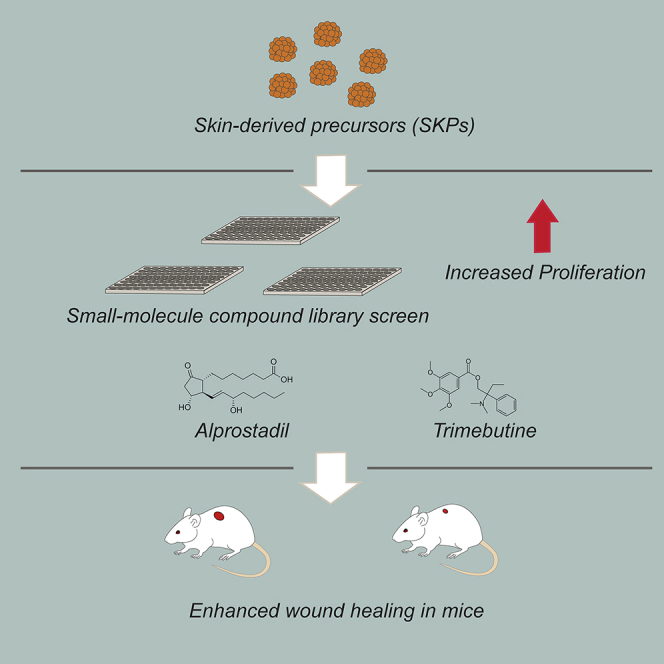
Summary
Here, we asked whether we could identify pharmacological agents that enhance endogenous stem cell function to promote skin repair, focusing on skin-derived precursors (SKPs), a dermal precursor cell population. Libraries of compounds already used in humans were screened for their ability to enhance the self-renewal of human and rodent SKPs. We identified and validated five such compounds, and showed that two of them, alprostadil and trimebutine maleate, enhanced the repair of full thickness skin wounds in middle-aged mice. Moreover, SKPs isolated from drug-treated skin displayed long-term increases in self-renewal when cultured in basal growth medium without drugs. Both alprostadil and trimebutine maleate likely mediated increases in SKP self-renewal by moderate hyperactivation of the MEK-ERK pathway. These findings identify candidates for potential clinical use in human skin repair, and provide support for the idea that pharmacological activation of endogenous tissue precursors represents a viable therapeutic strategy.
Graphical Abstract
Highlights
-
•
Small-molecule screens identify compounds that enhance SKP self-renewal
-
•
Alprostadil and trimebutine maleate both increase SKP self-renewal
-
•
Both compounds likely act by promoting activation of the MEK-ERK pathway
-
•
Both compounds activated dermal precursors in vivo to enhance wound healing
In this article, Kaplan, Miller, and colleagues performed screens to identify drugs already used in humans that enhance proliferation of skin-derived precursor (SKP) cells. They show that two of the compounds they identified, alprostadil and trimebutine maleate, promoted murine skin wound healing, likely by enhancing self-renewal of endogenous dermal precursors via activation of the MEK-ERK signaling pathway.
Introduction
Advances in adult tissue stem cell biology have led to the idea that pharmacological activation of resident stem cells might represent a therapeutic strategy for tissue repair (Miller and Kaplan, 2012). Indeed, pharmacological candidates that regulate tissue stem cells have been identified including, for example, metformin for neural precursors (Wang et al., 2012, Dadwal et al., 2015) and StemRegenin 1 for primary human hematopoietic stem cells (Boitano et al., 2010). Here, we asked whether this is a viable strategy for skin repair. Skin is a complex tissue with many endogenous tissue stem cells. These include epidermal stem cells (Hsu et al., 2014) and a population of dermal stem cells called skin-derived precursors (SKPs) (Toma et al., 2001, Toma et al., 2005). Cultured SKPs clonally generate mesenchymal progeny like dermal fibroblasts and adipocytes, and peripheral neural progeny like Schwann cells, consistent with the finding that they derive from both neural crest and mesodermal origins (Fernandes et al., 2004, McKenzie et al., 2006, Jinno et al., 2010, Krause et al., 2014), like the dermis itself. With regard to their in vivo function, cultured SKPs can clonally reconstitute the dermis and induce hair follicle morphogenesis (Biernaskie et al., 2009), suggesting key roles for the endogenous precursors in dermal maintenance and hair follicle biology.
Here, we have tested the idea that increasing the number and/or self-renewal of endogenous SKPs would enhance skin repair. To do so, we screened libraries of compounds that are used clinically in humans, looking for drugs that enhance SKP self-renewal. We identified two compounds, alprostadil and trimebutine maleate (TM), that increased SKP self-renewal, likely by activating the MEK-ERK pathway. Both compounds enhanced wound healing when applied topically. These findings provide proof of principle for the idea that compounds that regulate SKPs in culture have therapeutic efficacy in vivo, and identify potential drug candidates that can be repositioned for use in humans.
Results
Screens to Identify Compounds that Enhance Human and Rodent SKP Self-Renewal and Proliferation
We performed high-throughput proliferation screens using primary human foreskin SKPs and neonatal rat dorsal SKPs grown as spheres in serum-free growth medium containing 40 ng/ml fibroblast growth factor 2 (FGF2) and 20 ng/ml epidermal growth factor (EGF). This study was approved by the Hospital for Sick Children Animal Care Committee in accordance with CCAC guidelines, and for human tissues and cells, with the approval of the Research Ethics Board of the Hospital for Sick Children. We chose 3,000 human and 5,000 rat SKP cells per well as optimal cell numbers by robotically plating cells in 96-well plates, adding alamarBlue at 30 hr, and assessing its reduction 24 hr later as a measure of cell metabolic activity (Figure S1A) (Smith et al., 2010). We then performed two sets of screens. In one, spheres from four independent human SKP lines (Smith et al., 2010) were dissociated and treated with the Prestwick library of 1,120 compounds (primarily marketed drugs), and the LOPAC-Sigma library of 1,280 compounds with proven biological activity at 1 or 5 μM. In the second, rat or human SKPs were treated with the NIH Clinical Collection library of 446 highly drug-like compounds with known human safety profiles.
