Abstract
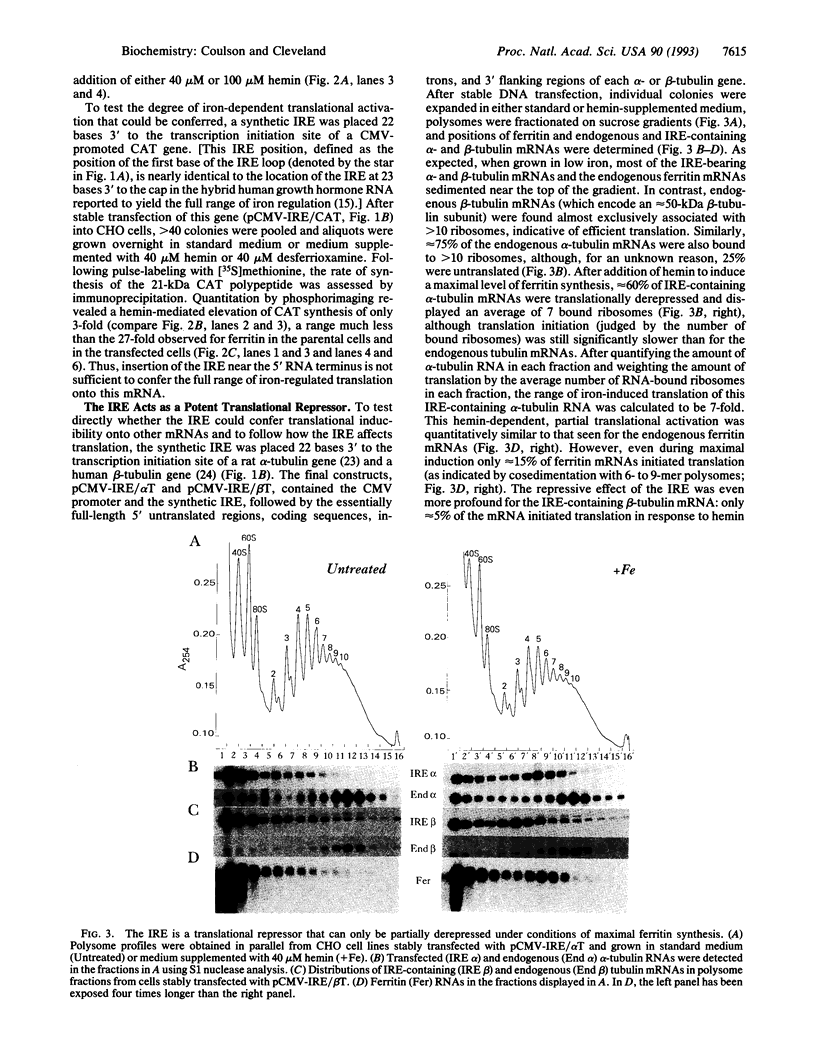
Ferritin synthesis, maintained at a very low basal rate when extracellular iron levels are low, is elevated up to 50-fold when iron levels are increased. Previous examinations of this iron-dependent activation have concluded that changes in ferritin synthesis results from selective translational activation conferred by an "iron response element" that lies near the 5' terminus of all known ferritin mRNAs. By placing an iron response element in an optimal position in other mRNAs, we find the iron response element to be a potent translational repressor whose influence can only partially be abrogated under optimal inducing conditions. Further, we show that the 25- to 50-fold iron-mediated increase in ferritin synthesis results from coupling a 5- to 6-fold iron-dependent translational derepression with a similar 5- to 6-fold nuclear-dependent increase in mRNA level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Caughman S. W., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. J Biol Chem. 1988 Dec 15;263(35):19048–19052. [PubMed] [Google Scholar]
- Dix D. J., Lin P. N., Kimata Y., Theil E. C. The iron regulatory region of ferritin mRNA is also a positive control element for iron-independent translation. Biochemistry. 1992 Mar 17;31(10):2818–2822. doi: 10.1021/bi00125a024. [DOI] [PubMed] [Google Scholar]
- Elliott E. M., Henderson G., Sarangi F., Ling V. Complete sequence of three alpha-tubulin cDNAs in Chinese hamster ovary cells: each encodes a distinct alpha-tubulin isoprotein. Mol Cell Biol. 1986 Mar;6(3):906–913. doi: 10.1128/mcb.6.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Hentze M. W. Position is the critical determinant for function of iron-responsive elements as translational regulators. Mol Cell Biol. 1992 May;12(5):1959–1966. doi: 10.1128/mcb.12.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., Ashwell G., van Renswoude J. Multiple post-transcriptional regulatory mechanisms in ferritin gene expression. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1801–1805. doi: 10.1073/pnas.86.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Eisenstein R. S. Translational control: the ferritin story. Curr Opin Cell Biol. 1989 Dec;1(6):1154–1159. doi: 10.1016/s0955-0674(89)80066-3. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Reynolds-Kohler C., Smith B. A. Negative and positive regulation by a short segment in the 5'-flanking region of the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1987 Nov;7(11):4125–4129. doi: 10.1128/mcb.7.11.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2277–2281. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Dancis A., Caughman W., Harford J. B., Klausner R. D. Influence of altered transcription on the translational control of human ferritin expression. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6335–6339. doi: 10.1073/pnas.84.18.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Sawada T., Cabral F. Expression and function of beta-tubulin isotypes in Chinese hamster ovary cells. J Biol Chem. 1989 Feb 15;264(5):3013–3020. [PubMed] [Google Scholar]
- Sisodia S. S., Gay D. A., Cleveland D. W. In vivo discrimination among beta-tubulin isotypes: selective degradation of a type IV beta-tubulin isotype following overexpression in cultured animal cells. New Biol. 1990 Jan;2(1):66–76. [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Thach R. E. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry. 1986 Apr 22;25(8):2033–2041. doi: 10.1021/bi00356a030. [DOI] [PubMed] [Google Scholar]