Abstract
We carried out a trans-ancestry genome-wide association and replication study of blood pressure phenotypes among up to 320,251 individuals of East Asian, European and South Asian ancestry. We find genetic variants at 12 new loci to be associated with blood pressure (P = 3.9 × 10−11 to 5.0 × 10−21). The sentinel blood pressure SNPs are enriched for association with DNA methylation at multiple nearby CpG sites, suggesting that, at some of the loci identified, DNA methylation may lie on the regulatory pathway linking sequence variation to blood pressure. The sentinel SNPs at the 12 new loci point to genes involved in vascular smooth muscle (IGFBP3, KCNK3, PDE3A and PRDM6) and renal (ARHGAP24, OSR1, SLC22A7 and TBX2) function. The new and known genetic variants predict increased left ventricular mass, circulating levels of NT-proBNP, and cardiovascular and all-cause mortality (P = 0.04 to 8.6 × 10−6). Our results provide new evidence for the role of DNA methylation in blood pressure regulation.
High blood pressure, which affects more than 1 billion people worldwide, is a major risk factor for myocardial infarction, stroke and chronic kidney disease. Approximately 9 million deaths each year are attributable to high blood pressure, including >50% of deaths from coronary heart disease and stroke1,2. High blood pressure is more prevalent in people of East Asian and South Asian ancestry and is a major contributor to their increased risk of stroke and coronary heart disease3,4. Genome-wide association studies (GWAS) have identified over 50 genetic loci influencing blood pressure in predominantly European populations5–16. A role for epigenetic mechanisms in blood pressure regulation has also been suggested17–20.
We carried out a GWAS in East Asians and South Asians, as well as Europeans, to seek both cosmopolitan and population-specific genetic effects for five blood pressure phenotypes: systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure, mean arterial pressure (MAP) and hypertension (Supplementary Fig. 1) (ref. 5). We then sought DNA coding and gene regulatory mechanisms, including DNA methylation and gene transcription, to help explain the relationships we observed between sequence variation and blood pressure.
RESULTS
Genome-wide association and replication testing
We used genome-wide association data from 99,994 individuals of East Asian (n = 31,516), European (n = 35,352) and South Asian (n = 33,126) ancestry. Characteristics of the participants and information on the genotyping arrays and imputation are summarized in Supplementary Tables 1–3. Phenotype-specific meta-analysis was carried out separately for East Asian, European and South Asian samples, followed by a meta-analysis across the three ancestral population groups.
The trans-ancestry genome-wide association results identified 4,077 variants with P < 1 × 10−4 against any blood pressure phenotype, distributed among 630 genetic loci. At each locus, we identified the sentinel SNP (the SNP with the lowest P value against any phenotype) and carried out combined analysis with phenotype-specific results from the International Consortium on Blood Pressure (ICBP) GWAS (maximum n = 87,205) (refs. 8,9). This analysis identified 19 previously unreported loci where the sentinel SNP had suggestive evidence for association with blood pressure (P < 1 × 10−7; Supplementary Table 4). We performed further testing of these 19 SNPs in additional samples of up to 133,052 individuals (48,268 East Asian, 68,456 European and 16,328 South Asian; Supplementary Table 5). Twelve of the 19 SNPs reached both P < 0.05 in replication testing and P < 1 × 10−9 in the combined analysis of data from across all stages (Table 1, Supplementary Figs. 2 and 3, and Supplementary Table 6). We set the threshold for genome-wide significance as P = 1 × 10−9 to provide a conservative Bonferroni correction for testing ~2.1 million SNPs against the 5 blood pressure phenotypes, in the 3 ancestry groups and overall.
Table 1.
Genetic loci newly identified to be associated with blood pressure
| Sentinel SNP | Chr. | Position (bp) | Candidate gene | EA | AA | EAF | Phenotype | n | Effect (mm Hg) | P |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1344653 | 2 | 19,730,845 | OSR1 n,m | A | G | 0.54 | PP | 220,853 | −0.27 (0.04) | 7.8 × 10−12 |
| rs1275988 | 2 | 26,914,364 | KCNK3 n,m | T | C | 0.50 | MAP | 236,311 | −0.37 (0.04) | 5.0 × 10−21 |
| rs2014912 | 4 | 86,715,670 | ARHGAP24 n,m | T | C | 0.16 | SBP | 242,456 | 0.62 (0.08) | 5.4 × 10−17 |
| rs13359291 | 5 | 122,476,457 | PRDM6 n,m | A | G | 0.31 | SBP | 229,584 | 0.53 (0.07) | 8.9 × 10−16 |
| rs9687065 | 5 | 148,391,140 | ABLIM3m, SH3TC2n,ns | A | G | 0.76 | DBP | 259,216 | 0.26 (0.04) | 7.4 × 10−11 |
| rs1563788 | 6 | 43,308,363 | TTBK1m, SLC22A7sv, ZNF318n,e | T | C | 0.31 | SBP | 220,757 | 0.51 (0.06) | 2.2 × 10−16 |
| rs2107595 | 7 | 19,049,388 | HDAC9 n | A | G | 0.24 | PP | 209,305 | 0.31 (0.05) | 3.9 × 10−11 |
| rs10260816 | 7 | 46,010,100 | IGFBP3 n,m,ns | C | G | 0.62 | PP | 207,070 | 0.32 (0.04) | 1.5 × 10−14 |
| rs751984 | 11 | 61,278,246 | LRRC10Bn, SYT7n,m | T | C | 0.76 | MAP | 233,082 | 0.33 (0.05) | 7.7 × 10−12 |
| rs12579720 | 12 | 20,173,764 | PDE3A n | C | G | 0.33 | DBP | 218,606 | −0.32 (0.04) | 2.2 × 10−16 |
| rs2240736 | 17 | 59,485,393 | C17orf82n, TBX2n,m,ns | T | C | 0.65 | MAP | 217,197 | 0.35 (0.04) | 2.2 × 10−16 |
| rs740406 | 19 | 2,232,221 | AMHm, DOT1Ln, PLEKHJ1n, SF3A2n | A | G | 0.85 | PP | 193,219 | −0.55 (0.07) | 3.1 × 10−15 |
Candidate genes are annotated by the nature of the variant: e, expression quantitative trait locus (eQTL); n, nearby gene (±10 kb); ns, nonsynonymous; sv, splicing variant; m, DNA methylation marker. Position is based on Build 37 of the reference genome. Effect is shown as unit change (mm Hg) in blood pressure (standard error, SE) per copy of the risk allele (SBP, DBP, PP (pulse pressure), MAP). SNPs rs751984, rs2240736 and rs740406 are near or in annotated microRNA genes. Chr., chromosome; EA, effect allele; AA, alternate allele; EAF, effect allele frequency; n, sample size.
Regional association plots for the 12 newly identified loci are shown in Figures 1–4 and Supplementary Figure 4; associations of the 12 sentinel SNPs with other blood pressure phenotypes are shown in Supplementary Figure 5 and Supplementary Table 7. There was little evidence for heterogeneity of effect between the ancestry groups in either the genome-wide association or replication data. We also replicated previously reported associations with blood pressure at 23 genetic loci at genome-wide significance; a further 17 loci were associated with blood pressure phenotypes at P < 0.05 (Supplementary Fig. 6 and Supplementary Table 8).
Figure 1.
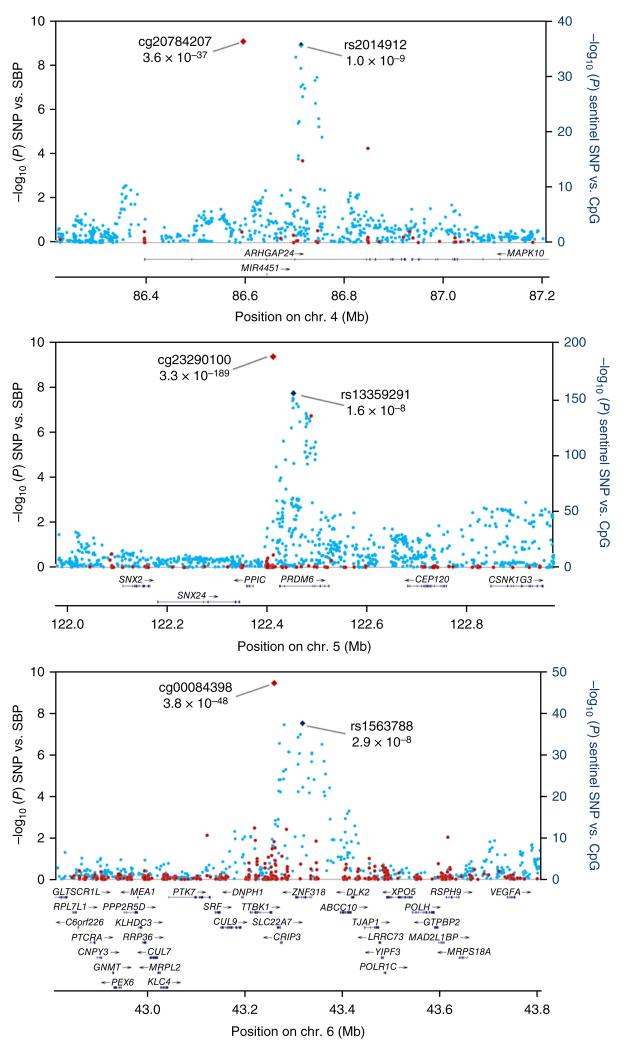
Regional plots for the three newly identified loci associated with SBP. Associations of SNPs with SBP in the trans-ancestry GWAS (blue markers; n = 99,994) and of sentinel SNP with methylation at nearby CpG sites (red markers; n = 2,664) are shown. The identities of the sentinel SNP and most closely associated CpG site are provided; correlations between markers are shown in Supplementary Figure 4.
Figure 2.
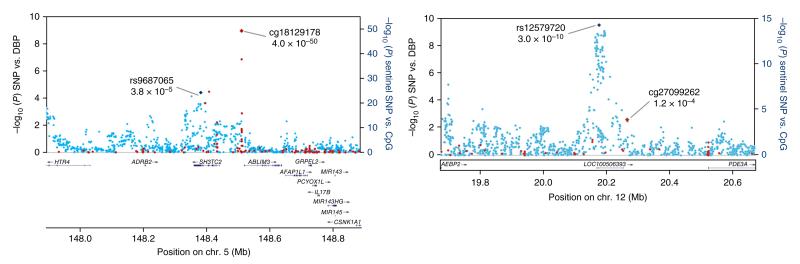
Regional plots for the two newly identified loci associated with DBP. Associations of SNPs with DBP in the trans-ancestry GWAS (blue markers; n = 99,994) and of sentinel SNPs with methylation at nearby CpG sites (red markers; n = 2,664) are shown. The identities of the sentinel SNP and most closely associated CpG site are provided; correlations between markers are shown in Supplementary Figure 4.