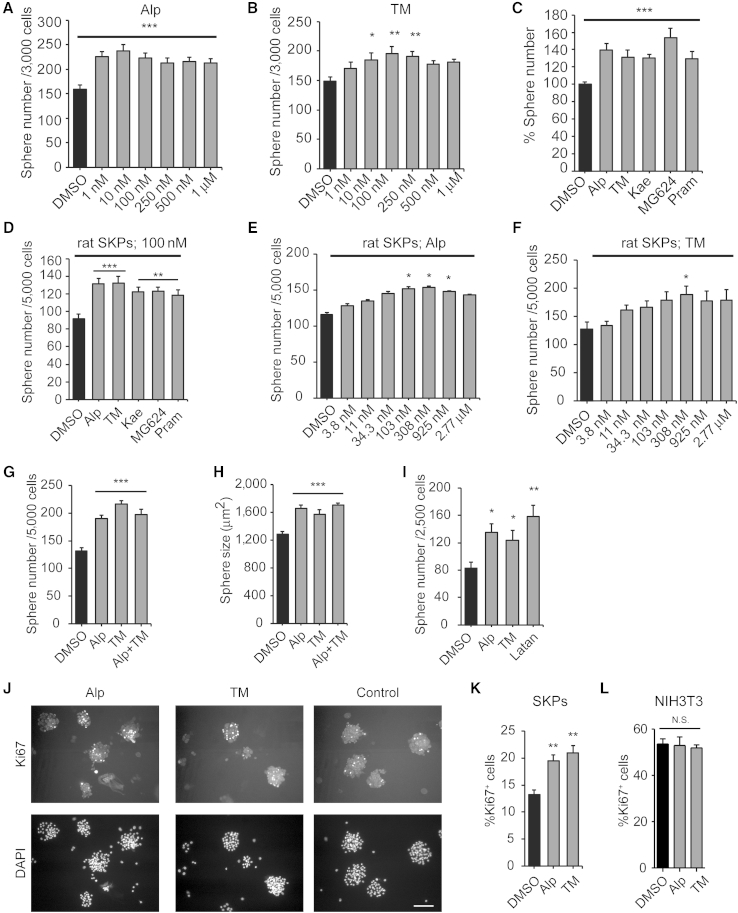
Hits were defined as (1) compounds with signals (B scores) shifted at least three SDs (99.73% confidence interval) from the mean of the general sample population (Figures S1B and S1C), or (2) compounds with a 20% or more increase in alamarBlue values relative to controls in at least three different human SKP lines. For secondary screens, we dissociated human SKPs from at least three independent lines, plated them in growth medium, added drugs on days 1 and 4, and on day 7 quantified spheres as an index of self-renewal. This analysis (Figures 1A and 1B; Figures S1D–S1F) confirmed five of the potential hits: alprostadil (prostaglandin E1 [PGE1]), used for erectile dysfunction (Hanchanale and Eardley, 2014); TM, a weak opioid receptor agonist and spasmolytic (Delvaux and Wingate, 1997); the natural flavonoid kaempferol (Calderón-Montaño et al., 2011); MG-624, an α7-nicotinic acetylcholine receptor antagonist used for postoperative vomiting (Gotti et al., 1998); and pramoxine, a local anesthetic (Fisher, 1998). All compounds have been used in humans, and all but MG-624 have been used topically. Three compounds, alprostadil, TM, and kaempferol, enhanced sphere number at doses as low as 1–10 nM (Figures 1A and 1B; Figure S1D), while others had effects at higher concentrations (Figures S1E and S1F). None of the compounds had toxic effects at concentrations up to 1 μM except for MG-624 (Figure S1E). A direct comparison showed that at 100 nM, all the drugs promoted sphere formation to approximately the same extent (Figure 1C). Similar results were obtained in secondary sphere formation assays with neonatal rat SKPs (Figure 1D).
Figure 1.
Identification of Compounds that Enhance Self-Renewal and Proliferation of Cultured SKPs
(A–C) Number of SKP spheres generated from secondary human SKPs grown for 7 days in varying concentrations of alprostadil (Alp) (A) or TM (B) or in 100 nM alprostadil, TM, kaempferol (Kae), MG-624, or pramoxine (Pram) (C). In (C) numbers are expressed relative to DMSO alone.
(D–F) Number of SKP spheres generated from secondary neonatal rat SKPs grown for 7 days in 100 nM of each of the five drugs (D), or in varying concentrations of alprostadil (E) or TM (F).
(G and H) Number (G) and size (H) of rat SKP spheres generated over 7 days in 100 nM alprostadil, TM, or both.
(I) Number of rat SKP spheres generated in 14-day clonal methylcellulose assays with 100 nM alprostadil, TM, or latanoprost (Latan).
(J and K) Secondary rat SKP spheres were grown for 4 days, and 100 nM alprostadil or TM was added for two additional days. (J) shows spheres immunostained for Ki67 (top) and counterstained with DAPI to show nuclei (bottom). (K) shows the percentage of Ki67-positive cells. Scale bar represents 100 μm.
(L) NIH 3T3 cells were treated for 24 hr with 100 nM alprostadil or TM, immunostained for Ki67, and the percentage of positive cells determined. N.S., not significant.
In all panels, results were pooled from 3 to 4 independent experiments with, in the human experiments, 3–4 different human SKP lines.
Error bars indicate SEM, and in all cases ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA with multiple comparison post hoc tests. See also Figure S1.
