Abstract
Background:
The medical management of hemorrhoids should include an integrated approach. This integrated approach can be achieved by polyherbal formulations containing anti-inflammatory, styptics, analgesics, and laxative effect which reduce inflammation, pain, and bleeding, and increase gastro-intestinal motility and soften stools. One such polyherbal kit is “Arshkeyt™, a 7 day kit,” which consists of oral tablets and powder along with topical cream.
Objective:
Efficacy and safety of Arshkeyt™, a 7 day kit, a marketed polyherbal formulation was evaluated in comparison with conventional therapy practiced in surgery outpatient departments.
Materials and Methods:
Patients (n = 90) with hemorrhoids were randomly allocated to receive either Arshkeyt™ or standard therapy (combination of oral Isabgul powder and 2% lidocaine gel) for 14 days. Assessment on the basis of rectal symptoms and proctoscopic examination was done on day 0, 7, and 14 to derive a “composite score” which ranged from 0 to 25 by a blinded evaluator. The primary endpoint was number of patients achieving composite score 0 at the end of therapy (day 14). Inter-group analysis was done using Chi-square test.
Results:
On day 14, the composite score of 0 was achieved in 15 patients of Arshkeyt™ group versus 6 patients receiving standard therapy. The symptoms and signs which showed significant improvement in Arshkeyt™ group compared to standard treatment group were the tenesmus (visual analog score) score (P = 0.047), anal sphincter spasm (P = 0.0495) and a decrease in the grade of hemorrhoids (P = 0.0205) on day 14. Arshkeyt™ was also more beneficial in case of bleeding hemorrhoids as compared to nonbleeding hemorrhoids (P < 0.05). The incidence of adverse drug reactions in both groups was comparable and no patient required any treatment for the same.
Conclusion:
“Arshkeyt™, a 7 day kit,” was effective in the treatment of hemorrhoids and had a good safety profile.
Keywords: Anal sphincter spasm, isabgul, rectal bleeding, tenesmus, 2% lidocaine gel
INTRODUCTION
An engorgement of the venous tissue in the anal region is called as a hemorrhoid. The prevalence of hemorrhoids in India according to recent surveys is around 40 million.[1] If left untreated, hemorrhoids can worsen and become complicated over time. The factors which aggravate the symptoms of hemorrhoids are chronic constipation and low fiber intake in diet. Some of the most commonly encountered symptoms associated with hemorrhoids are perianal pain, itching, tenesmus, bleeding, while defecation and mucous in stool.[2] Bleeding per rectum being the most common and troublesome symptom for the patients.
The management of hemorrhoids varies depending on the severity of the signs and symptoms with which the patient presents. The medical management includes symptomatic management of the local pain symptoms using local anesthetics, topical analgesics, and corticosteroid creams. It also includes oral supplementation with fibers and laxatives and certain dietary modifications. The surgical management includes nonoperative outpatient procedures such as sclerotherapy, cryotherapy, and rubber band ligation. While the surgical options include open, closed, or stapled hemorrhoidectomy.[2]
Thus medical management of hemorrhoids should include an integrated approach wherein drugs are needed to tackle the inflammation, pain, control bleeding, tighten the smooth muscle vasculature, and treat the aggravating factors. This integrated approach can be achieved by polyherbal formulations containing anti-inflammatory, styptics, analgesics, and laxative effect which reduce inflammation, pain, and bleeding, and increase gastrointestinal motility and soften stools. One such polyherbal marketed proprietary kit is “Arshkeyt™, a 7 day kit,” which consists of oral tablets and powder along with topical cream. The key ingredients of “Arshkeyt™ Tablet” are Arshoghni vati,[3] Triphala guggul,[4] Amorphallus campanulatus (Surana),[5] and Melia azedarach (Mahanimba)[6] which have styptic, analgesic, anti-inflammatory, antimicrobial, and wound healing properties.
