ABSTRACT
The reactive enamine 2-aminoacrylate (2AA) is a metabolic stressor capable of damaging cellular components. Members of the broadly conserved Rid (RidA/YER057c/UK114) protein family mitigate 2AA stress in vivo by facilitating enamine and/or imine hydrolysis. Previous work showed that 2AA accumulation in ridA strains of Salmonella enterica led to the inactivation of multiple target enzymes, including serine hydroxymethyltransferase (GlyA). However, the specific cause of a ridA strain's inability to grow during periods of 2AA stress had yet to be determined. Work presented here shows that glycine supplementation suppressed all 2AA-dependent ridA strain growth defects described to date. Depending on the metabolic context, glycine appeared to suppress ridA strain growth defects by eliciting a GcvB small RNA-dependent regulatory response or by serving as a precursor to one-carbon units produced by the glycine cleavage complex (GCV). In either case, the data suggest that GlyA is the most physiologically sensitive target of 2AA inactivation in S. enterica. The universally conserved nature of GlyA among free-living organisms highlights the importance of RidA in mitigating 2AA stress.
IMPORTANCE The RidA stress response prevents 2-aminoacrylate (2AA) damage from occurring in prokaryotes and eukaryotes alike. 2AA inactivation of serine hydroxymethyltransferase (GlyA) from Salmonella enterica restricts glycine and one-carbon production, ultimately reducing fitness of the organism. The cooccurrence of genes encoding 2AA production enzymes and serine hydroxy-methyltransferase (SHMT) in many genomes may in part underlie the evolutionary selection for Rid proteins to maintain appropriate glycine and one-carbon metabolism throughout life.
INTRODUCTION
Rid (RidA/YER057c/UK114) proteins are found throughout each kingdom of life, indicating that these proteins play fundamental roles in cellular processes. Bioinformatics analyses identified eight subgroups within the Rid protein family (1). The archetypal RidA subfamily is broadly distributed and includes the best-studied RidA enzyme from Salmonella enterica, while the remaining seven Rid subgroups are primarily restricted to bacteria. In S. enterica, RidA is known to preempt metabolic damage caused by the unbound reactive enamine 2-aminoacrylate (2AA) (2). In the absence of RidA, 2AA persisted in solution long enough to inactivate multiple target enzymes in vivo and in vitro (2–4). RidA proteins isolated from organisms spanning the tree of life displayed similar enamine and imine deaminase activities (5, 6), suggesting that RidA enzymes have a conserved role in preventing 2AA stress.
2AA stress arises as a consequence of some pyridoxal 5′-phosphate (PLP)-dependent enzyme activities. Previous analysis of S. enterica showed that PLP-dependent serine/threonine dehydratases (IlvA/TdcB; EC 4.3.1.19), cysteine desulfhydrase (CdsH; EC 4.4.1.1), and diaminopropionate ammonia-lyase (DpaL; EC 4.3.1.15) generated free 2AA from exogenous serine, cysteine, and diaminopropionate, respectively, leading to complete growth inhibition in the absence of RidA (7, 8; D. C. Ernst, M. E. Anderson, and D. M. Downs, submitted for publication). In the absence of exogenous 2AA precursors, basal IlvA activity produced sufficient 2AA from endogenous serine to cause a minor growth defect in ridA strains grown in minimal glucose medium (9). The minor growth defect coincided with measurable 2AA inactivation of several PLP-dependent target enzymes, including serine hydroxymethyltransferase (SHMT) (GlyA; EC 2.1.2.1), alanine racemases (Alr/DadX; EC 5.1.1.1), and transaminase B (IlvE; EC 2.6.1.42) (2–4). Isoleucine prevented IlvA-dependent 2AA production from serine (endogenous or exogenous) and restored growth of ridA strains to wild-type levels through feedback inhibition of IlvA (9). Isoleucine failed to restore growth to a ridA strain grown in the presence of exogenous cysteine or diaminopropionate (8) (Ernst et al., submitted) or when containing a feedback-resistant variant of IlvA (IlvAL447F) and grown in minimal glucose medium (10). Taken together, these data showed that isoleucine limitation alone could not explain the growth defects displayed by a ridA strain faced with 2AA stress.
Of the 2AA targets described to date, GlyA was inactivated to the greatest extent in ridA strains relative to the wild type (20% of wild-type activity in minimal glucose medium) (4). GlyA catalyzes the reversible transfer of a hydroxymethyl group from serine to tetrahydrofolate (THF), forming glycine and the one-carbon unit 5,10-methylene-tetrahydrofolate (5,10-mTHF) (Fig. 1). Glycine can be further catabolized by the glycine cleavage complex (GCV) to generate additional one-carbon units. Alternatively, glycine may serve as a proteinaceous amino acid, metabolic precursor, or regulatory signal (11). Work published by Flynn et al. demonstrated that ridA strains grown aerobically on glucose as the sole carbon source experienced a defect in one-carbon metabolism stemming from 2AA damage to GlyA (4). The one-carbon limitation diminished coenzyme A (CoA) biosynthesis, leading to pyruvate accumulation in the growth medium that correlated with a minor growth defect of the ridA strain. The CoA precursor pantothenate stimulated CoA production in a ridA strain but failed to restore wild-type growth. In contrast, glycine restored both growth and CoA production to wild-type levels in a ridA strain grown in minimal glucose medium (4). Building on this previous work, results presented herein demonstrate that glycine alleviates all ridA-dependent growth defects. However, the mechanism by which glycine suppresses these growth defects depends on the metabolic context. This work underlines the crucial role played by RidA in protecting GlyA from 2AA damage to facilitate efficient glycine and one-carbon metabolism. The ubiquity of GlyA homologs among free-living organisms may in part explain the broad conservation of RidA enzymes.
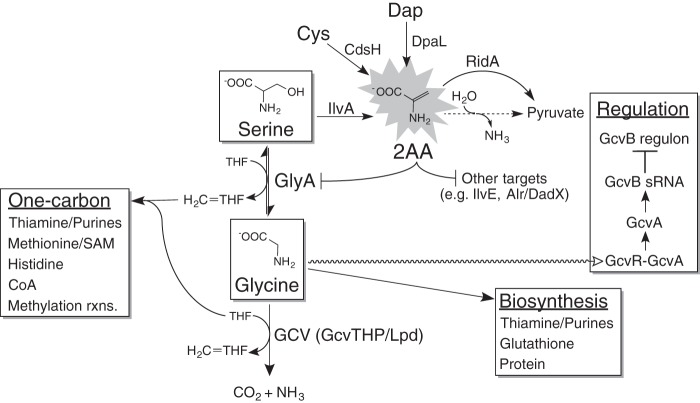
FIG 1.