We chose alprostadil and TM for more detailed characterization, since (1) they were efficacious in secondary screens, (2) they are used topically in humans, and (3) SKPs express the mRNAs encoding the EP(1–4) prostanoid receptors for alprostadil and the mu and kappa-opioid receptors for TM (data not shown). Initially, we performed dose-response curves with rat SKPs. Sphere numbers increased in a dose-dependent fashion to a maximum at 103–308 nM for alprostadil and 308 nM for TM (Figures 1E and 1F). Second, we added 100 nM of the drugs together or alone in sphere formation assays. Each drug alone increased sphere number and size (the latter a surrogate measure of proliferation), with no further increase with both (Figures 1G and 1H), suggesting that they might act via similar mechanisms. Third, we measured self-renewal using a colony formation assay in which rat SKPs were plated at clonal density (2.5 cells/μl) in medium containing 1.6% methylcellulose. For comparison, we also analyzed latanoprost, a prostaglandin (PGF2) that is bioactive in rodent and human skin (Sasaki et al., 2005). Clonal sphere numbers were significantly increased by all three drugs at 14 days (Figure 1I). Finally, we directly measured proliferation; rat SKPs were cultured for 3–4 days, treated with drugs daily for a further 2 days, and immunostained for the proliferation marker Ki67. Both drugs increased Ki67-positive sphere cells by approximately 60% (Figures 1J and 1K). These drugs are not general mitogens, since Ki67-positive NIH 3T3 cells were unaffected in similar assays (Figure 1L). Thus, alprostadil and TM increase SKP proliferation and self-renewal.
Alprostadil and TM Enhance Skin Repair in Middle-Aged Mice
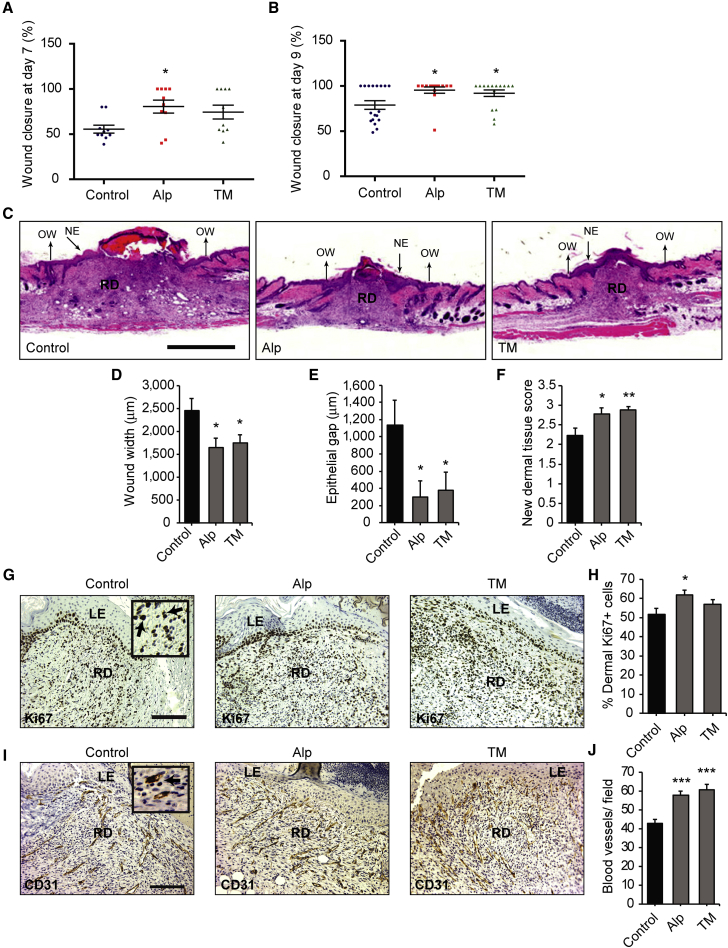
To ask whether alprostadil or TM promoted skin repair, we performed 6-mm diameter full thickness punch wounds on middle-aged (9 months old) mice, as we have previously described (Johnston et al., 2013), and applied the drugs daily for 9 days around the injury site, using 100 μM drug in a propylene glycol-ethanol-water mixture that enables dermal penetration (Tata et al., 1995). Wound closure was significantly accelerated in drug-treated mice. On day 7, almost half of the alprostadil- or TM-treated mice were fully healed, as opposed to none of the vehicle-treated controls (Figure 2A). By day 9, 70%–78% (11 of 14 for alprostadil and 10 of 14 for TM) of drug-treated mice were healed compared with 44% (8 of 18) of the vehicle-treated mice (Figure 2B).
Figure 2.
Topical Alprostadil or TM Promotes Skin Repair in Middle-Aged Mice
(A and B) Scatterplots showing wound closure at 7 days (A) and 9 days (B) after injury for 9-month-old mice treated daily with alprostadil (Alp), TM, or vehicle alone (Control). n = 10 or 14 mice per group, from three independent experiments.
(C–F) H&E-stained sections through the center of the wound bed 9 days after injury (C) were analyzed for wound width (D), epithelial gap (E), and new dermal tissue (F). n = 18, 14, and 17, vehicle-, alprostadil-, and TM-treated mice total, from three independent experiments. NE indicates the new epithelium, RD the regenerating dermis, and OW the borders of the wound. Scale bar represents 1 mm.
(G–J) Sections from the center of the wound bed of mice 7 days after injury were immunostained for Ki67 (G and H) or CD31 (I and J), counterstained with hematoxylin, and analyzed for the percentage of Ki67-positive cells (H) or the relative number of CD31-positive blood vessels (J) at the leading edge of the regenerating dermis. n = at least nine mice per group in (H) and three per group in (J). Insets in (G and I) show higher magnification images, with arrows denoting positive cells (black or dark brown). LE indicates the leading edge of the new epidermis, and RD the regenerating dermis. Scale bar represents 125 μm.
Error bars indicate SEM, and in all cases ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA with multiple comparison post hoc tests except for (E), where comparisons were made by pairwise Student's t tests.
Morphometric analyses of H&E-stained paraffin sections from the central portion of the wound bed on day 9 (Figure 2C) confirmed these results. Wound width (Figure 2D) and epithelial gap (Figure 2E) were both significantly smaller in alprostadil- or TM-treated mice. Dermal tissue regeneration was also enhanced, with a thicker layer of new dermal tissue (Figures 2C and 2F). In alprostadil-treated mice, this coincided with increased Ki67-positive proliferating dermal cells at the leading edge of the newly formed dermis 7 days after injury (Figures 2G and 2H). Treatment with alprostadil or TM also increased CD31-positive blood vessels in the same region (Figures 2I and 2J).
