Abstract
Objectives
Breathlessness is prevalent in older people. Symptom control at the end of life is important. This study investigated relationships between age, clinical characteristics and breathlessness sufficient to have people spend at least one half a day in that month in bed or cut down on their usual activities (restricting breathlessness) during the last year of life.
Design
Secondary data-analysis
Setting
General community
Participants
754 non-disabled persons, aged 70 and older. Monthly telephone interviews were conducted to determine the occurrence of restricting breathlessness. The primary outcome was the percentage of months with restricting breathlessness reported during the last year of life.
Results
Data regarding breathlessness were available for 548/589 (93.0%) decedents (mean age 86.7 years (range 71 to 106; males 38.8%). 311/548 (56.8%) reported restricting breathlessness at some time-point during the last year of life but no-one reported this every month. Frequency increased in the months closer to death irrespective of cause. Restricting breathlessness was associated with anxiety, (0.25 percentage point increase in months breathlessness per percentage point months reported anxiety, 95% CI 0.16 to 0.34, P<0.001), depression (0.14, 0.05 to 0.24, P=0.003) and mobility problems (0.07, 0.03 to 0.1, P<0.001). Percentage months of restricting breathlessness increased if chronic lung disease was noted at the most recent comprehensive assessment (6.62 percentage points, 95% CI 4.31 to 8.94, P<0.001), heart failure (3.34, 0.71 to 5.97, P<0.01), and ex-smoker status (3.01, 0.94 to 5.07, P=0.004), but decreased with older age (−0.19, −0.37 to −0.02, P=0.03).
Conclusion
Restricting breathlessness increased in this elderly population in the months preceding death from any cause. Breathlessness should be assessed and managed in the context of poor prognosis.
Keywords: dyspnea, breathlessness, prevalence, end of life, elderly
INTRODUCTION
Published estimates for the prevalence of breathlessness, medically known as dyspnea, in the general population vary between 9% to 59%, with a higher prevalence in older populations and in women.1–8 A large national primary care study found that one in three people seen by their family practitioner with breathlessness was over 75 years old.9 Common cardio-respiratory conditions such as cancer,10 chronic non-malignant lung disease, and heart failure increase in prevalence with age and are common causes of breathlessness (60% – 88% with heart failure and 90% – 95% with late stage chronic obstructive pulmonary disease).11 Breathlessness worsens in the advanced stages of disease,12 and in the weeks prior to death.13 Post-bereavement interviews among informal caregivers of people with lung cancer found that 78% of decedents had experienced breathlessness in their final year.14
Breathlessness is a frightening symptom which limits all aspects of life and is associated with poor clinical outcomes. People with breathlessness are more likely to be sent to hospital from primary care,9 admitted to hospital from the emergency department,15 experience an in-hospital serious event 16 and have a poorer prognosis.17–19 The relationship between breathlessness and mortality remains relevant for older age groups; in people aged 70 or over with breathlessness, both 2-year and 10-year survival are reduced.20 Good control of symptoms at the end of life is consistently rated as important by patients, caregivers, and health care providers.21 However, refractory breathlessness (breathlessness which persists despite optimal management of the underlying condition),22 is common and burdensome in many chronic cardio-respiratory conditions and cancer;12, 23 and is frequently neglected despite available effective interventions.24, 25 Breathlessness is likely to be particularly difficult for the elderly, where it adds burden to multiple chronic conditions with major effects on mobility, activities of daily living, confidence, independence, care needs and the ability to remain in their own homes in those older people nearing the end of life.
Despite associations between age and breathlessness, it is unknown whether breathlessness is primarily a feature of disease or of ageing.
Aim and Objectives
Using data from a cohort of community dwelling older people, we examined monthly patterns of reported difficulty breathing or shortness of breath sufficient to restrict activities (defined as stay in bed at least half a day, or cut down on their usual activities) during the last year of life (herein as restricting breathlessness). We investigated associations with clinical characteristics including “condition leading to death”, age and the dependent variable of restricting breathlessness reported longitudinally during the last year of life.
The study’s objectives were to: describe the prevalence and temporal patterns of restricting breathlessness during the last year; describe the temporal patterns of restricting breathlessness in relation to conditions leading to death; and to identify the clinical and demographic characteristics associated with restricting breathlessness.
Hypothesis
Based on the literature, we hypothesized that several factors would be associated with restricting breathlessness during the last year of life, including the presence of co-morbid disease, cause of death, smoking status, age, sex, anxiety and depression.
As this was a cohort of elderly people living at home (at study enrolment), this would include people who were medically fit but may have intercurrent illness, and those with chronic conditions causing breathlessness. We anticipated that during the last year of life, there would also be people who developed a condition which led to their death. Given the heterogeneity often observed in older persons, we also hypothesized that distinct trajectories of restricting breathlessness during the last year of life could be identified (no breathlessness; chronic [present at every month point]; intermittent; and crescendo breathlessness (started partway through the year and was continuously present until death).