Connections between 2-aminoacrylate (2AA) and the glycine metabolic node. 2AA is produced from serine, cysteine (Cys), and diaminopropionate (Dap) by IlvA, CdsH, and DpaL, respectively. In the absence of RidA, 2AA persists in solution long enough to inactivate target enzymes, including GlyA. Damage to GlyA restricts flux to glycine, simultaneously reducing the amount of tetrahydrofolate (THF) converted to one-carbon units (H2C = THF). Excess glycine can be converted to additional one-carbon units by the glycine cleavage complex (GCV) or can be incorporated into protein production or other biosynthetic pathways. Alternatively, glycine may serve as a regulatory molecule (undulating line), disrupting the complex formed between GcvR-GcvA, freeing GcvA to activate transcription of the GcvB sRNA and thereby leading to posttranscriptional regulation of the GcvB regulon. The spontaneous conversion from 2AA to pyruvate (dashed line) is slow enough to allow 2AA accumulation and damage to occur. rxns, reactions.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
All strains used for this study are derivatives of Salmonella enterica serovar Typhimurium LT2 and are listed with their respective genotypes in Table 1. Tn10d(Tc) is the transposition-defective mini-Tn10 (Tn10Δ16Δ17) described by Way et al. (12). Each strain was constructed for this study.
TABLE 1.
Salmonella strains used in this study
| Strain | Genotype |
|---|---|
| DM14828 | Wild-type LT2 |
| DM14829 | ridA1::Tn10d(Tc) |
| DM14830 | ilvA219 |
| DM14831 | ilvA219 ridA1::Tn10d(Tc) |
| DM14836 | gcvB32::Kn |
| DM14837 | ridA1::Tn10d(Tc) gcvB32::Kn |
| DM14838 | gcvP31::Cm |
| DM14839 | ridA1::Tn10d(Tc) gcvP31::Cm |
| DM14840 | ilvA219 gcvB32::Kn |
| DM14841 | ilvA219 ridA1::Tn10d(Tc) gcvB32::Kn |
| DM14842 | ilvA219 gcvP31::Cm |
| DM14843 | ilvA219 ridA1::Tn10d(Tc) gcvP31::Cm |
Minimal growth medium consisted of no-carbon E medium (NCE) supplemented with 1 mM MgSO4 (13) and trace elements (14). Glucose (11 mM) or pyruvate (50 mM) was provided as the sole carbon source where indicated below. Difco nutrient broth (NB; 8 g/liter) with sodium chloride (5 g/liter) was used as rich medium. Difco BiTek agar (15 g/liter) was added for solid medium. When added, final amino acid and vitamin concentrations were as follows: l-serine, 5 mM; l-cysteine, 0.25 mM; l-2,3-diaminopropionate, 0.1 mM; l-isoleucine, 1 mM; glycine, 0.67 mM; l-methionine, 1 mM; pantothenate, 0.1 mM; and thiamine, 0.1 μM. Antibiotics were added at the following final concentrations when necessary for rich and minimal media, respectively: 20 and 10 μg/ml of tetracycline, 20 and 5 μg/ml of chloramphenicol, and 50 and 12.5 μg/ml of kanamycin. l-2,3-Diaminopropionate was purchased from Chem-Impex International, Inc., Wood Dale, IL. All other chemicals were purchased from Sigma-Aldrich Chemical Company, St. Louis, MO.
Genetic techniques.
Strains were constructed by transductional crosses using the high-frequency general transducing mutant of bacteriophage P22 (HT105/1, int-201) (15). Methods used to perform transductions, remove phage contamination from cells, and identify phage-free recombinants were described previously (16, 17). Gene replacements were made using the λ red recombineering strategy described by Datsenko and Wanner (18). Primers gcvB_Wanner_For (5′-ACTTCCTGAGCCGGAACGAAAAGTTTTATCGGAATGCGTGGTGTAGGCTGGAGCTGCTTC-3′) and gcvB_Wanner_Rev (5′-AGCACCGCAATATGGCGGTGCTACATTAATCACTATGGACCATATGAATATCCTCCTTAG-3′) were used to insert a genetic marker at the gcvB locus, forming gcvB32::Kn. The gcvP locus was disrupted previously, forming gcvP31::Cm. All gene replacements were reconstructed in the appropriate isogenic strain backgrounds for this study.
Growth phenotypes were assessed in solid medium using agar overlays or in liquid medium using growth curves as described previously (19). Growth curves were performed as follows. An overnight NB culture of the relevant strain was pelleted and resuspended in an equal volume of saline (85 mM), and 5 μl was used to inoculate 195 μl of growth medium contained within each well of a 96-well microtiter plate (Corning). Microtiter plates were incubated at 37°C with shaking, and growth was monitored by the change in optical density at 650 nm (OD650) using a BioTek Elx808 plate reader. All growth experiments were performed in triplicate, and the resulting data were plotted using GraphPad Prism 5.0f, generating curves in log10-format that display the averages and standard deviations of the replicates. Specific growth rates (μ) were calculated according to the following equation: ln(X/X0)/T, where X is OD650, X0 is the starting OD650 of the exponential growth period monitored, and T is time in hours.
Transaminase B (IlvE) assays.
