both methods, each catching location was swept with 10 strokes from right to left and vice versa and the mosquitoes caught were pooled to make up the sample. To anesthetize the insects, the net was introduced into a plastic bag that contained a piece of cotton swab soaked with 2 ml ethyl acetate for approximately 2 min. Then the contents of the nets were emptied into a glass funnel of 50 cm diameter placed above a 500 ml beaker. After transportation to the laboratory the beakers were emptied and individual mosquitoes were stored at −70 °C until analyzed for anthrone content and colored bait in the gut.
2.4. Anthrone test for sugar and colored gut content analysis
To understand Ae. albopictus feeding behavior when ASB/ATSB and human baits are present together, we compared the proportion of mosquitoes positive for anthrone versus the proportion positive for food dye color in the gut. Mosquitoes without food dye in their gut were similarly evaluated to detect sugar meals from natural sources.
Gut sugar content was determined by a modified cold anthrone test for sucrose (Schlein and Jacobson, 1994). The reaction solution contained 0.15% anthrone (Sigma, St Louis MO, USA) w/v in 71.7% sulphuric acid. Each mosquito was placed in the well of a flat-bottomed microtiter plate and wetted with 20 μl of 100% ethanol. Aliquots of 200 μl reaction solution were added to the wells and the specimens were crushed with a glass rod that was repeatedly washed with water and wiped. After incubation for 60 min at 25 °C, the crushed mosquitoes were visually examined and meal size was estimated subjectively based on the degree of blue-green coloration (Schlein and Jacobson, 1999). To observe the ingested colored ASB or ATSB in the gut of Ae. albopictus, mosquitoes collected from the field were placed on glass slides and immersed in saline solution with a few drops of detergent and examined under a dissection microscope.
2.5. Statistical analysis
Statistical analysis was carried out using the GraphPad Prism 5.0 statistical package (GraphPad Software, Inc., San Diego, CA, USA). The numbers of female and male mosquitoes caught in the control site versus the treated site were analyzed using the unpaired one-tailed student's t-test (P < 0.05).
3. Results
3.1. Monitoring mosquitoes with human landing catches
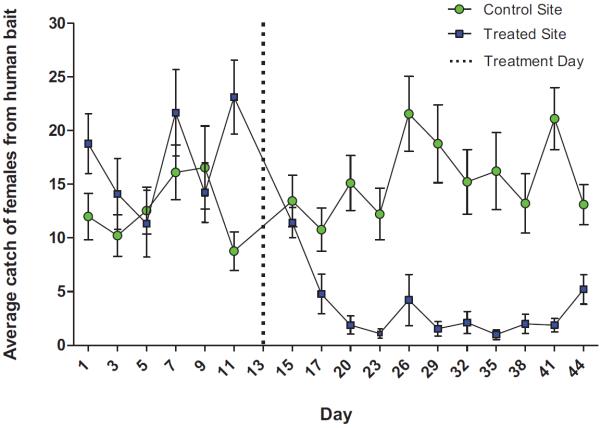
At the ATSB experimental site the average pre-treatment Ae. albopictus landing rate was 17.2 ± 8.7 landings per five minutes (Fig. 1). Two days after treatment the landing rate dropped to 11.4 ± 1.4, and continued to drop to an average of 2.6 ± 3.2 for the following 26 days. This drop in landing was significant (Fig. 1; P < 0.05). At the same time the control population was stable with an average landing rate of 12.7 ± 6.5 landings per five minutes that increased slightly to 13.4 ± 2.4 two days following treatment with ASB. The landing rate remained at an average of 15.7 ± 7.6 for the following 26 days. Moreover, there was no significant change in landing rate at the control site throughout the study period (Fig. 1).
Fig. 1.
Landing rates of Ae. albopictus ± SE on human bait at the control and experimental sites before and after treatment.