Of these ingredients, Triphala has been shown to possess analgesic, anti-inflammatory, and wound healing properties.[7] A. campanulatus, described as “Deepan” in Ayurvedic texts, has been used traditionally for the treatment of piles and shown to possess analgesic,[8] anti-bacterial, and anti-oxidant properties.[9] M. azedarach has been reported to contain triterpenoids, steroids, limonoids, flavonoid glycosides, and simple phenolics, which have been found to possess analgesic, anti-inflammatory, and anti-microbial activities.[10] The other constituents of the tablet-Cynodon dactylon known to be “Dahashamak” (subsides burning sensation) and “Raktasravhara” (stops bleeding) in traditional medicine has been shown to possess wound healing properties.[11] Mimosa pudica, described as “Sheet virya” (coolant) and “Raktasravhara” is also used to arrest bleeding and promote wound healing.[12] The flowers and leaves of Mesua ferrea, known as “Nagakesara,” is used in bleeding piles and has been shown to possess anti-inflammatory, anti-ulcer, and anti-microbial properties.[13] Bauhinia variegata described as “Vranaropak” (helps in wound healing) in Ayurvedic texts, has been reported to have anti-microbial property.[14] Pongamia pinnata described as “Arshahar” in Ayurveda, has been traditionally used in the treatment of piles and pharmacological studies have revealed antimicrobial and anti-inflammatory activities.[15]
“Arshkeyt™ Powder” in addition to Plantago ovata (Isabgul)[6] contains Ipomoea turpethum (Trivrit),[6] Glycyrrhiza glabra (Yashti),[16] and Cassia fistula (Aragvadha)[16] as its main ingredients. P. ovata is a high fiber bulk forming laxative shown to reduce the number of bleeding episodes in case of internal bleeding hemorrhoids.[17] The Cassia species described as “shoolhara” (pain killer) and “Stramsan” (mild laxative) has been shown to possess the same in addition to anti-inflammatory and anti-microbial properties.[18] G. glabra has been shown to help in wound healing.[11] Foeniculum vulgare, commonly known as Fennel, has been proven to have anti-inflammatory, anti-microbial, and laxative property.[19]
The chief ingredients of “Arshkeyt™ Cream” contain oil P. pinnata (Karanja Tel)[6] and oil Azadirachta indica (Nimba Tel),[6] both of which are described as “Arshohar” (used in treatment of piles) in Ayurveda and has been shown to exhibit broad spectrum of anti-microbial and anti-inflammatory properties.[15] The other ingredients of the “Arshkeyt™ Cream” Vateria indica, Berberisa ristata, C. dactylon, Curcuma longa, and M. pudica traditionally described as ‘Vranaropak’ (stops bleeding) has been shown to help in wound healing.[11] Yashada bhasma, traditional herbomineral preparation mainly comprising of zinc and Shuddha tankana, borax are used traditionally for local application on wounds as a disinfectant, has been shown to improves the binding power of the cells of skin, soft tissues, improves cell migration and cell regeneration, and hastens wound healing.[20] Hemidesmus indicus and Vitex negundo described as “Shothhara” (subsides swelling) and “Shoolhara” (pain killer) in Ayurvedic medicine has exhibited analgesic, anti-inflammatory, and anti-microbial activity.[21,22]
Although “Arshkeyt™, a 7 day kit” has been in the market for 1½ years, its efficacy and safety remains to be investigated in a scientific clinical study. Therefore, the present study was conducted to validate the efficacy and safety of “Arshkeyt™, a 7 day kit” in comparison with the “standard treatment” that is, combination of oral Isabgul powder and 2% lidocaine gel in treatment of hemorrhoids. As Arshkeyt™ contains Arshoghni vati, Triphala guggul, A. campanulatus, M. azedarach, C. dactylon, M. pudica, and M. ferrea which have hemostyptic, anti-inflammatory properties hence in addition its efficacy was also compared with standard treatment in a subset of patients presenting with bleeding hemorrhoids.
MATERIALS AND METHODS
The trial was conducted as a single center, prospective, randomized, controlled, parallel group, and assessor-blind study in patients of hemorrhoids. It was a pilot study. Study was conducted at Surgery outpatient Department (OPD) in collaboration with Department of Pharmacology of our institute. Following Ethics Committee approval (EC/PH-35/2011), recruitment was done over a period of 16 months (10/09/2012-26/12/2013).
Patients of either gender, aged ≥18 years, with hemorrhoids (Grades 1, 2 or 3) confirmed by proctoscopy, visiting the surgical OPD was enrolled in the study. Patients with Grade 4/prolapsed hemorrhoids, pregnant and lactating women, patients currently using other anti-hemorrhoidal drugs or having taken any herbal medications in the last 30 days or planning to undergo any surgical procedure for hemorrhoids were excluded from the study. Patients with major hepatic, renal, or cardiovascular ailments were also excluded from the study.
