Abstract
Objectives
To assess the relationship between comorbid depression and diabetes and cognitive decline among Mexican Americans age 65 and over.
Design
Retrospective cohort study with longitudinal analysis.
Setting
Texas, New Mexico, Colorado, Arizona, and California
Participants
Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE).
Measurements
Cognition was assessed using the Mini Mental State Examination (MMSE). Depression was defined as a score ≥16 on the Center for Epidemiologic Studies Depression Scale. Diabetes was defined as according to self-reported history or taking insulin or oral hypoglycemic medication.
Results
Participants with depression and diabetes, depression only, diabetes only, and neither condition declined an average of 6.5, 4.4, 7.8, and 4.2 points on the MMSE, respectively, across the six examination waves. Participants with diabetes or comorbid diabetes and depression declined an average of 0.18 points per year (P<0.01) and 0.25 points per year, respectively, on the MMSE compared to participants with neither condition. Depression was associated with significantly greater cognitive decline (β̂ =−0.11, P<0.05) after excluding participants with baseline cognitive impairment (MMSE≤17). The odds for developing severe cognitive impairment among participants with diabetes increased by 1.08 times per year (95% CI=1.03 – 1.122) and by 1.08 times per year (95% CI=1.01 – 1.15) for participants with comorbid depression and diabetes compared to participants with neither of these conditions.
Conclusion
Diabetes and comorbid depression and diabetes are risk factors for cognitive decline among older Mexican Americans. Interventions that reduce the prevalence of depression and diabetes among Mexican Americans may decrease the number of older adults who experience cognitive decline.
Keywords: Cognitive aging, Mexican Americans, Depression, Diabetes
INTRODUCTION
Depressed older adults are at an increased risk for diabetes [1] and diabetes-associated complications, including diabetic neuropathy [2] and hypoglycemia [3] compared to non-depressed older adults. Conversely, diabetic older adults are at greater risk for depression compared to non-diabetic older adults [4]. Older adults with comorbid depression and diabetes are at greater risk for stroke [5], are more likely to develop physical limitations [6] and have higher rates of mortality [7] compared to older adults with depression or diabetes only.
Depression and diabetes are both risk factors for cognitive impairment during old age [8, 9]. Diabetes is associated with an increased occurrence of cerebral infarctions and other brain abnormalities that contribute to the onset and progression of cognitive decline during old age [10]. Cardiovascular risk factors are also associated with damage to the frontal lobe and hippocampus [11], which are two brain regions critical for cognitive functioning. Depression may play a causal role in cognitive impairment by interacting with the neuropathological characteristics of Alzheimer’s disease, specifically amyloid plaques and neurofibrillary tangles [12, 13]. Also, depressed older adults have been observed to have smaller hippocampal volumes compared to non-depressed older adults [14].
Depression and diabetes have both been identified as independent risk factors for cognitive impairment among older Hispanics [15, 16], a population with higher rates of cognitive impairment compared to non-Hispanic whites [17]. High depressive symptoms and diabetes are also more common among Mexican Americans with cognitive impairment compared to non-Hispanic whites. In an analysis of 436 Mexican Americans and 633 non-Hispanic white adults, Mexican Americans diagnosed with either mild cognitive impairment or Alzheimer’s disease were more likely to be diagnosed with diabetes and exhibited more depressive symptoms compared to non-Hispanic whites [18]. Despite evidence that diabetes and depression are both risk factors for cognitive impairment, it is not known if older Mexican Americans with comorbid depression and diabetes exhibit greater cognitive decline compared to older adults with only one of these conditions.
The number of Hispanics age 65 and over has increased from 1.7 million (4.5% of total U.S. population) in 2000 to 2.8 million (5.7% of total U.S. population) in 2009 [19]. Hispanics are at an increased risk for diabetes compared to non-Hispanic whites [20]. The Centers for Disease Control and Prevention estimates the adjusted percentage of Hispanics with diabetes is 12.8% compared to 7.6% for non-Hispanic whites [21]. Prior research on differences in the prevalence of depression between Hispanics and non-Hispanic whites has produced conflicting results with Hispanics being reported to have similar [22], lower [23], and higher [24] depression prevalence compared to non-Hispanic whites.
The purpose of this study was to assess the relationship between comorbid depression and diabetes and cognitive decline among Mexican Americans age 65 and older. We hypothesize that older Mexican Americans with comorbid depression and diabetes will exhibit greater cognitive decline and be more likely to develop severe cognitive decline compared to participants with neither of these conditions or only one of these conditions.
METHODS
Sample Population
The Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE) is an ongoing longitudinal cohort study of community-dwelling Mexican Americans age 65 and over residing in Texas, New Mexico, Colorado, Arizona, and California [25]. The present analysis used demographic and health status data collected during the baseline examination (1993–94) and cognitive data collected during six examinations from 1993–94 to 2006–07. The Institutional Review Board of the University of Texas Medical Branch approved the study protocol of the H-EPESE.