3.2. Sugar feeding during human landing catches
Before treatment, no mosquitoes contained color in the gut, as the area had not yet been treated with stained baits. At the ASB control site, 10.0% of host- seeking females and 66.7% of males collected were positive for sugar feeding as indicated by a positive anthrone test (Table 1). A similar result was obtained at the ATSB treated site (pre-treatment) where 8.2% of females and 71.0% of males were positive for sugar.
Table 1.
Ae. albopictus collected from human bait. Sugar feeding on natural sources (verified by Anthrone test) compared to feeding on ASB and ATSB (verified by color stained bait in the gut).
| Pre-treatment control site (ASB) |
Pre-treatment experimental site (ATSB) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col gut | % Col gut | Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col gut | % Col gut |
| 3 | F | 90 | 120 | 15.56 | N/Aa | N/A | 3 | F | 90 | 8 | 8.89 | N/Aa | N/A |
| M | 11 | 7 | 63.64 | N/A | N/A | M | 11 | 5 | 45.46 | N/A | N/A | ||
| 5 | F | 100 | 9 | 9.00 | N/A | N/A | 5 | F | 100 | 11 | 11.00 | N/A | N/A |
| M | 17 | 12 | 70.59 | N/A | N/A | M | 17 | 12 | 70.59 | N/A | N/A | ||
| 9 | F | 120 | 8 | 6.67 | N/A | N/A | 9 | F | 120 | 9 | 7.50 | N/A | N/A |
| M | 15 | 10 | 66.67 | N/A | N/A | M | 15 | 13 | 86.67 | N/A | N/A | ||
| 11 | F | 70 | 7 | 10.00 | N/A | N/A | 11 | F | 70 | 4 | 5.71 | N/A | N/A |
| M | 8 | 5 | 62.50 | N/A | N/A | M | 8 | 6 | 75.00 | N/A | N/A | ||
| Total | F | 380 | 38 | 10.00 | N/A | N/A | Total | F | 380 | 31 | 8.16 | N/A | N/A |
| M | 51 | 34 | 66.67 | N/A | N/A | M | 51 | 36 | 71.00 | N/A | N/A | ||
| Post-treatment control site (ASB) |
Post-treatment experimental site (ATSB) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col gut | % Col gut | Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col gut | % Col gut |
| 17 | F | 90 | 7 | 7.78 | 4 | 4.44 | 17 | F | 40 | 4 | 10.00 | 0 | 0.00 |
| M | 11 | 5 | 45.46 | 5 | 45.46 | M | 2 | 1 | 50.00 | 0 | 0.00 | ||
| 23 | F | 100 | 3 | 3.00 | 5 | 5.00 | 23 | F | 10 | 0 | 0.00 | 1 | 10.00 |
| M | 9 | 3 | 33.33 | 4 | 44.44 | M | 0 | 0 | 0.00 | 0 | 0.00 | ||
| 29 | F | 160 | 5 | 3.13 | 7 | 4.38 | 26 | F | 35 | 5 | 14.29 | 0 | 0.00 |
| M | 20 | 2 | 40.00 | 5 | 25.00 | M | 1 | 0 | 0.00 | 0 | 0.00 | ||
| 35 | F | 130 | 9 | 6.92 | 4 | 3.08 | 35 | F | 8 | 1 | 12.50 | 0 | 0.00 |
| M | 14 | 6 | 42.86 | 4 | 28.57 | M | 0 | 0 | 0.00 | 0 | 0.00 | ||
| 41 | F | 150 | 6 | 4.00 | 6 | 4.00 | 41 | F | 15 | 2 | 13.33 | 0 | 0.00 |
| M | 19 | 3 | 36.84 | 4 | 47.37 | M | 2 | 1 | 50.00 | 0 | 0.00 | ||
| Total | F | 630 | 53 | 8.41 | 26 | 4.13 | Total | F | 108 | 12 | 11.11 | 1 | 0.93 |
| M | 73 | 29 | 39.72 | 22 | 30.13 | M | 5 | 2 | 40.00 | 0 | 0.00 | ||
N/A–not applicable; No ASB or ATSB sprayed pre-treatment.