Patients who provided a written informed consent and satisfying the selection criteria were enrolled in the study. At the baseline visit (day 0 - screening and randomization visit) patients were evaluated and scored by an independent surgeon (evaluator blinded to the treatment patient will receive) for the following symptoms: Tenesmus (visual analog score) [VAS] of 0–10 with 0 = No pain and 10 = severe pain, bleeding per rectum (with 0 = absent and 1 = present), pruritus (0= absent and 1 = present), mucous in stools (0 = absent and 1 = present), stool consistency (0–2, with 0 = soft, 1= firm, 2 = hard), and stool frequency (0 = regular, 1= irregular). On day 0 proctoscopic examination the following signs were noted: Proctitis (0= absent and 1= present), anal sphincter spasm (0 = absent, 1= mild, 2= moderate, 3 = severe spasm), ease of reduction (0= easily reduced, 1 = reduced with difficulty, 2= irreducible), and the degree of hemorrhoids (0 = absent, 1 = first degree, 2 = second degree, 3 = third degree). A composite score was calculated for all patients which included the addition of scores for all the rectal symptoms and signs of hemorrhoids and it ranged from minimum score of 0 to maximum score of 25 (higher score indicative of severe disease). According to the computer-generated randomization code the patients were randomized to receive the test product or comparator therapy for 14 days.
Both the test product and comparator for the study were supplied by Solumiks Herbaceuticals Limited, Mumbai. The “test drugs” were in the form of “Arshkeyt™, a 7 day kit,” consisting of three formulations. Its components were as follows:
“Arshkeyt™ Tablet” (seven strips of six tablets each) containing Arshoghni vati, Triphala guggul, A. campanulatus (Surana), M. azedarach (Mahanimba), C. dactylon (Durva), M. pudica (Lajjalu), M. ferrea (Nagakesara), B. variegata (Kanchanara), and P. pinnata (Karanja)
“Arshkeyt™ Powder: (seven sachets) in addition to P. ovata (Isabgul) contained I. turpethum (Trivrit), Cassia angustifolia (Svarnapatri), G. glabra (Yashti) and C. fistula (Aragvadha), and F. vulgare (Mishreya)
“Arshkeyt™ cream” (one lamitube with an applicator) contained oil of P. pinnata (Karanja Tel), oil of A. indica (Nimba Tel), V. indica (Sarja), processed borax (Shuddha tankana), processed zinc (Yashada bhasma), and extract of Berberi saristata (Rasanjana) processed in a decoction of C. dactylon (Durva), C. longa (Haridra), H. indicus (Shvetasariva), M. pudica (Lajjalu), Triphala (Terminalia chebula, Emblica officinalis, and Terminalia bellirica), and V. negundo (Nirgundi).
The patients had to take Arshkeyt™ tablets in a dose of two tablets thrice a day while the Arshkeyt™ powder (4 g) had to be taken once daily at night mixed with a glass (200 ml) of water. The Arshkeyt™ cream had to be applied before and after defecation. The comparator “Standard Kit” consisted of a tube containing 2% lidocaine gel and seven sachets containing Isabgul powder (6 g). The contents of the sachets were Isabgul husk (3 g), Senna extract (0.1 g), and excipients (q.s.). Each sachet had to be taken at night with its contents mixed with a glass of warm water and the lidocaine gel applied before and after defecation. Both test and standard group drug products were dispensed for 7 days on day 0 and day 7; thus all patients received the test and standard formulation for a total of 14 days.
Patients visited the surgical OPD on day 7 and day 14 for follow-up and were evaluated and scored for rectal symptoms and signs similar to the baseline visit by the same blinded evaluator. The composite score was calculated for each patient at each visit. Number of patients achieving a composite score of 0 (indicative of the absence of all rectal symptoms and signs) at the end of day 14 was the primary efficacy end-point. In addition, other efficacy variables included total number of patients with a given particular rectal symptom/sign at day 7 and day 14. All the patients who achieved Grade 0 hemorrhoids by day 14 were called on day 21 for evaluation of recurrence of hemorrhoids or bleeding per rectum (i.e., 7 days later after stopping the drug treatment).
Safety evaluation was done by recording adverse events and severe adverse events based on history and physical examination on day 7 and 14.
Statistical plan
The study being a pilot study, no formal sample size calculation was done. Considering the number of hemorrhoids patients coming to surgery OPD of study center (average 7–10 patients per month), we arbitrarily selected 90 patients to complete the trial within 1-year. Randomization schedule was generated by computer generated random code system so that each group contains an equal number of patients. All the data were tabulated using Microsoft Excel. The statistical analysis was done using statistics software GraphPad InStat 3.1. Values were expressed as a mean ± standard deviation. For inter-group analysis, Chi-square and Mann–Whitney test were applied while for intra-group data analysis Wilcoxon Rank sum test and McNemar test were applied. P < 0.05 was considered significant. Analysis was done using per protocol analysis. In addition, subgroup analysis was done in patients presenting with bleeding piles in both the group and their composite scores were compared using Mann–Whitney test.