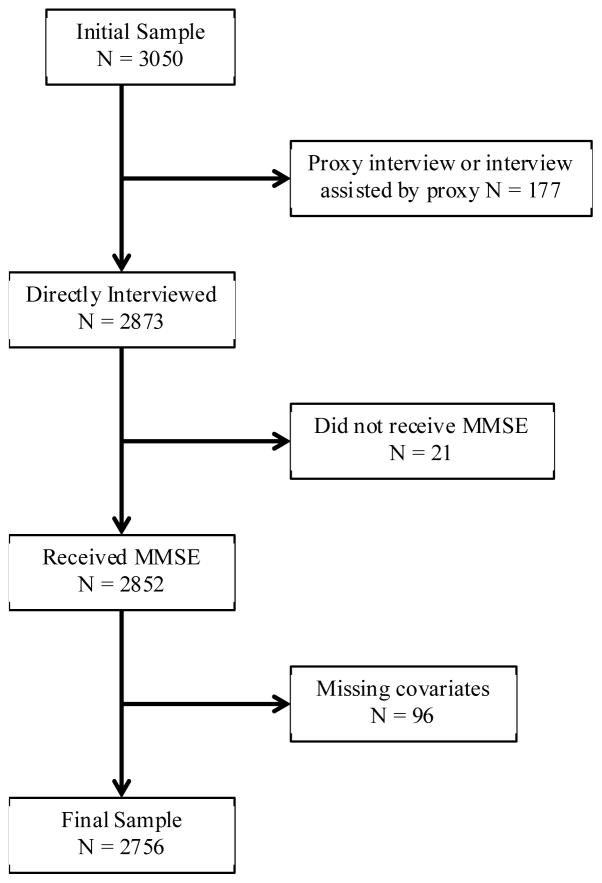
A visual representation for the selection of the final sample is provided in Figure 1. A total of 3050 participants were interviewed during the baseline examination, 2873 of which were interviewed without the assistance of a proxy, and 2852 participants completed the Mini-Mental State Examination (MMSE). Of the 2852 remaining participants, 2756 had baseline measures for educational attainment, nativity (being born in the U.S.), smoking, alcohol consumption, marital status, number of individuals living in the household, depressive symptoms, diabetes, hypertension, heart disease, stroke, activities of daily living (ADLs), and instrumental ADLS (IADLs). Participants interviewed with the assistance of a proxy, did not complete the baseline MMSE, or had missing baseline data for selected variables (n=294) were excluded from the final sample (n=2756). Participants excluded from the final sample were older, more likely to be male, to have lower education, to not consume alcohol, to never have been married, to have reported experiencing a stroke, to be unable to perform one or more ADLs and IADLs, and less likely to have hypertension than participants included in the final sample (P<0.05).
Figure 1.
Flow chart of selection of final sample Participants who required a proxy to complete or help complete the interview did not receive the MMSE.
Measures
Depression and diabetes
Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES-D) [26]. Participants who scored ≥16 on the CES-D Scale were determined to have high depressive symptoms and for the purposes of this study were classified as depressed. Diabetes status during the baseline examination was determined based on self-reported measures. Participants were classified as having diabetes if they responded yes to the question, “Have you ever been told by a doctor that you have diabetes, sugar in your urine or high blood sugar?” or if they were taking insulin, oral hypoglycemic or both. Previous research indicates this is a valid and reliable method to determine diabetes status [27]. This data was used to define depression and diabetes comorbidity status (no depression or diabetes, depression only, diabetes only, both depression and diabetes).
Cognition
Cognitive functioning was evaluated during each of the six examinations using the Spanish and English versions of the MMSE [28]. The MMSE is a frequently used cognitive assessment in epidemiological studies because it does not require an examiner to undergo extensive training and can be quickly and easily administered to the recipient [29]. The MMSE measures orientation to time and place, registration, attention and calculation, recall, language, constructional praxis, and ability to follow verbal commands. The maximum possible score is 30 points and minimum score is 0 points, with a higher score indicating more intact cognition.
Data from the MMSE was used to model trajectories of cognitive decline to detect differences according to diabetes and depression comorbidity status. Since these trajectories were meant to identify subtle changes in cognitive functioning that may not represent decline to a clinically meaningful level of cognitive impairment, a second analysis was conducted to determine if comorbid diabetes and depression was associated with severe cognitive decline. Participants who had a MMSE total score that declined below 18 points between any two consecutive examinations were defined as having severe cognitive decline. The number of incorrect responses on the MMSE is correlated with educational attainment [30] and previous research indicates that education specific cut offs are appropriate when using the MMSE to detect cognitive impairment in population studies [31]. Therefore, a score of 18 was chosen to account for the low educational attainment of the sample population and to prevent incorrectly classifying participants who had poor performance on the MMSE due to low educational attainment as having severe cognitive decline.
Covariates
Age, sex, and years of education (0, 1–4, 5–8, ≥9) were included as covariates in all analyses. Additional covariates assessed during the baseline examination included smoking, alcohol consumption, self-reported history of stroke and heart disease, nativity, marital status, number of people living in the household, ADLs, and IADLs. Marital status was categorized as never married, not married (separated, divorced, or widowed), and currently married. Smoking status was defined as never (<100 cigarettes lifetime), former (>100 cigarettes lifetime but not currently smoking), and current (>100 cigarettes lifetime and currently smoking). Alcohol consumption was defined as abstainer (never consumed alcohol), former (no alcohol consumed in the past year), and current (consumed alcohol in the past year). Stroke and heart disease were each assessed by self-report. Participants who responded yes to the question, “Has a doctor ever told you that you had a stroke, a blood clot in the brain, or brain hemorrhage?” or “Has a doctor ever told you that you had a heart attack, myocardial infarction, or coronary thrombosis?” were classified as having a stroke and heart disease, respectively. ADLs assessed included ability to walk across the room, bathing, personal grooming, dressing, eating, getting from a bed to a chair, and toileting. IADLs assessed included ability to use a telephone, drive a vehicle, go shopping, prepare meals, do housework, take medicine, manage money, do heavy house work, walk up and down stairs, and walk half a mile without help. Participants were dichotomized according to the reported inability to perform one or more ADLs (yes, no) or IADLs (yes, no) without assistance. The language (English or Spanish) in which the interview was administered was recorded during each observation.
Statistical analysis
Analyses were performed using R version 3.1.0 [32]. Descriptive characteristics of the final sample according to depression and diabetes comorbidity status were analyzed using analysis of variance and chi-square tests for continuous and categorical variables, respectively.