Topical Treatment of Mouse Skin with Alprostadil or TM in Vivo Causes Long-Term Enhancement of the Self-Renewal of SKPs Cultured from Treated Skin in the Absence of Drugs
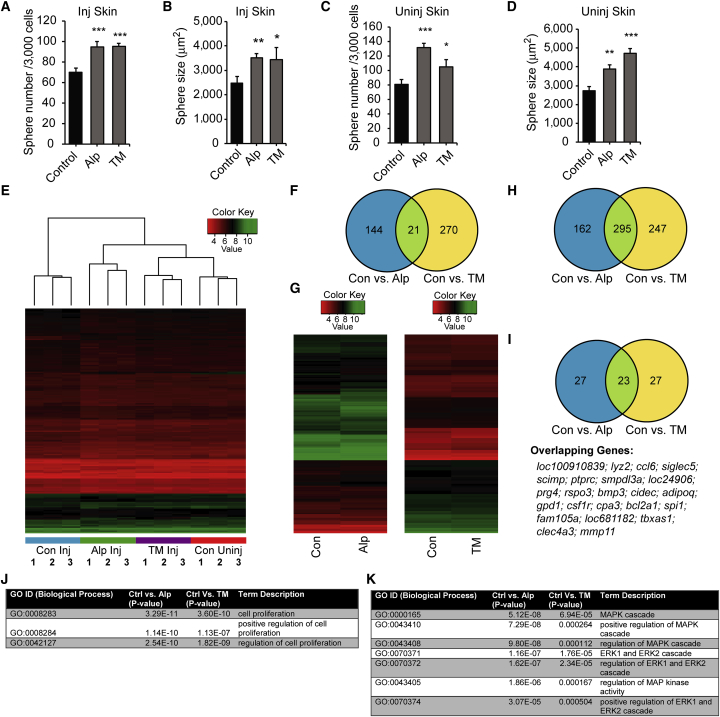
These data indicate that alprostadil and TM enhance SKPs self-renewal in culture and promote skin repair in vivo. To provide a link between these two activities, we performed punch wounds on 9-month-old mice, treated them with alprostadil, TM, or vehicle daily for 7 days, and then cultured SKPs from the regenerating skin without added drugs. For comparison, we analyzed uninjured mice following topical drug application for 7 days. The number and size of secondary SKP spheres were increased when they were isolated from either injured or uninjured drug-treated skin (Figures 3A–3D; Figure S2A), indicating that alprostadil and TM somehow regulated endogenous dermal precursors to affect their long-term self-renewal even when they were isolated and cultured without these drugs.
Figure 3.
Topical Treatment of Mouse Skin with Alprostadil or TM In Vivo Alters the Self-Renewal of SKPs Cultured from the Treated Skin in the Absence of Drug, and Acute Drug Treatment of Cultured SKPs Causes Changes in Gene Expression
(A–D) Primary SKPs were isolated from 9-month-old mice that had received punch wounds (Inj Skin; A and B) or that had intact skin (Uninj Skin; C and D) and that were then treated with topical vehicle (Control), alprostadil (Alp), or TM for 7 days. The primary SKPs were passaged into medium without drugs and the number (A and C) and size (B and D) of secondary spheres were quantified. n = at least three independent experiments each and error bars indicate SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < .001, one-way ANOVA with multiple comparison post hoc tests except for (B), where pairwise comparisons of control versus alprostadil or TM were made with Student's t test.
(E and F) mRNA from secondary SKP spheres generated as in (A–D) were analyzed on Affymetrix GeneChip Mouse Gene 2.0 ST arrays. (E) Heatmap and hierarchical clustering of all the samples based on probesets that differed by p < 0.01 (unadjusted) regardless of the fold change when comparing SKPs from vehicle-treated, injured skin versus alprostadil-treated injured skin. Three independent replicates of SKPs from vehicle-treated injured skin (Con Inj), alprostadil- or TM-treated injured skin (Alp Inj or TM Inj), and vehicle-treated uninjured skin (Con Uninj) were compared.
(F) Venn diagram comparing the overlap of genes that differed by p < 0.01 (unadjusted) from pairwise comparisons of SKPs from vehicle (Control) versus TM- or alprostadil-treated injured skin.
(G–K) Three independent preparations of primary neonatal rat SKPs were dissociated and cultured for 24 hr in alprostadil, TM, or vehicle (Con), and mRNA was isolated and analyzed on an Affymetrix GeneChip Rat Gene 2.0 ST array. (G) Heatmaps of pairwise comparisons of vehicle versus alprostadil- or TM-treated SKPs showing the average of the raw expression (log2) data for probesets that differed by p < 0.01 (unadjusted) with a fold change of greater than 1.1. (H and I) Venn diagrams comparing the overlap of all genes that differed by p < 0.01 (unadjusted) (H) or the top 50 most significantly changed genes (I) from pairwise comparisons of vehicle versus TM- or alprostadil-treated SKPs. The 23 overlapping genes in (I) are shown underneath. (J and K) Gene ontology (GO) enrichment analysis for gene categories that were significantly different in the comparisons of vehicle (Ctrl) versus TM- or alprostadil-treated SKPs showing highly significant enrichment for categories involving cell proliferation (J) and the MAP kinase (MAPK) pathway (K). See also Figure S2 and Tables S1, S2, S3, S4, S5, S6, S7, S8 and S9.
To ask about the nature of the drug-induced change(s), we performed transcriptome analysis on the different secondary SKP sphere populations, isolating RNA from three independent biological replicates of each and using Affymetrix GeneChip Mouse Gene 2.0 ST Arrays. Unbiased hierarchical clustering (using the complete linkage method) of a Euclidean distance matrix of log2 normalized expression data demonstrated that, while the different biological replicates for each treatment population clustered together, the different populations were all highly similar to each other (Figure 3E). Intriguingly, the clustering also showed that SKPs from alprostadil- and TM-treated injured skin were more similar to each other and to those from uninjured skin than to SKPs from vehicle-treated injured skin, potentially reflecting the accelerated wound healing caused by these two drugs.