Figure 3.
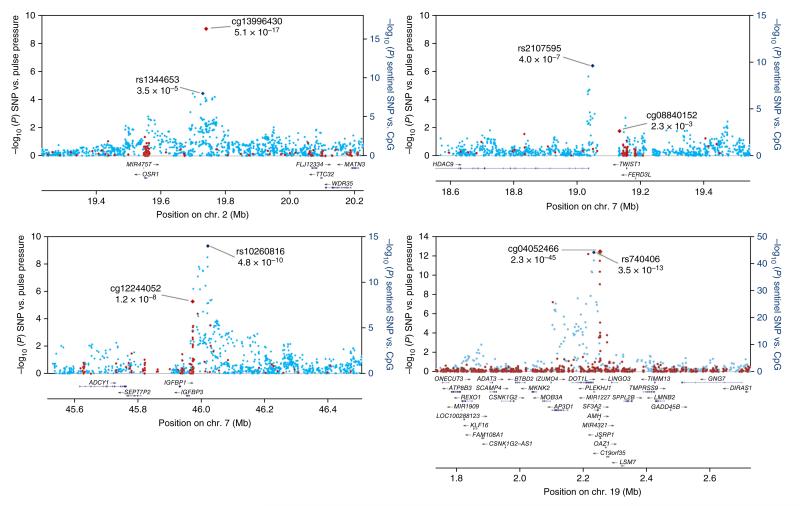
Regional plots for the four newly identified loci associated with pulse pressure. Associations of SNPs with pulse pressure in the trans-ancestry GWAS (blue markers; n = 99,994) and of sentinel SNPs with methylation at nearby CpG sites (red markers; n = 2,664) are shown. The identities of the sentinel SNP and most closely associated CpG site are provided; correlations between SNPs and between methylation markers are shown in Supplementary Figure 4.
Figure 4.
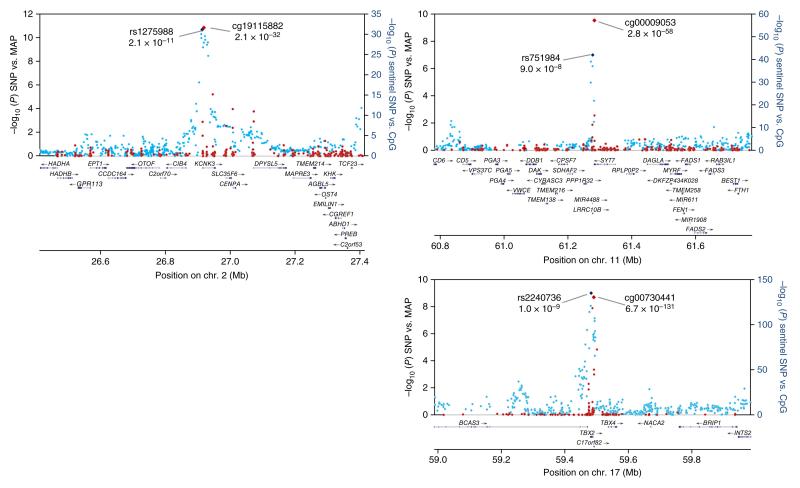
Regional plots for the three newly identified loci associated with MAP. Associations of SNPs with MAP in the trans-ancestry GWAS (blue markers; n = 99,994) and of sentinel SNPs with methylation at nearby CpG sites (red markers; n = 2,664) are shown. The identities of the sentinel SNP and most closely associated CpG site are provided; correlations between markers are shown in Supplementary Figure 4.
In population-specific analyses, we identified two further SNPs (rs9425586 in East Asians and rs13395018 in Europeans) that reached P < 1 × 10−7 against a blood pressure phenotype in their respective discovery meta-analyses. We carried out ancestry-specific testing in the East Asian and European replication samples. Neither SNP reached P < 0.05 in replication testing or P < 1 × 10−9 in combined analysis with the discovery data (Supplementary Table 6).
Candidate sequence variants and genes at new loci
Taking advantage of trans-ancestry differences in linkage disequilibrium (LD), we used MANTRA and varLD21,22 to narrow the 99% credible SNP sets and facilitate future efforts to identify the causal variants underlying blood pressure variability (Supplementary Figs. 7 and 8, and Supplementary Table 9).
Next, we searched for genetic variants at the newly identified blood pressure loci that might influence protein coding or gene transcription and that were in high LD (r2 >0.8) with sentinel blood pressure SNPs. We identified SNPs that were nonsynonymous (n = 9) or splicing variants (n = 2) and/or were present in regulatory regions (including transcription factor binding sites, promoter and enhancer regions, DNase I hypersensitivity sites, regulatory motifs and CpG islands; n = 825; Supplementary Table 10) (refs. 23,24).
Analysis of coding variation and gene regulatory signatures (Supplementary Tables 10 and 11) identified 20 genes as possible candidates underlying the associations with blood pressure at the newly identified loci (Table 1). Current knowledge on gene function for all 20 candidates is summarized in Supplementary Table 12.
Association of sentinel SNPs with DNA methylation
We investigated the relationships of the sentinel blood pressure SNPs with local DNA methylation (within 1 Mb of each SNP) in 1,904 South Asians with whole-genome methylation data available (peripheral blood; Illumina HumanMethylation450 BeadChip (450K) array; Supplementary Table 13). We found a ~2-fold enrichment for association between sequence variation and DNA methylation in comparison with expectations under the null hypothesis (P = 0.01; Supplementary Fig. 9). Twenty-eight of the 35 sentinel blood pressure SNPs were associated with one or more methylation markers at P < 3.8 × 10−6 (P < 0.05 after Bonferroni correction for the 13,275 SNP-CpG association tests; Supplementary Table 14); the 28 leading CpG sites (the CpG sites with the lowest P value for association with each sentinel blood pressure SNP) are summarized in Table 2. All 28 leading CpG sites showed replication in further testing among 4,780 European and South Asian samples (P < 0.05 and consistent direction of effect; Supplementary Table 15). Regional plots of DNA methylation are shown in Figures 1–4. There was little evidence for heterogeneity of effect of SNPs on methylation between Europeans and South Asians (Supplementary Fig. 10).
Table 2.
CpG sites associated in cis with the sentinel blood pressure SNPs
| Sentinel SNP | Chr. | EA | Lead CpG | CpG position (bp) |
SNP-CpG distance (bp) |
SNP–CpGa |
Nearest gene to CpG |
Relation to gene (CpG) |
CpG–eQTLb |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | P | Effect | P | ||||||||
| rs880315 | 1 | T | cg02903756 | 10,750,680 | 46,186 | −0.17 | 7.0 × 10−24 | CASZ1 | Body | 0.09 | 2.5 × 10−2 |
| rs12567136 | 1 | T | cg05228408 | 11,865,352 | 18,379 | 0.6 | 2.8 × 10−248 | MTHFR | 5′ UTR | 2.34 | 6.5 × 10−4 |
| rs1344653 | 2 | A | cg13996430 | 19,741,587 | −10,742 | −0.12 | 7.0 × 10−14 | OSR1 | Intergenic | 0.20 | 2.4 × 10−1 |
| rs1275988 | 2 | T | cg19115882 | 26,919,145 | −4,781 | −0.3 | 1.8 × 10−74 | KCNK3 | Body | 0.25 | 1.5 × 10−4 |
| rs7629767 | 3 | T | cg02108620 | 42,002,230 | 41,279 | 0.57 | 2.1 × 10−741 | ULK4 | 5′ UTR | −0.1 | 4.4 × 10−1 |
| rs13149993 | 4 | A | cg05452645 | 81,117,647 | 40,898 | −0.26 | 3.7 × 10−47 | PRDM8 | 5′ UTR | 0.03 | 5.8 × 10−1 |
| rs2014912 | 4 | T | cg20784207 | 86,597,598 | 118,072 | −0.27 | 9.7 × 10−51 | ARHGAP24 | Body | −0.51 | 2.4 × 10−1 |
| rs7733331 | 5 | T | cg24363955 | 32,788,467 | 40,379 | −0.22 | 1.6 × 10−41 | NPR3 | Upstream | 0.09 | 5.9 × 10−1 |
| rs13359291 | 5 | A | cg23290100 | 122,435,626 | 40,831 | −0.88 | 6.8 × 10−372 | PRDM6 | Body | −0.05 | 4.4 × 10−1 |
| rs9687065 | 5 | A | cg18129178 | 148,520,854 | −129,714 | −0.45 | 2.0 × 10−138 | ABLIM3 | TSS | −0.07 | 3.5 × 10−1 |
| rs11960210 | 5 | T | cg22790839 | 157,883,933 | −66,299 | −0.28 | 3.1 × 10−65 | EBF1 | Intergenic | −0.11 | 1.7 × 10−1 |
| rs1563788 | 6 | T | cg00084398 | 43,249,983 | 58,380 | −0.42 | 5.0 × 10−139 | TTBK1 | Body | 0.06 | 5.3 × 10−1 |
| rs17080102 | 6 | C | cg02784464 | 151,121,916 | −117,146 | 0.27 | 7.2 × 10−29 | PLEKHG1 | Body | 0 | 3.0 × 10−2 |
| rs10260816 | 7 | C | cg12244052 | 45,961,469 | 48,631 | −0.08 | 4.6 × 10−6 | IGFBP3 | Upstream | 0.59 | 7.6 × 10−15 |
| rs731141 | 10 | A | cg10751070 | 96,143,568 | −244,887 | 0.14 | 8.3 × 10−16 | TBC1D12 | Intergenic | 0.1 | 5.2 × 10−2 |
| rs11191375 | 10 | T | cg07119830 | 104,412,306 | 52,351 | 0.97 | 3. × 10−746 | TRIM8 | Body | 0.08 | 2.5 × 10−2 |
| rs2484294 | 10 | A | cg05575054 | 115,804,968 | −12,906 | −0.26 | 2.7 × 10−49 | ADRB1 | Body | −0.23 | 1.7 × 10−1 |
| rs751984 | 11 | T | cg00009053 | 61,283,865 | −5,619 | 0.46 | 1.2 × 10−167 | SYT7 | 3′ UTR | 0.1 | 5.1 × 10−1 |
| rs2055450 | 11 | A | cg05925497 | 100,734,094 | −183,677 | 0.19 | 1.2 × 10−30 | ARHGAP42 | Body | −0.09 | 2.7 × 10−5 |
| rs10894192 | 11 | A | cg03927812 | 130,271,903 | −5,786 | −0.41 | 5.1 × 10−136 | ADAMTS8 | Intergenic | −0.07 | 4.3 × 10−1 |
| rs11105354 | 12 | A | cg00757033 | 89,920,650 | 105,873 | −0.76 | 9.6 × 10−452 | GALNT4 | Intergenic | 1.02 | 2.1 × 10−7 |
| rs3184504 | 12 | T | cg10833066 | 111,807,467 | 96,904 | −0.59 | 4.8 × 10−222 | FAM109A | Intergenic | −0.02 | 6.7 × 10−1 |
| rs1378942 | 15 | A | cg02696790 | 75,250,997 | −173,630 | 0.53 | 3.1 × 10−223 | RPP25 | Intergenic | −0.23 | 1.7 × 10−1 |
| rs8032315 | 15 | A | cg06330618 | 91,428,456 | −10,159 | 0.45 | 3.0 × 10−493 | FES | Body | −3.19 | 1.3 × 10−7 |
| rs2301597 | 17 | T | cg19407385 | 43,099,144 | 74,129 | −0.72 | 6.0 × 10−1257 | DCAKD | Intergenic | 0.74 | 7.8 × 10−6 |
| rs7405452 | 17 | T | cg22053945 | 46,651,360 | 23,310 | −0.72 | 4.0 × 10−358 | HOXB3 | 5′ UTR | −0.07 | 3.3 × 10−1 |
| rs2240736 | 17 | T | cg00730441 | 59,483,863 | 1,530 | 0.65 | 1.4 × 10−330 | TBX2 | Body | −0.06 | 3.1 × 10−1 |
| rs740406 | 19 | A | cg04052466 | 2,251,061 | −18,840 | −0.46 | 3.7 × 10−71 | AMH | Body | −0.08 | 1.5 × 10−2 |
Results are shown for SNP–CpG associations reaching both P < 3.8 × 10−6 in discovery (Bonferroni correction for 13,275 SNP–CpG marker tests) and P < 0.05 with consistent direction of effect in replication testing (Supplementary Table 15). For each sentinel SNP, the lead CpG site is provided (lowest P value for association of the SNP with the CpG; PSNP–CpG), along with the genomic context of the CpG site. The gene nearest to the CpG site is listed, as well as the P value for association between the CpG site and expression of the nearest gene (PCpG–eQTL). Chr., chromosome; EA, effect allele; NA, not available.