Trajectories of cognitive decline according to baseline depression and diabetes comorbidity status were modeled using mixed-effects regression [33]. This method provides valid estimates when data is unbalanced due to differences in the number and timing in which participants are observed. Time was defined as the number of years following the baseline examination. Random effects for time and intercept were included in all models to allow for the estimated slope and intercept values for cognition to vary for each participant. A two-way interaction term between time and comorbid depression and diabetes status was included in all mixed-effects regression models to assess differences in the trajectory of cognitive decline according to depression and diabetes comorbidity status. The beta coefficients for the two-way interaction term are interpreted as the average change per year on the MMSE according to comorbidity status. Participants who did not have diabetes or depression were the reference category in all analyses.
Generalized mixed effects models were used to examine the relationship between baseline depression and diabetes comorbidity status and severe cognitive decline. Time was defined as the number of years following the baseline examination. Two-way interaction terms between time and comorbid depression and diabetes status were used to determine if the odds for severe cognitive decline differed according to depression and diabetes comorbidity status. The odds ratios for the two-way interaction term are interpreted as the increase or decrease in odds per year for developing severe cognitive impairment according to comorbidity status. Participants who did not have depression or diabetes were the reference category in all analyses.
Three separate linear mixed effects and generalized mixed effects models were conducted. Model 1 controlled for the effects of age, sex, and education. Model 2 included age, sex, and education, plus smoking, alcohol consumption, self-reported history of stroke, heart disease, hypertension, nativity, marital status, number of people living in the household, ability to perform ADLs and IADLs, and the language in which the interviews were conducted. Model 2 was used to determine if these additional covariates mediate the relationship between comorbid depression and diabetes status and cognitive decline. A limitation of the MMSE is it underestimates the rate of cognitive decline for older adults with low cognitive functioning [34]. Therefore, a third model was used that included all covariates from Model 1 and Model 2, but excluded participants who scored ≤17 on the MMSE during the baseline examination (baseline cognitive impairment).
All generalized mixed effects models controlled for baseline cognitive functioning and the number of years a participant remained in the H-EPESE. These variables were included as covariates because participants who remained in the study longer will experience more cognitive decline as a result of advancing age and therefore be more likely to decline below 18 points. All independent variables in the linear mixed-effects and generalized mixed effects models were treated as time invariant.
RESULTS
A description of the baseline characteristics of the final sample according to depression and diabetes comorbidity status is provided in Table 1. A total of 1626 (59.0%) participants had no depression or diabetes, 458 (16.6%) had depression only, 474 (17.2%) had diabetes only, and 198 (7.2%) had both depression and diabetes. During the baseline examination, participants with no depression or diabetes had higher educational attainment, were more likely to be male, to be a current smoker, to consume alcohol, to never have been married, to be unable to perform one or more ADLs or IADLs, and were less likely to have a self-reported history of hypertension, heart disease, and stroke (P <0.05). Participants with depression only or comorbid depression and diabetes had significantly lower baseline cognitive functioning compared to participants with no depression and no diabetes (P <0.05).
Table 1.
Descriptive characteristics of final sample according to baseline depression and diabetes comorbidity status.
| Characteristic | Neither (n=1626) | Depression (n=458) | Diabetes (n=474) | Both (n=198) | P-value |
|---|---|---|---|---|---|
| Age, mean (±SD) | 73.3 (±6.5) | 73.5 (±6.7) | 72.7 (±5.9) | 72.6 (±6.3) | 0.10 |
| Sex, n (%) | < 0.01 | ||||
| Male | 733 (63.9) | 137 (11.9) | 217 (18.9) | 61 (5.3) | |
| Female | 893 (55.5) | 321 (20.0) | 257 (16.0) | 137 (8.5) | |
| Education, n (%) | 0.02 | ||||
| 0 years | 269 (58.9) | 71 (15.5) | 81 (17.7) | 36 (7.9) | |
| 1–4 years | 569 (56.3) | 196 (19.4) | 168 (16.6) | 77 (7.6) | |
| 5–8 years | 495 (59.3) | 137 (16.4) | 140 (16.8) | 63 (7.5) | |
| 9+ years | 293 (64.5) | 54 (11.9) | 85 (18.7) | 22 (4.8) | |
| Smoking, n (%) | < 0.01 | ||||
| Never | 908 (56.4) | 300 (18.6) | 278 (17.3) | 123 (7.6) | |
| Former | 493 (62.1) | 100 (12.6) | 150 (18.9) | 51 (6.4) | |
| Current | 225 (63.7) | 58 (16.4) | 46 (13.0) | 24 (6.8) | |
