Abstract
Background:
The prevalence of mental depression has increased in recent years, and has become a serious health problem in most countries of the world, including India. Due to the high cost of antidepressant synthetic drugs and their accompanying side effects, the discovery of safer antidepressant herbal remedies is on the rise. Moringa oleifera (MO) (drumstick) has been used in traditional folk medicine, and in Ayurveda, it is considered as a valuable remedy for treating nervous system disorders as well as memory enhancing agent.
Objective:
The present study was designed to evaluate the acute and chronic behavioral and antidepressant effects of alcoholic extracts of MO leaves in standardized mouse models of depression.
Materials and Methods:
Alcoholic extracts of MO (MOE) leaves were prepared, and phytoconstituents were determined using appropriate chemical analytical methods. Following preliminary dose-finding toxicity studies, the biological activity of MOE was tested in Swiss albino mice. Animals were divided into six groups: Groups 1 and 2 served as vehicle control and fluoxetine (20 mg/kg) standard control, respectively. Groups 3 and 4 served as treatment groups and were orally administered ethanolic MOE at doses of 100 mg/kg and 200 mg/kg, respectively. Groups 5 and 6, respectively, received combination doses of MOE 100 mg/kg + 10 mg fluoxetine, and MOE 200 mg/kg + 10 mg/kg fluoxetine. Following acute and 14 days chronic treatments, all animals were tested using behavioral models of depression, such as forced swim test (FST), tail suspension test (TST), and locomotor activity test (LAT).
Results:
Significant changes in all tested activities (FST, TST, LAT) of chronically dosed mice were observed, especially in animals given simultaneously combined doses of 200 mg/kg/day MOE + 10 mg/kg/day fluoxetine for 14 days. The antidepressant effect of MOE may have been invoked through the noradrenergic-serotonergic neurotransmission pathway, which is the hallmark of selective serotonin reuptake inhibitors (SSRI) class of drugs.
Conclusion:
The results obtained in this study suggest that combined administration of MOE with low doses of fluoxetine or other SSRI drugs seems to have promising potential.
Keywords: Fluoxetine, forced swim test in mice, locomotor activity, Moringa oleifera, tail suspension test
INTRODUCTION
Depression has become a wide spread mental disorder worldwide. According to global depression statistics, it is estimated that about 121 million people suffer from mental disorders. Currently, 12.3% of world population suffer from depression, and it is predicted that by 2020, the number may rise to 15%.[1] Ravindran and da Silva[2] have reported that about 7% of the Indian population suffers from mood and anxiety disorders.
A constant state of depression results from continuous stress or central nervous system (CNS) neurochemical imbalance.[3] Oxidative stress (OS) is considered to be a major factor in the causation of anxiety and depression, and these CNS disorders appear to result from the imbalance in oxidation-reduction reactions (redox-state). It is characterized by the reduced ability of the antioxidant defense mechanism of the CNS to efficiently remove the excess of oxygen-derived free radicals, which are known to produce detrimental effects in the CNS. A growing body of evidence suggests that OS causes imbalance between the production of oxygen-derived free radicals and the antioxidant ability of neuronal cells and tissues, consequently contributing to the neuropathology and psychiatric diseases, including mild and major depression.[4]
The antipsychotic drugs available for treating depression include tricyclic antidepressants, selective monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRI), and specific serotonin-noradrenaline reuptake inhibitors. Although most of these medications are safe and efficacious, yet in some patients, prolonged usage may cause a variety of minor side effects such as dry mouth, mydriasis, constipation, sleepiness, fatigue, restlessness, and headaches.[5] In some patients, prolonged therapy with fluoxetine may induce serious side effects, such as suicidal tendencies. Thus, the alternative therapies are needed which could be efficacious but produce lesser side effects.
Fluoxetine (prozac, sarafem) belongs to the SSRI class of antipsychotic drugs. It is recommended for the treatment of major depressive disorders in both children and adults, bulimia nervosa, and panic disorder as well as premenstrual dysphoric disorder. In adult humans, an initial oral daily dose of fluoxetine is 20 mg, and in some patients, maximum daily dose may be escalated to 80 mg after several weeks if sufficient clinical improvement does not occur.