The P value shown is for combined analysis of discovery and replication data for SNP–CpG association.
Statistical significance inferred at P < 1.8 × 10−3 (Bonferroni correction for 26 CpG–eQTL tests).
We found evidence of replication of the relationships of the sentinel blood pressure SNPs with methylation of their respective leading CpG sites in genomic DNA from cord blood (P = 4.0 × 10−4, binomial test for directionally consistent effects, n = 237 samples; Supplementary Table 16). The presence of these associations at an early stage of life, before substantial environmental exposure, lends support to the view that the sequence variants have a direct effect on DNA methylation and argues against reverse causation. We separately showed that association of sentinel SNPs with local DNA methylation is generalizable across multiple phenotypic traits and not unique to blood pressure phenotypes (Supplementary Fig. 11).
Sequence variation, DNA methylation and blood pressure
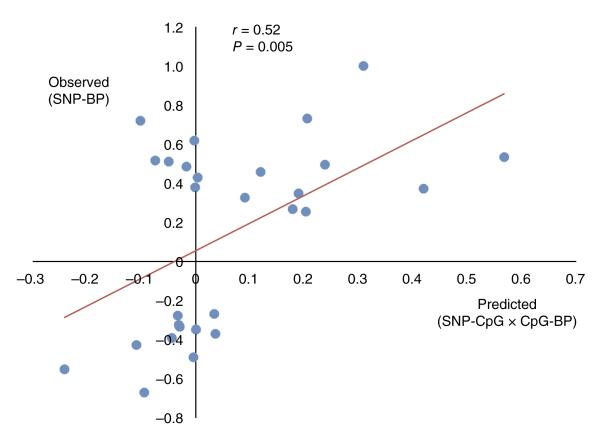
We used genetic association and the concept of Mendelian randomization to test whether DNA methylation might contribute, at least in part, to the relationship of the sentinel SNPs with blood pressure. For the 28 sentinel SNPs that were associated with methylation of cis CpG sites (Supplementary Table 15), we quantified the three-way relationships between the sentinel SNPs, their leading CpG sites and blood pressure among the 6,757 Europeans and South Asians with DNA methylation data available (Supplementary Table 17). Across all 28 loci, we found that the observed effects of SNPs on blood pressure were correlated with the effects predicted through association with methylation (r = 0.52; P = 0.005; Fig. 5). Of the 14 sentinel SNPs with the highest predicted genetic effects (above the median for the distribution), 13 were directionally consistent (P = 1.2 × 10−4, sign test), with a close correlation between the observed and predicted effects (r = 0.72; P = 0.004). Our results support the view that DNA methylation may be involved in the regulatory pathway linking DNA sequence variants to blood pressure.
Figure 5.
DNA methylation as a potential mediator of the relationship between sentinel SNPs and blood pressure at the loci reaching genome-wide significance in our study. Results are shown for the 28 sentinel SNPs that are associated with methylation at P < 0.05 after Bonferroni correction for multiple tests. Predicted effects on blood pressure are based on the relationship of sentinel SNPs with methylation and the relationship of methylation with blood pressure (BP); observed effects represent the direct relationship between the sentinel SNPs and blood pressure (discovery phenotype). The P value is for the correlation of the observed versus predicted effects (solid line).
Fine mapping the association of SNPs and DNA methylation
The 450K methylation array assays ~2% of the estimated ~30 million CpG sites in the human genome. To further evaluate the relationship between the sentinel blood pressure SNPs and DNA methylation at the 19p13.3 locus near AMH, we used next-generation sequencing to fine map DNA methylation at all CpG sites within 1 kb on either side of the leading 450K CpG site in 168 samples. We successfully quantified DNA methylation at 34 CpG sites, of which only 2 are assayed by the 450K array (Supplementary Fig. 12). The sentinel blood pressure SNP at the AMH locus (rs740406) had a directionally consistent effect on methylation at 29 of the 34 CpG sites assayed (P = 4 × 10−5, sign test; Supplementary Fig. 13), consistent with published data suggesting that clusters of adjacent CpG sites are co-regulated25,26. Of the 34 CpG sites assayed, we found that 28 had a positive relationship with blood pressure (P = 2 × 10−4, sign test), and 10 were associated with blood pressure at P < 0.05 (P = 5 × 10−7 for enrichment; Supplementary Fig. 13).
Cross-tissue patterns of DNA methylation
DNA methylation can show tissue-specific patterns that contribute to differences in transcriptional regulation and cellular differentiation27. We investigated the cross-tissue patterns of DNA methylation at the 26 leading CpG sites associated with the sentinel blood pressure SNPs in the present study. Using data from 7 tissue samples (including muscle, liver, and subcutaneous and visceral fat), we showed that DNA methylation in blood at the 26 CpG sites was closely correlated with methylation in a wide range of tissues (Pearson correlation coefficient, 0.61–0.97; P = 1.2 × 10−4 to 1.3 × 10−47; Supplementary Figs. 14 and 15). Our findings support the view that, for the CpG sites examined, methylation levels in blood provide a surrogate for patterns of methylation in other tissues.
Clinical relevance of our findings
We tested whether the genetic variants singly or in aggregate contribute to risk of clinical phenotypes associated with high blood pressure. In single-variant tests, we found that the 35 (known and new) sentinel SNPs were enriched for variants associated with adiposity, type 2 diabetes, coronary heart disease and kidney function in published GWAS (P = 2.5 × 10−3 to 1.8 × 10−11; Supplementary Table 18). We further showed that weighted genetic risk scores comprising known and new variants predicted increased left ventricular mass by electrocardiographic criteria, circulating levels of NT-proBNP (a marker of heart function), clinical coronary heart disease, and cardiovascular and all-cause mortality (P = 0.04 to 8.6 × 10−6; Supplementary Table 19). Our findings provide evidence that the genetic loci associated with blood pressure contribute to cardiovascular outcomes.
DISCUSSION
Our genome-wide association and replication study in 320,251 people identifies 12 new genetic loci influencing blood pressure phenotypes in 3 ancestry groups. Among the genetic loci and candidate genes identified, several have been implicated in other cardiovascular and metabolic phenotypes through genome-wide association. IGFBP3, KCNK3, PDE3A and PRDM6 have a role in vascular smooth muscle cell biology. PDE3A is a phosphodiesterase involved in cyclic GMP (cGMP) metabolism, vascular smooth muscle contraction and cardiovascular function28. Pharmacological inhibitors of PDE3A lower blood pressure29. KCNK3 is a potassium channel involved in the regulation of vascular tone; mutations in KCNK3 are associated with pulmonary hypertension30. PRDM6 acts as an epigenetic regulator of vascular smooth muscle cell phenotypic plasticity by suppressing differentiation and maintaining proliferative potential. Genetic variants near PRDM6 are associated with intracranial aneurysm31. IGFBP3 modulates the actions of insulin-like growth factors (IGFs), circulating hormones that influence vascular smooth muscle cell function. Serum levels of IGFBP3 are associated with cardiovascular disease32. We also note several candidate genes that are involved in renal function, a determinant of blood pressure. ARHGAP24 influences podocyte formation33, OSR1 encodes a transcription factor that influences renal mass and function34, and SLC22A7 encodes a key renal solute transporter35; genetic variants at TBX2 are determinants of renal function and chronic kidney disease36.
The mechanisms underlying the associations between common genetic variants and blood pressure are incompletely understood. The majority of the loci identified do not contain common or low-frequency coding variants to account for the association between the sentinel SNP and blood pressure. Using both the 450K methylation array and fine mapping through targeted bisulfite sequencing, we show that SNPs influencing blood pressure are associated with methylation at multiple local CpG sites and that DNA methylation is associated with blood pressure. Using genetic association and the concept of Mendelian randomization, we further show that the observed effect of SNPs on blood pressure is closely correlated with the effect predicted through association with methylation. The effects of genetic variation on methylation can be demonstrated in the newborn, in the absence of substantial adverse environmental exposures, further supporting a causal relationship. Our results suggest that DNA methylation may be involved in the regulatory pathway linking common genetic variants with blood pressure at some of the loci identified, consistent with findings from experimental models of hypertension37. We note an effect of genome-wide associated sentinel SNPs on DNA methylation for traits in addition to blood pressure, suggesting that DNA methylation might have a wider role in linking common genetic variation to multiple phenotypes.
URLs
Sequenom EpiDesigner BETA, http://www.epidesigner.com/.