| U.S. born, n (%) | 0.09 | ||||
| Yes | 914 (59.1) | 236 (15.3) | 277 (17.9) | 120 (7.8) | |
| No | 712 (58.9) | 222 (18.4) | 197 (16.3) | 78 (6.5) | |
| Alcohol consumption, n (%) | < 0.01 | ||||
| Abstainer | 845 (56.1) | 284 (18.8) | 257 (17.1) | 121 (8.0) | |
| Former | 396 (58.9) | 86 (12.8) | 133 (19.8) | 57 (8.5) | |
| Current | 385 (66.7) | 88 (15.3) | 84 (14.6) | 20 (3.5) | |
| Marriage, n (%) | < 0.01 | ||||
| Married | 915 (59.5) | 225 (14.6) | 296 (19.2) | 102 (6.6) | |
| Not married | 624 (57.6) | 214 (19.7) | 160 (14.8) | 86 (7.9) | |
| Never married | 87 (64.9) | 19 (14.2) | 18 (13.4) | 10 (7.5) | |
| Number of residents in home, mean (±SD) | 2.6 (±1.7) | 2.4 (±1.5) | 2.6 (±1.5) | 2.4 (±1.4) | 0.14 |
| Language, n (%) | 0.37 | ||||
| English | 371 (61.2) | 88 (14.5) | 107 (17.7) | 40 (6.6) | |
| Spanish | 1255 (58.4) | 370 (17.2) | 367 (17.1) | 158 (7.3) | |
| Heart disease, n (%) | < 0.01 | ||||
| Yes | 116 (47.5) | 36 (14.8) | 58 (23.8) | 34 (13.9) | |
| No | 1510 (60.1) | 422 (16.8) | 416 (16.6) | 164 (6.5) | |
| Stroke, n (%) | < 0.01 | ||||
| Yes | 61 (42.1) | 26 (17.9) | 39 (26.9) | 19 (13.1) | |
| No | 1565 (59.9) | 432 (16.5) | 435 (16.7) | 179 (6.9) | |
| Hypertension, n (%) | < 0.01 | ||||
| Yes | 963 (55.5) | 291 (16.8) | 325 (18.7) | 156 (9.0) | |
| No | 663 (64.9) | 167 (16.4) | 149 (14.6) | 42 (4.1) | |
| Impairment ADLs, n (%) | < 0.01 | ||||
| Yes | 107 (35.1) | 85 (27.9) | 56 (18.4) | 57 (18.7) | |
| No | 1519 (62.0) | 373 (15.2) | 418 (17.1) | 141 (5.8) | |
| Impairment IADLs, n (%) | < 0.01 | ||||
| Yes | 706 (49.8) | 299 (21.1) | 255 (18.0) | 157 (11.1) | |
| No | 920 (68.7) | 159 (11.9) | 219 (16.4) | 41 (3.1) | |
| Baseline MMSE, mean (±SD) | 25.2 (±4.3) | 24.0 (±4.8) | 25.1 (±4.5) | 23.4 (±5.2) | < 0.01 |
Analysis of variance and chi-square tests used for continuous and categorical variables, respectively. Bold indicates statistical significant P < 0.05.
Activities of daily living (ADLs); Instrumental activities of daily living (IADLs)
A total of 130 participants had impaired baseline cognitive functioning (MMSE ≤17). These participants were significantly older, had lower educational attainment, more likely to have been born in the U.S., to consume alcohol, to be currently married, to reported having a stroke, to have high depressive symptoms, and to have inability to perform one or more ADLs or IADLs, and on average had more people living in the household compared to participants with non-impaired cognitive functioning.
Trajectories of Cognitive decline According to Comorbid Depression and Diabetes Status
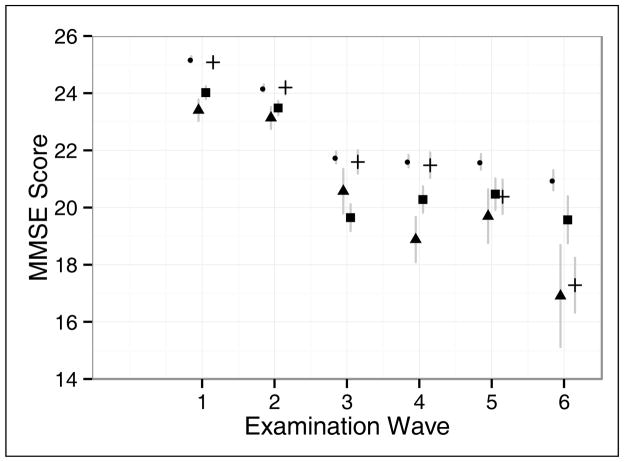
A visual representation of the average MMSE scores for each examination wave according to baseline depression and diabetes comorbidity status is provided in Figure 2. In general, participants with comorbid depression and diabetes had lower scores on the MMSE at each examination wave compared to the other comorbidity categories and these differences appeared to increase over time. Over the course of the six observation waves, participants with comorbid depression and diabetes declined 6.5 points on the MMSE, whereas participants with depression declined 4.4 points, participants with diabetes declined 7.8 points, and participants without depression or diabetes declined 4.2 points.
Figure 2.
Average score on the MMSE for each examination wave according to depression and diabetes comorbidity status. The grey vertical lines represent the 95% confidence interval for each estimated value. The small standard errors for the estimated values for participants with neither diabetes nor depression (circle) and for participants with diabetes only (cross) during examination waves one and two prevent the confidence intervals from being visible. Participants with depression only are represented with squares and participants with comorbid depression and diabetes are represented with triangles.
- ● neither
- ▲ both
- ■ depression only
- + diabetes only
Significant differences in the baseline cognitive functioning according to comorbid depression and diabetes status were detected after adjusting for covariates (Table 2). Based on the results from Model 1, participants with only depression scored 0.88 (standard error [S.E.]=0.20, P<0.01) points lower on the MMSE than participants with no depression or diabetes during the baseline observation. Participants with comorbid depression and diabetes scored 1.13 (S.E.=0.29, P<0.01) points lower on the MMSE during the baseline observation than participants with neither of these conditions. These differences were reduced and no longer statistically significant after adjusting for additional covariates (Model 2) and excluding participants with impaired baseline cognition (Model 3). However, participants with only diabetes had significantly higher baseline cognition compared to participants with no depression or diabetes after adjusting for additional covariates (Model 2) and removing participants with impaired baseline cognition (Model 3).
Table 2.