IlvE activity was measured in crude lysates as previously described (8). Cultures were grown at 37°C with aeration to stationary phase in 2 ml of NB. Cells were pelleted and resuspended in an equal volume of saline (85 mM NaCl), and 100 μl was used to inoculate 5 ml of minimal glucose medium. Where appropriate, isoleucine (1 mM) or glycine (0.67 mM) was included in the growth medium. Cultures were returned to 37°C and incubated for ∼6 to 8 h with shaking until an OD650 of 1 was achieved. Cultures were chilled on ice and then pelleted (8,000 × g) at 4°C. Cell pellets were gently resuspended in NCE medium (1 ml), then harvested by centrifugation as described above, and frozen at −20°C until assayed. Upon thawing, cell pellets were resuspended in 250 μl of 50 mM potassium phosphate buffer (pH 8.0). An aliquot of the cell suspension (30 μl) was added to 150 μl of buffer containing 50 nM PLP, 10 nM 2-ketoglutarate, and 10% PopCulture (Merck). Reactions were preequilibrated for 10 min at 37°C and then initiated by adding 20 μl of 0.2 mM isoleucine. After 20 min, the reactions were terminated by adding 2,4-dinitrophenylhydrazine (DNPH), and the amount of 2-ketomethylvalerate (2KMV) produced was quantified to assess IlvE activity. Following derivatization with DNPH, the relevant hydrazone was extracted with toluene, washed with hydrochloric acid (0.5 N), and treated with sodium hydroxide (1.5 N) to allow chromophore formation. The absorbance of the chromophore-containing aqueous layer was measured spectrophotometrically at 540 nm using a Spectramax M2 and compared to a standard curve of 2KMV generated using the same extraction and chromophore development procedure. Cell extracts were generated and the amount of protein in each extract was quantified using the bicinchoninic acid (BCA) assay (Pierce). IlvE activity is reported as nanmoles of 2KMV formed per milligram of protein per minute. The data are displayed as the averages and standard deviations from three independent experiments. Statistical significance (P < 0.01) was determined by conducting one-way analysis of variance (ANOVA) and Tukey's posttest using GraphPad Prism 5.0f.
RESULTS AND DISCUSSION
Glycine alleviates growth arrest caused by 2AA.
The effect exogenous glycine had on growth of a ridA strain in minimal glucose medium was reported previously (4). Glycine suppressed the minor growth defect of a ridA strain and prevented pyruvate accumulation, suggesting that glycine limitation may contribute to alternative ridA phenotypes. Consistent with previous reports, the addition of serine, cysteine, or diaminopropionate to cultures of a ridA strain grown in minimal glucose medium completely prevented growth as a consequence of 2AA production (7, 8; Ernst et al., submitted) (Table 2). Additionally, a ridA strain did not grow with pyruvate as the sole carbon source (10) (Table 2). The impact of exogenous glycine on these phenotypes was quantified in liquid cultures containing minimal glucose medium and the respective 2AA precursors or in minimal medium containing pyruvate as the sole carbon source. The addition of glycine (0.67 mM) to cultures of a ridA strain (DM14829) containing 5 mM serine restored growth (Fig. 2), resulting in a specific growth rate (μ) of 0.34 h−1 (Table 2). Although the same final cell density was achieved, the ridA strain grew slower than the wild type (DM14828; μ = 0.60 h−1) in the presence of serine and glycine, suggesting that additional metabolic deficiencies persisted in the ridA strain. Growth was similarly restored when glycine was added to ridA cultures containing 0.25 mM cysteine (μ = 0.30 h−1) or 0.1 mM diaminopropionate (μ = 0.43 h−1) or when pyruvate served as the carbon source (μ = 0.33 h−1) (Table 2). In each case, the growth rate of ridA cultures supplemented with glycine remained lower than those of the respective wild-type cultures, but the final cell densities were equivalent (data not shown). Additional amino acids and vitamins were tested for the ability to suppress ridA strain growth defects caused by 2AA stress induced by serine or cysteine added to the growth medium. Previous reports described the stimulatory effect threonine and isoleucine had on growth of ridA strains inhibited by exogenous serine (9, 20). None of the remaining common amino acids or coenzyme precursors, including pyridoxal, thiamine, or pantothenate, significantly stimulated growth of the ridA strain in the presence of serine or cysteine (data not shown). These observations suggest that the perturbation of glycine metabolism was the most sensitive defect encountered by ridA strains challenged with 2AA stress and showed that glycine significantly alleviated all ridA strain growth defects identified to date.
TABLE 2.
Glycine improves growth rates of ridA strains inhibited by 2AA
| Growth mediumb | Growth ratea (h−1) of: |
|||||
|---|---|---|---|---|---|---|
| DM14828 (Wt) | DM14829 (ridA) | DM14838 (gcvP) | DM14839 (ridA gcvP) | DM14836 (gcvB) | DM14837 (ridA gcvB) | |
| Min-glucose | 0.56 | 0.42 | 0.52 | 0.33 | 0.51 | 0.42 |
| +Gly | 0.52 | 0.52 | 0.38 | 0.23 | 0.59 | 0.51 |
| +Ser | 0.57 | NGc | 0.59 | 0.28d | 0.59 | NG |
| +Ser and Gly | 0.60 | 0.34 | 0.58 | 0.18 | 0.59 | NG |
| +Cys | 0.50 | NG | 0.54 | NG | 0.57 | NG |
| +Cys and Gly | 0.57 | 0.30 | 0.52 | NG | 0.54 | 0.31 |
| +Dap | 0.58 | NG | 0.52 | NG | 0.56 | NG |
| +Dap and Gly | 0.57 | 0.43 | 0.45 | NG | 0.49 | 0.51 |
| Min-pyruvate | 0.41 | NG | 0.40 | NG | 0.44 | NG |
| +Gly | 0.42 | 0.33 | 0.35 | NG | 0.41 | 0.35 |
Specific growth rates (μ) were calculated as ln(X/X0)/T, where X represents OD650, X0 is the initial OD650 of the exponential growth period monitored, and T is time in hours. Data are the averages for three independent cultures. The standard deviation was less than 0.05 in all cases. Growth was assessed within 20 h following inoculation. Wt, wild type.
Glucose (11 mM) and pyruvate (50 mM) were provided as the carbon source in minimal media as indicated (Min-glucose and Min-pyruvate, respectively). Amino acid supplements were added at the following final concentrations: 0.67 mM glycine (Gly), 5 mM serine (Ser), 0.25 mM cysteine (Cys), and 0.1 mM diaminopropionate (Dap).
Average growth rates less than 0.05 h−1 were designated as no growth (NG).
The displayed growth rate reflects the secondary growth phase of a biphasic growth pattern; the primary growth rate was 0.06 h−1.
FIG 2.