The new approach for the treatment of depression with plant-derived remedies may prove to more economical in developing countries. Many plant products are used for the treatment of depression, like Hypericum perforatum Linn., Areca catechu Linn., Bacopa monnieri, Centella asiatica, Ginkgo biloba, etc.[6] Some herbal drugs as well as nutraceuticals containing flavonoids and polyphenolic compounds have proven to be similar in efficacy to that of synthetic antidepressants. The most common example is St. John Wort (H. perforatum) that is used for treating minor and mild depression. A limited number of clinical trials have shown that St. John Wort has antidepressant activity similar to SSRI drugs like fluoxetine and paroxetine.[7] However, St. John's Wort is a well-known inducer of drug metabolizing enzymes (cytochrome P-450) and drug transporters (P-glycoprotein) in the gastrointestinal tract and modifies the disposition and pharmacokinetics of orally administered drugs.[8,9]
The present studies were designed to assess the antidepressant activity of Moringa oleifera (MO) in the mouse model. MO is commonly known as drumstick and belongs to the family Moringacae. In India, it is used as food and for medicinal purposes. It is widely grown in different parts of the world. MO possesses antioxidant, antidiabetic, antibacterial, antifungal, anti-inflammatory, antiulcer, and cholesterol lowering properties. Chemical analyses show that MO contains Vitamins A, B, C, flavonoids, oleic, palmitic and stearic acid, saponins, glycoside, gum, protein, calcium, magnesium, potassium, and iron.[10,11] The leaves have shown to possess strong antioxidant and anti-inflammatory properties, and thus could be used in the treatment of depression caused by OS or inflammation.[11]
This investigation was done in mice dosed with ethanolic extract of MO (MOE) either alone or in combination with fluoxetine in various experiments of depression, and MO-induced effects were compared with reference antidepressant drug, fluoxetine.
MATERIALS AND METHODS
Preparation of plant extract
Leaves of MO were purchased from a local market in Mumbai. They were identified and authenticated by the Department of Life Sciences, Ramanarain Ruia College, Mumbai. The voucher specimen was deposited at their herbarium. The dried leaves were powdered, and their different physicochemical parameters such as extractive values, ash values, foreign organic matter, and loss on drying were recorded. Detailed macroscopic and microscopic studies were conducted by standard procedures according to the Indian Pharmacopoeia 2010. Following crude characterization, the leaves were defatted using petroleum ether. The defatted leaves were then placed for extraction by heating at 65–70°C and refluxed with 95% ethanol for 6–12 h. The mixture was filtered by suction filtration, and the filtrate was concentrated by rotary evaporator. The yield of extraction was about 12% (w/w).
Animal husbandry of experimental animals
All studies were conducted after obtaining prior approval from the institutional animal ethical committee (Approval no: CPCSEA/SPTM/P-09/2012). Swiss albino mice of either sex (25–30 g) were used in all experiments. Mice were purchased from Bharat serums and vaccines limited, Thane, Maharashtra. Animals were housed and maintained in the Animal House of School of Pharmacy and Technology, NMIMS (temperature 25°C ± 2°C; relative humidity 75% ±5%). During the experiments, animals were provided with standard feed and drinking water ad libitum in polycarbonate feeder bottles with a stainless steel nipple.
Acute toxicity study
The dose-finding acute toxicity study of ethanolic MOE was carried out in mice using the OECD Guidelines 423.[8] Mice were randomly divided into different treatment groups with three animals in each group. The extract was orally administered at a dose of 2000 mg/kg/day and distilled water as vehicle. A 2% w/v solution of the extract was prepared in distilled water with 1% w/v sodium carboxymethyl cellulose. The prepared MO formulation was administered orally by gavage in a dosing volume of 1 ml. The control group received distilled water in a dosing volume of 1 ml. Following the administration of once daily single dose of MO, the overt general behavior of mice was continuously monitored for 1 h after dosing, and then periodically during the first 24 h with special attention given during the first 4 h, and daily thereafter, for a total of 14 days. Changes in normal activity of mice and their body weights were monitored and the time at which signs of toxicity or death appeared was recorded.
Drugs and preparations
Fluoxetine was used as a standard drug while MO ethanolic extract was used as a test agent. A 2% w/v solution of the standard drug fluoxetine as well as the ethanolic MOE was prepared by dissolving them in 1.0% w/v sodium carboxymethyl cellulose just before administration. These solutions were administered orally by gavage in a dosing volume not exceeding 1 ml in both cases.