METHODS
The study was approved by the human investigational committee at Yale University and participants provided verbal informed consent.
Design
This is a secondary analysis of data relating to the last year of life among decedents who had participated in a longitudinal cohort study of initially non-disabled, community-living persons 70 years of age or older when enrolled.26
Study population
The study cohort (n = 754) was identified from a computerized list of 3157 members of a large health plan in the United States of America. Sequential screening and enrollment was conducted between March 1998 and October 1999. Data collection is ongoing. Eligible plan members, 75.2% of whom agreed to participate, were English-speaking and could bathe, walk, dress, and transfer from a chair to standing without assistance at baseline. Those who did not agree to participate were not significantly different in terms of age or sex. Members were excluded if they had: a diagnosis of a terminal illness with an expected prognosis of less than 12 months; plans to move out of the study area during the next 12 months; or significant cognitive impairment with no available proxy.
Sample for analysis
Of the 589 participants who had died between October 1998 and June 2013, 41 had withdrawn from the study, leaving 548 (93%) with data on breathlessness in the last year of life. Of these, 50 (9.1%) died within the first year of follow up and therefore had less than one year of data.
Baseline data collection
Comprehensive home-based assessments were completed at baseline and at 18-month intervals by trained nurses who used standard instruments to perform all interviews and assessments. Data were collected on demographic characteristics and nine self-reported chronic conditions diagnosed by a physician (hypertension; myocardial infarction; congestive heart failure; stroke; diabetes; arthritis; hip fracture, chronic lung disease, cancer), cognition (Folstein Mini-Mental State Examination) and frailty.27
Follow up data collection
Telephone interviews were conducted monthly. For participants with significant cognitive impairment, data were collected from a proxy. Proxy assessments are valid for many measures, including breathlessness. 28, 29 Data were used from the comprehensive assessment that pre-dated death by at least one year and from the monthly interviews during the last year of life. Data for this current secondary analysis were collected until June 2013.
Condition leading to death
Decedents were identified through local obituary reviews, from the next of kin or another suitable informant during a subsequent telephone interview, or both. The classification of conditions leading to death is described elsewhere.30 The approach used by Lunney31 was modified by adding advanced dementia as a condition leading to death, and including chronic kidney disease and cirrhosis to the category of ‘organ failure’. Frailty, as a condition leading to death, was defined by the phenotype described by Fried et al32 using data from the comprehensive assessments. Where data from death certificates were obtained, diagnoses were the ‘immediate’ or ‘underlying cause’ of death.
Assessment of restricting breathlessness
This analysis relates to difficulty breathing that caused restriction in activity during the previous month. During the monthly interviews, participants were asked if they had stayed in bed at least half a day or cut down on their usual activities due to an illness, injury, or other problem in the preceding month. Those who said ‘yes’ to either question (i.e. had restricted activity) were asked about a series of problems including whether they had experienced “difficulty breathing or shortness of breath since we last talked” and whether or not that problem caused restricted activity. Restricting activity has face validity as an important concern for elderly people, recognised over 30 years ago in the US Surgeon General’s Healthy People Report.33–35 It has been extensively used in the elderly care literature where it has been a key outcome measure in several clinical trials of interventions.35–39
Statistical analysis
Using date of death as the anchor, descriptive statistics presented the number and percentage of participants reporting breathlessness at each month before death. We calculated the total number of months participants reported restricting breathlessness during the last year of life and evaluated its relationship with the condition leading to death, plotting this relationship as a percentage of the total possible number of months. If participants died during the first year of follow-up, the denominator was the number of months with available data.
We used percentage of months with restricting breathlessness during the last year as the outcome variable rather than a binary outcome (presence/absence) to provide a quantitative measure of restricting breathlessness. We investigated the relationships between percentage months of restricting breathlessness and possible associated variables recorded before the final year (the most recent comprehensive assessment prior to the last year of life), using two sample t tests or linear regression. From the monthly follow-up data during the last year of life we calculated the percentage of months for which possible associated variables, and proxy measures were recorded and tested their associations with months of restricting breathlessness using multiple regression. Candidate variables were drawn from the literature and can be seen in table 2.
Table 2.