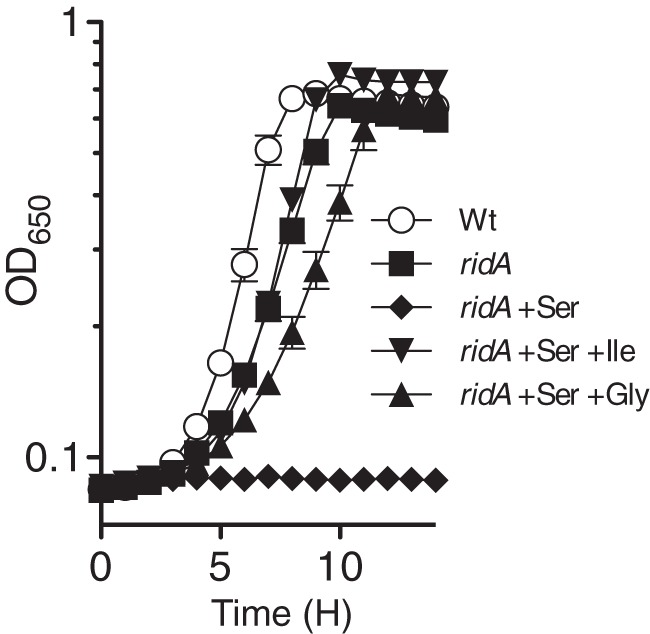
Glycine restores growth to ridA strains inhibited by 2AA produced from serine. Growth of the wild-type strain (Wt; DM14828) in minimal glucose medium is compared to growth of the ridA strain (DM14829) in minimal glucose medium containing no addition, 5 mM serine, 5 mM serine and 1 mM isoleucine, or 5 mM serine and 0.67 mM glycine. The data represent the averages and standard deviations from three independent cultures. Growth of the wild-type strain was not significantly improved by exogenous serine, glycine, or isoleucine.
It was possible that glycine suppressed the growth defect of the ridA strain by preventing 2AA formation and/or accumulation. To test this possibility, the influence of glycine on transaminase B (IlvE) activity was assessed in a ridA background. Previous studies demonstrated the susceptibility of IlvE to free 2AA inactivation by covalent modification of the enzyme (2, 9), rendering IlvE a reliable proxy for endogenous 2AA levels. IlvE activity was measured in crude lysates of wild-type and ridA strains grown to stationary phase (OD650 ≈ 1) in minimal glucose medium containing the appropriate additions. IlvE activity was significantly reduced (P < 0.01) in a ridA background (234 nmol/min/mg) relative to that of the wild-type control (370 nmol/min/mg) when grown in minimal glucose medium, while isoleucine (1 mM) added to the growth medium preserved IlvE activity in the ridA strain (333 nmol/min/mg) compared to that in the wild type (334 nmol/min/mg) (Fig. 3), consistent with previous reports (9). When glycine (0.67 mM) was present in the growth medium, IlvE activity in a ridA strain (232 nmol/min/mg) remained significantly reduced (P < 0.01) relative to that in the wild type (395 nmol/min/mg) (Fig. 3). These data showed that exogenous glycine did not prevent 2AA accumulation or concomitant damage caused to IlvE. Thus, a simple scenario suggested that glycine restored growth to a ridA strain under 2AA stress by bypassing a target of that stress (i.e., GlyA).
FIG 3.
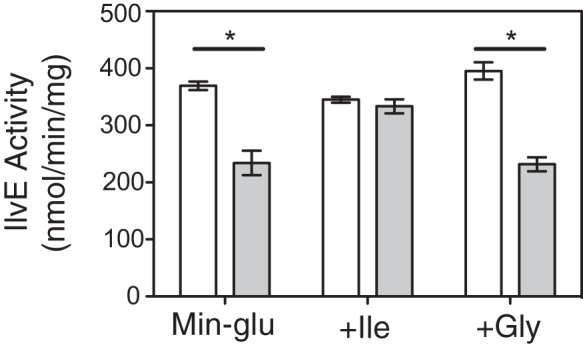
Glycine does not directly prevent IlvA-dependent 2AA production from endogenous serine in vivo. IlvE activity was measured as a proxy for 2AA accumulation in wild-type (DM14828; white bars) and ridA (DM14829; gray bars) strains grown in minimal glucose medium alone (Min-glu), with 1 mM isoleucine added (+Ile), or with 0.67 mM glycine added (+Gly). The data are reported as nanomoles of 2-ketomethylvalerate produced per minute per milligram of crude lysate. Experiments were performed in triplicate, and data are reported as the averages and standard deviations of the means. The data were analyzed by one-way ANOVA and Tukey's test using GraphPad Prism 5.0f to identify significantly reduced activities (P < 0.01) for a given growth condition, indicated by the asterisks.
GcvB, but not GCV, is required to suppress ridA strain sensitivity to exogenous serine.
Glycine serves as an amino acid building block in protein biosynthesis throughout life. Glycine can also function as a metabolic precursor contributing to the production of one-carbon units and a variety of core metabolites (Fig. 1). Additionally, glycine influences the regulation of metabolism, coordinating the production of metabolic enzymes with the nutrient state of the cell (21). A genetic approach was taken to tease apart the relative benefit of glycine to a ridA strain experiencing 2AA stress. A genetic marker inserted in gcvP, which encodes the P protein of the glycine cleavage complex (GCV), prevented further catabolism of glycine to 5,10-mTHF, carbon dioxide, and ammonia. If the benefit of glycine to a ridA strain was due to restored one-carbon units, the lesion in gcvP was predicted to abolish glycine rescue of a ridA strain grown in the presence of exogenous serine. Instead, the ridA gcvP double mutant strain (DM14839) grew biphasically in minimal glucose medium containing 5 mM serine even in the absence of exogenous glycine (Fig. 4). The biphasic growth consisted of a primary (∼15 h) slow growth phase (μ = 0.06 h−1), followed by a faster secondary growth phase (μ = 0.28 h−1) (Table 2). Growth of the gcvP single-mutant strain (DM14838) was similar to that of the wild-type strain under the conditions tested (Table 2). These data indicated that glycine accumulation in the absence of GCV directly suppressed the ridA strain growth defect (Fig. 4). The addition of glycine to ridA gcvP (DM14839) cultures containing serine circumvented the initial slow growth phase and enabled monophasic growth, achieving a growth rate (μ = 0.18 h−1) intermediate to the biphasic growth rates observed in the absence of glycine (Table 2; Fig. 5A). Together, these data demonstrated that GCV was not essential for glycine suppression of ridA strain sensitivity to exogenous serine.
FIG 4.