On day 13, sites were treated with the colored solutions. At the end of the study period, (day 41) color was present in 4.1% of females and 30.1% of males indicating they fed on the ASB (Table 1). During that same time period, a total of 8.4% of ASB control site females were sugar positive while 39.7% of males from this area were sugar positive. This indicated that almost half of all female mosquitoes and nearly all males fed on the ASB despite the presence of natural sugar sources in the area.
At the end of the study period, the experimental site yielded significantly fewer male and female mosquitoes were (P < 0.05; Table 1). Of those females caught 11.1% were positive for sugar and only one of those had dye in the gut. Only five males were caught during this same period, two were positive for sugar and none had dye in the gut from the toxic bait.
3.3. Sugar feeding of mosquitoes in vegetation in the absence of human bait
Before treatment, 55.1% of females and 74.4% of males had fed on natural sugars at the control ASB site (Table 2). During this same period at the experimental site we also found that 62.5% of the females and 69.2% of males had similarly fed on natural sugars.
Table 2.
Ae. albopictus collected in vegetation. Sugar feeding on natural sources verified by Anthrone test compared to feeding on ASB & ATSB verified by color stained bait in the gut.
| Pre-treatment control site (ASB) |
Pre-treatment experimental site (ATSB) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col Gut | % Col Gut | Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col gut | % Col gut |
| 4 | F | 36 | 21 | 58.33 | N/Aa | N/A | 4 | F | 55 | 38 | 69.01 | N/Aa | N/A |
| M | 28 | 18 | 64.29 | N/A | N/A | M | 39 | 29 | 74.36 | N/A | N/A | ||
| 6 | F | 42 | 20 | 47.62 | N/A | N/A | 6 | F | 27 | 15 | 55.56 | N/A | N/A |
| M | 33 | 25 | 75.76 | N/A | N/A | M | 19 | 15 | 78.95 | N/A | N/A | ||
| 10 | F | 45 | 24 | 53.33 | N/A | N/A | 10 | F | 60 | 42 | 70.00 | N/A | N/A |
| M | 29 | 18 | 62.07 | N/A | N/A | M | 48 | 30 | 62.50 | N/A | N/A | ||
| 12 | F | 62 | 37 | 59.67 | N/A | N/A | 12 | F | 82 | 45 | 54.88 | N/A | N/A |
| M | 43 | 38 | 88.37 | N/A | N/A | M | 53 | 36 | 67.92 | N/A | N/A | ||
| Total | F | 185 | 102 | 55.14 | N/A | N/A | Total | F | 224 | 140 | 62.50 | N/A | N/A |
| M | 133 | 99 | 74.43 | N/A | N/A | M | 159 | 110 | 68.18 | N/A | N/A | ||
| Post-treatment control site (ASB) |
Post-treatment experimental site (ATSB) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col Gut | % Col Gut | Day | Sex | # Tested | # Anthrone + | % Anthrone + | # Col gut | % Col gut |
| 18 | F | 40 | 15 | 37.50 | 17 | 42.50 | 18 | F | 44 | 7 | 15.91 | 4 | 9.09 |
| M | 32 | 13 | 40.63 | 8 | 25.00 | M | 19 | 8 | 42.11 | 2 | 10.53 | ||
| 24 | F | 51 | 21 | 41.18 | 15 | 29.41 | 24 | F | 19 | 5 | 26.32 | 1 | 5.26 |
| M | 45 | 18 | 40.00 | 13 | 28.89 | M | 8 | 4 | 50.00 | 1 | 12.50 | ||
| 30 | F | 43 | 16 | 37.12 | 9 | 20.93 | 30 | F | 35 | 15 | 42.86 | 5 | 14.29 |
| M | 29 | 7 | 24.14 | 11 | 37.93 | M | 13 | 7 | 53.84 | 3 | 23.08 | ||
| 36 | F | 35 | 12 | 34.29 | 8 | 22.86 | 36 | F | 15 | 9 | 60.00 | 1 | 6.67 |
| M | 28 | 15 | 53.57 | 5 | 17.86 | M | 7 | 3 | 42.86 | 0 | 0.00 | ||
| 42 | F | 45 | 15 | 33.33 | 10 | 22.22 | 42 | F | 9 | 5 | 55.56 | 2 | 22.22 |
| M | 37 | 8 | 21.62 | 7 | 18.92 | M | 4 | 3 | 75.00 | 1 | 25.00 | ||
| Total | F | 214 | 79 | 36.16 | 59 | 27.57 | Total | F | 122 | 41 | 33.61 | 13 | 10.66 |
| M | 171 | 61 | 35.67 | 44 | 25.73 | M | 51 | 25 | 49.02 | 7 | 13.72 | ||
N/A–not applicable; No ASB or ATSB sprayed pre-treatment.