The conclusion that these different SKP populations were all similar was further supported by differential gene expression analysis with the limma bioconductor package (Ritchie et al., 2015). Only 165 and 291 annotated genes were differentially expressed in SKPs from alprostadil and TM versus vehicle-treated injured skin, respectively (p < 0.01 for both comparisons) (Figure 3F). These differentially expressed genes were largely non-overlapping (Tables S1 and S2), with only 21 shared between the alprostadil and TM groups (Figure S2B). Thus, transcriptionally similar SKP populations are present from treated or untreated skin, and somehow the drug treatments enhance their long-term self-renewal when these SKPs are cultured in vitro.
Alprostadil and TM Regulate Genes in Cultured SKPs that Are Associated with Cell Proliferation and the MAP Kinase Pathway
One potential explanation for the modest gene expression changes observed in the long-term experiments was the long lag time between drug exposure and analysis. We therefore performed acute drug exposure experiments. Primary rat SKPs were passaged, acutely treated with drugs for 24 hr, and analyzed via microarrays as for the long-term experiments. This analysis identified 457 and 545 differentially expressed genes in the pairwise comparisons of control versus alprostadil or TM treatment, respectively (p < 0.01 for both comparisons) (Tables S3 and S4). The changes were generally of higher magnitude for alprostadil than for TM (Figure 3G), but more than half of the significantly changed genes (295) were shared between the two groups (Figure 3H; Table S5). In addition, of the top 50 differentially expressed genes in the two pairwise comparisons (defined as highest significance; Table S6 and S7), 23 were shared (Figure 3I), suggesting mechanistic convergence between alprostadil and TM.
Gene ontology (GO) enrichment analysis using the GOstats bioconductor package (Falcon and Gentleman, 2007) showed that, consistent with the biological effects of alprostadil and TM, genes associated with cell proliferation were significantly enriched (Figure 3J) for both drugs. Of the genes that were associated with proliferation (Table S8), 25 overlapped between TM and alprostadil, and a number encoded either growth factors (such as LIF, IL6, and FGF10) or growth factor receptors (such as LIF and EGF receptors). Intriguingly, of all the signaling pathways, GO terms indicating positive regulation of the MAP kinase pathway were most highly enriched for both drugs (Figure 3K; Table S9), suggesting that they might increase SKP self-renewal by activating this pathway.
Alprostadil and TM Likely Regulate SKPs by Stimulating a MEK-ERK Self-Renewal Pathway
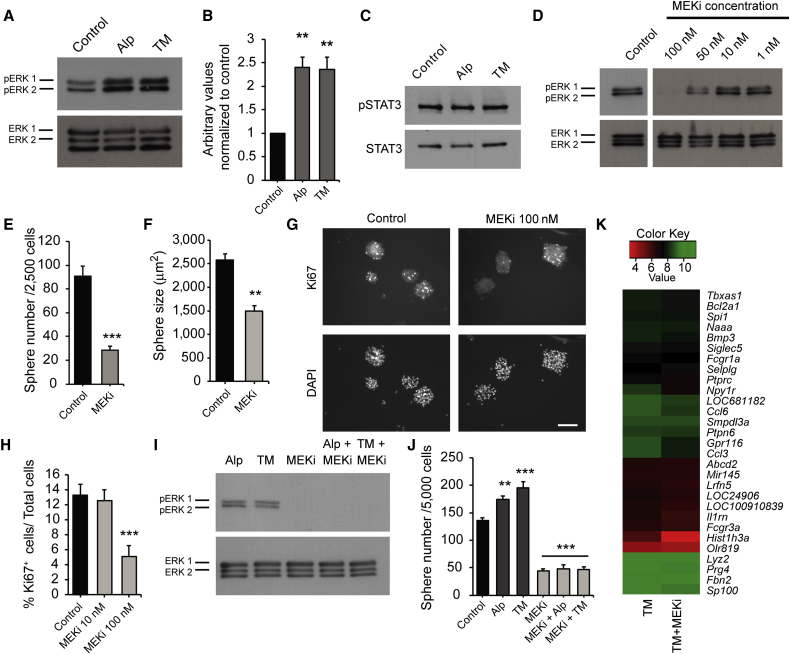
On the basis of these findings, we asked whether alprostadil or TM activated the MEK-ERK pathway in cultured SKPs. We cultured dissociated SKPs for 24 hr, added 100 nM alprostadil or TM for 10 min, and performed western blots for phosphorylated, activated ERK1/2. SKPs grown in FGF2 and EGF had basal levels of ERK1/2 phosphorylation that were increased approximately 2-fold by treatment with either drug (Figures 4A and 4B). In contrast, neither drug increased the phosphorylated activated forms of STAT3, GSK3beta, Akt1, or CREB (Figure 4C and S.N., F.D.M., and D.R.K., unpublished data).
Figure 4.
Signaling via the MEK-ERK Pathway Is Necessary for Basal and Drug-Induced SKPs Self-Renewal
(A–C) SKPs were treated with alprostadil (Alp), TM, or vehicle (Control) for 10 min, and lysates probed on western blots with anti-phosphorylated ERK1/2 T202/Y204 (pERK) (A) or anti-phosphorylated STAT3 (C) and reprobed for total ERK1/2 or total STAT3. (B) shows quantification by scanning densitometry of three experiments as in (A), where pERK was normalized to total ERK1/2.
(D) Western blot of SKPs treated with varying concentrations of trametinib (MEKi) or DMSO (Control) for 30 min, probed for pERK1/2 and reprobed for total ERK1/2.
(E and F) Number (E) and size (F) of SKP spheres generated in clonal methylcellulose assays from secondary neonatal rat SKPs grown 14 days in 100 nM trametinib or DMSO.