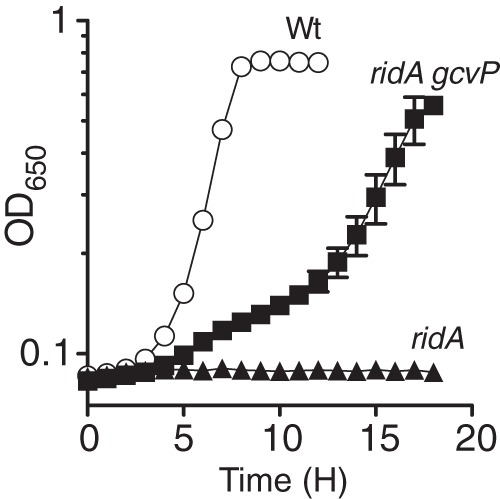
The disruption of gcvP, encoding a component of GCV, stimulates growth of a ridA strain inhibited by exogenous serine. Growth curves were performed in minimal glucose medium containing 5 mM serine. The data show the averages and standard deviations for three independent cultures. The relevant genotypes of wild-type (Wt; DM14828), ridA (DM14829), and ridA gcvP (DM14839) strains are indicated. Growth of the gcvP single-mutant strain (DM14838; not shown) was indistinguishable from that of the wild type.
FIG 5.
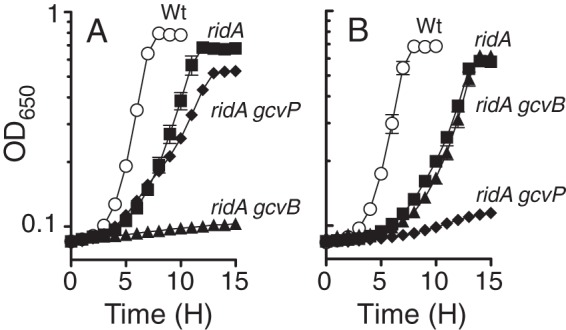
The metabolic components required for glycine to suppress the growth defect of a ridA strain are based on the source of metabolic stress. Growth of the indicated strains was assessed in minimal glucose glycine (0.67 mM) media containing 5 mM serine (A) or 0.25 mM cysteine (B). The averages and standard deviations for three independent cultures of wild-type (Wt; DM14828), ridA (DM14829), ridA gcvB (DM14837), and ridA gcvP (DM14839) strains are plotted. Growth of the gcvB (DM14836) and gcvP (DM14838) single-mutant strains was identical to that of the wild type (data not shown).
A genetic marker inserted into the gcvB locus to prevent transcription of the GcvB small RNA (sRNA) was used to assess whether glycine was required for regulatory purposes. The GcvB sRNA was previously shown to negatively regulate translation of a number of amino acid uptake and biosynthesis enzymes in response to glycine accumulation in S. enterica (22). The regulatory cascade leading to GcvB production is initiated when glycine disrupts the protein complex formed between GcvA and GcvR, freeing GcvA to activate transcription of gcvB and other target genes (Fig. 1). The ridA gcvB double mutant (DM14837) did not grow in minimal medium containing 5 mM serine, while growth of the gcvB single mutant (DM14836) was indistinguishable from that of the wild type (DM14828) (Table 2). Including glycine in the growth medium did not stimulate growth of the ridA gcvB strain (DM14837) in minimal medium containing serine (Table 2; Fig. 5A), demonstrating that the GcvB sRNA was essential for glycine suppression of serine sensitivity in a ridA strain. Genetic experiments showed that deleting the major serine transporter regulated by GcvB, SstT, had no impact on serine sensitivity or glycine suppression phenotypes in a ridA background (data not shown).
GCV is necessary and sufficient for glycine effect in a moderate 2AA stress scenario.
The impact of gcvP or gcvB on additional ridA phenotypes was tested to determine if the mechanism of glycine suppression was conserved. In contrast to the results seen with exogenous serine, the ridA gcvP strain (DM14389) was unable to grow when cysteine or diaminopropionate was included in the growth medium despite the addition of glycine (Table 2; Fig. 5B). Similarly, glycine failed to stimulate growth of the ridA gcvP strain when pyruvate was provided as the sole carbon source (Table 2). Disruption of the GcvB sRNA had no impact on the growth response of a ridA strain to cysteine, diaminopropionate, or pyruvate (Table 2). Growth of the ridA gcvB double-mutant strain (DM14837) was restored to roughly the same rate as that of the ridA single-mutant strain when glycine was added to cultures containing cysteine (μ = 0.31 h−1) (Fig. 5B) or diaminopropionate (μ = 0.51 h−1) or when pyruvate served as the sole carbon source (μ = 0.35 h−1). These data demonstrated that glycine suppression of ridA strain sensitivity to cysteine, diaminopropionate, or pyruvate relied on an intact GCV but was unaffected by the GcvB sRNA. The conclusion drawn from these data is that the stimulation of one-carbon production by GCV-mediated catabolism of glycine was required to support growth of a ridA strain inhibited by 2AA produced from cysteine or diaminopropionate or when pyruvate served as the carbon source.
A genetic strategy was devised to address the contradicting mechanisms of glycine suppression observed for a ridA strain inhibited by exogenous serine compared to the alternative sources of growth inhibition (i.e., cysteine, diaminopropionate, and pyruvate). The ilvA219 allele encoding an IlvA variant (IlvAL447F) resistant to feedback inhibition by isoleucine (23) was introduced into the appropriate strain backgrounds to exacerbate 2AA production from endogenous serine, as previously described (9, 10). Consistent with previous reports, the uncontrolled activity of IlvAL447F in an ilvA219 ridA background (DM14831) inhibited growth in minimal glucose medium (Fig. 6A), achieving a final cell density (final OD650 = 0.22) more than 2-fold less than that of the ilvA219 single-mutant strain (DM14830) (final OD650 = 0.57) (Table 3). These data were consistent with a nutrient limitation caused by increased 2AA stress in the ilvA219 ridA strain. Adding glycine to the growth medium stimulated growth of the ilvA219 ridA strain, allowing it to reach the same final cell density as that of the ilvA219 single mutant (final OD650 = 0.58). A lesion in gcvP prevented glycine from stimulating growth of the ilvA219 ridA gcvP mutant strain (DM14843), while the inactivation of gcvB in the ilvA219 ridA gcvB strain (DM14841) did not affect glycine stimulation (Fig. 6B). The data showed that glycine suppressed the growth defect of a ridA strain caused by endogenous serine in a GCV-dependent manner, counter to the GcvB sRNA requirement for glycine suppression of the growth defect caused by exogenous serine described above. These data suggest that there is no inherent difference in the metabolic consequences encountered by a ridA strain experiencing 2AA stress, regardless of the source of 2AA. The different mechanistic determinants of glycine suppression observed between endogenous and exogenous serine stress conditions likely reflects the disparate 2AA burdens caused by unequal levels of serine accumulation inside the cell.