At the end of the study, in the control area post-ASB treatment, 36.2% of females and 35.7% of males were positive for sugars in their gut (Table 2). Of those females, 27.6% had fed on the ASB while 25.7% of the males had fed on the bait.
At the ATSB treated site, significantly fewer mosquitoes were caught in the surrounding vegetation than at the ASB control site (P < 0.05). About 34% of the females collected had fed on a sugar source with 10.7% of the individuals having fed on the ATSB (Table 2). Nearly 50% of the males had fed on a sugar source while 13.7% of them had fed on the toxic bait, as shown by the presence of dye in the gut.
4. Discussion
In the late 1960s, the toxicity of garlic oil was discovered (Reznik and Imbs, 1965) and later demonstrated against third-instar larvae of several Culex and Aedes mosquito species (Amonkar and Reeves, 1970; Amonkar and Banerji, 1971). More recently, the insecticidal properties of garlic oil have been revisited. It was shown to be an effective pesticide against pear psyllids (Cacopsylla chinensis), nasal botflies (Cephalopina titillator) and ticks (Boophilus annulatus) when adults or larvae were subjected to immersion in the essential oil (Zhao et al., 2013; Khater, 2014; Aboelhadid et al., 2013). When garlic oil was fed to sandflies (Phlebotomus papatasi), on artificial membranes, a 1% solution caused 100% mortality (Valerio and Maroli, 2005).
We have demonstrated for the first time here that U.S. EPA exempt microencapsulated garlic oil, when used as an oral toxin, is effective at controlling adult Ae. albopictus in an ATSB formulation. The public is increasingly wary about conventional pesticides and their potential negative side effects on people and the environment. With access to the internet, a wealth of information on these effects is available to the average consumer. The effects of pesticides on human health depend on the type of pesticide but some, such as organophosphates and carbamates, affect the nervous system while others may be carcinogenic (DiPaolo and Elis, 1967). Some pesticides may simply irritate the skin or eyes while others may affect the hormone or endocrine system (Colborn et al., 1993). It is therefore not surprising that environmentally friendly methods for pest control and low-risk active ingredients, especially if based on food-grade compounds, are in high demand (Ignacimuthu and Jayaraj, 2005).
In our study, the mosquito population collapsed about 4 days after treatment (and continued to drop steadily for 27 days through the end of the study). By coloring the baits, we confirmed that this formulation was highly attractive and mosquitoes fed on the bait about as much as they did on natural sugar sources in the gardens and surrounding parkland.
Relatively few sugar fed females (8–10%) approached the human bait while anthrone tests showed relatively small amounts of sugar within their crops. This is in itself not surprising because Foster (1995) found that recent large sugar meals inhibited blood feeding behavior by competing for space in the digestion system. After sugar digestion, it is assumed that mosquitoes would consequently search for blood meals again. However, we found that the microencapsulated garlic kills relatively slowly and some mosquitoes may survive up to 30 h after ingestion (unpublished data). The almost complete absence of ATSB fed females from human bait collections (1/108) can probably be explained by the fact that initially, the large sugar meals inhibit the quest for blood and some time later the effect of the gut toxin kicks in, resulting in behavioral changes which continue to keep the mosquitoes away from humans before they finally die. Xue et al. (2006) made similar observations in their trials using boric acid baits against Ae. albopictus and Cx. nigripalpus in laboratory and semi-field trials.