Trajectories of cognitive decline according to comorbid depression and diabetes status
| Model One | Model Two | Model Three | ||||
|---|---|---|---|---|---|---|
| Comorbidity Status | β̂ | SE | β̂ | SE | β̂ | SE |
| Time | **−0.55 | 0.86 | **−0.55 | 0.02 | **−0.56 | 0.02 |
| No depression, no diabetes (ref) | ~ | ~ | ~ | ~ | ~ | ~ |
| Depression, no diabetes | **−0.88 | 0.20 | *−0.55 | 0.19 | −0.33 | 0.18 |
| Diabetes, no depression | 0.20 | 0.19 | *0.45 | 0.19 | *0.41 | 0.17 |
| Depression and diabetes | **−1.13 | 0.29 | −0.28 | 0.28 | −0.03 | 0.27 |
| Comorbidity status x time | ||||||
| No depression, no diabetes (ref) | ~ | ~ | ~ | ~ | ~ | ~ |
| Depression, no diabetes | −0.08 | 0.05 | −0.08 | 0.05 | −0.11 | *0.05 |
| Diabetes, no depression | **−0.18 | 0.05 | **−0.18 | 0.05 | **−0.19 | 0.05 |
| Depression and diabetes | **−0.25 | 0.08 | **−0.25 | 0.08 | **−0.26 | 0.09 |
Model one controlled for the effects of age, and educational attainment
Model two included the covariates from model one, nativity, smoking status, alcohol consumption status, marital status, number of residents living in the home, hypertension, heart disease, stroke, ability to perform ADLs and IADLs, and language.
Model three included all covariates from model two and excluded participants with baseline MMSE ≤ 17.
Statistical significance P < 0.05;
Statistical significance P < 0.01.
SE (Standard Error)
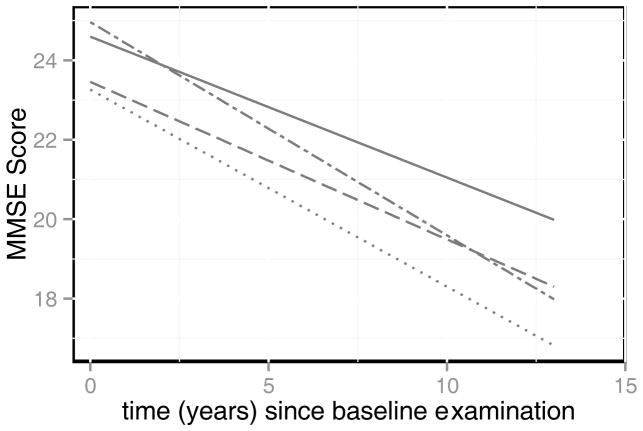
A visual representation of the cognitive trajectories according to depression and diabetes comorbidity status adjusted for age, sex, and educational attainment is provided in Figure 3 and the adjusted coefficients are provided in Table 2. Compared to participants with no depression or diabetes, those with only diabetes declined 0.18 (S.E.=0.05, P<0.01) points more per year on the MMSE and participants with comorbid depression and diabetes declined 0.25 (S.E.=0.08, P<0.01) points more per year on the MMSE. Participants with depression only did not exhibit significantly greater cognitive decline compared to participants who did not have depression or diabetes (β̂ =−0.08 points per year, S.E.=0.05, P=0.12).
Figure 3.
Adjusted trajectories of cognitive functioning according to depression and diabetes comorbidity status. Predicted trajectories of cognitive decline according to depression and diabetes comorbidity status based on linear mixed effects model that controlled for the effects of age, gender, and educational attainment. These trajectories represent the average decline per year on the MMSE according to comorbidity status while adjusting for sociodemographic characteristics.
- — neither
- ···· both
- – - depression only
- - – · diabetes only
Findings from Model 1 did not change substantially after adjusting for additional covariates (Model 2) and after excluding participants with impaired baseline cognition (Model 3). However, participants with only depression declined 0.11 (S.E.=0.05, P=0.05) points more per year compared to participants without depression and diabetes when cognitively impaired participants were excluded (Model 3). In addition, participants with comorbid depression and diabetes declined 0.17 points more per year on the MMSE compared to participants with depression only, but this difference only approached statistical significance (S.E.=0.09, P=0.06). Participants with comorbid depression and diabetes did not exhibit significantly greater cognitive decline compared to participants with diabetes only (β̂ =−0.08, S.E.=0.09, P=0.39).
Severe Cognitive Decline According to Comorbid Depression and Diabetes Status
A total of 768 participants declined below 18 points on the MMSE between any two consecutive examinations. Participants classified as having severe cognitive decline were significantly older, were more likely to be male, had lower education, were less likely to have been born in the U.S., to consume alcohol, to be married, and were more likely to have had a stroke, to have diabetes, to be depressed, and to be unable to perform one or more ADLs and IADLs, compared to participants who did not develop severe cognitive decline (all P<0.05).
The odds for severe cognitive decline over time according to comorbid depression and diabetes status are summarized in Table 3. Participants with diabetes or comorbid depression and diabetes were more likely to develop severe cognitive decline over time compared to participants with neither condition. After adjusting for age, sex, and education (Model 1), participants with only diabetes had 1.07 times higher odds per year (95% C.I.=1.03 – 1.12) for severe cognitive decline compared to participants with no depression or diabetes. Participants with comorbid depression and diabetes had 1.08 times higher odds per year (95% C.I.=1.01 – 1.15) for severe cognitive decline compared to participants with neither of these conditions. Participants with only depression did not have significantly higher odds for severe cognitive decline (OR=1.02, 95% CI=0.97 – 1.06). These findings remained consistent after adjusting for additional covariates (Model 2) and after excluding participants with a baseline MMSE of ≤17 (Model 3). However, the increased odds for severe cognitive decline among participants with comorbid depression and diabetes was no longer significant after excluding participants with low baseline cognitive functioning (Model 3).
Table 3.