RESULTS
Total number of patients recruited in the study were 90, of which 45 patients received “Arshkeyt™, a 7 day kit” and 45 patients received “standard treatment.” Total number of patients completing the study as per protocol were 40 (5 patients lost to follow-up) in the standard treatment group, while 41 (4 patients lost to follow-up) patients from the Arshkeyt™ treatment group. Figure 1 describes the flow of the study participants.
Figure 1.
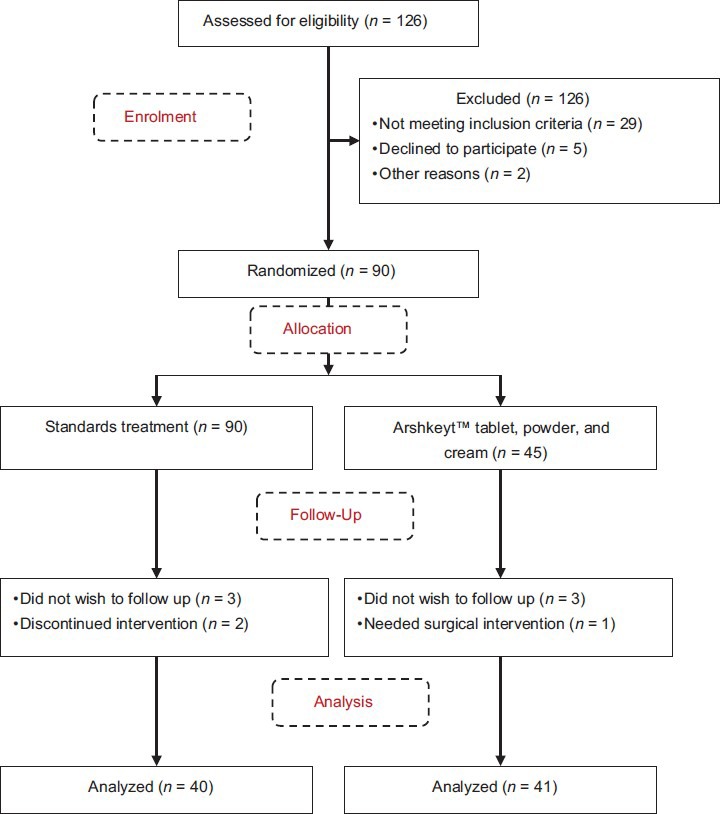
Flow diagram of study
The average age of patients belonging to the standard treatment group was 39.13 ± 10.33 years, while that of patients belonging to Arshkeyt™ group was 42.42 ± 11.27 years (P > 0.05). The number of male and female patients belonging to each group was comparable, so was the grade-wise distribution in both the groups (P > 0.05).
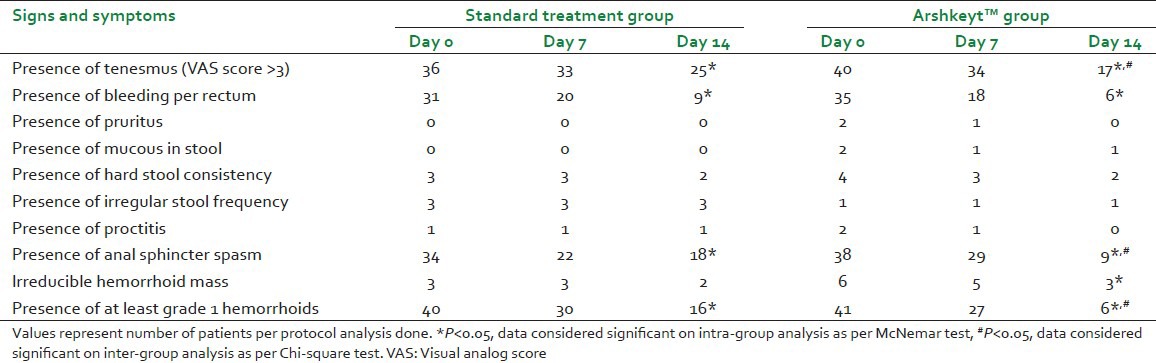
Within group analysis (i.e., both in standard therapy and Arshkeyt™) revealed statistical improvement in tenesmus, bleeding per rectum, anal sphincter spasm, and degree of hemorrhoids by day 14 (number of patients presenting with this symptom/sign decreased by day 14 in comparison to day 0). While other symptoms and signs such as the presence of mucous in stool, hard stool consistency, irregular stool frequency, proctitis, and irreducible hemorrhoid mass were seen only in few patients and were comparable in both the groups. On inter-group analysis the symptoms and signs which showed significant improvement in Arshkeyt™ group compared to standard treatment group were the tenesmus (P = 0.047), anal sphincter spasm (P = 0.0495), and a presence of at least Grade 1 hemorrhoid (P = 0.0205) on day 14 [Table 1].