Males of several Aedes species, including Ae. albopictus, seek out hosts for the sole purpose of finding females for mating (Jaenson, 1985; Li et al., 2012). The high proportion of sugar positive males (around 60–70 %) caught near our human bait pre-treatment may indicate that males were feeding on sugar for mating related behavior. This number dropped to around 40% post-treatment at both sites. Since there was no toxin in the ASB, the drop at this site suggests that the widely available sugar meal in the form of the ASB spray inhibited the mate seeking behavior of males. At the ATSB site, only five males were caught, two of which were sugar positive, and the drop here may be the result of mortality caused by ingesting the toxin.
The natural sugar and/or sugar bait feeding status of mosquitoes collected from vegetation was very different. A much higher proportion of females were sugar positive, an average of about 55% pre-treatment. After treatment with colored ASB about 36% of the 214 females collected had consumed sugar meals from natural sources and about 28% from the colored bait. The number of males getting sugar from natural sources and ASB were similar to the number of females feeding on these sources.
From the vegetation treated with the toxic bait, we recovered significantly fewer (about 10–14%) males and females stained by ATSB than at the ASB-treated control site. This might indicate that the toxic baits may also change the resting behavior of the poisoned mosquitoes within the vegetation. This is important because the availability of sugar sources in the local environment is a key factor in regulating most aspects of mosquito biology and therefore their vector potential (Gu et al., 2011).
Though heavy rains would probably wash away much of the ATSB formula on top of the leaves while bait on the underside is protected in part, it is worth noting that light rain occurred on 6 days throughout this study period yet the ATSB still reduced the mosquito landing rate more than 6.5 fold. To overcome this problem, the ATSB could be applied to the underside of vegetation and more than once per month during periods of heavy rain or could be used in bait stations with a cover that protects from rain.
Extensive testing with different active ingredients has shown that ASB can be combined with a wide spectrum of toxins from different classes that are orally ingested (Allan, 2011; Müller et al., 2010c; Beier et al., 2012, Qualls et al., 2014). The impact of ATSB using similar low-risk insecticides, such as dinotefuran and boric acid, on non-target organisms was evaluated in several trials and the results suggest very low impact on pollinators and close to no effect on predatory insects (Khallaayoune et al., 2013; Revay et al., 2014; Qualls et al., 2014).
This first successful field study of a commercial ATSB formulation, with garlic oil encapsulated in beta-cyclodextrin as the active ingredient, provides a strong indication that this method could be very effective at controlling vector mosquitoes such as Ae. albopictus. The ATSB method differs from traditional control methods, which focus on indoor-feeding and resting mosquitoes, because it is effective in outdoor habitats, it kills all physiological states of females, and also kills male mosquitoes.