Odds for severe cognitive decline according to comorbidity status.
| Model One | Model Two | Model Three | ||||
|---|---|---|---|---|---|---|
| Comorbidity Status | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Time | **1.13 | 1.11 – 1.16 | **1.14 | 1.12 – 1.17 | **1.15 | 1.12 – 1.18 |
| No depression, no diabetes (ref) | ~ | ~ | ~ | ~ | ~ | ~ |
| Depression, no diabetes | 0.99 | 0.70 – 1.41 | 0.95 | 0.67 – 1.36 | 1.01 | 0.71 – 1.44 |
| Diabetes, no depression | 0.83 | 0.58 – 1.19 | 0.79 | 0.55 – 1.13 | 0.77 | 0.53 – 1.12 |
| Depression and diabetes | 0.91 | 0.55 – 1.52 | 0.82 | 0.49 – 1.36 | 0.83 | 0.48 – 1.41 |
| Comorbidity status x time | ||||||
| No depression, no diabetes (ref) | ~ | ~ | ~ | ~ | ~ | ~ |
| Depression, no diabetes | 1.02 | 0.97 – 1.06 | 1.01 | 0.97 – 1.06 | 1.01 | 0.96 – 1.06 |
| Diabetes, no depression | **1.07 | 1.03 – 1.12 | **1.08 | 1.03 – 1.12 | **1.08 | 1.03 – 1.13 |
| Depression and diabetes | *1.08 | 1.01 – 1.15 | *1.08 | 1.03 – 1.12 | 1.07 | 0.99 – 1.15 |
Model one controlled for age, educational attainment, baseline MMSE, and time (years) of follow-up.
Model two included the covariates from model one plus nativity, smoking status, alcohol consumption status, marital status, number of residents living in the home, hypertension, heart disease, stroke, ability to perform ADLs and IADLs, and language.
Model three included the covariates from model two, but excluded participants with baseline MMSE ≤ 17.
Statistical significance P < 0.05;
Statistical significance P < 0.01.
Coefficient estimates were exponentiated to be interpreted as odds ratios.
OR (Odds Ratio); CI (Confidence Interval)
DISCUSSION
This study presents new evidence that older Mexican Americans with diabetes or comorbid depression and diabetes exhibit significantly greater cognitive decline over an 11-year period and are more likely to develop severe cognitive impairment compared to those with neither of these conditions, but not compared to Mexican Americans with depression or diabetes only. The greater cognitive decline observed for participants with diabetes only or comorbid depression and diabetes was robust to confounding as indicated by the consistent findings between the three models presented in Table 2. Participants with high depressive symptoms exhibited significantly greater cognitive decline but only after participants with impaired baseline cognition were excluded (Model 3).
The findings from this study indicate there are statistically significant differences in the rate of cognitive decline according to depression and diabetes comorbidity status, but these differences may be difficult to detect in a clinical setting. However, these small differences in cognitive decline can be of clinical importance as previous research indicates that subtle changes in cognition have been observed among older adults who are in the preclinical stages of Alzheimer’s disease [35]. In addition, Figure 3 indicates the between group differences in cognitive functioning increase over time and it is likely that differences in cognitive functioning according to comorbid depression and diabetes status have greater clinical significance among older adults who survive to advanced ages.
Findings from the generalized mixed effects model that adjusted for age, sex, and education (Model 1) indicated Mexican Americans with diabetes or comorbid depression and diabetes are more likely to develop severe cognitive decline compared to Mexican Americans with neither condition. However, comorbid depression and diabetes was no longer significantly associated with severe cognitive decline when excluding participants with baseline cognitive impairment. Depression and diabetes are associated with hypertension [4], stroke [36], and heart disease [37] and these health conditions are risk factors for cognitive impairment [38–40]. Also, it is likely that the observed relationship between comorbid depression and diabetes and severe cognitive decline is mediated by social and behavioral mechanisms.
Longitudinal studies that have examined the association between late life depression and risk for dementia report that there is a close temporal relationship between the onset of depressive symptoms and dementia [41] and that depression may be an early symptom of dementia [42]. In addition the rate of cognitive decline has been observed to be greater among older adults with comorbid depression and dementia compared to older adults with dementia only [43]. In Model 3 we observed that depressed non-diabetics exhibited significantly greater cognitive decline, but only after excluding participants with impaired baseline cognition (MMSE < 17). This provides evidence that depression is associated with greater cognitive decline among older Mexican Americans who are not cognitively impaired. This finding could also be a consequence of a floor effect of the MMSE [34], which limits the ability of the MMSE to detect cognitive decline among older adults with cognitive impairment [44]. Additional studies that use more comprehensive measures of cognitive functioning are necessary before any definitive conclusions on the relationship between depressive symptoms and cognitive decline among older Mexican Americans can be made.
The findings from the present analysis have significant public health and clinical implications. Diabetes is highly prevalent among older Mexican Americans with depression [45]. Prior research indicates Mexican Americans poorly manage depression and diabetes and are less likely to receive treatment for these conditions than non-Hispanic whites [46]. Cultural differences, attitudes toward seeking treatment for chronic illnesses, and limited access to medical care all play a significant role in the decreased use of health care services by Mexican Americans [47]. Promoting the use of health care services for the treatment and prevention of chronic conditions such as depression and diabetes may decrease the number of older Mexican Americans that experience cognitive decline later in life.
Our findings are consistent with those reported in previous research on non-Hispanic older adults. Two separate studies have observed that adults with comorbid depression and diabetes are at a greater risk for dementia compared to adults with diabetes only [48, 49]. Also, in a study of non-demented adults, participants with comorbid depression and diabetes had lower overall cognitive functioning, as well as worse performance on measures of attention, verbal memory, visual memory, and executive functioning compared to non-depressed diabetics [50].