Unifactorial analysis of candidate predictors
| Candidate predictor variables, and source | coefficient | 95% confidence intervals | Unifactorial analysis (P value) |
|---|---|---|---|
| Factors from most recent comprehensive assessment prior to last year of life | |||
| Age | −0.32 | −0.53 to −0.12 | 0.002 NB – inverse relationship |
|
|
|||
| Frailty* | −0.56 | −3.13 to 2.02 | 0.7 |
|
|
|||
| Mobility ** | 0.080 | 0.042 to 0.118 | <0.001 |
|
|
|||
| Gender(female) | 0.79 | −1.70 to 3.78 | 0.5 |
|
|
|||
| Proxy measures | 1.18 | −1.64 to 4.00 | 0.41 |
|
|
|||
| Peak expiratory flow rate | −0.04 | −0.08 to 0.01 | 0.1 |
|
|
|||
| Smoking status | 4.40 | 1.90 to 6.91 | 0.001 |
|
|
|||
| Congestive Heart failure | 3.68 | 0.39 to 6.97 | 0.03 |
| Chronic Lung disease | 8.73 | 6.00 to 11.45 | <0.001 |
| Heart attack | 2.24 | −0.56 to 5.04 | 0.1 |
| Cancer | 1.67 | −1.12 to 4.46 | 0.2 |
| Stroke | 3.62 | 0.30 to 6.95 | 0.03 |
| Arthritis | 1.43 | −0.99 to 3.85 | 0.2 |
| Hip fracture | 0.38 | −3.52 to 4.27 | 0.9 |
| Hypertension | −0.69 | −3.20 to 1.81 | 0.6 |
| Diabetes mellitus | 0.25 | −2.60 to 3.10 | 0.9 |
|
|
|||
| Condition leading to death: | |||
| cancer | 1.17 | −3.11 to 5.45 | < 0.001 |
| dementia | −5.35 | −9.66 to −1.04 | |
| organ failure | 5.46 | 1.31 to 9.60 | |
| sudden death | −7.18 | −15.20 to 0.84 | |
| frailty | −2.30 | −6.25 to 1.66 | |
|
|
|||
| Educational level | −0.05 | −0.47 to 0.36 | 0.8 |
|
|
|||
| “White” Ethnicity | −0.24 | −0.50 to 0.02 | 0.07 |
|
|
|||
| Depression (Center for Epidemiologic Studies-Depression Scale) yes/no | 1.35 | −1.42 to 4.13 | 0.3 |
|
|
|||
| Factors from monthly interviews during the last year of life | |||
|
|
|||
| Self report anxiety, present or absent | 0.44 | 0.38 to 0.50 | < 0.001 |
|
|
|||
| Self report depression, present or absent | 0.49 | 0.33 to 0.46 | < 0.001 |
|
|
|||
| Problems with mobility | 0.08 | 0.04 to 0.12 | < 0.001 |
|
|
|||
Frailty = >3 of: Slow gait, weight loss, exhaustion, inactivity and reduced grip strength
Mobility problems= the need for personal assistance or unable to walk one quarter of a mile or unable to climb one flight of stairs
We used multiple regression to investigate demographic and associated clinical factors, which had an effect identified in the unifactorial analysis and others with a plausible biological rationale or have been previously reported in the literature.40 These were included in a multiple regression analysis using stepwise analysis with backwards elimination. At each stage, the variable with the largest non-significant P value was removed and the step repeated until there were no non-significant associated factors.
To assess the effect of using a mixture of participant and proxy measures, the stepwise regression model included the proportion of proxy measures as an associated factor.
The appropriateness of the regression model was tested by examining residuals. Histograms and scatter plots were inspected for non-Normal distribution and non-linearity. Analyses were carried out by MB and MJ using Stata 12 (StataCorp, 2011, College Station, Texas).
RESULTS
Of the 548 decedents in the analytic sample, 61.2% were women. Ethnicity was coded as “white” (91.2%) or “other” (8.8%). The mean age at death was 86.7 years (SD 6.0; range 71 to 106; median age 87; IQR 83 to 91). Most were ex-smokers (59%) or current smokers (6%). This sample was representative of the main New Haven elderly cohort (64.6% women; 90.5% “white”), but has more “white” people and, as expected in an older group, more women than in the general population in that region (79.3% “white”; 51.7% female).41
Restricting breathlessness by condition leading to death
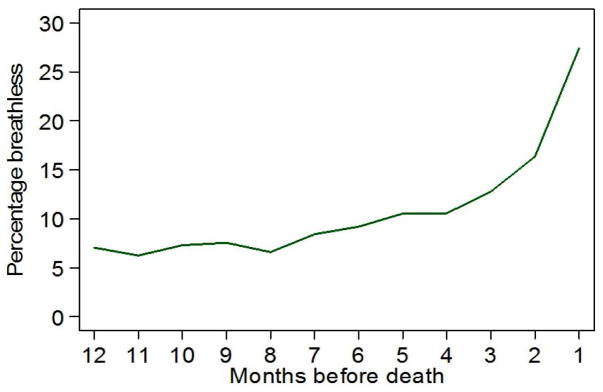
The proportion of participants reporting restricting breathlessness at each month time-point during the last year of life (Figure 1) increases closer to death.