References
- Aboelhadid SM, Kamel AA, Arafa, Shokier WM, KA Effect of Allium sativum and Allium cepa oils on different stages of Boophilus annulatus. Parasitol. Res. 2013;112:1883–1890. doi: 10.1007/s00436-013-3344-0. [DOI] [PubMed] [Google Scholar]
- Allan SA. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. J. Vector Ecol. 2011;36:59–67. doi: 10.1111/j.1948-7134.2011.00141.x. [DOI] [PubMed] [Google Scholar]
- Amonkar SV, Banerji A. Isolation and characterization of the larvicidal principle of garlic. Science. 1971;174:1343–1344. doi: 10.1126/science.174.4016.1343. [DOI] [PubMed] [Google Scholar]
- Amonkar SV, Reeves EL. Mosquito control with active principle of garlic, Allium sativum. J. Econ. Entomol. 1970;63:1172–1175. doi: 10.1093/jee/63.4.1172. [DOI] [PubMed] [Google Scholar]
- Ashbel D. Bio- Climatic Atlas of Israel. Meteorology Department of the Hebrew University; Jerusalem: 1951. p. 151. in Hebrew and English. [Google Scholar]
- Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favored sugar-source blossoms. Malar. J. 2012;11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin H, Erler F, Yanikoglu A. Larvicidal activity of a botanical natural product, AkseBio2, against Culex pipiens. Fitoterapia. 2004;75:724–728. doi: 10.1016/j.fitote.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Colborn T, Vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo JA, Elis J. The comparison of teratogenic and carcinogenic effects of some carbamate compounds. Cancer Res. 1967;27:1696–1701. [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Gu W, Müller GC, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS One. 2011;6(1):e15996. doi: 10.1371/journal.pone.0015996. http://dx.doi.org/10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 1988:1–40. Supplement #1. [PubMed] [Google Scholar]
- Ignacimuthu S, Jayaraj S, editors. Narosa Publishing House Pvt. Ltd.; New Deli, India: 2005. p. 324. [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. [Google Scholar]
- Jaenson TGT. Attraction to mammals of male mosquitoes with special reference to Aedes diantaeus in Sweden. J. Am. Mosq. Control Assoc. 1985;1:195–198. [PubMed] [Google Scholar]
- Khallaayoune K, Qualls W, Revay E, Allan S, Arheart KL, Kravchenko VD, Xue R, Schlein Y, Beier JC, Müller GC. Attractive toxic sugar baits: Control of mosquitoes with the low-risk active ingredient dinotefuran and potential impacts on nontarget organisms in Morocco. Environ. Entomol. 2013;42:1040–1045. doi: 10.1603/EN13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater HF. Bioactivities of some essential oils against the camel nasal botfly, Cephalopina titillator. Parasitol. Res. 2014;113:593–605. doi: 10.1007/s00436-013-3688-5. [DOI] [PubMed] [Google Scholar]
- Kimbaris AC, Kioulos E, Koliopoulos G, Polissiou MG, Michaelakis A. Coactivity of sulfide ingredients: a new perspective of the larvicidal activity of garlic essential oil against mosquitoes. Pest Manage. Sci. 2009;65:249–254. doi: 10.1002/ps.1678. [DOI] [PubMed] [Google Scholar]
- Li C, Yan T, Dong Y, Zhao T. Identification and quantitative analysis of genes encoding odorant binding proteins in Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2012;49:573–580. doi: 10.1603/me11239. [DOI] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Sugar questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int. J. Parasitol. 2006;36:1077–1080. doi: 10.1016/j.ijpara.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Efficacy of toxic sugar baits against adult cistern-dwelling Anopheles claviger. Trans. R. Soc. Trop. Med. Hyg. 2008;102:480–484. doi: 10.1016/j.trstmh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Müller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive, toxic (Spinosad), sugar bait stations in an oasis. J. Am. Mosq. Control Assoc. 2008;24:147–149. doi: 10.2987/8756-971X(2008)24[147:DOASAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malaria J. 2010a;9 doi: 10.1186/1475-2875-9-210. 210, PMID: 20663142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Junnila A, Qualls W, Revay EE, Kline DL, Allan S, Schlein Y, Xue RD. Control of Culex quinquefasciatus in a storm drain system in Florida with attractive toxic sugar baits (ATSB) Med. Vet. Entomol. 2010b;24:346–351. doi: 10.1111/j.1365-2915.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- Müller GC, Junnila A, Schlein Y. Effective control of adult Culex pipiens by spraying an attractive toxic sugar bait solution in the vegetation near larval developmental sites. J. Med. Entomol. 2010c;47:63–66. doi: 10.1603/033.047.0108. [DOI] [PubMed] [Google Scholar]