Table 1.
Comparison of patients of hemorrhoids in Arshkeyt™ and standard treatment groups
Within group analysis revealed significant decrease in composite scores from 12.98 ± 5.88 (day 0) to 5.53 ± 2.87 (day 14) in the standard group (P < 0.0001), and while in Arshkeyt group the composite score decreased from 14.20 ± 5.81 (day 0) to 4.31 ± 2.92 (day 14); P < 0.0001. However, the day 14 composite score of patients on standard therapy and Arshkeyt therapy were comparable. The mean composite scores on baseline, day 7, and day 14 have been described in Figure 2.
Figure 2.
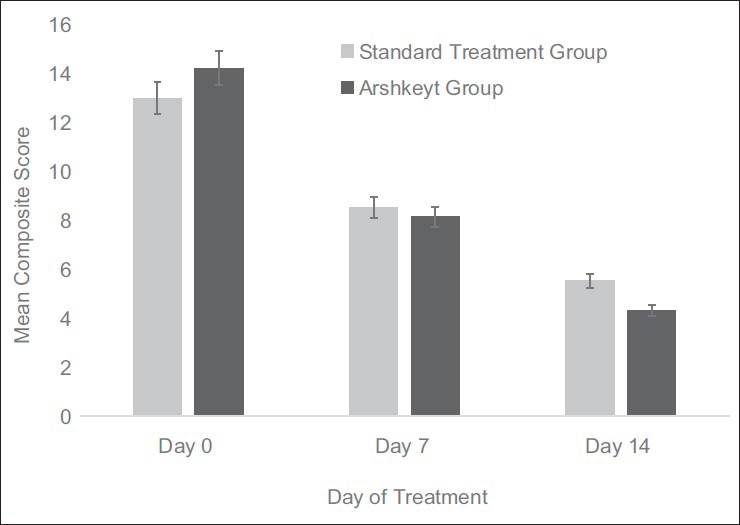
Comparison of mean composite scores on days 0, 7, and 14 in both treatment groups
On day 14, the number of patients achieving composite score 0 in Arshkeyt™ group was 15 which was significantly more in contrast to 6 patients from standard treatment group who achieved composite score 0 (P < 0.05).
Similarly, day 14 analysis also revealed that 24 patients (16 patients out of 40 had at least Grade 1 hemorrhoid) from standard treatment group had achieved Grade 0 hemorrhoids, while 35 patients (6 patients out of 41 had at least Grade 1 hemorrhoid) from Arshkeyt™ group achieved Grade 0 hemorrhoid (P < 0.05). These patients were followed up on day 21 for recurrence of hemorrhoids and rectal bleeding. No patient belonging to Arshkeyt™ group showed recurrence in bleeding (P = 0.0161) as well as hemorrhoids on day 21 while 7 patients in the standard treatment group showed a recurrence in bleeding and 2 patients showed a recurrence of hemorrhoids [Table 2].
Table 2.
Comparison of patients with bleeding and nonbleeding hemorrhoids receiving standard and Arshkeyt™ therapy
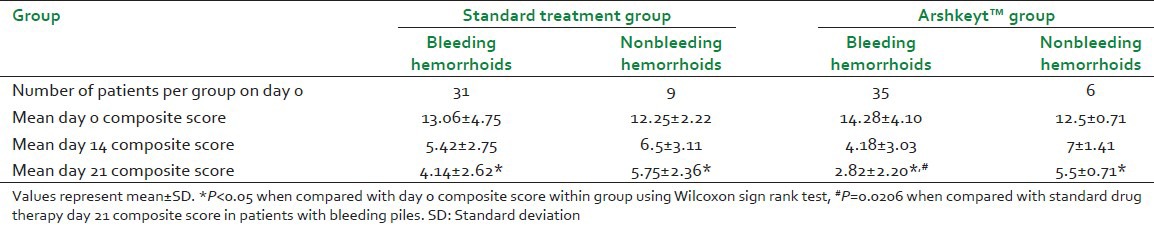
In addition, during subgroup analysis, the patients in both groups were stratified according to the presence of rectal bleeding as bleeding piles and absence as nonbleeding piles. On day 0, 31/40 patients presented with bleeding piles in standard group and 35/41 presented with bleeding piles in Arshkeyt™ group. On intra-group analysis, the composite scores on day 14 and 21 were significantly lower compared to day 0 in both, the standard treatment group and Arshkeyt group in patients with bleeding and nonbleeding hemorrhoids. However, there was no difference between composite scores of patients with bleeding and nonbleeding hemorrhoids within the group or between groups on day 14. But on day 21, the composite score of patients with bleeding hemorrhoids given Arshkeyt™ was significantly lower compared to those given standard treatment (P < 0.05). Such difference was not found with respect to scores for nonbleeding hemorrhoids [Table 2].