Treatment plan for acute dosing
The groups assigned for acute dose study were as follows:
Group 1: Control group (distilled water)
Group 2: Fluoxetine alone (20 mg/kg)
Group 3: MOE-1 (100 mg/kg)
Group 4: MOE-2 (200 mg/kg)
Group 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg)
Group 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg)
In the acute treatment study, a single dose was administered 30 min prior to testing. For the chronic treatment study, a single dose was administered daily for 14 days. In groups receiving the combination of standard drug and extract, the respective doses were administered concomitantly. In the chronic dose study, the behaviors of all groups were assessed for antidepressant activity 30 min after the last treatment dose on the 14th day. Different standardized depression models were used for behavioral tests to evaluate the antidepressant activity, such as forced swim test (FST), tail suspension test (TST), and locomotor activity test.
Forced swim test
FST was carried out according to the method described by Porsolt et al.[12]
Tail suspension test
TST was carried out according to the method described by Porsolt et al. with slight modifications.[12,13]
Locomotor activity test
LAT was performed to assess CNS inhibitory or stimulatory activity of the extract.[14]
Statistical analysis
The differences between experimental and control groups were determined using the Graph Pad INSTAT 3.0 (Graphpad Software Inc.) software for windows. Comparisons among different groups were performed by analysis of variance test. Statistically significant differences between control and experimental groups were assessed by Student's t-test. All data are expressed as mean ± standard error of mean. P < 0.05 was considered to be significant.
RESULTS
In acute toxicity studies, 2000 mg/kg dose of MO did not cause any mortality, or overt clinical signs of toxicity observed over 14 days period. Thus, 2000 mg/kg dose was considered as a safe dose of MOE in mice.
Acute study
Forced swim test
The results indicated that after 30 min MOE administration, there was no significant decrease in the immobility time, but both fluoxetine and test substance treated animals showed slight reductions in immobility time compared to the vehicle controls [Figure 1].
Figure 1.
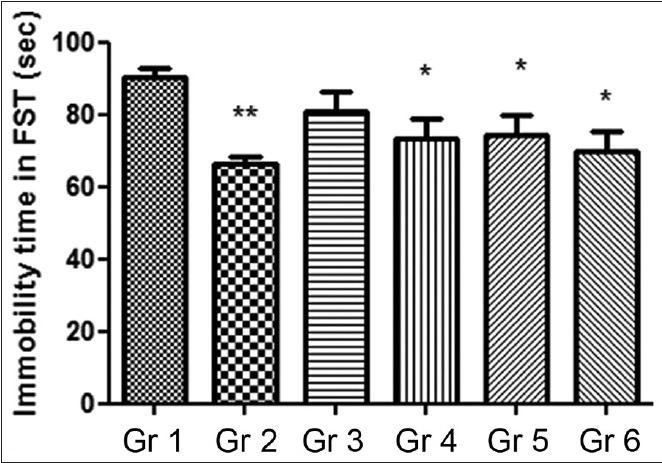
Effect of Moringa oleifera extract on duration of immobility forced swim test. Gr 1: Normal Control, Gr 2: Standard Fluoxetine (20 mg/kg), Gr 3: MOE-1 (100 mg/kg), Gr 4: MOE-2 (200 mg/kg), Gr 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg), Gr 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg). Results are represented as mean ± standard error of mean significantly different at *P < 0.05 and **P < 0.01 compared to vehicle control
Tail suspension test
No decrease in the immobility time was observed after administering 100 mg/kg MOE, whereas the immobility time was markedly shortened (180 ± 13.3 min vs. 277 ± 2.5 min, P < 0.05) in 200 mg/kg MOE treated animals. Significant reductions in the immobility time, ranging from 35% to 46% (P < 0.05) were also noted in Groups 2 and 6 in comparison with the vehicle control [Figure 2].
Figure 2.