ONLINE METHODS
Populations and phenotypes
Details of the participating cohorts are summarized in Supplementary Table 1 and in the Supplementary Note. Phenotype definitions were based on the published literature6. SBP, DBP, pulse pressure and MAP were continuous variables measured in millimeters of mercury. SBP and DBP were directly measured in millimeters of mercury, and pulse pressure and MAP were calculated by SBP – DBP and (2 × DBP + SBP)/3, respectively. SNP associations for SBP, DBP, pulse pressure and MAP were tested by linear regression with age and sex using an additive genetic model. For individuals being treated with blood pressure–lowering medication, the following adjustments to the blood pressure values were made before performing the regression analysis: SBP (+15), DBP (+10), pulse pressure (+5) and MAP (+11.667). For hypertension, logistic regression with sex as a covariate was applied, with cases and controls defined as follows: cases: (i) SBP ≥160 mm Hg or DBP ≥100 mm Hg or on antihypertensive treatment and (ii) age of onset ≤65 years; controls: (i) SBP <130 mm Hg and DBP <85 mm Hg and not on antihypertensive treatment and (ii) age ≥50 years). Data and sample collection by the cohorts participating in the study was approved by respective research ethics committees, and all research participants gave written consent to take part.
Genome-wide association
Genome-wide association was analyzed in a total of 99,994 subjects, of whom 31,516 were of East Asian ancestry, 35,352 were of European ancestry and 33,126 were of South Asian ancestry. Imputation was carried out using haplotypes from HapMap Phase 2. Details of genotyping arrays and imputation are summarized in Supplementary Table 2. Quality control checks included a check of the distribution of effect sizes across phenotypes and comparison of allele frequencies against those expected from HapMap populations. There were between 2,127,883 (SBP) and 2,166,286 (hypertension) SNPs for analysis after quality control. Genomic control inflation factors ranged from 1.01 to 1.09 in the ancestry-specific meta-analyses and from 1.05 to 1.12 in global analyses (Supplementary Table 3).
Genome-wide significance was inferred at P < 1 × 10−9. This conservative choice fully corrects for the ~10 million SNP-phenotype combinations tested, in 3 ancestry groups and overall, and makes no adjustment for the potential correlations between the SNPs or phenotypes tested. We adopted this strategy to ensure that the results reported are robust and to reduce the risk of spurious findings in out multi-stage trans-ancestry GWAS.
Associations of SNPs with phenotype were tested in each cohort separately in single-marker tests, using regression analysis and an additive genetic model. Principal components and other study-specific factors were included as covariates to account for population substructure as described in Supplementary Table 2. Test statistics from each cohort were then corrected for their respective genomic control inflation factor to adjust for residual population substructure; the genomic control inflation factors are summarized in Supplementary Table 3. We then performed inverse variance meta-analysis of the results from the individual cohorts; meta-analysis was carried out among East Asian, European and South Asian populations separately. SNPs with information score <0.5 and minor allele frequency (MAF) <1% (weighted average across the cohorts) as well as sample size <50% of the maximum n for the phenotype were removed. We also removed SNPs showing heterogeneity of effect (Phet < 1 × 10−8) within any one of the three ancestry groups.
Finally, we carried out inverse variance meta-analyses of the results from the three ancestry groups. There was little evidence for inflation of test statistics at SNPs not known to be associated with blood pressure phenotypes, and genomic control was not applied to the final meta-analysis results.
Identification of candidate SNPs
We identified all common genetic variants that were in LD with one or more of the sentinel SNPs at r2 >0.8. LD was calculated using pooled haplotypes for (i) European and East Asian samples in the 1000 Genomes Project data set (March 2012 release) and (ii) 168 South Asians with whole-genome sequence data. We annotated the sentinel SNPs and their proxies for regulatory regions (promoter and enhancer histone marks, DNase I hypersensitivity, protein binding and regulatory motifs) with HaploRegv2 (Broad Institute)24. VEP (Variant Effect Predictor) was used for the identification of transcription factor binding sites and nonsynonymous and splicing variants23. EpiExplorer and the UCSC Genome Browser were used to annotate CpG islands38.
Identification of candidate genes
We considered the nearest gene and any other gene located within 10 kb of the sentinel SNP to be candidates for mediating the association with the blood pressure phenotype, along with any gene containing a SNP predicted to be nonsynonymous or affecting a splice site. We also examined the associations of the sentinel SNPs and their proxies with eQTL data from Zeller et al., consisting of data from circulating monocytes in 1,490 unrelated individuals39. SNPs were tested for association with the expression of nearby genes (within 1 Mb of the sentinel SNP; P < 1 × 10−5). Finally, for significant SNP-methylation associations, the gene nearest the leading CpG site was also included as a candidate.
Association between sentinel SNPs, DNA methylation and phenotype
The associations of the 36 sentinel blood pressure SNPs with DNA methylation were first examined among 1,904 South Asian individuals from the LOLIPOP cohort. Bisulfite conversion of genomic DNA was performed using the EZ DNA methylation kit according to the manufacturer’s instructions (Zymo Research). Methylation of genomic DNA was quantified using the Illumina HumanMethylation450 array according to the manufacturer’s instructions. To facilitate the comparison of effects between CpG sites, methylation levels were z-transformed for all analyses; the scale for methylation is thus ‘standard deviations’. Whole-genome genotyping was carried out using the Illumina 317, 610 or OmniExome microarray, with genomic DNA and according to the manufacturer’s instructions. SNPs and samples with low call rates (<98%) were excluded, as were SNPs with departure from Hardy-Weinberg equilibrium (P < 1 × 10−6). We used IMPUTE2 to predict (impute) unmeasured genotypes, using phased haplotypes from the whole-genome sequencing of 168 Indian Asians as a reference panel.
The association of the sentinel blood pressure SNPs with cis DNA methylation (within 1 Mb) was tested by linear regression and an additive genetic model. We used an analytic strategy validated to reduce batch and other technical confounding effects in quantification of DNA methylation and adjusted for the white blood cell composition of blood40–42. We inferred statistical significance at P < 3.8 × 10−6 (Bonferroni correction for 13,275 SNP–CpG marker tests). We identified the leading CpG site (having the lowest P value for association with the sentinel SNP) at each blood pressure locus. We then carried out replication testing of the leading SNP-CpG associations among independent samples of South Asians (LOLIPOP, n = 1,373) and Europeans (LOLIPOP, n = 166; LifeLines Deep, n = 752; RS-BIOS, n = 762; KORA, n = 1,727; Supplementary Table 13).
Next, we quantified the relationship of the 28 leading CpG sites with blood pressure (Supplementary Tables 15 and 17). We then calculated the predicted effect of each SNP on blood pressure as the product of the regression coefficients between (i) the SNP and methylation (n = 6,684) and (ii) methylation and blood pressure (n = 6,757). We used linear regression and sign tests to compare the predicted effect of a SNP on blood pressure via methylation with the directly observed effect of this SNP on blood pressure in genome-wide association (Fig. 5).
Association of methylation with gene expression
The relationship between methylation and the expression of nearest genes was investigated in samples from LOLIPOP (n = 1,082; 907 South Asians and 175 Europeans) and the EnviroGenoMarkers project, a nested case-control study of incident breast cancer and B cell leukemia (n = 638 Europeans)43,44.
LOLIPOP
Details of the LOLIPOP cohort and methylation analysis have been provided above. Gene expression analysis was performed with the Illumina HumanHT-12 v4 BeadChip according to the manufacturer’s protocol. Background correction using negative controls was performed, and data were subsequently quantile normalized and log2 transformed. Linear models were fitted with log-transformed gene expression as the response variable and quantile normalized with β values (methylation), age, sex, the top 24 control probe principal components from methylation measurement and technical covariates related to the measurement of expression, including RNA integrity number (RIN), RNA extraction batch, RNA conversion batch, scanning batch, array and array position. Analyses were conducted separately in South Asians and Europeans, followed by inverse variance–weighted meta-analysis. Calculations were performed using R, version 3.0.1.
EnviroGenoMarkers
Methylation and gene expression were quantified in the baseline blood samples collected 1–17 years before disease onset. Transcriptomic profiles were obtained using the Agilent 4x44K Whole Human Genome microarray and subjected to extensive quality control procedures45. DNA methylation profiles were obtained using the Illumina Infinium HumanMethylation450 BeadChip according to the manufacturer’s protocol. Bisulfite conversion was carried out using the Zymo EZ DNA Methylation kit. Probes that had missing values in more than 20% of the samples were excluded. We used linear regression to determine the association between methylation and gene transcription.
Enrichment of reported sentinel SNPs for association with DNA methylation
SNPs reported to be associated with phenotype were retrieved from the National Human Genome Research Institute (NHGRI) GWAS catalog. We considered studies with a sample size greater than 1,000 and retained SNPs with association P < 5 × 10−8. For simplicity, we removed data for Crohn’s disease and ulcerative colitis (both represented by inflammatory bowel disease) and obesity (represented by body mass index (BMI)). To account for biases due to LD, SNPs were pruned for each trait on the basis of a 1-Mb flanking window (by consecutively selecting the SNP with the lowest P value and removing any variant within 1 Mb). Traits were then ranked by the number of significant associations, and the top 20 traits were tested for enrichment with methylation quantitative trait locus (methQTL) SNPs. For this purpose, we derived 1 million sets of matched background SNPs for each trait. These background SNPs were chosen randomly but had properties matched to the associated SNPs (MAF ±2%, distance to gene ±10 kb, CpGs in cis ±200 kb). The proportion of cis methQTLs among the associated SNPs was then compared to the proportion among each of the 1 million sets of background SNPs, thereby deriving an empirical P value.
Cross-tissue methylation
Publicly available data (GSE48472) were downloaded from the Gene Expression Omnibus (GEO)46. Briefly, the data set consisted of 41 samples from blood, liver, muscle, pancreas, subcutaneous fat, omentum and spleen analyzed on the 450K methylation array. Data from the 28 CpG sites of interest were extracted and plotted using the heatmap.2 function in the gplots library with R. Mean methylation levels for each CpG site across all samples within each tissue type were used to test for pairwise correlation between tissue types.
Relationship of sentinel SNPs with methylation in cord blood
We tested the relationship of sentinel SNPs with methylation for the 28 SNP-CpG pairs of interest in cord blood to investigate whether reverse causation might account for the observed associations between SNPs and methylation. This analysis was conducted in the GUSTO (Growing Up in Singapore Toward Healthy Outcomes) study47. Extracted DNA from cord blood (n = 237 samples) was genotyped using the Illumina OmniExpress + exome array, and DNA methylation profiling was performed using the Infinium HumanMethylation450 BeadChip. Data were processed as described48. Both data sets have been described previously and are deposited in GEO under accessions GSE53816 and GSE54445 (ref. 49). Genotype data were imputed with reference to HapMap 2 East Asian populations. SNPs with MAF <1% in GUSTO and CpGs that failed quality control were excluded from further analysis. Linear regression was used to quantify SNP-CpG associations, adjusting for sex.