Figure 1.
Percentage of participants reporting restricting breathlessness at each month during the last year of life
Temporal patterns of restricting breathlessness
Overall, 311/548 (56.8%) participants were reported to have restricting breathlessness at some point during the last year of life. Table 1 shows the participants who reported restricting breathlessness in the month before death and who reported this symptom in consecutive months prior to death, that is, those who presented with new breathlessness during the last year of life and continued with this symptom until death. No participant had restricting breathlessness reported at every month, and very few fitted the category “breathlessness that commenced partway through the year and continued”, for example, only 4 participants developed breathlessness during the last year and continued with reported breathlessness until death.
Table 1.
The Month of First Restricting Breathlessness which continued until the Month of Death
| frequency | percentage | ||
|---|---|---|---|
| Not breathless in last month | 400 | 73.0 | |
|
|
|||
| Breathless reported in the last month | 148 | 27.0 | |
|
| |||
| Breathless for 4 months before death | 4 | 0.7 | |
|
| |||
| Breathless for 3 months before death | 12 | 2.2 | |
|
| |||
| Breathless for 2 months before death | 29 | 5.3 | |
|
| |||
| Breathless for month in which death occurred but not previous month | 103 | 18.9 | |
|
|
|||
| Total | 548 | 100.0 | |
|
|
|||
Some of these participants may have had earlier restricting breathlessness episodes as well. Only about half (148/311) of those who reported restricting breathlessness at some time in the last year, were restricted by breathlessness one month before death. From the original hypotheses regarding temporal patterns of breathlessness during the last year, only “intermittent” and “crescendo” were observed.
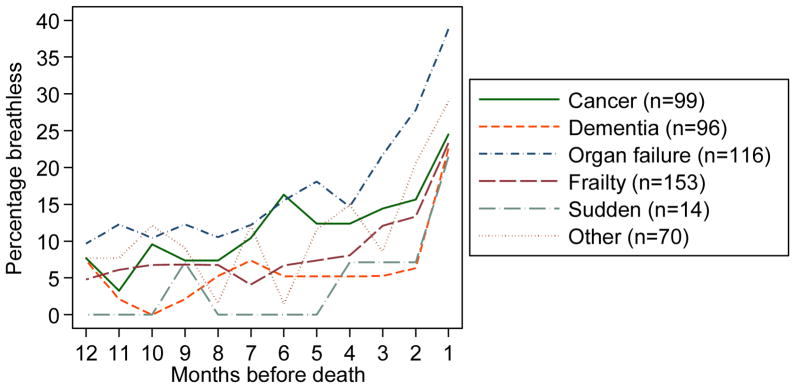
When displayed as restricting breathlessness per month according to the condition leading to death, breathlessness was most commonly reported for those who died from organ failure. This can be seen graphically in Figure 2, which shows increasing prevalence as death nears.
Figure 2.
The percentage of participants reporting restricting breathlessness at each month during the last year of life by condition leading to death
Selection of potential variables associated with restricting breathlessness during the last year of life: unifactorial analyses
Candidate factors with a plausible rationale and a statistically significant association with breathlessness in the last year of life on unifactorial analysis were: i) (when a problem was identified in the monthly report) mobility problems; depression; anxiety and, from death certificate data, the condition leading to death; and ii) from the most recent comprehensive assessment prior to the last year of life: age; frailty; gender; physicians’ diagnoses; peak expiratory flow rate (PEFR); smoking status. The condition leading to death was a six-level factor (cancer, dementia, organ failure, frailty, sudden, other) and was tested together, rather than by individual t tests. Smoking was a three-level factor (current, ex-smoker, never) and was also treated as a composite. Educational level was included due to its plausible impact and reported association with breathlessness in previous work.40. Other physician-diagnosed conditions such as arthritis, hip fracture, hypertension or diabetes mellitus had no rationale, nor unifactorial significance and were excluded. Ethnicity was not significant on unifactorial analysis. (See Table 2)
Predictors of restricting breathlessness during the last year of life: multifactorial analysis
The final multifactorial model is shown in Table 3. Percentage months of restricting breathlessness was associated with percentage months of anxiety, depression, and mobility problems reported during the last year of life, and a history of chronic lung disease, congestive heart failure, being an ex-smoker from the most recent comprehensive assessment, and having cancer, organ failure or other categories as the condition leading to death rather than frailty, dementia or sudden death. Percentage months of restricting breathlessness decreased with higher educational attainment and age at death.
Table 3.