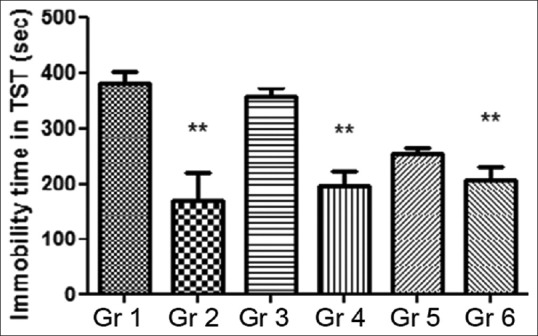
Effect of Moringa oleifera extract on duration of immobility in tail suspension test. Gr 1: Normal Control, Gr 2: Standard Fluoxetine (20 mg/kg), Gr 3: MOE-1 (100 mg/kg), Gr 4: MOE-2 (200 mg/kg), Gr 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg), Gr 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg). Results are represented as mean ± standard error of mean significantly different at *P < 0.05 and **P < 0.01 compared to vehicle control
Locomotor activity test
In order to assess the antidepressant activity of the extract, the locomotor activity of mice was recorded using actophotometer.
Locomotor activity of treated and untreated mice was assessed from their locomotor behavior, and the results obtained were compared with the vehicle controls. Statistically significant increase in locomotor activities (ranging from 28% to 30%, P < 0.05) was found in animals dosed with 200 mg/kg MOE, fluoxetine alone (20 mg/kg), and those administered combination of 200 mg/kg MOE + 10 mg/kg fluoxetine [Figure 3].
Figure 3.
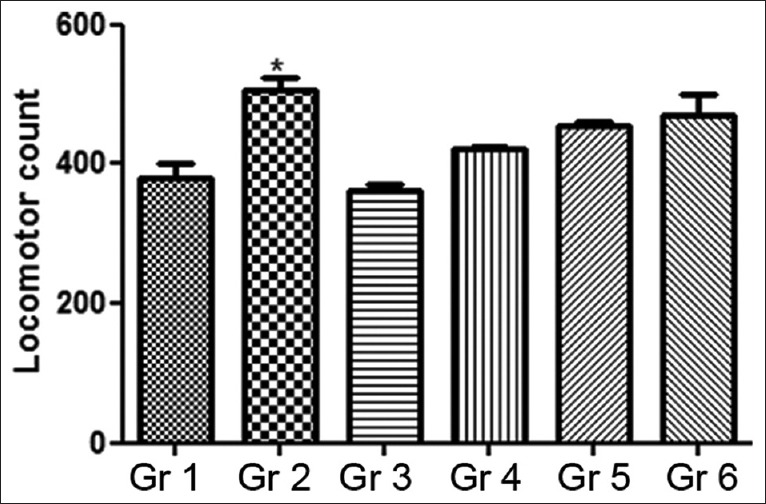
Effect of Moringa oleifera extract on run score in locomotor activity. Gr 1: Normal Control, Gr 2: Standard Fluoxetine (20 mg/kg), Gr 3: MOE-1 (100 mg/kg), Gr 4: MOE-2 (200 mg/kg), Gr 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg), Gr 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg). Results are represented as mean ± standard error of mean significantly different at *P < 0.05 and **P < 0.01 compared to vehicle control
Chronic study findings
Forced swim test
In the subacute/chronic investigation, the animals were treated either with MOE alone or in combination with fluoxetine for 14 consecutive days. Results summarized in Figure 4 show that oral administration of ethanolic MOE at both dose levels and in combinations with fluoxetine caused reductions in FST immobility time in mice. Standard fluoxetine dose of 20 mg/kg displayed a significant decrease in the immobility time. The group treated with the combination of 200 mg/kg MOE + 10 mg/kg fluoxetine dose exhibited a 30% decrease in the immobility time compared to the vehicle control.
Figure 4.