Targeted resequencing for regional methylation
The 450K array assays <2% of the estimated ~30 million CpG sites in the human genome. To better describe the patterns of regional methylation, we carried out resequencing of the AMH locus in 168 samples. We used sequence capture and next-generation sequencing to assay 34 predicted CpG sites within 1 kb of the sentinel methylation marker at the AMH locus (chr. 19, 2,250,061–2,252,061). Primers were designed using Sequenom EpiDesigner BETA. Target DNA enrichment was carried out using the Fluidigm 48.48 Access Array IFC system, followed by PCR to attach sequence-specific adaptors and sample barcodes. Pooled sequencing was performed using the Illumina MiSeq platform (300-bp paired-end runs). We then used Burrows-Wheeler Aligner to map the directional, paired-end Illumina sequencing reads to the reference genome (hg19 build) and quantified methylation from the frequencies of converted and unconverted cytosine residues observed in reads mapped to each CpG site.
Fine mapping
To take advantage of any variation in LD structure between ancestry groups, we used MANTRA and varLD for further trans-ancestry fine mapping21,22. MANTRA, a Bayesian approach, allows for heterogeneity in effect sizes between ancestry or ethnic groups, which arises as a result of underlying differences in LD patterns but with a shared underlying causal variant across diverse populations that cannot be accommodated in fixed-effects meta-analysis. At each locus, 99% credible SNP sets were also constructed, which can be interpreted in a similar way to confidence intervals in a frequentist statistical framework21,50.
Genetic risk scores
We calculated weighted genetic risk scores for each of the 5 blood pressure phenotypes, using all 35 sentinel SNPs reaching genome-wide significance or the 12 sentinel SNPs from the newly identified genetic loci; this yielded 10 genetic risk scores per person. Each score was calculated as the sum of the effect allele counts weighted by β coefficients for association with the respective phenotype. To facilitate comparisons between genetic risk scores, each score was then standardized. We examined the relationships between genetic risk scores and phenotypes relevant to blood pressure in three cohorts—LOLIPOP, LifeLines and PREVEND—using regression analysis, including age and sex as covariates. Results were combined across cohorts by inverse variance meta-analysis where necessary. Where possible, we also used the in silico approach from T. Johnson for comparison8.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the use of data from the International Consortium for Blood Pressure Genome-Wide Association Studies8,9.
AASC.This work was supported by Grants for Scientific Research (24390084, 21390099 and 20390185) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Science and Technology Incubation Program in Advanced Regions, Japanese Science and Technology Agency; the Japanese Atherosclerosis Prevention Fund; the Takeda Medical Research Foundation; and National Cardiovascular Research Grants.
AIDHS/SDS. This study was supported by US National Institutes of Health (NIH) grants R01DK082766 (D.K.S.) funded by the National Institute of Diabetes and Digestive and Kidney Diseases and NOT-HG-11-009 (D.K.S.) funded by the National Human Genome Research Institute (D.K.S.) and by a VPR bridge grant (D.K.S.) from the University of Oklahoma Health Sciences Center.
BIOS-consortium. The BIOS-consortium is funded by BBMRI-NL, a research infrastructure financed by the Netherlands Organization for Scientific Research (NWO project 184.021.007).
CAGE-Amagasaki. We acknowledge the outstanding contributions of the employees of the National Center for Global Health and Medicine who provided technical and infrastructural support for this work. Above all, we thank the participants who made this work possible and who gave it value. We also thank T. Ogihara, Y. Yamori, A. Fujioka, C. Makibayashi, S. Katsuya, K. Sugimoto, K. Kamide, R. Morishita and the many physicians of the participating hospitals and medical institutions in the Amagasaki Medical Association for their assistance in collecting the DNA samples and accompanying clinical information. This work was supported by Grants for Scientific Research (22390186, 24591060, 25253059 and 25461127) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CAGE-Fukuoka. This work was supported by Grants-in-Aid for the 21st Century Center of Excellence Program (Kyushu University) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Grants-in-Aid for Scientific Research (category A) from the Japanese Society for the Promotion of Science. We are grateful to all participants of this study. We also especially thank S. Kono for his management of the DNA samples and clinical information.
CAGE_GWAS1. The CAGE Network studies were supported by grants for Core Research for Evolutional Science and Technology (CREST) from the Japanese Science and Technology Agency; the Program for the Promotion of Fundamental Studies in Health Sciences, National Institute of Biomedical Innovation Organization (NIBIO); KAKENHI (Grant-in-Aid for Scientific Research) on Priority Area ‘Applied Genomics’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the National Center for Global Health and Medicine. N.K. is a recipient of the Okinaga Scholarship and thanks S. Okinaga, H. Okinaga and other staff at Teikyo University, Japan, for their considerable support of doctoral work.
CAGE-KING. This study was supported in part by Grants-in-Aid for Scientific Research, including ones from categories A and B and the NEXT program of the Japanese Society for the Promotion of Science and by Grants-in-Aid on Priority Areas ‘Comprehensive Genomics’ and ‘Applied Genomics’, from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CAGE-Vietnam. The CAGE-Vietnam study was supported by Grants for International Health Research (17C-1 and 20S-6) from the Ministry of Health, Labour and Welfare of Japan, grants for the National Center for Global Health and Medicine (22S-10 and 25S-1) and the Manpei Suzuki Diabetes Foundation. We acknowledge the following investigators and institutions for their substantial contribution to the current study: T. Sasazuki, M. Noda, N. Kato, S. Kanagawa, T. Mizoue, H. Ohara and Y. Takahashi (Japanese investigators); T. Quy, N. Lan Viet, P. Thi Hong Hoa, N. Hoa Dieu Van, N. Thi Lam, L. Bach Mai, N. Quang Bay, P. Thi Phuong Thuy and B. Minh Duc (Vietnamese investigators); the National Center for Global Health and Medicine (Japan), Bach Mai Hospital (Vietnam), the Vietnam National Institute of Nutrition and the NCGM-BMH Medical Collaboration Center.
Cilento. We thank the populations of Cilento, Italy, for their participation in the study. This work was supported by grants from the Italian Ministry of Universities (FIRB-RBNE08NKH7, Interomics Flag project), the Assessorato Ricerca Regione Campania, the Fondazione con il SUD (2011-PDR-13) and the Fondazione Banco di Napoli to M.C.
CLHNS. We thank the Office of Population Studies Foundation research and data collection teams for the Cebu Longitudinal Health and Nutrition Survey. This work was supported by National Institutes of Health grants DK078150, TW05596, HL085144 and TW008288 and pilot funds from RR20649, ES10126 and DK56350.
DIABNORD. We are grateful to the study participants who dedicated their time and samples to these studies. We also thank the VHS, the Swedish Diabetes Registry and the Umeå Medical Biobank staff for biomedical data and DNA extraction. We also thank M. Sterner, M. Juhas and P. Storm for their expert technical assistance with genotyping and genotype data preparation. The current study was funded by Novo Nordisk, the Swedish Research Council, Påhlssons Foundation, the Swedish Heart Lung Foundation and the Skåne Regional Health Authority (all to P.W.F.).
EGCUT. EGCUT received targeted financing from the Estonian government (SF0180142s08), the Center of Excellence in Genomics (EXCEGEN) and the University of Tartu (SP1GVARENG). We acknowledge EGCUT technical personnel, especially V. Soo and S. Smit. Data analyses were carried out in part at the High-Performance Computing Center of the University of Tartu.
FINCAVAS. This work was supported by Competitive Research Funding from Tampere University Hospital (grants 9M048 and 9N035), the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, Finland, and the Tampere Tuberculosis Foundation. The authors thank the staff of the Department of Clinical Physiology for collecting the exercise test data.
GEMS. This work was partially supported by US NIH grants R01CA107431 and P42ES10349 to H. Ahsan. We would like to thank the study participants, as well as the staff of UChicago Research Bangladesh.
GeneBank. The Cleveland Clinic GeneBank study is supported by National Heart, Lung, and Blood Institute grants P01HL098055, P01HL076491, R01HL103866, P20HL113452 and R01HL103931. H. Allayee was supported by grant R01ES021801 from the National Institute of Environmental Health Sciences.
GenSalt. The Genetic Epidemiology Network of Salt Sensitivity is supported by research grants (U01HL072507, R01HL087263 and R01HL090682) from the National Heart, Lung, and Blood Institute, US NIH.
GLACIER-exome. We are indebted to the study participants who dedicated their time and samples to these studies. We thank J. Hutiainen and Å. Ågren (Umeå Medical Biobank) for data organization and K. Enquist and T. Johansson (Västerbottens County Council) for technical assistance with DNA extraction. We also thank M. Sterner, M. Juhas and P. Storm for their expert technical assistance with genotyping and genotype data preparation. The current study was funded by Novo Nordisk, the Swedish Research Council, Påhlssons Foundation, the Swedish Heart Lung Foundation and the Skåne Regional Health Authority (all to P.W.F.).
GLACIER Metabochip. We are indebted to the study participants who dedicated their time and samples to these studies. We also thank the VIP and Umeå Medical Biobank staff for biomedical data collection and preparation. We specifically thank J. Hutiainen, Å. Ågren and S. Nilsson (Umeå Medical Biobank) for data organization, K. Enquist and T. Johansson (Västerbottens County Council) for expert technical assistance with DNA preparation, and D. Hunter, P. Soule and H. Ranu (Harvard School of Public Health) for expert assistance with planning and undertaking genotyping of GLACIER samples. The current study was funded by Novo Nordisk, the Swedish Research Council, Påhlssons Foundation, the Swedish Heart Lung Foundation and the Skåne Regional Health Authority (all to P.W.F.).
Health2006. The Health2006 study was financially supported by grants from the Velux Foundation; the Danish Medical Research Council, Danish Agency for Science, Technology and Innovation; the Aase and Ejner Danielsens Foundation; and ALK-Abello (Hørsholm, Denmark), Timber Merchant Vilhelm Bangs Foundation, MEKOS Laboratories Denmark and Research Centre for Prevention and Health, the Capital Region of Denmark. This project was also funded by the Lundbeck Foundation and produced by the Lundbeck Foundation Centre for Applied Medical Genomics in Personalised Disease Prediction, Prevention and Care (LuCamp; http://www.lucamp.org/). The Novo Nordisk Foundation Centre for Basic Metabolic Research is an independent Research Centre at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation (http://www.metabol.ku.dk/).