Final Regression Model investigating Factors Associated with the Presence of Percentage Months of Reported Breathlessness during the Last Year of Life
| Coefficient | Standard Error | t | P>|t| | [95% Confidence Interval] | |
|---|---|---|---|---|---|
| Predictive factors from monthly interviews during the last year of life | |||||
| Depression* (Self report, present or absent) | 0.14 | 0.05 | 3.02 | 0.003 | 0.05 to 0.24 |
|
|
|||||
| Mobility problems* (Self report, present or absent) | 0.07 | 0.02 | 3.85 | <0.001 | 0.03 to 0.11 |
|
|
|||||
| Anxiety* (Self report, present or absent) | 0.25 | 0.05 | 5.24 | <0.001 | 0.16 to 0.34 |
| PREDICTIVE FACTORS FROM MOST RECENT COMPREHENSIVE ASSESSMENT PRIOR TO LAST YEAR OF LIFE | |||||
| Educational attainment | −0.47 | 0.17 | −2.76 | 0.006 | −0.80 to −0.13 |
|
|
|||||
| Chronic lung disease | 6.62 | 1.18 | 5.62 | <0.001 | 4.31 to 8.94 |
|
|
|||||
| Congestive heart failure | 3.34 | 1.34 | 2.50 | 0.013 | 0.71 to 5.97 |
| Condition leading to death | |||||
| Cancer | 3.25 | 1.53 | 2.12 | 0.034 | 0.24 to 6.25 |
|
|
|||||
| Other condition | 3.64 | 1.71 | 2.12 | 0.034 | 0.27 to 7.00 |
|
|
|||||
| Dementia | −0.81 | 1.49 | −0.54 | 0.586 | −3.75 to 2.12 |
|
|
|||||
| Organ failure | 4.19 | 1.41 | 2.97 | 0.003 | 1.41 to 6.96 |
|
|
|||||
| Sudden death | 0.32 | 3.26 | 0.10 | 0.923 | −6.08 to 6. |
|
|
|||||
| Age one year prior to death | −0.19 | 0.09 | −2.17 | 0.030 | −0.37 to −0.02 |
|
|
|||||
| Smoking status | |||||
|
|
|||||
| Ex-smoker | 3.01 | 1.05 | 2.86 | 0.004 | 0.94 to 5.07 |
|
|
|||||
| Never smokers | 1.28 | 2.17 | 0.59 | 0.554 | −2.97 to 5.54 |
|
|
|||||
| constant | 16.48 | 8.02 | 2.06 | 0.73 to 32.24 | |
|
|
|||||
percentage months reported during the last year of life
P = probability
For an explanation of the meaning of the co-efficient, see text
For associated factors identified from the comprehensive assessment recorded as present or absent e.g. chronic lung disease, the coefficient represents the change in percentage of months with breathlessness if the diagnosis is present. For associated factors recorded as percentage of months where the factor is reported during the last year of life, the coefficient represents the change in percentage months with breathlessness for each percentage point of months with this report. Thus an increase of one percentage point in months of anxiety is related to an increase of 0.25 in percentage months of breathlessness. Age is in years, so an increase of one year is related to a 0.23 reduction in percentage months with breathlessness. Testing of the regression model confirmed acceptable analyses.
Proxy measures for restricting breathlessness were obtained for 418 participants, including 177 with partial proxy data and 241 with complete proxy data. In the last year of life, the proportion of proxy measures increased slowly for the first nine months from (37% to 45%), and more rapidly over the last three months of life (49%; 57%; 70%). To assess the effect of using a mixture of participant and proxy measures, stepwise regression included the proportion of proxy measures as an associated factor. It did not contribute to the final model. We also regressed the percentage of months with breathlessness on proportion of proxies but the relationship was not significant (P=0.40). It was felt that, in such a regression analysis, this approach was an appropriate and sufficiently robust method to assess the influence of proxy measurement and demonstrated that the analysis was not materially affected by proxy measures.
DISCUSSION
These data show that during the last year of life, over 50% of community-living elderly people experienced breathlessness severe enough to restrict activity. Only half of those who reported breathlessness at some point during their last year had breathlessness during their last month. Few people (10%) had restricting breathlessness until a few months before their death. This prevalence may be considered lower than expected given previous community studies of older people (e.g. 32.3 to 37%)4, 6, but these studies used an outcome of MRC ≥3 (”On level ground, I walk slower than people of the same age because of breathlessness, or have to stop for breath when walking at my own pace”) which is likely to represent a much less severe level of breathlessness than that measured in this study.