FIG 6.
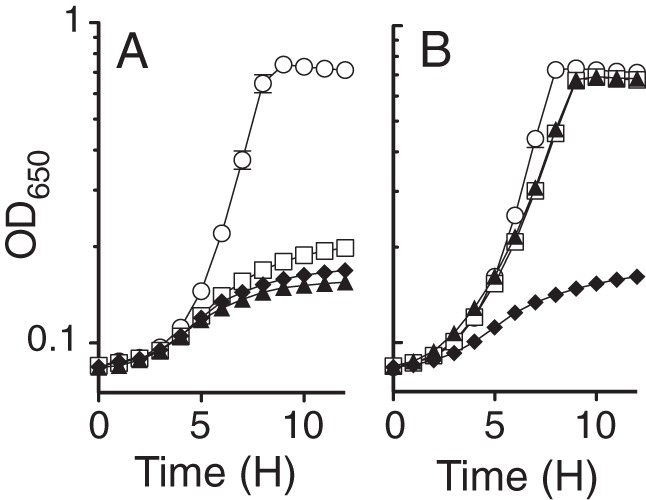
GCV is necessary for glycine to stimulate growth of a ridA strain inhibited by 2AA produced from endogenous serine. Strains were grown in minimal glucose medium lacking additions (A) or supplemented with 0.67 mM glycine (B). The strains include ilvA219 (DM14830; circles), ilvA219 ridA (DM14831; squares), ilvA219 ridA gcvB (DM14841; triangles), and ilvA219 ridA gcvP (DM14843; diamonds) strains. The data reflect the averages and standard deviations for three independent cultures.
TABLE 3.
Metabolites that require one-carbon units for their production bypass the need to restore one-carbon production via GCV in ilvA219 ridA strains grown in minimal glucose medium
| Strain | Genotype | Final cell densitya (OD650) in: |
||||
|---|---|---|---|---|---|---|
| Min-glu | +Gly | +Thi | +Pan | +Met | ||
| DM14830 | ilvA219 | 0.70 | 0.70 | 0.73 | 0.76 | 0.74 |
| DM14831 | ilvA219 ridA | 0.20 | 0.67 | 0.58 | 0.71 | 0.62 |
| DM14842 | ilvA219 gcvP | 0.66 | 0.68 | 0.70 | 0.75 | 0.76 |
| DM14843 | ilvA219 ridA gcvP | 0.17 | 0.17 | 0.48 | 0.68 | 0.64 |
| DM14840 | ilvA219 gcvB | 0.67 | 0.69 | 0.72 | 0.75 | 0.75 |
| DM14841 | ilvA219 ridA gcvB | 0.15 | 0.68 | 0.57 | 0.70 | 0.62 |
The data represent the average cell densities of cultures grown in triplicate based on OD650 readings taken after 15 h of growth. Standard deviations were less than 0.03 in all cases. Growth was assessed in minimal glucose medium alone (Min-glu) and in minimal glucose medium containing the following concentrations of the indicated supplements: 0.67 mM glycine (Gly), 0.1 μM thiamine (Thi), 0.1 mM pantothenate (Pan), and 1 mM methionine (Met).
GCV is dispensable for growth of an ilvA219 ridA strain when downstream products of one-carbon metabolism are provided directly.
The necessity of GCV to allow glycine to stimulate growth of the ilvA219 ridA strain suggested that products of one-carbon metabolism were limiting. In S. enterica, one-carbon units are required for DNA methylation reactions and the biosynthesis of purines, thiamine, methionine, pantothenate, and histidine (24). The impact of these metabolites on growth of the ilvA219 ridA strain in minimal glucose medium was compared to that of glycine (Table 3). Thiamine (0.1 μM), pantothenate (0.1 mM), and methionine (1 mM) independently restored growth of the ilvA219 ridA strain to the same final cell density as the glycine-supplemented cultures (Table 3). Furthermore, the disruption of gcvP in the ilvA219 ridA gcvP strain (DM14843) had little impact on the growth suppression afforded by these metabolites, suggesting that the need for an intact GCV was bypassed by directly feeding downstream products requiring one-carbon units instead of glycine. None of the products of one-carbon metabolism (i.e., thiamine, pantothenate, or methionine) stimulated growth of a ridA (DM14828) strain inhibited by exogenous serine, cysteine, or diaminopropionate (data not shown), perhaps reflecting altered growth requirements caused by different levels of 2AA damage inside the cell. Histidine had no impact on growth of any of the strains grown under the conditions described for Table 3 (data not shown). The addition of adenine as a purine source further diminished the already low level of growth displayed by the ilvA219 ridA strain in minimal glucose medium (data not shown), possibly through feedback inhibition of the shared pathway for thiamine production. These data suggest that in an ivA219 ridA background, 2AA damage to GlyA limits the production of one-carbon units required for the biosynthesis of thiamine, pantothenate, or methionine. The ability of these metabolites to suppress the growth defect independently may reflect a one-carbon sparing phenomenon. Alternatively, because coenzyme A and methionine are known to stimulate production of thiamine in S. enterica (25), these data may indicate that a thiamine limitation is the key to the growth defect observed for the ilvA219 ridA strain.
Conclusions.
The aim of this study was to determine why strains of S. enterica lacking RidA were unable to grow when challenged with 2AA. The data support a model in which the growth-limiting defect of ridA strains is 2AA inactivation of GlyA, which causes a disruption in glycine and one-carbon production. Exogenous glycine suppressed the growth defects of a ridA strain caused by cysteine, diaminopropionate, or pyruvate only when gcvP was intact, suggesting that glycine was used to generate additional one-carbon units via GCV. Similarly, when 2AA was produced from endogenous serine by an isoleucine-insensitive variant of IlvA, glycine suppression required a functional GCV.