We observed that participants with comorbid depression and diabetes had greater cognitive decline compared to participants with depression alone, but this difference only approached statistical significance. Diabetes and other cardiovascular disease risk factors may contribute to a subtype of depression called vascular depression [51]. Vascular depression is characterized by the presence of white matter hyperintensities along with depressive symptoms [52]. Patients diagnosed with vascular depression also present with impaired executive functioning [53] and have been observed to have lower cognitive functioning compared to older adults with nonvascular depression [54].
This study has limitations that need to be acknowledged. Participants were classified as being diabetic according to self-report and some participants may have been incorrectly classified. It should also be noted that a score ≥16 on the CES-D does not represent a clinical diagnosis of depression and participants may have been incorrectly classified as depressed or not depressed. Future research can address these limitations by using patient medical records or biomarkers to validate diabetes and depressive status. Also, the measure for cognitive functioning was limited to the English and Spanish versions of the MMSE. The MMSE has been used in other studies of older Hispanics [55, 56], but ethnic and cultural biases of the MMSE that decrease the accuracy of this measure for assessing the cognitive functioning of older Mexican Americans have been reported [57]. These biases may complicate the interpretation of the findings for the trajectories of cognitive decline presented Figure 2 and for the increased odds for severe cognitive decline associated with diabetes and comorbid depression and diabetes. Therefore, our findings that diabetes and comorbid depression and diabetes are associated with greater cognitive decline need to be replicated with data collected using cognitive assessments that have been specifically created for measuring cognition in minority populations, in particular older Mexican Americans.
Conclusion
In summary, diabetes is associated with greater cognitive decline and increased odds for severe cognitive decline among older Mexican Americans. Mexican Americans with comorbid diabetes and depression experienced greater cognitive decline compared to those with neither of these conditions, but were not more likely to develop severe cognitive impairment after adjusting for several covariates. Depression may be a risk factor for greater cognitive decline among Mexican Americans with no cognitive impairment. We did not detect sufficient evidence that indicates Mexican Americans with comorbid depression and diabetes experience greater cognitive decline or are more likely to develop severe cognitive impairment compared to Mexican Americans with depression or diabetes only. Interventions and clinical treatments that reduce the prevalence and incidence of depression and diabetes among older Mexican Americans may decrease the number of older adults who experience cognitive decline.
Acknowledgments
This work was supported by the National Institute on Aging contracts 5T32 AG000270-16 (to BD and BNV) and 5R01 AG010939-20 (to MR, SAS, and KSM) and P30 AG024832 (to SAS).
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Downer, Al Snih, Markides: study conception and design. Downer: data analysis. Markides: data acquisition. All authors were involved in the interpretation of data, substantive edits, and manuscript preparation. All authors have given final approval of this version of the manuscript.
Sponsor’s Role: The sponsors did not play any role in the design, methods, subject recruitment, data collection, analysis, or preparation of this manuscript.
References
- 1.Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 2.de Groot M, Anderson R, Freedland KE, et al. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Katon W, Young BA, Russo J, et al. Association of depression with increased risk of severe hypoglycemic episodes in patients with diabetes. Ann Fam Med. 2013;11:245–250. doi: 10.1370/afm.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman JA, Mast BT, Miles T, et al. Vascular risk and depression in the Hispanic Established Population for the Epidemiologic Study of the Elderly (EPESE) Int J Geriatr Psychiatry. 2009;24:409–416. doi: 10.1002/gps.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: A prospective cohort study. Diabetes Care. 2010;33:264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 7.Katon W, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 8.Lenoir H, Dufouil C, Auriacombe S, et al. Depression history, depressive symptoms, and incident dementia: The 3C Study. J Alzheimers Dis. 2011;26:27–38. doi: 10.3233/JAD-2011-101614. [DOI] [PubMed] [Google Scholar]
- 9.Strachan MW, Reynolds RM, Marioni RE, et al. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 10.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 13.Rapp MA, Schnaider-Beeri M, Purohit DP, et al. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry. 2008;16:168–174. doi: 10.1097/JGP.0b013e31816029ec. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 15.Raji MA, Reyes-Ortiz CA, Kuo YF, et al. Depressive symptoms and cognitive change in older Mexican Americans. J Geriatr Psychiatry Neurol. 2007;20:145–152. doi: 10.1177/0891988707303604. [DOI] [PubMed] [Google Scholar]
- 16.Wu JH, Haan MN, Liang J, et al. Impact of diabetes on cognitive function among older Latinos: A population-based cohort study. J Clin Epidemiol. 2003;56:686–693. doi: 10.1016/s0895-4356(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 17.Langa KM, Kabeto M, Weir D. Report on Race and Cognitive Impairment using HRS. 2010 Alzheimer’s Disease Facts and Figures [Internet] 2009 Available from: http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf.