Out of total 81 patients who completed the study, 5 patients from the standard treatment group and 3 patients from Arshkeyt™ group experienced adverse events. Patients from standard treatment group experienced local itching and tingling (n = 2), bloating and diarrhea (n = 2), and 1 patient experienced both. Two patients from Arshkeyt™ group experienced bloating and diarrhea, while 1 patient experienced bloating and diarrhea along with dyspepsia.
DISCUSSION
Isabgul (P. ovata) and Senna (C. angustifolia) are commonly used for treating hemorrhoids, but they mainly relieve only constipation and do not adequately reduce other symptoms and signs associated with hemorrhoids. Various other Ayurvedic drugs are claimed to have a beneficial effect in patients of hemorrhoids, but such claims are not validated through systematic randomized clinical trials. Arshkeyt™ though a proprietary formulation did contain multiple ingredients which collectively possessed hemostyptic, anti-inflammatory, analgesic, wound healing properties as Ayurveda textbooks. In addition, it also had Isabgul to relieve constipation the most common contributing factor for hemorrhoids.
In the current study, the number of patients achieving composite score 0 in Arshkeyt™ group on day 14 was 15 which was significantly more in contrast to 6 patients from standard treatment group who achieved composite score as 0. This statistical difference could be attributed to all the additional ingredients present in Arshkeyt™ like Arshoghni vati, Triphala guggul,[4] A. campanulatus,[5] M. azedarach,[6] C. dactylon, M. pudica,[11] M. ferrea, B. variegate, and P. pinnata[15] which conferred hemostyptic, anti-inflammatory, analgesic, wound healing properties which lacked in the standard treatment.
In addition, the effect of Arshkeyt™ product on improving individual symptoms was also statistically significant. The number of patients presenting with near absence of tenesmus (reduction of VAS <3 on day 14), was significantly higher in Arshkeyt™ treated group as compared to Standard treatment (P < 0.05). The benefit can be attributed especially to Arshkeyt™ Tablets containing Triphala guggul,[4] A. campanulatus,[5] and M. azedarach,[6] which possess anti-inflammatory and analgesic effect.
Similarly, the number of patients showing improvement in grade of anal sphincter spasm was significantly higher in Arshkeyt™ treatment group as compared to standard treatment (P < 0.05). This can be attributed to Arshkeyt™ Cream containing oil of P. pinnata (Karanja Tel)[6] and oil of A. indica (Nimba Tel)[6] have potential to relax the smooth muscles, reduce pruritus, and has a soothing effect.
When patients were evaluated for a presence of at least Grade 1 hemorrhoids on day 14, significantly more number of patients were (16/40) in standard treatment in contrast to 6/41 patients on Arshkeyt™ treatment had presence of Grade 1 hemorrhoids (P < 0.05). This can be explained as “Arshkeyt™ Tablets” contains ingredients like Arshoghni vati[3] and Triphala guggul[4] which have potential to shrink the hemorrhoid mass. “Arshkeyt™ Powder” in addition to P. ovata (Isabgul) contains I. turpethum,[6] C. angustifolia,[6] G. glabra, and C. fistula to regulate smooth bowel movement, thus relieving the straining factor in hemorrhoids.