(G and H) Secondary rat SKPs were cultured for 4 days, and 10 or 100 nM trametinib added for 2 more days. (G) shows spheres immunostained for Ki67 (top) and counterstained with DAPI (bottom). (H) shows the percentage of Ki67-positive cells. Scale bar represents 100 μm.
(I) Western blot of SKPs treated with 100 nM trametinib for 30 min and coincidently stimulated with 100 nM alprostadil or TM for the final 10 min, probed for pERK1/2, and reprobed for total ERK1/2.
(J) Number of rat SKP spheres generated over 7 days in 100 nM alprostadil or TM with or without 100 nM trametinib.
(K) Heatmap comparing microarray data for three independent preparations of dissociated rat SKPs cultured for 24 hr in TM with or without trametinib, focusing on 29 of the top 50 genes that were altered by TM relative to control (see Figure 3), and that were significantly normalized by MEK inhibition. The heatmap shows the average of the raw expression (log2) data for probesets that differed by p < 0.01 (unadjusted). In all cases, results are pooled from at least three independent experiments.
Error bars indicate SEM, and ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA with multiple comparison post hoc tests, except (E and F), which were analyzed by Student's t test.
We next asked whether MEK-ERK activity was important for SKP self-renewal, using the highly selective MEK1/2 inhibitor trametinib (GSK1120212) (Gilmartin et al., 2011), after first demonstrating that it inhibited basal ERK1/2 phosphorylation in cultured SKPs (Figure 4D). To ask about self-renewal, we used the methylcellulose colony formation assay. Trametinib decreased both the number (Figure 4E) and size (Figure 4F) of clonal spheres grown in FGF2 and EGF. MEK inhibition also decreased SKPs proliferation, as monitored by Ki67 immunostaining of SKP spheres cultured for 48 hr with or without trametinib, but not survival, as monitored by DAPI staining for nuclear morphology (Figures 4G and 4H).
Three lines of evidence indicated that MEK-ERK activity was also important for drug-induced self-renewal. First, trametinib suppressed the ability of alprostadil and TM to increase ERK1/2 phosphorylation in SKPs cultured for 24 hr (Figure 4I). Second, in 7-day sphere assays, inhibition of MEK with 100 nM trametinib robustly decreased sphere number and neither drug could compensate for this decrease (Figure 4J). Third, microarrays showed that trametinib decreased, in part, the downstream gene expression changes seen when SKPs were cultured in TM for 24 hr. Specifically, of the top 50 genes changed by TM treatment, over half (29) had a reduced fold change when trametinib was also included in the cultures (Figure 4K).
Discussion
The experiments presented here identify compounds that promote SKP self-renewal and proliferation in culture, and provide evidence that these compounds activate dermal precursors in vivo to promote skin repair. They also define the MEK-ERK pathway as a key self-renewal pathway for SKPs, and suggest that molecules targeting distinct cell surface receptors enhance self-renewal by converging on this intracellular signaling pathway. These data therefore provide support for the concept that pharmacological activation of endogenous tissue stem cells provides a valid therapeutic approach (Miller and Kaplan, 2012).
How do alprostadil, TM, and SKP growth factors activate the MEK-ERK pathway to enhance SKP proliferation and self-renewal? FGF2 and EGF signal via receptor tyrosine kinases expressed by SKPs, and are well-known activators of the MEK-ERK pathway. Alprostadil (PGE1) binds to the EP1–4 isoforms of the EP family of receptors (Breyer et al., 2001), which are known to stimulate ERK1/2 (Yu et al., 2008). TM binds peripheral mu and kappa-opioid receptors (Kaneto et al., 1990), which also activate ERK1/2 (Gutstein et al., 1997). Thus, three distinct cell surface cues converge on a single intracellular pathway to enhance SKP self-renewal.
How do SKP self-renewal drugs promote skin repair? We previously showed that SKPs arise from, and are similar to, endogenous dermal precursors, and that transplantation of either population led to dermal reconstitution, dermal repair, and hair follicle morphogenesis (Biernaskie et al., 2009). We therefore propose that alprostadil and TM enhance skin repair by promoting proliferation and self-renewal of endogenous dermal precursors. Support for this idea comes from the data showing that SKPs isolated from drug-treated regenerating skin displayed a long-term increase in self-renewal. We suggest that alprostadil and TM enhanced the self-renewal and numbers of dermal precursors in vivo, and that this resulted in an increase in SKP sphere formation in culture. Precedent for this idea comes from studies where transient embryonic exposure to IL-6 led to an increase in adult forebrain neural precursors in vivo, and a persistent increase in sphere formation when these adult precursors were cultured (Gallagher et al., 2013). However, while our findings suggest that activation of dermal precursors underlies the enhanced skin repair, they do not exclude the possibility that alprostadil and/or TM also regulate other endogenous cells to promote wound healing.
Chronic skin wounds are a major medical problem (Eming et al., 2014), and there is a paucity of safe and effective approaches for promoting wound healing. Our findings suggest that targeting endogenous dermal precursors by repurposing of drugs already known to be safe in humans provides a new therapeutic approach for this largely unmet medical need.
Experimental Procedures
Animals
This study was approved by the Hospital for Sick Children Animal Care Committee, in accordance with CCAC guidelines. Sprague-Dawley rats were purchased from Charles River. Punch wounds were performed on 9-month-old C57/Bl6 mice as described in the Supplemental Experimental Procedures (Biernaskie et al., 2009, Johnston et al., 2013). 100 μl of drugs or vehicle were applied topically around the wound or on uninjured shaved skin daily for 7–9 days, as indicated.
Cell Cultures
Neonatal rat SKPs were cultured from dorsal skin as previously described (Biernaskie et al., 2009, Fernandes et al., 2004) and in the Supplemental Experimental Procedures. For human SKPs, anonymized foreskin tissue from voluntary circumcisions was obtained with approval of the Research Ethics Board of the Hospital for Sick Children, and grown as described (Toma et al., 2005, Krause et al., 2014) and in the Supplemental Experimental Procedures. NIH 3T3 cells were grown as described in the Supplemental Experimental Procedures.