HEXA. This work was supported by grants from the Korean Centers for Disease Control and Prevention (4845-301, 4851-302 and 4851-307) and an intramural grant from the Korean National Institute of Health (2012-N73002-00), Republic of Korea.
HPS. The Heart Protection Study (ISRCTN48489393) was funded by the UK Medical Research Council, the British Heart Foundation, Merck & Co., and Roche Vitamins, Ltd. Genotyping and analysis were supported by a grant to the University of Oxford and the Centre National de Génotypage from Merck & Co. and the Oxford British Heart Foundation Centre of Research Excellence. J.C.H. acknowledges support from the British Heart Foundation (FS/14/55/30806).
GOYA. This study was conducted as part of the activities of the Gene-Diet Interactions in Obesity project (GENDINOB; http://www.gendinob.dk/) and the Medical Research Council Centre for Causal Analyses in Translational Epidemiology (MRC CAiTE). We thank the staff of the Copenhagen City Heart Study for their skillful examination of the study subjects in the collection of baseline and follow-up data. T.S.A. was also funded by the GENDINOB project and acknowledges the same.
GUSTO. The GUSTO study group includes P. Agarwal, A. Biswas, C. Looi Bong, B.F.P. Broekman, S. Cai, J.K.Y. Chan, Y.H. Chan, C.Y.I. Chee, H.Y.H. Chen, Y.B. Cheung, A. Chia, A. Chinnadurai, C.K. Chng, M.F.-F. Chong, S.C. Chong, M.C. Chua, C.M. Ding, E.A. Finkelstein, D. Fok, M. Fortier, A.E.N. Goh, Y.T.D. Goh, J.J. Gooley, W.M. Han, M. Hanson, C.J. Henry, C.-Y. Hsu, H. Inskip, J. Kapur, K. Kwek, I.Y.-M. Lau, B.W. Lee, N. Lek, S.B. Lim, Y.-L. Low, I. Magiati, L. Mary Daniel, C. Ngo, K. Naiduvaje, W.W. Pang, A. Qiu, B.L. Quah, V.S. Rajadurai, M. Rauff, S.A. Rebello, J.L. Richmond, A. Rifkin-Graboi, L.P.-C. Shek, A. Sheppard, B. Shuter, L. Singh, W. Stunkel, L.L. Su, O.H. Teoh, H.P.S. van Bever, R.M. van Dam, I.B.Y. Wong, P.C. Wong and G.S.H. Yeo. This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore-NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, A*STAR, Singapore.
INGI-VB. The research was supported by funds from Compagnia di San Paolo (Torino, Italy); Fondazione Cariplo, Italy, and the Ministry of Health, Ricerca Finalizzata 2008 and CCM 2010, and Telethon, Italy, to D.T. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank the inhabitants of Val Borbera who made this study possible, the local administrations, the Tortona and Genova archdioceses, and the ASL-22, Novi Ligure (Al), for support. We also thank C. Camaschella for the supervision of data collection and organization of the clinical data collection, F. Viganò for technical help, and C. Masciullo and M. Cocca for building the analysis platform.
Inter99. Inter99 was initiated by T.J. (principal investigator), K. Borch-Johnsen (co-principal investigator), H. Ibsen and T.F. Thomsen. The steering committee comprises the first two and C. Pisinger. The study was financially supported by research grants from the Danish Research Council, the Danish Centre for Health Technology Assessment, Novo Nordisk, the Research Foundation of Copenhagen County, the Ministry of Internal Affairs and Health, the Danish Heart Foundation, the Danish Pharmaceutical Association, the Augustinus Foundation, the Ib Henriksen Foundation, the Becket Foundation and the Danish Diabetes Association. This project was also funded by the Lundbeck Foundation and produced by LuCamp (http://www.lucamp.org/). The Novo Nordisk Foundation Centre for Basic Metabolic Research is an independent Research Centre at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation (http://www.metabol.ku.dk/).
InterAct. We are grateful to all participants who gave their time and effort to the study. We are also extremely grateful to all persons who contributed to the data collection across the study sites. This study was supported by funding from the European Union (integrated project LSHM-CT-2006-037197 in the Sixth Framework Programme of the European Community) and the Medical Research Council, UK.
JMGP. The JMGP study group is composed of the following individuals; JMGP-Ohasama: T. Ohkubo, M. Satoh, R. Inoue, T. Hirose, H. Metoki, M. Kikuya and Y. Imai; JMGP-Yokohama: N. Hirawa, K. Yatsu, T. Shiwa, M. Ogawa and S. Umemura; JMGP-Shigaraki and Takashima: Y. Kita, Y. Nakamura, N. Takashima and H. Ueshima; and JMGP-Nomura: Y. Tabara, R. Kawamoto, K. Kohara and T. Miki (chairperson).
This work was supported by Grants-in-Aid for Scientific Research (Priority Areas ‘Medical Genome Science (Millennium Genome Project)’ and ‘Applied Genomics’), the Leading Project for Personalized Medicine and Scientific Research (20390185, 21390099, 19659163, 16790336, 12204008, 15790293, 16590433, 17790381, 17790381, 18390192, 18590265, 18590587, 18590811, 19590929, 19650188, 19790423, 17390186, 20390184 and 21390223) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Grants-in-Aid (H15-Longevity-005, H17-Longevity-003, H16-Kenko-001, H18-Longevity (kokusai), H11-Longevity-020, H17-Kenkou-007, H17-Pharmaco-common-003, H18-Junkankitou[Seishuu]-Ippan-012 and H20-Junkankitou[Seishuu]-Ippan-009, 013) from the Ministry of Health, Labor and Welfare of Japan, Health and Labor Sciences Research Grants, Japan; a Science and Technology Incubation Program in Advanced Regions, the Japanese Science and Technology Agency; Grants-in-Aid from Japanese Society for the Promotion of Science fellows (16.54041, 18.54042, 19.7152, 20.7198, 20.7477 and 20.54043); Health Science Research Grants and Medical Technology Evaluation Research Grants from the Ministry of Health, Labour and Welfare of Japan; the Japanese Atherosclerosis Prevention Fund; the Uehara Memorial Foundation; the Takeda Medical Research Foundation; National Cardiovascular Research Grants; Biomedical Innovation Grants; and the Japanese Research Foundation for Clinical Pharmacology.
KARE. This work was supported by grants from the Korean Centers for Disease Control and Prevention (4845-301, 4851-302 and 4851-307) and an intramural grant from the Korean National Institute of Health (2012-N73002-00), Republic of Korea.
KORA. KORA was initiated and financed by the Helmholtz Zentrum München–German Research Center for Environmental Health and supported by grants from the German Federal Ministry of Education and Research (BMBF), the Federal Ministry of Health and the Munich Center of Health Sciences (MC Health) as part of LMUinnovativ. This research was supported by a grant from the German-Israeli Foundation for Scientific Research and Development, by the European Union’s Seventh Framework Programme (FP7-HEALTH-F5-2012) under grant agreement 305280 (MIMOmics), by Helmholtz-Russia Joint Research Group (HRJRG) 310 and by the German Center for Diabetes Research (DZD). We thank all members of the field staff who were involved in the planning and conduct of the MONICA/KORA Augsburg studies. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. C.G. is supported by EU-FP7-HEALTH grant 602936: CARTARDIS–Identification and Validation of Novel Pharmaceutical Drug Targets for Cardiovascular Disease and BMBF e:Med project e:AtheroSysMed–Systems Medicine of Myocardial Infarction and Stroke.
LBC1921. We thank the cohort participants and team members who contributed to these studies. Phenotype collection was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC), the Royal Society and the Chief Scientist Office of the Scottish government. Genotyping was funded by the BBSRC. The work was undertaken by the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the BBSRC and Medical Research Council, UK, is gratefully acknowledged.
LBC1936. We thank the cohort participants and team members who contributed to these studies. Phenotype collection was supported by Age UK (The Disconnected Mind project). Genotyping was funded by the BBSRC. The work was undertaken by the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the BBSRC and Medical Research Council, UK, is gratefully acknowledged.
LifeLines. The LifeLines Cohort Study and the generation and management of GWAS genotype data for the LifeLines Cohort Study are supported by the Netherlands Organization for Scientific Research (NWO; grant 175.010.2007.006), the Economic Structure-Enhancing Fund (FES) of the Dutch government, the Ministry of Economic Affairs, the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the Northern Netherlands Collaboration of Provinces (SNN), the province of Groningen, University Medical Center Groningen, the University of Groningen, the Dutch Kidney Foundation and the Dutch Diabetes Research Foundation. We thank B. Alizadeh, A. Boesjes, M. Bruinenberg, N. Festen, I. Nolte, L. Franke and M. Valimohammadi for their help in creating the GWAS database and R. Bieringa, J. Keers, R. Oostergo, R. Visser and J. Vonk for their work related to data collection and validation. The authors are grateful to the study participants, the staff of the LifeLines Cohort Study and Medical Biobank Northern Netherlands, and the participating general practitioners and pharmacists. LifeLines Scientific Protocol Preparation: R. de Boer, H. Hillege, M. van der Klauw, G. Navis, H. Ormel, D. Postma, J. Rosmalen, J. Slaets, R. Stolk and B. Wolffenbuttel; LifeLines GWAS Working Group: B. Alizadeh, M. Boezen, M. Bruinenberg, N. Festen, L. Franke, P. van der Harst, G. Navis, D. Postma, H. Snieder, C. Wijmenga and B. Wolffenbuttel.
LOLIPOP. The LOLIPOP study is supported by the NIHR Comprehensive Biomedical Research Centre Imperial College Healthcare NHS Trust, the British Heart Foundation (SP/04/002), the Medical Research Council, UK (G0601966 and G0700931), the Wellcome Trust (084723/Z/08/Z), the NIHR (RP-PG-0407-10371), the European Union’s Seventh Framework Programme (EpiMigrant, 279143) and Action on Hearing Loss (G51). We thank the participants and research staff who made the study possible.
LURIC. We extend our appreciation to the participants of the LURIC study; without their collaboration, this report would not have been written. We thank the LURIC study team who were either temporarily or permanently involved in patient recruitment as well as sample and data handling, in addition to the laboratory staff at the Ludwigshafen General Hospital, the University of Freiburg and the University of Ulm, Germany. LURIC has received funding from the Sixth Framework Programme (integrated project Bloodomics, grant LSHM-CT-2004-503485) and from the Seventh Framework Programme (Atheroremo, grant agreement 201668 and RiskyCAD, grant agreement 305739) of the European Union as well as from the INTERREG IV Oberrhein Program (project A28, Genetic Mechanisms of Cardiovascular Diseases) with support from the European Regional Development Fund (ERDF) and the Wissenschaftsoffensive TMO.