When the patients were evaluated on day 21 for recurrence of bleeding, significantly more number of patients showed recurrence when standard treatment was stopped at day 14, while none of the patients treated with Arshkeyt™ showed recurrence of bleeding (P < 0.05). This could be explained due to presence of ingredients like M. pudica, used to arrest bleeding and promote wound healing;[12] M. ferrea used as anti-inflammatory, anti-ulcer, and anti-microbial agent;[13] and P. pinnata used as antimicrobial and anti-inflammatory agents in hemorrhoids.[15] It also appears from the results that Arshkeyt™ is more beneficial in case of bleeding hemorrhoids as compared to nonbleeding hemorrhoids (P < 0.05). This can be especially attributed to “Arshkeyt™ Tablet” containing Arshoghni vati, Triphala guggul,[4] A. campanulatus,[5] M. azedarach,[6] C. dactylon,[6] M. pudica (Lajjalu),[6] and M. ferrea which have hemostyptic, anti-inflammatory, analgesic, anti-microbial, and wound healing property.
It is worth noting that patients showed carry forward effects as well as maintenance of efficacy when treated with of “Arshkeyt™, a 7 day kit” as compared to “standard treatment.” These benefits effectively and significantly prevented recurrence of overall symptoms of hemorrhoids, bleeding, spasm, and pain.
The adverse events in both the groups were comparable and none of them required any treatment for adverse events. None of the complaints were unexpected, and there were no Serious Adverse Events (SAEs) reported throughout the study duration in both the groups. The drug appears to be safe.
A 6 weeks study conducted by Mehra et al. using Triphala guggul in hemorrhoid patients showed positive results. Triphala showed good wound healing properties as well as confirmed its anti-inflammatory effects. The study showed relief in 55 off its 129 patients suffering from hemorrhoids,[23] that is, success rate was 42% which was similar to our study wherein 15 out of 41 patients receiving Arshkeyt™ product had achieved composite score of 0 and thus complete relief by day 14 demonstrating a 37% success rate.
Two studies conducted by Perez-Miranda et al. and Kecmanovic et al. assessing the use of P. ovata (Isabgul) in the treatment of bleeding hemorrhoids showed good results in cessation of bleeding in hemorrhoids patients. These results were obtained in a short span of less than a week of using this supplement.[17,24]
The above-mentioned studies demonstrate the use of herbal preparations in the treatment of hemorrhoids. It also corroborates our findings that the use of “Arshkeyt™, a 7 day kit,” in treating hemorrhoids was more effective than the use of the “standard treatment” option of lidocaine gel and Isabgul powder. This unique combination of a polyherbal oral and local medication provides an integral approach for the management of hemorrhoids.
CONCLUSION
Our study confirms that the use of polyherbal formulation “Arshkeyt™, a 7 day kit” was more effective in the treatment of hemorrhoids than Isabgul powder and 2% lidocaine gel. It was also a safe treatment option. This kit also showed carry forward effect by preventing the recurrence of bleeding in these patients. However, a long-term follow-up study in a large population is required to confirm these findings.
Acknowledgments
The authors thank the doctors, residents and OPD staff from the Department of General Surgery of Seth G.S. Medical College and KEM Hospital, Parel, Mumbai and the study participants for their assistance, support and cooperation in the conduct of this study. We express our sincere thanks to Dr. A.K. Gwalani (Professor and Head), Dr. A.N. Dalvi (Professor) and Dr. Aparna Deshpande (Professor) from the Department of General Surgery for facilitating the conduct of the study.
Financial support and sponsorship
Support for the conduct of the trial is Solumiks Herbaceuticals Limited.
Conflicts of interest
All authors have no competing interest in the trial of drug product.
REFERENCES
- 1. [Last accessed on 2014 Jul 10]. Available from: http://www.rightdiagnosis.com/h/hemorrhoids/stats-country.htm .