Drug Composition and Reagents
Drugs were reconstituted in DMSO (Sigma) at 50 mM for culture studies, and at 100 μM in propylene glycol-ethanol-water (40%:20%:40%) for topical application. All drugs were obtained from Sigma except latanoprost (Cayman Chemical) and trametinib (MedChemexpress).
SKP Screens
Screens using alamarBlue were performed as previously described (Smith et al., 2010) and in the Supplemental Experimental Procedures.
Sphere Formation Assays
Unless otherwise indicated, secondary or tertiary rat or human SKP spheres were seeded at 3,000–5,000 cells/well in 96-well plates, and compounds were added upon plating and at 3 days with fresh medium. At 7 days, cells were fixed, nuclei were stained with Hoechst 33258, sphere numbers were counted manually, and size was determined using Northern Eclipse software. For clonal sphere assays, SKPs were plated at 2,500 cells/ml in medium containing 1.6% methylcellulose and cultured for 14 days, as previously (Jinno et al., 2010, Biernaskie et al., 2009), with compounds added on days 1, 4, 8, and 12.
Tissue Preparation and Morphometric Analyses
Morphometric analyses were performed on paraffin-embedded sections, as described previously (Johnston et al., 2013) and in the Supplemental Experimental Procedures. Ki67-positive cells or CD31-positive blood vessels were determined from five representative 150-μm2 fields randomly distributed over the leading edge of the regenerating dermis bordering the new epithelium, using Northern Eclipse software (Empix). All morphometric analyses were performed in a blinded manner. Antibodies are described in the Supplemental Experimental Procedures.
Western Blot Analysis
Secondary SKP spheres were dissociated and seeded at 106 cells/well in 6-well plates (Costar), cultured for 24 hr, treated as indicated, and analyzed on western blots as described (Naska et al., 2010). Densitometry was performed after subtracting the background using ImageJ software. Antibodies are described in the Supplemental Experimental Procedures.
Microarray Analysis
For microarrays of neonatal rat SKPs, secondary spheres were dissociated, treated with 100 nM drugs for 24 hr. For in vivo drug treatments, secondary SKP spheres were generated from drug-treated murine skin in medium without additional drugs. In both cases, RNA was isolated using the RiboPure Kit (Ambion by Life Technologies) and quality assessed by an Agilent BioAnalyzer. cDNA was generated with the Ambion Whole Transcript Expression Kit (Applied Biosystems) and was hybridized to the Affymetrix GeneChip Mouse Gene 2.0 ST or Rat Gene 2.0 ST arrays. The hybridized microarray image was scanned with the GeneChip Scanner 3000 7G (Affymetrix). Raw probe intensity values were background corrected, normalized with quantile normalization, transformed into the log2 scale, and summarized into probesets with the Robust Multichip Analysis algorithm using the Oligo bioconductor package in R (Carvalho and Irizarry, 2010). For calculation of differential gene expression, the limma bioconductor package was used to calculate Bayesian statistics. In these analyses, any annotated gene with an expression change of p < 0.01 was considered statistically significant. For the hierarchical clustering and heatmap analysis, all the genes with p < 0.01 when comparing the injured skin and the alprostadil groups were used to perform the cluster and heatmap analysis on all groups using the heatmap.2 function of the gplots package in R. In this case, the complete linkage method of a Euclidian distance matrix was used to perform the cluster analysis. For acutely treated rat SKPs, heatmaps were produced by averaging log2 expression data for the three replicates for the appropriate groups and these data were displayed using the heatmap.2 function as described above. GO analysis was performed using the GOstats bioconductor package in R. The gene universe in this analysis was all of the mapped keys in the org.Mm.egGO and org.Ra.egGO databases for the Mouse Gene 2.0 ST and Rat Gene 2.0 ST arrays, respectively, while the differentially expressed genes (computed as described above) served as the enrichment samples. The threshold p value in the GO analysis was set at p < 0.001.
Statistical Analysis
Except for microarrays, data were expressed as the mean plus or minus the SEM and were tested for statistical significance with one-way ANOVA plus Dunnett's, Newman-Keuls’, or Tukey's post hoc multiple comparison tests, unless otherwise indicated. All studies were performed with at least three independent biological replicates.
Author Contributions
S.N. designed, performed, and analyzed many experiments and co-wrote the paper, S.A.Y. performed the microarray analyses and co-wrote the paper, A.P.W.J. performed many experiments and co-wrote the paper, S.P. participated in many of the experiments, M.V.S. assisted with the delivery formulation and some data interpretation, K.M.S. and M.P. validated the hits, K.M.S. and A.D. designed and performed screens, and F.D.M. and D.R.K. conceptualized the experiments, analyzed the data, and co-wrote the manuscript. All authors read the manuscript.
Acknowledgments
This work was funded by CIHR grant MOP-64211 to F.D.M. and a Canadian Stem Cell Network Global Research grant to F.D.M. and D.R.K. S.A.Y. is funded by an Ontario Stem Cell Initiative fellowship. F.D.M. and D.R.K. hold Canada Research Chairs, and F.D.M. is an HHMI Senior International Research Scholar. We thank Darius Bagli for providing the human foreskin tissue, Tatiana Kroupnik, Natalie Grinshtein, Anastassia Voronova, and Guang Yang for advice and assistance.
Published: December 24, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, two figures, and nine tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.12.002.
Contributor Information
Freda D. Miller, Email: fredam@sickkids.ca.
David R. Kaplan, Email: dkaplan@sickkids.ca.
Accession Numbers
Microarray data have been submitted to the NCBI GEO database under the accession number GEO: GSE73329.