- Orni E, Efrat E. Geography of Israel. 4th revised ed Israel University Press; Jerusalem: 1980. p. 455. [Google Scholar]
- Pietrobelli M. Importance of Aedes albopictus in veterinary medicine. Parassitologia. 2008;50:113–115. [PubMed] [Google Scholar]
- Qualls WA, Müller GC, Revay EE, Allan SA, Arheart KL, Beier JC, Smith ML, Scott JM, Kravchenko VD, Hausmann A, Yefremova ZA, Xue RD. Evaluation of attractive toxic sugar bait (ATSB)- barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta Trop. 2014;131:104–110. doi: 10.1016/j.actatropica.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revay EE, Müller GC, Qualls WA, Kline DL, Naranjo DP, Arheart KL, Kravchenko VD, Yefremova Z, Hausmann A, Beier JC, Schlein Y, Xue R. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014;113:73–79. doi: 10.1007/s00436-013-3628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik PA, Imbs YG. Ixodid ticks and phytoncides. Zool. Zh. 1965;44:1861–1864. In Russian. [Google Scholar]
- Schlein Y, Jacobson RL. Mortality of Leishmania major in Phlebotomus papatasi caused by plant feeding of the sand flies. Am. J. Trop. Med. Hyg. 1994;50:20–27. [PubMed] [Google Scholar]
- Schlein Y, Jacobson RL. Sugar meals and longevity of the sandfly Phlebotomus papatasi in an arid focus of Leishmania major in the Jordan Valley. Med. Vet. Entomol. 1999;13:65–71. doi: 10.1046/j.1365-2915.1999.00138.x. [DOI] [PubMed] [Google Scholar]
- Schlein Y, Müller GC. An approach to mosquito control: using the dominant attraction of flowering Tamarix jordanis trees against Culex pipiens. J. Med. Entomol. 2008;45:384–390. doi: 10.1603/0022-2585(2008)45[384:aatmcu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (United States Environmental Protection Agency) [Accessed Aug. 30, 2014];Sets of scientific issues being considered by the Environmental Protection Agency regarding: session I—implementation plan for probabilistic ecological assessment: a consultation; session II—insect repellent product performance testing guideline evaluation [Internet] 2000 Available from the Environmental Protection Agency, Arlington, VA. http://www.epa.gov/oscpmont/sap/meetings/2000/april/freportapril572000.pdf.
- U.S. EPA (United States Environmental Protection Agency) [Accessed Aug. 30, 2015];Active Ingredients Allowed in Minimum Risk Pesticide Products. 2015 http://www2 epa.gov/minimum-risk-pesticides/active-ingredients-allowed-minimum-risk-pesticide-products.
- Valerio L, Maroli M. Evaluation of repellent and antifeeding effect of garlic (Allium sativum) (Aglio Plus Sinergix®) against the bite of phlebotimine sand flies (Diptera: Psychodidae) Ann. 1st Super Sanità. 2005;41:253–256. In Italian. [PubMed] [Google Scholar]
- Xue RD, Kline DL, Ali A, Barnard DR. Application of boric acid baits to plant foliage for adult mosquito control. J. Am. Mosq. Control Assoc. 2006;22:497–500. doi: 10.2987/8756-971X(2006)22[497:AOBABT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Xue RD, Ali A, Kline DL, Barnard DR. Field evaluation of boric acid and fipronil-based bait stations against adult mosquitoes. J. Am. Mosq. Control Assoc. 2008;24:415–418. doi: 10.2987/5683.1. [DOI] [PubMed] [Google Scholar]
- Xue RD, Müller GC, Kline DL, Barnard DR. Effect of application rate and persistence of boric acid sugar baits applied to plants for control of Aedes albopictus. J. Am. Mosq. Control Assoc. 2011;27:56–60. doi: 10.2987/10-6069.1. [DOI] [PubMed] [Google Scholar]
- Yuval B. The other habit: sugar feeding by mosquitoes. Bull. Soc. Vector Ecol. 1992;17:150–156. [Google Scholar]
- Zhao NN, Zhang H, Zhang XC, Luan XB, Zhou C, Liu QZ, Shi WP, Liu ZL. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae) J. Econ. Entomol. 2013;106:1349–1354. doi: 10.1603/ec12191. [DOI] [PubMed] [Google Scholar]