- 18.O'Bryant SE, Johnson L, Balldin V, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2013;33:373–379. doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau; Bureau USC, editor. Table 9: Resident Population by Race, Hispanic Origin, and Age: 2000 and 2009. Washington D.C: 2011. [Google Scholar]
- 20.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 22.Steffens DC, Fisher GG, Langa KM, et al. Prevalence of depression among older Americans: The Aging, Demographics and Memory Study. Int Psychogeriatr. 2009;21:879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riolo SA, Nguyen TA, Greden JF, et al. Prevalence of depression by race/ethnicity: Findings from the National Health and Nutrition Examination Survey III. Am J Public Health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945–1952. doi: 10.2105/ajph.93.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markides KS, Rudkin L, Angel RJ, et al. Health Status of Hispanic Elderly. In: Martin LG, Soldo BJ, editors. Racial and Ethnic Differences in the Health of Older Americans. Washington DC: National Academy Press; 1997. pp. 285–300. [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1(3 Summer):385–401. [Google Scholar]
- 27.El Fakiri F, Bruijnzeels MA, Hoes AW. No evidence for marked ethnic differences in accuracy of self-reported diabetes, hypertension, and hypercholesterolemia. J Clin Epidemiol. 2007;60:1271–1279. doi: 10.1016/j.jclinepi.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State" A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Cameron J, Worrall-Carter L, Page K, et al. Screening for mild cognitive impairment in patients with heart failure: Montreal cognitive assessment versus Mini Mental State Exam. Eur J Cardiovasc Nurs. 2013;12:252–260. doi: 10.1177/1474515111435606. [DOI] [PubMed] [Google Scholar]
- 30.Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391.31. [PubMed] [Google Scholar]
- 31.Uhlmann RF, Larson EB. Effect of education on the Mini-Mental State Examination as a screening test for dementia. J Am Geriatr Soc. 1991;39:876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 32.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 33.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. 2. Hoboken: John Wiley & Sons; 2011. [Google Scholar]
- 34.Tombaugh TN, McIntyre NJ. The mini-mental state examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 35.Riley KP, Jicha GA, Davis D, et al. Prediction of preclinical Alzheimer's disease: Longitudinal rates of change in cognition. J Alzheimers Dis. 2011;25:707–717. doi: 10.3233/JAD-2011-102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katon W. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 37.Egede LE. Effect of comorbid chronic diseases on prevalence and odds of depression in adults with diabetes. Psychosom Med. 2005;67:46–51. doi: 10.1097/01.psy.0000149260.82006.fb. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein FC, Levey AI, Steenland NK. High blood pressure and cognitive decline in mild cognitive impairment. J Am Geriatr Soc. 2013;61:67–73. doi: 10.1111/jgs.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linden T, Skoog I, Fagerberg B, et al. Cognitive impairment and dementia 20 months after stroke. Neuroepidemiology. 2004;23:45–52. doi: 10.1159/000073974. [DOI] [PubMed] [Google Scholar]
- 40.Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–145. doi: 10.2147/CLEP.S30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirza SS, de Bruijn RF, Direk N, et al. Depressive symptoms predict incident dementia during short- but not long-term follow-up period. Alzheimers Dement. 2014;10(5 Suppl):S323–S329. doi: 10.1016/j.jalz.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapp MA, Schnaider-Beeri M, Wysocki M, et al. Cognitive decline in patients with dementia as a function of depression. Am J Geriatr Psychiatry. 2011;19:357–363. doi: 10.1097/JGP.0b013e3181e898d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galasko DR, Gould RL, Abramson IS, et al. Measuring cognitive change in a cohort of patients with Alzheimer's disease. Stat Med. 2000;19:1421–1432. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1421::aid-sim434>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 45.Mier N, Bocanegra-Alonso A, Zhan D, et al. Clinical depressive symptoms and diabetes in a binational border population. J Am Board Fam Med. 2008;21:223–233. doi: 10.3122/jabfm.2008.03.070255. [DOI] [PubMed] [Google Scholar]
- 46.Thackeray R, Merrill RM, Neiger BL. Disparities in diabetes management practice between racial and ethnic groups in the United States. Diabetes Educ. 2004;30:665–675. doi: 10.1177/014572170403000418. [DOI] [PubMed] [Google Scholar]
- 47.De Jesus M, Xiao C. Predicting health care utilization among latinos: Health locus of control beliefs or access factors? Health Educ Behav. 2014;41:423–430. doi: 10.1177/1090198114529130. [DOI] [PubMed] [Google Scholar]
- 48.Katon W, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: A prospective cohort study. J Gen Intern Med. 2010;25:423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katon W, Lyles CR, Parker MM, et al. Association of depression with increased risk of dementia in patients with type 2 diabetes: The Diabetes and Aging Study. Arch Gen Psychiatry. 2012;69:410–417. doi: 10.1001/archgenpsychiatry.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watari K, Letamendi A, Elderkin-Thompson V, et al. Cognitive function in adults with type 2 diabetes and major depression. Arch Clin Neuropsychol. 2006;21:787–796. doi: 10.1016/j.acn.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Alexopoulos GS, Meyers BS, Young RC, et al. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 52.Baldwin RC, O'Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157–160. doi: 10.1192/bjp.180.2.157. [DOI] [PubMed] [Google Scholar]
- 53.Mast BT, Yochim B, MacNeill SE, et al. Risk factors for geriatric depression: The importance of executive functioning within the vascular depression hypothesis. J Gerontol A Biol Sci Med Sci. 2004;59:1290–1294. doi: 10.1093/gerona/59.12.1290. [DOI] [PubMed] [Google Scholar]
- 54.Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 55.Perrino T, Mason CA, Brown SC, et al. Longitudinal relationships between cognitive functioning and depressive symptoms among Hispanic older adults. J Gerontol B Psychol Sci Soc Sci. 2008;63:P309–317. doi: 10.1093/geronb/63.5.p309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acevedo A, Loewenstein DA, Agron J, et al. Influence of sociodemographic variables on neuropsychological test performance in Spanish-speaking older adults. J Clin Exp Neuropsychol. 2007;29:530–544. doi: 10.1080/13803390600814740. [DOI] [PubMed] [Google Scholar]
- 57.Ortiz IE, LaRue A, Romero LJ, et al. Comparison of cultural bias in two cognitive screening instruments in elderly Hispanic patients in New Mexico. Am J Geriatr Psychiatry. 1997;5:333–338. doi: 10.1097/00019442-199700540-00008. [DOI] [PubMed] [Google Scholar]