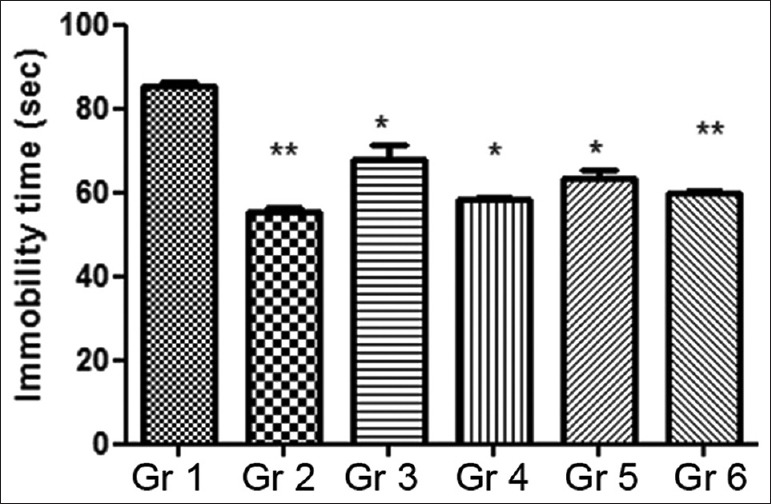
Effect of chronic administration of Moringa oleifera extract on duration of immobility in forced swim test. Gr 1: Normal Control, Gr 2: Standard Fluoxetine (20 mg/kg), Gr 3: MOE-1 (100 mg/kg), Gr 4: MOE-2 (200 mg/kg), Gr 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg), Gr 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg). Results are represented as mean ± standard error of mean significantly different at *P < 0.05 and **P < 0.01 compared to vehicle control
Tail suspension test
Results of the 14 days chronic study revealed that there was an inverse relationship between the dose of the extract and the immobility time, that is, an increase in the MOE dose produced a corresponding reduction in the immobility time in comparison with the control group [Figure 5]. In addition, repeated administration of standard fluoxetine (20 mg/kg/day) showed a profound decrease (100 ± 6.6 min vs. 255 ± 7.6 min, P < 0.01) in the mean immobility period. Similarly, the average immobility time in mice treated with the MOE either alone (200 mg/kg/day) or the combined administration of MOE (200 mg/kg/day) and fluoxetine (10 mg/kg/day) was significantly shortened (P < 0.001) as opposed to vehicle control. The respective groups (Groups 4 and 6) showed approximately 41% and 57% decrease in the immobility period compared to the control.
Figure 5.
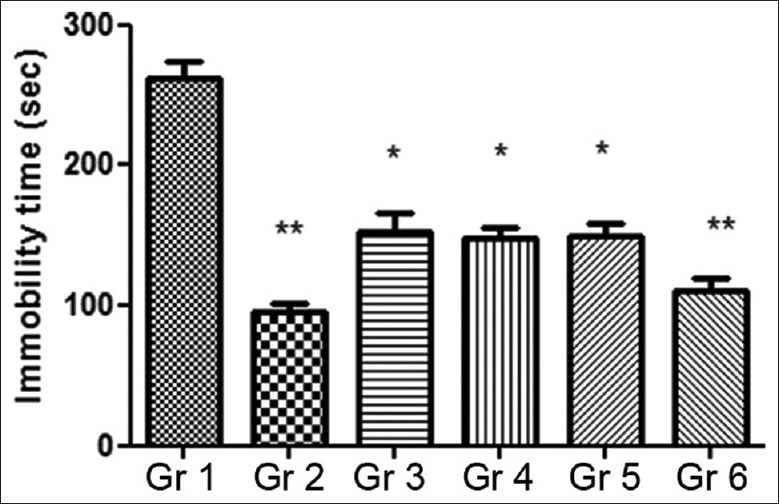
Effect of chronic administration of Moringa oleifera extract on duration of immobility in tail suspension test. Gr 1: Normal Control, Gr 2: Standard Fluoxetine (20 mg/kg), Gr 3: MOE-1 (100 mg/kg), Gr 4: MOE-2 (200 mg/kg), Gr 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg), Gr 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg). Results are represented as mean ± standard error of mean significantly different at *P < 0.05 and **P < 0.01 compared to vehicle control
Overall, 14 days repeated administration of MOE showed a significant decrease in the immobility activity in both FST and TST animal models. The combination dosing of MOE (200 mg/kg/day) and fluoxetine (10 mg/mg/day) resulted in producing more profound (P < 0.01) actions leading to approximately 57% reduction in the immobility time compared to the vehicle control. It appears that in both FST and TST mouse models, the combined administration of MOE and fluoxetine had produced an additive effect on these parameters, but the underlying mechanism of action remains unknown.