The prevalence of breathlessness increased in the last few months prior to death for all conditions leading to death, including dementia and sudden death. Sudden death due to cardiac arrhythmia is associated with heart failure and an increase in breathlessness could reflect worsening of the heart condition. The finding that breathlessness increased prior to death in people dying with dementia is consistent with a retrospective study of nursing home residents with advanced dementia which found the presence of breathlessness to be a poor prognostic indicator.42 Even in the setting of dementia, the impact of increasing breathlessness speaks to the prevalence of this symptom irrespective of the underlying etiology, and the importance of breathlessness as a prognostic factor.
Although the prevalence of breathlessness is known to be higher in older people when compared with younger people, within this elderly population, age was inversely related to restricting breathlessness during their last year. Restricting breathlessness was also associated with a diagnosis of heart and lung disease, mobility problems, anxiety and depression, smoking status and with cancer as a condition leading to death. It may be that those living to older old age have lived to such an age because they do not have the medical conditions associated with restricting breathlessness.
The strong association between anxiety documented during monthly interviews and restricting breathlessness during the last year is consistent with other literature which describes anxiety as both a driver and a consequence of breathlessness,43 although causality cannot be inferred from these data. The similarly strong association seen for depression noted at monthly interviews and restricting breathlessness during the last year, was not found for depression reported at the last comprehensive assessment prior to death. This may indicate that breathlessness contributes to depression in this population. Depression is prevalent in people with chronic conditions and cancer, adversely impacting on quality of life, adherence with management and days at home.44 These new data confirm the importance of diagnosis and management of this potentially reversible co-morbidity.
Although there are reports that women report more dyspnea for the same workload than men,40, 45, 1, 8 we did not find an excess of reported breathlessness in women.
Mobility problems were associated with breathlessness; it is interesting to note that there is trial evidence to support the use of mobility aids to improve breathlessness.46
Implications for clinical practice and future research
The increased prevalence of restricting breathlessness in the months prior to death in each of the etiological sub-groups suggests that breathlessness is part of a final common pathway at the end of life. The presence, therefore, of this symptom should be systematically identified, assessed and managed in order to ensure optimal symptom management at the end of life, and optimal care planning in the light of its association with poor prognosis. However, although a trajectory pattern of escalating breathlessness was seen in some individuals, it was not inevitable that a participant reporting restricting breathlessness would continue with this symptom until death.
Strengths and Limitations
Strengths of the present study include its prospective, repeated-measures design with standardized and comprehensive assessments including monthly reports with high rates of participation and follow up. Prospective assessment of self-reported symptoms, rather than a reliance on administrative or registry based data, is vital as older persons may not seek medical attention despite severe and prolonged symptomatology.49
With the exception of ethnicity, the cohort demographics reflect the US general population well.26 The study cohort oversampled people at enrollment with an intermediate and high risk for disability, but rates of disability or symptoms were not affected.50
“Restricting breathlessness” is not a familiar outcome in the palliative care or respiratory literature, in which measures of intensity, mastery or exercise tolerance are more often used such as the MRC dyspnea scale. 47 Like the MRC scale, it is a subjective measure and relative to what the participant counts as usual daily activities. These results indicate that restricted activity is a practical way to quantify the impact on people who experience restricting breathlessness. Functional status is known to be a useful prognostic indicator,48 and further work is indicated to explore further whether breathlessness sufficient to affect function is also a useful marker of deterioration.
The dataset has proved immensely valuable in increasing understanding of the experience of the elderly over time, and the effect of many health issues on their independence and ability to conduct activities of daily living. Furthermore, we specifically wished to investigate breathlessness which was bad enough to potentially affect independent living, and thus this definition of restricting breathlessness was fit for our purpose.
We cannot comment on the number of days with restricted activity or the symptom severity, except that all reports relate to breathlessness sufficient to restrict activities, therefore all reports are of direct relevance to the respondents.
CONCLUSION
This analysis of data from a longstanding longitudinal study adds to the understanding of clinically relevant breathlessness in the elderly in the last year of life. Restricting breathlessness increased in this elderly population in the months preceding death from any cause. The data suggest that ageing per se may not cause breathlessness in the absence of other pathology. Data may indicate that depression was a response to restricting breathlessness. These data suggest that breathlessness may be part of a final common pathway very late in life. This symptom should therefore be systematically assessed and managed in the likely context of poor prognosis.
Acknowledgments
Funding: National Institute on Ageing (NIA)(R37AG17560). Data were collected, in part, through the support of the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging.
Funding: The work for this article was funded by a grant from the National Institute on Ageing (NIA)(R37AG17560). Thomas M. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. The PEP study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342).
Footnotes
Conference presentation: Oral presentation. American Thoracic Society May 2015 International Conference, Denver, Colorado.
Sponsor’s Role: The sponsors did not have any role in the design, conduct, interpretation, review, approval, or control of this article.
Conflict of Interest: David C. Currow and Miriam J. Johnson are clinical consultants for Mayne Pharma (institutional payment). Thomas M. Gill is the holder of the NIA grant which funded the PEP study. No other authors have conflicts of interests to declare.