Surprisingly, the ability of glycine to allow growth of a ridA strain in the presence of exogenous serine required the regulatory GcvB small RNA but not a functional GCV. The concentration of exogenous serine (5 mM) supplied to cultures was 20 to 50 times greater than that of cysteine (0.25 mM) or diaminopropionate (0.1 mM) and approximately 70 times greater than levels of endogenous serine (based on intracellular serine concentrations determined for E. coli) (26). Therefore, it is reasonable to predict that exogenous serine resulted in a large pool of endogenous 2AA, leading to broad inactivation of target enzymes, including but not limited to GlyA. In this scenario, gcvP-dependent one-carbon production alone was insufficient to overcome the metabolic needs of the cell despite glycine supplementation. We propose that ridA strains grown in the presence of exogenous serine experience substantial damage to targets aside from GlyA, eliciting one or more nutritional requirements in addition to glycine. The addition of glycine serves to rewire the metabolic network by eliciting a GcvB-dependent regulatory response to overcome 2AA stress caused by exogenous serine. The GcvB regulon consists of more than 50 targets in S. enterica (22), and it is likely that growth in the presence of high 2AA stress required the cumulative effect of genes in the regulon. Significantly, the lack of GcvB would result in derepression of the major serine transporter (SstT) and indirectly (via Lrp) reduce the expression of sdaA (27). SdaA (EC 4.3.1.17) is an Fe-S cluster serine dehydratase that degrades serine without releasing the reactive 2AA species (G. Y. Chen and D. M. Downs, unpublished data; G. Grant, personal communication). Thus, the lack of GcvB could contribute to enhanced serine uptake and similarly prevent efficient degradation of serine by a nontoxic route (i.e., SdaA), thereby exacerbating 2AA stress to the point where one-carbon production by GCV would be insufficient to restore growth to a ridA strain.
In the absence of exogenous serine, glycine was required to suppress alternative ridA growth defects by stimulating one-carbon production, catalyzed by GCV. The phenotypic data presented herein are consistent with biochemical data presented by Flynn et al. that showed that GlyA activity was reduced (20% of wild-type activity) in ridA strains grown in minimal glucose medium, leading to a downstream deficiency in one-carbon units (4). In the absence of an extreme 2AA burden (i.e., exogenous serine), the stimulation of one-carbon production by GCV and glycine was sufficient to restore growth in the presence of exogenous cysteine, diaminopropionate, or pyruvate or when 2AA was produced from endogenous serine by the isoleucine-insensitive variant of IlvA, suggesting that a one-carbon limitation is the primary defect encountered by ridA strains under the growth conditions tested. Because thiamine, methionine, and pantothenate individually stimulated growth of an ilvA219 ridA strain, consistent with one-carbon units being spared, it remains to be seen if one of these metabolites alone is primarily limiting for growth.
Insights gained from our work in S. enterica inform our understanding of the RidA paradigm in other organisms. Serine hydroxymethyltransferase (SHMT; EC 2.1.2.1) is one of only two PLP-dependent enzymes encoded in the genomes of all free-living organisms, the other being aspartate aminotransferase (EC 2.6.1.1) (28). Future work is needed to address whether SHMT inactivation by 2AA elicits similar glycine or one-carbon requirements in other organisms. It is feasible that alternative metabolic configurations may preclude SHMT inactivation from drastically impacting growth given that alternative strategies of glycine production from threonine have been described (29, 30). In such cases, different targets of 2AA inactivation (e.g., aspartate aminotransferase) may have a greater impact on organism fitness. Nonetheless, several organisms produce one or more isozymes of SHMT, and in some cases, SHMT is predicted to colocalize with RidA homologs throughout the cell. For example, the RidA homolog (At3g20390) from Arabidopsis thaliana is targeted to the plastid (6), while SHMT (AtSHMT3) activity can also be detected there (31). In Saccharomyces cerevisiae, the SHM1 isozyme of SHMT and the yeast RidA homolog (Yil051cp) both localize to the mitochondria (32, 33). In some mammalian cell lines, RidA-like UK114 is produced in the cytoplasm (34), while SHMT isozymes are found in both the cytosol and mitochondria (35). Developing a better understanding of the link between RidA homologs and SHMT may lead to better chemotherapeutics to combat diseases such as cancer, as tumor cells rely heavily on flux through SHMT to meet the rampant demand for glycine and one-carbon units necessary for rapid cell growth and division (36–38). Interestingly, UK114 serves as a cytotoxic tumor antigen in some forms of cancer (39), perhaps reflecting a reliance by cancer cells on UK114 to preserve efficient SHMT activity. Future investigation of connections between Rid enzymes and glycine and one-carbon metabolism are warranted given the importance of this node of metabolism throughout life.