Locomotor activity test
Repeated administration of the MOE resulted in enhanced locomotor activity in mice. More prominent effects were seen in groups treated with the combination of MOE at the dose of 200 mg/kg/day and fluoxetine (10 mg/kg/day). As mentioned earlier, these treatment groups exhibited nearly 37% and 41% increase in the locomotor activity compared to the vehicle control. Results are summarized in Figure 6.
Figure 6.
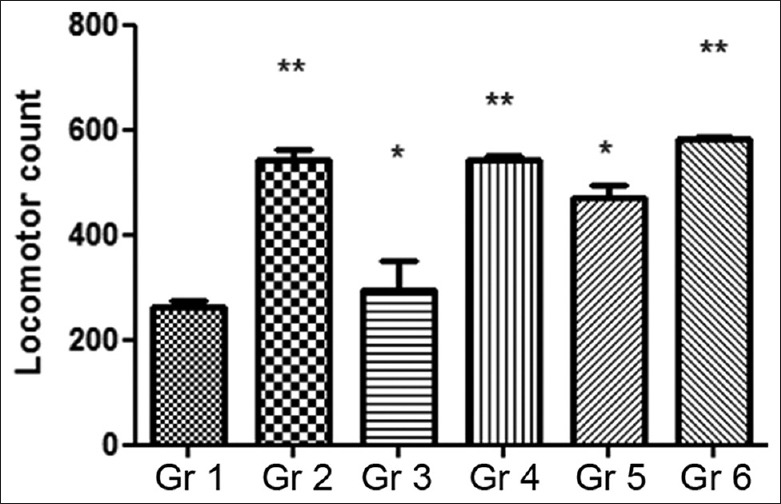
Effect of chronic administration of Moringa oleifera extract on the run score in locomotor activity. Gr 1: Normal Control, Gr 2: Standard Fluoxetine (20 mg/kg), Gr 3: MOE-1 (100 mg/kg), Gr 4: MOE-2 (200 mg/kg), Gr 5: MOE-1 (100 mg/kg) + fluoxetine (10 mg/kg), Gr 6: MOE-2 (200 mg/kg) + fluoxetine (10 mg/kg). Results are represented as mean ± standard error of mean significantly different at *P < 0.05 and **P < 0.01 compared to vehicle control
DISCUSSION
The present study was designed to investigate the antidepressant activity of ethanolic MOE in mice using well-tried-out and standardized behavioral tests of depression. MO is often used as antidiabetic, anti-inflammatory, antimicrobial, and antiulcer remedy.[15] However, its neuropharmacological activity invoking antidepressant-like action is unknown. It is reported that MO has a strong antioxidant and anti-inflammatory activity,[16] thereby suggesting that it could be useful in treating depression caused by OS or inflammation-induced due the neurochemical imbalance in the brain.[17] The antidepressant potential of MOE was assessed in mice exposed to FST, TST, and locomotor activity. The study was divided into two parts, namely acute dose study and subacute or chronic dose study when the animals were repeatedly dosed orally for 14 consecutive days. The purpose was to know the toxicological effects, if any, and antidepressant effects of MO when given as a single dose and when administered in repeated dosing. Combination effects of MO were also assessed with standard antidepressant drug fluoxetine. The rationale of evaluating the combined dosing is, whether or not the antidepressant dose of fluoxetine may be reduced to circumvent its minor and major side effects experienced by patients.
MO contains many constituents such as flavonoids and phenols, which are vital antioxidants.[18] The polyphenolic compounds are purported to exert neuroprotective and anti-inflammatory effects in the CNS.[19] The significant reduction in the immobility time observed in the FST following the acute [Figure 1] and chronic [Figure 4] co-administration of 10 mg/kg fluoxetine + 200 mg/kg MOE suggests the additive interaction of fluoxetine with MOE, and consequently enhancing the antidepressant action of MOE. The potential additive interaction may be through the noradreno-serotonergic pathway as is known for SSRI class of drugs.[20]
There was a significant reduction in the immobility time in the TST groups administered 200 mg/kg/day MOE alone and its combination with 10 mg fluoxetine for 14 days [Figure 5]. The combination produced a more pronounced effect than MOE alone, thereby suggesting an additive interaction between these agents.