NFBC86. We thank P. Rantakallio (launch of NFBC1986 and initial data collection), S. Vaara (data collection), T. Ylitalo (administration), M. Koiranen (data management), and O. Tornwall and M. Jussila (DNA biobanking). Financial support was provided by the Academy of Finland (project grants 104781, 120315, 129269 Center of Excellence in Complex Disease Genetics), University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), the European Commission (EURO-BLCS, Fifth Framework Programme award QLG1-CT-2000-01643), National Heart, Lung, and Blood Institute grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01), the US NIH/National Institute of Mental Health (5R01MH63706:02), the ENGAGE project and grant agreement HEALTH-F4-2007-201413 and the Medical Research Council, UK (grants G0500539, G0600331 nad PrevMetSyn). DNA extraction, sample quality control, biobank upkeep and aliquotting were performed at the National Public Health Institute, Biomedicum Helsinki, Finland, and supported financially by the Academy of Finland and Biocentrum Helsinki. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
NHAPC. The authors thank all of the participants in this study. The authors also thank the Bio-X Institute, Shanghai Jiao Tong University and the Chinese National Human Genome Center in Shanghai for performing DNA microarray analysis.
POPGEN. The POPGEN study was supported by the German Ministry of Education and Research (BMBF) through the National Genome Research Network (NGFN) and the Ministry of Science, Commerce and Transportation of the state of Schleswig-Holstein. The project has also received infrastructure support through the DFG excellence cluster ‘Inflammation at Interfaces’. The POPGEN 2.0 network is supported by a grant from the German Ministry of Education and Research (01EY1103).
PREVEND. PREVEND genetics is supported by the Dutch Kidney Foundation (grant E033), European Union project grant GENECURE (FP-6 LSHM CT 2006 037697), the US NIH (grant 2R01LM010098), the Netherlands Organization for Health Research and Development (NWO-Groot grant 175.010.2007.006, NWO VENI grant 916.761.70 and ZonMw grant 90.700.441) and the Dutch Interuniversity Cardiology Institute Netherlands (ICIN).
PROMIS. Genotyping in PROMIS was supported by the Wellcome Trust and Pfizer. Some core support to PROMIS was provided by the British Heart Foundation. The Cardiovascular Epidemiology Unit at the University of Cambridge is underpinned by the Medical Research Council, UK (G0800270), the British Heart Foundation (SP/09/002), the British Heart Foundation Cambridge Cardiovascular Centre of Excellence and the NIHR Cambridge Biomedical Research Centre.
PROSPER. The PROSPER study was supported by an investigator-initiated grant obtained from Bristol-Myers-Squibb. J.W.J. is an Established Clinical Investigator of the Netherlands Heart Foundation (grant 2001 D 032). Support for genotyping was provided by the Seventh Framework Programme of the European Commission (grant 223004) and by the Netherlands Genomics Initiative (Netherlands Consortium for Healthy Aging grant 050-060-810).
RHS. RHS was supported by a grant from the National Center for Global Health and Medicine.
Rotterdam Study. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, the Netherlands Organization for Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the municipality of Rotterdam. The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists. The generation and management of the Illumina 450K methylation array data (EWAS data) for the Rotterdam Study were executed by the Human Genotyping Facility of the Genetic Laboratory of the Department of Internal Medicine, Erasmus Medical Center, the Netherlands. The EWAS data were funded by the Genetic Laboratory of the Department of Internal Medicine, Erasmus Medical Center, and by the Netherlands Organization for Scientific Research (NWO; project 184021007) and were made available as a Rainbow Project (RP3; BIOS) of BBMRI-NL. We thank M. Verbiest, M. Jhamai, S. Higgins and M. Verkerk for their help in creating the methylation database.
SCES. SCES is funded by the Biomedical Research Council of Singapore (grant 08/1/35/19/550) and the NMRC, Singapore (grants STaR/0003/2008 and CG/SERI/2010). The National University Health System Tissue Repository and the Genome Institute of Singapore (A*STAR, Singapore) provided services for tissue archiving and genotyping, respectively.
SCHS. We would like to thank S.-H. Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study and K. Arakawa for development of the cohort study database. The founding, longstanding principal investigator of the Singapore Chinese Health Study is M.C. Yu. Significant contributions to the GWAS substudy of SCHS were made by W.-P. Koh, J.-M. Yuan, R. Wang, Z. Chen, M. Seielstad, A.O. Odegaard, E.S. Tai, Y.-Y. Teo, J. Liu, B. Thyagarajan and R. Koratkar. Funding sources included Genetic and Environmental Determinants of Type 2 Diabetes in Chinese Singaporeans, grant R01DK080720 from the US NIH. Additional support came from the NMRC of Singapore under the individual research grants scheme, from the Genome Institute of Singapore, the NMRC of Singapore under its individual research grants and clinician scientist award scheme, and from A*STAR, Singapore. The Singapore Chinese Health Study primary cohort was supported by US NIH/National Cancer Institute grants RO1CA55069, R35CA53890, R01CA80205 and R01CA144034.
SCHS_MI. SCHS was supported by the US NIH (NCI RO1CA55069, R35CA53890, R01CA80205 and R01CA144034), the NUS-HUJ CREATE Programme of the National Research Foundation, Singapore (project 370062002) and a grant from the NMRC, Singapore (NMRC/1270/2010).
SiMES. SiMES is funded by the NMRC, Singapore (grants 0796/2003, IRG07nov013, IRG09nov014, STaR/0003/2008 and CG/SERI/2010) and the Biomedical Research Council of Singapore (grant 09/1/35/19/616). The Singapore Tissue Network and the Genome Institute of Singapore (A*STAR, Singapore) provided services for tissue archiving and genotyping, respectively.
SINDI. SINDI is funded by the Biomedical Research Council of Singapore (grant 08/1/35/19/550) and the NMRC, Singapore (grants STaR/0003/2008 and CG/SERI/2010). The National University Health System Tissue Repository and the Genome Institute of Singapore (A*STAR, Singapore) provided services for tissue archiving and genotyping, respectively.
SMART. This research was financially supported by BBMRI-NL, a research infrastructure financed by the Dutch government (NWO 184.021.007).
SMHS. The study was supported by grants RO1CA82729 and UM1CA173640 from the US NIH. The authors thank the participants and staff members of the SMHS research team for their important contributions.
SMSS. SMSS was supported by the National Natural Science Foundation of China (grant 81172761) and the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
SP2. This project acknowledges the support of the Yong Loo Lin School of Medicine, the National University Health System and the Life Sciences Institute of the National University of Singapore. We also acknowledge the support of the National Research Foundation of Singapore (NRF-RF-2010-05), the Biomedical Research Council of Singapore (under the individual research grants scheme) and the NMRC, Singapore, under the individual research grant and the clinician scientist award schemes).
SRS. This work was supported by the Chinese National Key Program for Basic Research (973 grants: 2004CB518603, 2006CB503804 and 2009CB521905), the Chinese National High-Tech Program (863 grants: 2009AA022703 and 2006AA022179) and the Ministry of Science and Technology, National Natural Science Foundation of China (30871361).
SWHS. This research was supported by US NIH research grant R37CA70867. The authors thank the participants and staff members of SWHS for their important contributions.
TWSC. We gratefully acknowledge the members of the Translational Resource Center (TRC) (NSC102-2325-B-001-040) and the National Center for Genome Medicine (NSC102-2319-B-001-001) at Academia Sinica for their support in subject recruitment, genotyping and statistical analysis. The TWSC study was supported by the Academia Sinica Genomic Medicine Multicenter Study, Taiwan (40-05-GMM).
WHII. The WHII study is supported by grants from the Medical Research Council, UK (G0902037), the British Heart Foundation (RG/07/008/23674), the Stroke Association, the National Heart, Lung, and Blood Institute (5RO1HL036310), the National Institute on Aging (5RO1AG13196), the Agency for Health Care Policy Research (HS06516) and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-Economic Status and Health.
YFS. The Young Finns Study has been financially supported by the Academy of Finland through grants 286284 (T.L.), 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi) and 41071 (Skidi); the Social Insurance Institution of Finland; Kuopio, Tampere and Turku University Hospital Medical Funds (grant X51001 for T.L.); the Juho Vainio Foundation; the Paavo Nurmi Foundation; the Finnish Foundation of Cardiovascular Research (T.L.); the Finnish Cultural Foundation; the Tampere Tuberculosis Foundation (T.L.); the Emil Aaltonen Foundation (T.L.); and the Yrjö Jahnsson Foundation (T.L.).