Supplemental Information
References
- Biernaskie J., Paris M., Morozova O., Fagan B.M., Marra M., Pevny L., Miller F.D. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano A.E., Wang J., Romeo R., Bouchez L.C., Parker A.E., Sutton S.E., Walker J.R., Flaveny C.A., Perdew G.H., Denison M.S. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer R.M., Bagdassarian C.K., Myers S.A., Breyer M.D. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Calderón-Montaño J.M., Burgos-Morón E., Pérez-Guerrero C., López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- Carvalho B.S., Irizarry R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadwal P., Mahmud N., Sinai L., Azimi A., Fatt M., Wondisford F.E., Miller F.D., Morshead C.M. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep. 2015;5:166–173. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaux M., Wingate D. Trimebutine: mechanism of action, effects on gastrointestinal function and clinical results. J. Int. Med. Res. 1997;25:225–246. doi: 10.1177/030006059702500501. [DOI] [PubMed] [Google Scholar]
- Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon S., Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23:257–258. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- Fernandes K.J.L., McKenzie I.A., Mill P., Smith K.M., Akhavan M., Barnabé-Heider F., Biernaskie J., Junek A., Kobayashi N.R., Toma J.G. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell. Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- Fisher A.A. The safety of pramoxine hydrochloride when used as a topical (surface) anesthetic. Cutis. 1998;62:122–123. [PubMed] [Google Scholar]
- Gallagher D., Norman A.A., Woodard C.L., Yang G., Gauthier-Fisher A., Fujitani M., Vessey J.P., Cancino G.I., Sachewsky N., Woltjen K. Transient maternal IL-6 mediates long-lasting changes in neural stem cell pools by deregulating an endogenous self-renewal pathway. Cell Stem Cell. 2013;13:564–576. doi: 10.1016/j.stem.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Gilmartin A.G., Bleam M.R., Groy A., Moss K.G., Minthorn E.A., Kulkarni S.G., Rominger C.M., Erskine S., Fisher K.E., Yang J. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin. Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- Gotti C., Balestra B., Moretti M., Rovati G.E., Maggi L., Rossoni G., Berti F., Villa L., Pallavicini M., Clementi F. 4-Oxystilbene compounds are selective ligands for neuronal nicotinic alphaBungarotoxin receptors. Br. J. Pharmacol. 1998;124:1197–1206. doi: 10.1038/sj.bjp.0701957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein H.B., Rubie E.A., Mansour A., Akil H., Woodgett J.R. Opioid effects on mitogen-activated protein kinase signaling cascades. Anesthesiology. 1997;87:1118–1126. doi: 10.1097/00000542-199711000-00016. [DOI] [PubMed] [Google Scholar]
- Hanchanale V., Eardley I. Alprostadil for the treatment of impotence. Expert Opin. Pharmacother. 2014;15:421–428. doi: 10.1517/14656566.2014.873789. [DOI] [PubMed] [Google Scholar]
- Hsu Y.-C., Li L., Fuchs E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno H., Morozova O., Jones K.L., Biernaskie J.A., Paris M., Hosokawa R., Rudnicki M.A., Chai Y., Rossi F., Marra M.A., Miller F.D. Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins. Stem Cells. 2010;28:2027–2040. doi: 10.1002/stem.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.P.W., Naska S., Jones K., Jinno H., Kaplan D.R., Miller F.D. Sox2-mediated regulation of adult neural crest precursors and skin repair. Stem Cell Rep. 2013;1:38–45. doi: 10.1016/j.stemcr.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H., Takahashi M., Watanabe J. The opioid receptor selectivity for trimebutine in isolated tissues experiments and receptor binding studies. J. Pharmacobiodyn. 1990;13:448–453. doi: 10.1248/bpb1978.13.448. [DOI] [PubMed] [Google Scholar]
- Krause M.P., Dworski S., Feinberg K., Jones K., Johnston A.P.W., Paul S., Paris M., Peles E., Bagli D., Forrest C.R. Direct genesis of functional rodent and human Schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014;3:85–100. doi: 10.1016/j.stemcr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie I.A., Biernaskie J., Toma J.G., Midha R., Miller F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F.D., Kaplan D.R. Mobilizing endogenous stem cells for repair and regeneration: are we there yet? Cell Stem Cell. 2012;10:650–652. doi: 10.1016/j.stem.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Naska S., Lin D.C., Miller F.D., Kaplan D.R. p75NTR is an obligate signaling receptor required for cues that cause sympathetic neuron growth cone collapse. Mol. Cell. Neurosci. 2010;45:108–120. doi: 10.1016/j.mcn.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Hozumi Y., Kondo S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp. Dermatol. 2005;14:323–328. doi: 10.1111/j.0906-6705.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Smith K.M., Datti A., Fujitani M., Grinshtein N., Zhang L., Morozova O., Blakely K.M., Rotenberg S.A., Hansford L.M., Miller F.D. Selective targeting of neuroblastoma tumour-initiating cells by compounds identified in stem cell-based small molecule screens. EMBO Mol. Med. 2010;2:371–384. doi: 10.1002/emmm.201000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata S., Flynn G.L., Weiner N.D. Penetration of minoxidil from ethanol/propylene glycol solutions: effect of application volume and occlusion. J. Pharm. Sci. 1995;84:688–691. doi: 10.1002/jps.2600840605. [DOI] [PubMed] [Google Scholar]
- Toma J.G., Akhavan M., Fernandes K.J., Barnabé-Heider F., Sadikot A., Kaplan D.R., Miller F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Toma J.G., McKenzie I.A., Bagli D., Miller F.D. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- Wang J., Gallagher D., DeVito L.M., Cancino G.I., Tsui D., He L., Keller G.M., Frankland P.W., Kaplan D.R., Miller F.D. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Yu L., Wu W.K.K., Li Z.J., Wong H.P.S., Tai E.K.K., Li H.T., Wu Y.C., Cho C.H. E series of prostaglandin receptor 2-mediated activation of extracellular signal-regulated kinase/activator protein-1 signaling is required for the mitogenic action of prostaglandin E2 in esophageal squamous-cell carcinoma. J. Pharmacol. Exp. Ther. 2008;327:258–267. doi: 10.1124/jpet.108.141275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.