This phenomenon also suggested the involvement of noradrenergic and serotonergic pathways in the induction of antidepressant activity.[20]
In order to assess the occurrence of false positive results exhibited in the TST and FST due to CNS stimulant action of MOE, mice were subjected to open field tests.[21] A significant increase in the locomotor activity was noticed in MOE treated animals as compared to the vehicle controls. Similarly, the locomotor activity was also markedly enhanced in animals dosed with a combination of MOE and fluoxetine. The findings suggested a nonspecific antidepressant action of the MOE.[22] These observations implied that the dynamics of the locomotors activity may be due to involvement of CNS stimulatory action.
The preliminary pharmacological screening with acute dosing exhibited the antidepressant activity of MOE, but its antidepressant activity was more enhanced after repeated dosing. In comparison with the acute studies, chronic dose studies displayed a significant antidepressant manifestation in the behavioral patterns when compared to the vehicle controls. This effect was far more significantly pronounced in animals treated with MOE alone at a dose of 200 mg/kg/day as well as in combination with 10 mg/kg/day dose of fluoxetine.
Several investigations suggest that some minor and major depressive disorders can be ameliorated with flavonoids.[23] It has been reported that flavonoids act through their antioxidant mechanism as well as through neurogenesis.[24] Many mental disorders such as Alzheimers disease,[25] epilepsy,[26] schizophrenia,[27] and depressions[28] are also related to impaired neurogenesis. Antioxidants and flavonoids assist in the scavenging of free radicals and counteract the deleterious interaction of free radicals with cell membranes and DNA.[27,28] It is noteworthy that a high level of saponin content in MO may aid in the antidepressant effect.[29] The radical-scavenging ability of MO-derived antioxidants and polyphenolic compounds too may be responsible for its neuroprotective and anti-inflammatory activity.[30]
MO-derived ingredients have been reported to possess anti-inflammatory activity. These ingredients may contribute to the antidepressant activity of MO owing to reduction of stress mediated through the inflammatory pathway.[31] However, the neuroprotective and anti-inflammatory potential of MO-derived antioxidants and flavonoids remains to be verified.
CONCLUSIONS
The results of the present investigation showed the antidepressant activity of ethanolic MOE in mice. The highest activity was observed when 200 mg/kg doses of MOE were administered in combination with 10 mg/kg of fluoxetine, suggesting an additive interaction between these two agents. However, the possibility of additive interactive mechanism remains to be elucidated. The plausible mechanism of action of MO appears to be partially due to the reduction in CNS OS accompanied by its influence on noradrenergic and serotonergic neurotransmission pathways. There could also be an involvement of the SSRI mechanism as is seen in SSRI class of drugs. The combined administration of MOE with low doses of fluoxetine and other SSRI psychotropic drugs seems to have a promising potential for the development of alternative therapies for treating depression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pedersen ME, Szewczyk B, Stachowicz K, Wieronska J, Andersen J, Stafford GI, et al. Effects of South African traditional medicine in animal models for depression. J Ethnopharmacol. 2008;119:542–8. doi: 10.1016/j.jep.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran AV, da Silva TL. Complementary and alternative therapies as add-on to pharmacotherapy for mood and anxiety disorders: A systematic review. J Affect Disord. 2013;150:707–19. doi: 10.1016/j.jad.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Umadevi P, Murugan S, Jennifer S. Evaluation of antidepressant like activity of Cucurbita pepo seed extracts in rats. Int J Curr Pharm Res. 2011;3:108–13. [Google Scholar]
- 4.Yadav R, Kaushik R. A study of phytochemical constituents and pharmacological actions of Trigonella foenum-graecum: A review. Int J Pharm Technol. 2011;3:1022–8. [Google Scholar]
- 5.Rojas P, Serrano-García N, Medina-Campos ON, Pedraza-Chaverri J, Ogren SO, Rojas C. Antidepressant-like effect of a Ginkgo biloba extract (EGb761) in the mouse forced swimming test: Role of oxidative stress. Neurochem Int. 2011;59:628–36. doi: 10.1016/j.neuint.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Jawai T, Gupta R, Siddiquie Z. A review on herbal plants showing antidepressant activity. Int J Pharm Sci Res. 2011;2:3051–60. [Google Scholar]