REFERENCES
- 1.Niehaus TD, Gerdes S, Hodge-Hanson K, Zhukov A, Cooper AJL, ElBadawi-Sidhu M, Fiehn O, Downs DM, Hanson AD. 2015. Genomic and experimental evidence for multiple metabolic functions in the RidA/YjgF/YER057c/UK114 (Rid) protein family. BMC Genomics 16:382. doi: 10.1186/s12864-015-1584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecht JA, Schmitz GE, Downs DM. 2013. RidA proteins prevent metabolic damage inflicted by PLP-dependent dehydratases in all domains of life. mBio 4:e00033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn JM, Downs DM. 2013. In the absence of RidA, endogenous 2-aminoacrylate inactivates alanine racemases by modifying the pyridoxal 5′-phosphate cofactor. J Bacteriol 195:3603–3609. doi: 10.1128/JB.00463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn JM, Christopherson MR, Downs DM. 2013. Decreased coenzyme A levels in ridA mutant strains of Salmonella enterica result from inactivated serine hydroxymethyltransferase: ridA mutants are deficient in one carbon metabolism. Mol Microbiol 89:751–759. doi: 10.1111/mmi.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrecht JA, Flynn JM, Downs DM. 2012. Conserved YjgF protein family deaminates reactive enamine/imine intermediates of pyridoxal 5′-phosphate (PLP)-dependent enzyme reactions. J Biol Chem 287:3454–3461. doi: 10.1074/jbc.M111.304477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niehaus TD, Nguyen TND, Gidda SK, ElBadawi-Sidhu M, Lambrecht JA, McCarty DR, Downs DM, Cooper AJL, Fiehn O, Mullen RT, Hanson AD. 2014. Arabidopsis and Maize RidA proteins preempt reactive enamine/imine damage to branched-chain amino acid biosynthesis in plastids. Plant Cell 7:3010–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enos-Berlage JL, Langendorf MJ, Downs DM. 1998. Complex metabolic phenotypes caused by a mutation in yjgF, encoding a member of the highly conserved YER057c/YjgF family of proteins. J Bacteriol 180:6519–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst DC, Lambrecht JA, Schomer RA, Downs DM. 2014. Endogenous synthesis of 2-aminoacrylate contributes to cysteine sensitivity in Salmonella enterica. J Bacteriol 196:3335–3342. doi: 10.1128/JB.01960-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz G, Downs DM. 2004. Reduced transaminase B (IlvE) activity caused by the lack of yjgF is dependent on the status of threonine deaminase (IlvA) in Salmonella enterica serovar Typhimurium. J Bacteriol 186:803–810. doi: 10.1128/JB.186.3.803-810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopherson MR, Schmit GE, Downs DM. 2008. YjgF is required for isoleucine biosynthesis when Salmonella enterica is grown on pyruvate medium. J Bacteriol 190:3057–3062. doi: 10.1128/JB.01700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stauffer G. 19 May 2004, posting date Regulation of serine, glycine, and one-carbon biosynthesis. EcoSal Plus 2004. doi: 10.1128/ecosalplus.3.6.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Way JC, Davis MA, Morisato D, Roberts DE, Kleckner N. 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32:369–379. doi: 10.1016/0378-1119(84)90012-X. [DOI] [PubMed] [Google Scholar]
- 13.Vogel HJ, Bonner DM. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 14.Balch WE, Wolfe RS. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmieger H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet 119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 16.Chan RK, Botstein D, Watanabe T, Ogata Y. 1972. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology 50:883–898. [DOI] [PubMed] [Google Scholar]
- 17.Downs DM, Petersen L. 1994. apbA, a new genetic locus involved in thiamine biosynthesis in Salmonella typhimurium. J Bacteriol 176:4858–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen L, Enos-Berlage J, Downs DM. 1996. Genetic analysis of metabolic crosstalk and its impact on thiamine synthesis in Salmonella typhimurium. Genetics 143:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christopherson MR, Lambrecht JA, Downs D, Downs DM. 2012. Suppressor analyses identify threonine as a modulator of ridA mutant phenotypes in Salmonella enterica. PLoS One 7:e43082. doi: 10.1371/journal.pone.0043082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modi SR, Camacho DM, Kohanski MA, Walker GC, Collins JJ. 2011. Functional characterization of bacterial sRNAs using a network biology approach. Proc Natl Acad Sci U S A 108:15522–15527. doi: 10.1073/pnas.1104318108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma CM, Papenfort K, Pernitzsch SR, Mollenkopf H-J, Hinton JCD, Vogel J. 2011. Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol Microbiol 81:1144–1165. doi: 10.1111/j.1365-2958.2011.07751.x. [DOI] [PubMed] [Google Scholar]
- 23.LaRossa RA, Van Dyk TK, Smulski DR. 1987. Toxic accumulation of alpha-ketobutyrate caused by inhibition of the branched-chain amino acid biosynthetic enzyme acetolactate synthase in Salmonella typhimurium. J Bacteriol 169:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews RG. 1996. One-carbon metabolism, p 506–513. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coliandSalmonella typhimurium: cellular and molecular biology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 25.Palmer LD, Dougherty MJ, Downs DM. 2012. Analysis of ThiC variants in the context of the metabolic network of Salmonella enterica. J Bacteriol 194:6088–6095. doi: 10.1128/JB.01361-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. 2009. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol 5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tani TH, Khodursky A, Blumenthal RM, Brown PO, Matthews RG. 2002. Adaptation to famine: a family of stationary-phase genes revealed by microarray analysis. Proc Natl Acad Sci U S A 99:13471–13476. doi: 10.1073/pnas.212510999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Percudani R, Peracchi A. 2003. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4:850–854. doi: 10.1038/sj.embor.embor914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman EB, Kapoor V, Potter R. 1976. Role of l-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol 126:1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa H, Gomi T, Fujioka M. 2000. Serine hydroxymethyltransferase and threonine aldolase: are they identical? Int J Biochem Cell Biol 32:289–301. doi: 10.1016/S1357-2725(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Sun K, Sandoval FJ, Santiago K, Roje S. 2010. One-carbon metabolism in plants: characterization of a plastid serine hydroxymethyltransferase. Biochem J 430:97–105. doi: 10.1042/BJ20100566. [DOI] [PubMed] [Google Scholar]
- 32.Kastanos EK, Woldman YY, Appling DR. 1997. Role of mitochondrial and cytoplasmic serine hydroxymethyltransferase isozymes in de novo purine synthesis in Saccharomyces cerevisiae. Biochemistry 36:14956–14964. doi: 10.1021/bi971610n. [DOI] [PubMed] [Google Scholar]
- 33.Kim J-M, Yoshikawa H, Shirahige K. 2001. A member of the YER057c/yjgf/Uk114 family links isoleucine biosynthesis and intact mitochondria maintenance in Saccharomyces cerevisiae. Genes Cells 6:507–517. doi: 10.1046/j.1365-2443.2001.00443.x. [DOI] [PubMed] [Google Scholar]
- 34.Chong CL, Huang SF, Hu CP, Chen YL, Chou HY, Chau GY, Shew JY, Tsai YL, Chen CT, Chang C, Chen ML. 2008. Decreased expression of UK114 is related to the differentiation status of human hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 17:535–542. doi: 10.1158/1055-9965.EPI-07-0506. [DOI] [PubMed] [Google Scholar]
- 35.Tibbetts AS, Appling DR. 2010. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr 30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 36.Locasale JW. 2013. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. 2014. Serine and glycine metabolism in cancer. Trends Biochem Sci 39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labuschagne CF, van den Broek NJF, Mackay GM, Vousden KH, Maddocks ODK. 2014. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep 7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 39.Bartorelli A, Bussolati B, Millesimo M, Gugiotta P, Bussolati G. 1996. Antibody-dependent cytotoxic activity of human cancer cells expressing UK 114 membrane antigen. Int J Oncol 8:543–548. [DOI] [PubMed] [Google Scholar]