Author Contributions
David C. Currow and Miriam J. Johnson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: David C. Currow, Miriam J. Johnson, Magnus Ekström
Acquisition of data and funding: Thomas M. Gill.
Analysis and interpretation of data: Martin Bland, Miriam J. Johnson
Statistical expertise: Martin Bland
Drafting of the manuscript: Miriam J. Johnson
Critical revision of the manuscript for important intellectual content: All authors
References
- 1.Guenette JA, Jensen D, Webb KA, et al. Sex differences in exertional dyspnea in patients with mild COPD: Physiological mechanisms. Respir Physiol Neurobiol. 2011;177:218–227. doi: 10.1016/j.resp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Currow DC, Plummer JL, Crockett A, et al. A community population survey of prevalence and severity of dyspnea in adults. J Pain Symptom Manage. 2009;38:533–545. doi: 10.1016/j.jpainsymman.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Frostad A, Soyseth V, Haldorsen T, et al. Respiratory symptoms and 30 year mortality from obstructive lung disease and pneumonia. Thorax. 2006;61:951–956. doi: 10.1136/thx.2006.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho SF, O’Mahony MS, Steward JA, et al. Dyspnoea and quality of life in older people at home. Age Ageing. 2001;30:155–159. doi: 10.1093/ageing/30.2.155. [DOI] [PubMed] [Google Scholar]
- 5.Shin C, Lee S, Abbott RD, et al. Relationships between respiratory symptoms and FEV1 in men and women with normal lung function: The Korean Health and Genome Study. Lung. 2005;183:301–309. doi: 10.1007/s00408-004-2543-y. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen F, Mehlsen J, Raymond I, et al. Evaluation of dyspnoea in a sample of elderly subjects recruited from general practice. Int J Clin Pract. 2007;61:1481–1491. doi: 10.1111/j.1742-1241.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MJ, Currow DC, Booth S. Prevalence and assessment of breathlessness in the clinical setting. Expert Rev Respir Med. 2014;8:151–161. doi: 10.1586/17476348.2014.879530. [DOI] [PubMed] [Google Scholar]
- 8.Ofir D, Laveneziana P, Webb KA, et al. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol (1985 ) 2008;104:1583–1593. doi: 10.1152/japplphysiol.00079.2008. [DOI] [PubMed] [Google Scholar]
- 9.Currow DC, Clark K, Mitchell GS, et al. The characteristics of adults and their consultations as they present with breathlessness to general practice in Australia. PLOS ONE. 2013 doi: 10.1371/journal.pone.0074814. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7:233–243. doi: 10.1007/s005200050255. [DOI] [PubMed] [Google Scholar]
- 11.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Moens K, Higginson IJ, Harding R. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48:660–677. doi: 10.1016/j.jpainsymman.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Currow DC, Smith J, Davidson PM, et al. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39:680–690. doi: 10.1016/j.jpainsymman.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Edmonds P, Karlsen S, Khan S, et al. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med. 2001;15:287–295. doi: 10.1191/026921601678320278. [DOI] [PubMed] [Google Scholar]
- 15.Saracino A, Weiland TJ, Jolly B, et al. Verbal dyspnoea score predicts emergency department departure status in patients with shortness of breath. Emerg Med Australas. 2010;22:21–29. doi: 10.1111/j.1742-6723.2009.01254.x. [DOI] [PubMed] [Google Scholar]
- 16.Banzett RB, Howell MD, O’Donnell CR, et al. Dyspnea Prevalence And Risk Of Adverse Event In A General Hospital Population. American Journal of Respiratory and Critical Care Medicine 2013; American Thoracic Society International Conference Abstract Issue(B27. PATIENT-REPORTED OUTCOMES: SYMPTOMS AND QUALITY OF LIFE). [Google Scholar]
- 17.Frostad A, Soyseth V, Andersen A, et al. Respiratory symptoms as predictors of all-cause mortality in an urban community: A 30-year follow-up. J Intern Med. 2006;259:520–529. doi: 10.1111/j.1365-2796.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- 18.Geraci JM, Tsang W, Valdres RV, et al. Progressive disease in patients with cancer presenting to an emergency room with acute symptoms predicts short-term mortality. Support Care Cancer. 2006;14:1038–1045. doi: 10.1007/s00520-006-0053-6. [DOI] [PubMed] [Google Scholar]
- 19.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1.064. 004 men and women. Am J Public Health Nations Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed T, Steward JA, O’Mahony MS. Dyspnoea and mortality in older people in the community: a 10-year follow-up. Age Ageing. 2012;41:545–549. doi: 10.1093/ageing/afs049. [DOI] [PubMed] [Google Scholar]