AUTHOR CONTRIBUTIONS
Participant recruitment, characterization and data generation. Anti-aging study cohort: K. Kohara, M. Igase; Asian Indian Diabetic Heart Study/Sikh Diabetes Study: Y.T., A.B., D.K.S., G.S.W., R. Sarju, R. Saxena, T.R.B.; Biobank-based Integrative Omics Studies Consortium: A.I., B.T.H., M.J.B.; CAGE-Amagasaki: H.R., M. Isono, T.K., F.T., N.K.; CAGE Network: E.N., S.Y., T. Nabika, T. Sugiyama, F.T., N.K.; CAGE-Fukuoka: K.A., K.O., K.Y., R.T.; CAGE-KING: M.N., M.Y., S.I., T. Matsubara; CAGE-Vietnam: H.K., L.D.D., M. Kishimoto, Q.N.N., S.T.P., Y.M.; Cebu Longitudinal Health and Nutrition Survey: K.L.M., L.S.A., N.R.L., Y. Wu; CHD: A.F.R.S., N.J.S., P.D.; INGI Cilento: D.R., M.C., R. Sorice, T. Nutile; EnviroGenoMarkers: G.C., J.C.S.K., M.C.-H., P.V., S.A.K.; Estonian Genome Center of the University of Tartu: A.M., E.M., L.M., T.E.; Finnish Cardiovascular Study: K.N., M. Kähönen, L.-P.L., V. Turjanmaa; Gene Environment Multiphenotype Study: A.A., H. Ahsan, L.T., M.G.K., M.R., M.S.B.; GeneBank Study: H. Allayee, J. Hartiala, S.L.H., W.H.W.T.; Genetic Epidemiology Network of Salt Sensitivity: D.G., J.E.H., Jiang He, T.N.K.; Genetics of Extremely Overweight Young Adults: T.I.A.S., L.P.; Gene × Lifestyle Interactions and Complex Traits Involved in Elevated Disease Risk: I.B., P.W.F., R.W.K., O.R.; DIABNORD: P.W.F., R.W.K., O.R.; Growing Up in Singapore Towards Healthy Outcomes: A.L.T., J.D.H., K. Kwek, Y.-S.C.; Health Examinee (HEXA) shared control study: J.-Y.L., Y.J.K., Y.K.K.; Health2006: A.L., J.M.J., L.L.N.H., T.H., T.S.A.; Heart Protection Study: J.C.H., R. Clarke, R. Collins, S.P.; INGI Val Borbera: C.F.S., D.T., M.T.; Inter99: N.G., O.P., T. Sparsø, T.J., T.S.A.; InterAct: J. Luan, R.A.S.; Japanese Millennium Genome Project: Y.T., H.U., S.U., T. Miki, T.O.; KORA: A.P., C.G., M.M.-N., M.W., R.W., S.W., T. Meitinger; Korea Association Resource study: B.-J.K., J.-Y.H., M.J.G.; LifeLines Cohort Study: B.H.W.W., C.W., D.J.v.V., H.S., L.F., P.v.d.H.; London Life Sciences Population study: B.L., J.A., J.C.C., J.G., J.S., J.S.K., M.L., P.E., R.M., S.-T.T., U.A., W.R.S., W. Zhang, A.W.D., M.I.M.; Lothian Birth Cohorts: D.C.M.L., G. Davies, I.J.D., J.M.S., S.E.H.; Ludwigshafen Risk and Cardiovascular Health Study: G. Delgado, M.E.K., T.B.G., W.M.; Northern Finland Birth Cohort 1986: A.D.S.C.A., A.-L.H., M.-R.J., M.V., S.D., S.F.; Nutrition and Health of Aging Population in China: H.L., X.L., X.Y., Y. Wang; Pakistan Risk Of Myocardial Infarction Study: D.S., J.D., R.A., R.D.Y.; POPGEN study: A.F., I.A., S.S., W.L.; Prevention of Renal and Vascular End-Stage Disease: I.M.L., N.V., R.T.G., W.H.v.G.; Prospective Study of Pravastatin in the Elderly at Risk: D.J.S., I.P., S.T., J.W.J.; Ragama Health Study: A.K., A.R.W., K.S., M.J.P.; Rotterdam Study: A.D., A.G.U., A.H., J.B.J.v.M., L.S., O.H.F.; Secondary Manifestations of Arterial Disease: F.W.A., P.A.D., V. Tragante, W.S.; Shanghai Men’s and Women’s Health Studies: H.C., Jing He, R. Courtney, T.L.E., W. Zheng, X.-O.S., Y.-B.X., Y.-T.G.; Shanghai-Ruijin Study: D.Z., W.H., X.Z., Yi Zhang; Singapore Chinese Eye Study: C.-C.K., C.-Y.C., J. Liu, T.-Y.W.; Singapore Chinese Health Study: C.H., D.O.S., M.A.P., M.D.G., C.-K.H., J.-M.Y., R.D., R.M.v.D., W.-P.K., Y.F.; Singapore Indian Eye Study: E.S.T., E.V., J. Liao, T.A.; Singapore Malay Eye Study: Y.-Y.T.; Singapore Prospective Study Program: J. Lee, P.C., T.L.Y., X.W.; Suzhou Metabolic Syndrome Study: A.W., H.P., Yonghong Zhang, Z.G.; Taiwan Super Control Study: C.-H.C., J.-Y.W., L.-C.C., Y.-T.C.; Tartu: S.K.; Whitehall II study: J.W., M. Kivimaki, M. Kumari; Young Finns Study: J.S.V., N.M., O.T.R., T.L.
Functional genomics and targeted resequencing: M.L., H.K.N., M.A.R., Z.Y.M., R. Soong, N.S.S. Statistical analyses: M.L., F.T., N.V., X.W., W. Zhang, B.L., I.M.L., N.K., J.C.C.
Steering and manuscript writing committee: N.K., M.L., F.T., T.N.K., Y.-Y.T., Jiang He, P.E., E.S.T., P.v.d.H., J.S.K., J.C.C.
Footnotes
Accession codes. GWAS summary statistics and next-generation sequencing data have been deposited in the European Genome-phenome Archive (EGA) under study accession EGAS00001001427.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Lim SS, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imano H, et al. Trends for blood pressure and its contribution to stroke incidence in the middle-aged Japanese population: the Circulatory Risk in Communities Study (CIRCS) Stroke. 2009;40:1571–1577. doi: 10.1161/STROKEAHA.108.538629. [DOI] [PubMed] [Google Scholar]
- 3.Ueshima H, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia. 2006;49:2580–2588. doi: 10.1007/s00125-006-0393-2. [DOI] [PubMed] [Google Scholar]
- 5.Ganesh SK, et al. Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum. Mol. Genet. 2013;22:1663–1678. doi: 10.1093/hmg/dds555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato N, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat. Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson T, et al. Blood pressure loci identified with a gene-centric array. Am. J. Hum. Genet. 2011;89:688–700. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Consortium for Blood Pressure Genome-Wide Association Studies Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wain LV, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton-Cheh C, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat. Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomaszewski M, et al. Genetic architecture of ambulatory blood pressure in the general population: insights from cardiovascular gene-centric array. Hypertension. 2010;56:1069–1076. doi: 10.1161/HYPERTENSIONAHA.110.155721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adeyemo A, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5:e1000564. doi: 10.1371/journal.pgen.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschini N, et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am. J. Hum. Genet. 2013;93:545–554. doi: 10.1016/j.ajhg.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padmanabhan S, et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 2010;6:e1001177. doi: 10.1371/journal.pgen.1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong KW, et al. Genetic variations in ATP2B1, CSK, ARSG and CSMD1 loci are related to blood pressure and/or hypertension in two Korean cohorts. J. Hum. Hypertens. 2010;24:367–372. doi: 10.1038/jhh.2009.86. [DOI] [PubMed] [Google Scholar]
- 17.Archer SL, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellavia A, et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J. Am. Heart Assoc. 2013;2:e000212. doi: 10.1161/JAHA.113.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu XF, Cheng F, Du LZ. Epigenetic regulation of pulmonary arterial hypertension. Hypertens. Res. 2011;34:981–986. doi: 10.1038/hr.2011.79. [DOI] [PubMed] [Google Scholar]
- 20.Turcot V, et al. LINE-1 methylation in visceral adipose tissue of severely obese individuals is associated with metabolic syndrome status and related phenotypes. Clin. Epigenetics. 2012;4:10. doi: 10.1186/1868-7083-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris AP. Transethnic meta-analysis of genomewide association studies. Genet. Epidemiol. 2011;35:809–822. doi: 10.1002/gepi.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong RT, Teo YY. varLD: a program for quantifying variation in linkage disequilibrium patterns between populations. Bioinformatics. 2010;26:1269–1270. doi: 10.1093/bioinformatics/btq125. [DOI] [PubMed] [Google Scholar]
- 23.McLaren W, et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26:2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby M, Gohrbandt S, Clausse V, Brons NH, Muller CP. Interindividual variability and co-regulation of DNA methylation differ among blood cell populations. Epigenetics. 2012;7:1421–1434. doi: 10.4161/epi.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sofer T, Schifano ED, Hoppin JA, Hou L, Baccarelli AA. A-clustering: a novel method for the detection of co-regulated methylation regions, and regions associated with exposure. Bioinformatics. 2013;29:2884–2891. doi: 10.1093/bioinformatics/btt498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lokk K, et al. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol. 2014;15:r54. doi: 10.1186/gb-2014-15-4-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beca S, et al. Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium ATPase type 2a signaling complexes in mouse heart. Circ. Res. 2013;112:289–297. doi: 10.1161/CIRCRESAHA.111.300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo SK, Kang WK, Kwon KI. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans. Clin. Pharmacol. Ther. 2002;71:246–252. doi: 10.1067/mcp.2002.122474. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, et al. A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis CA, et al. PRISM/PRDM6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol. Cell. Biol. 2006;26:2626–2636. doi: 10.1128/MCB.26.7.2626-2636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanraj L, et al. IGFBP-3 inhibits cytokine-induced insulin resistance and early manifestations of atherosclerosis. PLoS ONE. 2013;8:e55084. doi: 10.1371/journal.pone.0055084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akilesh S, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 2011;121:4127–4137. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, et al. A variant OSR1 allele which disturbs OSR1 mRNA expression in renal progenitor cells is associated with reduction of newborn kidney size and function. Hum. Mol. Genet. 2011;20:4167–4174. doi: 10.1093/hmg/ddr341. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Vapurcuyan A, Shahidullah M, Aleksunes LM, Pelis RM. Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab. Dispos. 2012;40:617–624. doi: 10.1124/dmd.111.042036. [DOI] [PubMed] [Google Scholar]
- 36.Chambers JC, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat. Genet. 2010;42:373–375. doi: 10.1038/ng.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flister MJ, et al. Identifying multiple causative genes at a single GWAS locus. Genome Res. 2013;23:1996–2002. doi: 10.1101/gr.160283.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halachev K, Bast H, Albrecht F, Lengauer T, Bock C. EpiExplorer: live exploration and global analysis of large epigenomic datasets. Genome Biol. 2012;13:R96. doi: 10.1186/gb-2012-13-10-r96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeller T, et al. Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS ONE. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehne B, et al. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 2015;16:37. doi: 10.1186/s13059-015-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houseman EA, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers JC, et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3:526–534. doi: 10.1016/S2213-8587(15)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palli D, et al. A molecular epidemiology project on diet and cancer: the EPIC–Italy Prospective Study. Design and baseline characteristics of participants. Tumori. 2003;89:586–593. doi: 10.1177/030089160308900602. [DOI] [PubMed] [Google Scholar]
- 44.Hallmans G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand. J. Public Health Suppl. 2003;61:18–24. doi: 10.1080/14034950310001432. [DOI] [PubMed] [Google Scholar]
- 45.Hebels DG, et al. Performance in omics analyses of blood samples in long-term storage: opportunities for the exploitation of existing biobanks in environmental health research. Environ. Health Perspect. 2013;121:480–487. doi: 10.1289/ehp.1205657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slieker RC, et al. Identification and systematic annotation of tissue-specific differentially methylated regions using the Illumina 450k array. Epigenetics Chromatin. 2013;6:26. doi: 10.1186/1756-8935-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soh SE, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int. J. Epidemiol. 2014;43:1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 48.Pan H, et al. Measuring the methylome in clinical samples: improved processing of the Infinium Human Methylation450 BeadChip Array. Epigenetics. 2012;7:1173–1187. doi: 10.4161/epi.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teh AL, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24:1064–1074. doi: 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellcome Trust Case Control Consortium Bayesian refinement of association signals for 14 loci in 3 common diseases. Nat. Genet. 2012;44:1294–1301. doi: 10.1038/ng.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.