- 2.Williams NS. The anus and anal canal. In: Russell RC, Williams NS, Bulstrod CJ, editors. Bailey and Love's Short Practice of Surgery. 24th ed. London: Edward Arnold; 2004. pp. 1255–62. [Google Scholar]
- 3.Ayurveda Pharmacopia of India, Part II, Government of India. Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homeopathy. 2000. Vati and Gutika: 10:3 [Google Scholar]
- 4.Tripathi B. Sharangdhar Samhita of Sharangadharacharya; Madhyam Khanda. 3rd ed. Varanasi: Chaukhamba Sanskrit Sansthan; 1998. Vatakkalpana; p. 206. [Google Scholar]
- 5.Mishra B, Vaishya R. Bhavprakash Nighantu of Bhavamishra. 9thed. Varanasi: Chaukhamba Sanskrit Sansthan; 1999. Shaka varga; p. 693. [Google Scholar]
- 6.Mishra B, Vaishya R. Bhavprakash Nighantu of Bhavamishra. 9th ed. Varanasi: Chaukhamba Sanskrit Sansthan; 1999. Guduchyadi varga; pp. 331–467. [Google Scholar]
- 7.Baliga MS, Meera S, Mathai B, Rai MP, Pawar V, Palatty PL. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: A review. Chin J Integr Med. 2012;18:946–54. doi: 10.1007/s11655-012-1299-x. [DOI] [PubMed] [Google Scholar]
- 8.Shilpi JA, Ray PK, Sarder MM, Uddin SJ. Analgesic activity of Amorphophallus campanulatus tuber. Fitoterapia. 2005;76:367–9. doi: 10.1016/j.fitote.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Ansil PN, Wills PJ, Varun R, Latha MS. Cytotoxic and apoptotic activities of Amorphophallus campanulatus (Roxb.) Bl. tuber extracts against human colon carcinoma cell line HCT-15. Saudi J Biol Sci. 2014;21:524–31. doi: 10.1016/j.sjbs.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang WM, Liu JQ, Peng XR, Wan LS, Zhang ZR, Li ZR, et al. Triterpenoids and sterols from the leaves and twigs of Melia azedarach. Nat Prod Bioprospect. 2014;4:157–62. doi: 10.1007/s13659-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas TK, Mukherjee B. Plant medicines of Indian origin for wound healing activity: A review. Int J Low Extrem Wounds. 2003;2:25–39. doi: 10.1177/1534734603002001006. [DOI] [PubMed] [Google Scholar]
- 12.Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124:311–5. doi: 10.1016/j.jep.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Mazumder R, Dastidar SG, Basu SP, Mazumder A, Kumar S. Emergence of mesua ferrea linn. Leaf extract as a potent bactericide. Anc Sci Life. 2003;22:160–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra A, Sharma AK, Kumar S, Saxena AK, Pandey AK. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed Res Int 2013. 2013 doi: 10.1155/2013/915436. 915436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Muqarrabun LM, Ahmat N, Ruzaina SA, Ismail NH, Sahidin I. Medicinal uses, phytochemistry and pharmacology of Pongamia pinnata (L.) Pierre: A review. J Ethnopharmacol. 2013;150:395–420. doi: 10.1016/j.jep.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Mishra B, Vaishya R. 9th ed. Varanasi: Chaukhamba Sanskrit Sansthan; 1999. Haritakyadi varga. In: Bhavprakash Nighantu of Bhavamishra; pp. 1–122. [Google Scholar]
- 17.Perez-Miranda M, Gomez-Cedenilla A, León-Colombo T, Pajares J, Mate-Jimenez J. Effect of fiber supplements on internal bleeding hemorrhoids. Hepatogastroenterology. 1996;43:1504–7. [PubMed] [Google Scholar]
- 18.Bhalodia NR, Shukla VJ. Antibacterial and antifungal activities from leaf extracts of Cassia fistula L.: An ethnomedicinal plant. J Adv Pharm Technol Res. 2011;2:104–9. doi: 10.4103/2231-4040.82956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimi R, Ardekani MR. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin J Integr Med. 2013;19:73–9. doi: 10.1007/s11655-013-1327-0. [DOI] [PubMed] [Google Scholar]
- 20.Datta HS, Mitra SK, Patwardhan B. Wound healing activity of topical application forms based on ayurveda. Evid Based Complement Alternat Med. 2011;2011:134378. doi: 10.1093/ecam/nep015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng CJ, Li HQ, Ren SC, Xu CL, Rahman K, Qin LP, et al. Phytochemical and Pharmacological Profile of Vitex negundo. Phytother Res. 2015;29:633–47. doi: 10.1002/ptr.5303. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Bisht SS. The bioactive and therapeutic potential of Hemidesmus indicus R. Br. (Indian Sarsaparilla) root. Phytother Res. 2013;27:791–801. doi: 10.1002/ptr.4788. [DOI] [PubMed] [Google Scholar]
- 23.Mehra R, Makhija R, Vyas N. A clinical study on the role of Ksara Vasti and Triphala Guggulu in Raktarsha (Bleeding piles) Ayu. 2011;32:192–5. doi: 10.4103/0974-8520.92572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kecmanović D, Pavlov M, Ceranić M, Sepetkovski A, Kovacevi P, Stamenković A. Phlebodia (diosmine): A role in the management of bleeding non-prolapsed hemorrhoids. Acta Chir Iugosl (Sweden) 2005;52:115–6. doi: 10.2298/aci0501115k. [DOI] [PubMed] [Google Scholar]