- 21.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 22.Abernethy AP, Currow DC, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327:523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bausewein C, Booth S, Gysels M, et al. Understanding breathlessness: Cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13:1109–1118. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 24.Farquhar MC, Prevost A, McCrone P, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med. 2014;12:194. doi: 10.1186/s12916-014-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higginson IJ, Bausewein C, Reilly C, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: A randomised controlled trial. Lancet Respir Med. 2014;2:979–987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 26.Gill TM, Desai MM, Gahbauer EA, et al. Restricted activity among community-living older persons: Incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 27.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 28.Higginson I, Priest P, McCarthy M. Are bereaved family members a valid proxy for a patient’s assessment of dying? Soc Sci Med. 1994;38:553–557. doi: 10.1016/0277-9536(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 29.Quinn C, Dunbar SB, Higgins M. Heart failure symptom assessment and management: Can caregivers serve as proxy? J Cardiovasc Nurs. 2010;25:142–148. doi: 10.1097/JCN.0b013e3181bf93a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill TM, Gahbauer EA, Han L, et al. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 33.National Health Interview Survey, National Center for Health Statistics. Current Estimates from the National Health Interview Survey, United States. Hyattsville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of Health Research, Statistics, and Technology, National Center for Health Statistics; 1986. DHHS publication no. PHS 86–1584. [Google Scholar]
- 34.Healthy People: The Surgeon General’s Report on Health Promotion and Disease Prevention, 1979. Rockville, MD: U. S. Department of Health, Education, and Welfare, Public Health Service, Office of the Assistant Secretary for Health and Surgeon General; 1979. DHEW (PHS) publication no. 79-55071. [Google Scholar]
- 35.Institute of Medicine. Health Outcomes for Older People: Questions for the Coming Decade. Washington, DC: National Academy Pr; 1996. [PubMed] [Google Scholar]
- 36.Wagner EH, LaCroix AZ, Grothaus L, et al. Preventing disability and falls in older adults: A population-based randomized trial. Am J Public Health. 1994;84:1800–1806. doi: 10.2105/ajph.84.11.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leveille SG, Wagner EH, Davis C, et al. Preventing disability and managing chronic illness in frail older adults: A randomized trial of a community-based partnership with primary care. J Am Geriatr Soc. 1998;46:1191–1198. doi: 10.1111/j.1532-5415.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- 38.Nevitt MC, Thompson DE, Black DM, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med. 2000;160:77–85. doi: 10.1001/archinte.160.1.77. [DOI] [PubMed] [Google Scholar]
- 39.Reuben DB, Frank JC, Hirsch SH, et al. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. J Am Geriatr Soc. 1999;47:269–276. doi: 10.1111/j.1532-5415.1999.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 40.Gronseth R, Vollmer WM, Hardie JA, et al. Predictors of dyspnoea prevalence: Results from the BOLD study. Eur Respir J. 2014;43:1610–1620. doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.United States Census Bureau. Quickfacts census. New Haven County; Connecticut: 2014. [28-5-2015]. http://quickfacts.census.gov/qfd/states/09/09009.html. [Google Scholar]
- 42.Mitchell SL, Miller SC, Teno JM, et al. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304:1929–1935. doi: 10.1001/jama.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neuman A, Gunnbjornsdottir M, Tunsater A, et al. Dyspnea in relation to symptoms of anxiety and depression: A prospective population study. Respir Med. 2006;100:1843–1849. doi: 10.1016/j.rmed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. 2002;53:859–863. doi: 10.1016/s0022-3999(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 45.Bowden JA, To TH, Abernethy AP, et al. Predictors of chronic breathlessness: A large population study. BMC Public Health. 2011;11:33. doi: 10.1186/1471-2458-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crisafulli E, Costi S, De BF, et al. Effects of a walking aid in COPD patients receiving oxygen therapy. Chest. 2007;131:1068–1074. doi: 10.1378/chest.06-2108. [DOI] [PubMed] [Google Scholar]
- 47.Dorman S, Jolley C, Abernethy A, et al. Researching breathlessness in palliative care: consensus statement of the National Cancer Research Institute Palliative Care Breathlessness Subgroup. Palliat Med. 2009;23:213–227. doi: 10.1177/0269216309102520. [DOI] [PubMed] [Google Scholar]
- 48.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 49.Rakowski W, Julius M, Hickey T, et al. Daily symptoms and behavioral responses. Results of a health diary with older adults. Med Care. 1988;26:278–297. doi: 10.1097/00005650-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Gill TM, Gahbauer EA, Han L, et al. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci. 2011;66:1238–1243. doi: 10.1093/gerona/glr142. [DOI] [PMC free article] [PubMed] [Google Scholar]