- 7.Zanoli P. Role of hyperforin in the pharmacological activities of St.John's wort. CNS Drug Rev. 2004;10:203–18. doi: 10.1111/j.1527-3458.2004.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou S, Chan E, Pan SQ, Huang M, Lee EJ. Pharmacokinetic interactions of drugs with St John's wort. J Psychopharmacol. 2004;18:262–76. doi: 10.1177/0269881104042632. [DOI] [PubMed] [Google Scholar]
- 9.Komoroski BJ, Zhang S, Cai H, Hutzler JM, Frye R, Tracy TS, et al. Induction and inhibition of cytochromes P450 by the St.John's wort constituent hyperforin in human hepatocyte cultures. Drug Metab Dispos. 2004;32:512–8. doi: 10.1124/dmd.32.5.512. [DOI] [PubMed] [Google Scholar]
- 10.Leelavinothan P, Magdalena K. Antioxidant activity of the crude extracts of Moringa oleifera and Scoparia dulcis L. leaves. Pol J Food Nutr Sci. 2007;57:203–8. [Google Scholar]
- 11.Bhoomika R, Babita G, Goyal R. Phyto-pharmacology of Moringa Oleifera Lam an overview. Nat Prod Radiance. 2007;6:347–53. [Google Scholar]
- 12.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 13.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: An experimental study. Phytomedicine. 2000;7:463–9. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 15.Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–34. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 16.Kurmi R, Ganeshpurkar A, Bansal D, Agnihotri A, Dubey N. Ethanol extract of Moringa oliefera prevents in vitro glucose induced cataract on isolated goat eye lens. Indian J Ophthalmol. 2014;62:154–7. doi: 10.4103/0301-4738.116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunello N, Mendlewicz J, Kasper S, Leonard B, Montgomery S, Nelson J, et al. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur Neuropsychopharmacol. 2002;12:461–75. doi: 10.1016/s0924-977x(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal D, Kumar Rai P, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123:392–6. doi: 10.1016/j.jep.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Pemminati S, Gopalakrishna H, Shenoy A, Sahu S, Mishra S, Meti V, et al. Antidepressant activity of aqueous extract of fruits emblica offcinalis in mice. Int J Appl Biol Pharm Technol. 2012;1:449–54. [Google Scholar]
- 20.Huang QJ, Jiang H, Hao XL, Minor TR. Brain IL-1 beta was involved in reserpine-induced behavioral depression in rats. Acta Pharmacol Sin. 2004;25:293–6. [PubMed] [Google Scholar]
- 21.Parekar RR, Jadhav KS, Marathe PA, Rege NN. Effect of Saraswatarishta in animal models of behavior despair. J Ayurveda Integr Med. 2014;5:141–7. doi: 10.4103/0975-9476.140469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priyanka B, Sridhar Y, Shankaraiah P. Antidepressant and muscle relaxant activity of Cardiospermum halicacabum Linn. Roots in mice. J Adv Pharm Sci. 2012;2:193–200. [Google Scholar]
- 23.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–26. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, Samhan-Arias AK, Bueno C, Garcia-Martinez V. Neuroprotective actions of flavonoids. Curr Med Chem. 2011;18:1195–212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- 25.Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Porter BE. Neurogenesis and epilepsy in the developing brain. Epilepsia. 2008;49 Suppl 5:50–4. doi: 10.1111/j.1528-1167.2008.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reif A, Schmitt A, Fritzen S, Lesch KP. Neurogenesis and schizophrenia: Dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci. 2007;257:290–9. doi: 10.1007/s00406-007-0733-3. [DOI] [PubMed] [Google Scholar]
- 28.Elder GA, De Gasperi R, Gama Sosa MA. Research update: Neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med. 2006;73:931–40. [PubMed] [Google Scholar]
- 29.Sharma V, Paliwal R. Isolation and characterization of saponins from Moringa oleifera pods. Int J Pharm Pharm Sci. 2013;5:179–83. [Google Scholar]
- 30.Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr. 2009;64:303–11. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- 31.Catena-Dell’Osso M, Bellantuono C, Consoli G, Baroni S, Rotella F, Marazziti D. Inflammatory and neurodegenerative pathways in depression: A new avenue for antidepressant development? Curr Med Chem. 2011;18:245–55. doi: 10.2174/092986711794088353. [DOI] [PubMed] [Google Scholar]


