Abstract
Standardization of herbal drugs is essential to certify their quality and purity. Kshara (alkaline substance) of Apamarga (Achyranthes aspera Linn.) is an important constituent in many Ayurvedic formulations, but its standard manufacturing process (SMP) is not attempted till date. This study is aimed to establish SMP for Apamarga kshara. In pharmaceutical process; generally the sediments of ash obtained at the end of washes will be discarded. However, in the study, we attempted to wash the sediments repeatedly by adding water to extract more Kshara. Apamarga was collected from the local area and authenticated. Kshara was prepared by following standard methods and the preliminary physicochemical profile was developed. It is observed that the ash yields Kshara even in the consecutive washes. First wash yielded 21.23% w/w Kshara, while the second and third washes yielded 9.38% w/w and 4.76% w/w, respectively. Repeated washes yield more Kshara. Hence, it is advocated to wash the ashes repeatedly. As the findings are encouraging, similar experiments can be extended to all other Kshara preparations.
Keywords: Achyranthes aspera, Apamarga, Apamarga kshara, standardization
INTRODUCTION
In the present era, plant-derived products are gaining importance as medicinal products, nutraceuticals and cosmetics.[1,2] Herbal medicines are widely used in health-care in both developed and developing countries. According to an estimate of the World Health Organization, about 80% of the world population still uses herbs and other traditional medicines for their primary health care needs.[3] The use of herbal medicines has increased remarkably in line with the global trend of people returning to natural therapies.[4] Ayurveda utilizes different forms of herbs in therapeutics. Kshara is one among such forms.
Ksharas are alkaline substances obtained from the water soluble ashes of herbal drugs. Several Ksharas have been explained in Ayurveda and Apamarga kshara is one among them. Different opinions exist regarding specifications for the nature of vessels, the proportion of water, time for settling, cloth folding, etc. Regarding method of preparation in Sushruta Samhita,[5] Sharngadhara Samhita,[6] Rasatarangini,[7] Dravyaguna Vigyana[8] and Ayurveda Sara Samgraha[9] were widely described. However, none of the classics has mention about the repetition of washings. Considering this, it has been attempted to subject the ashes for repeated washings and prepare Kshara and develop preliminary physicochemical profile of A. Kshara.
MATERIALS AND METHODS
Collection of Apamarga panchanga
Fresh Apamarga panchanga (whole plant of Achyranthes aspera) was collected during October to November 2012. Authentication of Apamarga was done on the basis of pharmacognostical characters.[10,11]
Vessel for Kshara nirmana
Generally Ksharajala is prepared by using cylindrical earthen, steel or plastic vessel. Collecting supernatant layer of Ksharajala from these vessels is inconvenient and difficult; hence, a new vessel has been designed for this purpose.
Cylindrical steel vessel of 10 L capacity with 23.9 cm of depth and 22.2 cm diameter was taken. After few trials; an outlet was made in the wall of the vessel about 3.2 cm above from the bottom that allows draining of clear liquids leaving the sediment in the container [Figure 1].
Figure 1.
Collection of Apamarga, preparation of Apamarga ash and vessel for Kshara nirmana. (a) A fully matured fresh Apamarga plant. (b) Drying of Apamarga Panchanga under sunlight. (c) Ignited of dried Panchanga in a big iron pan. (d) Completely burnt Panchanga. (e) Collection of prepared ash. (f) Specially designed vessel for Kshara preparation
Preparation of Apamarga kshara
Apamarga kshara has been prepared by following classical methods.[7] Whole process was divided into three phases.
Preparation of ash
Matured Apamarga Panchanga was collected and dried completely in sunlight for 8 days. After removing the physical impurities; dried Panchanga was burnt completely by placing it in a big iron pan. After the self-cooling, white ashes were collected [Figure 1].
Preparation of Ksharajala
One part of ash was collected in a specially designed steel vessel and 4 times of water was added to it. The contents were mashed thoroughly with hands and left undisturbed for 3 h. After that, the clear supernatant liquid layers were collected through the outlet and filtered through three layered cotton cloth. The residual ash was again mashed with 6 L of potable water and kept undisturbed for the next 3h, followed by a collection of the second filtrate. A similar method was followed for the 3rd time to collect third filtrate [Figure 2].
Figure 2.
Preparation of Apamarga Kshara. (a) Measured Apamarga ash. (b) Ash dissolved in water. (c) Filtaration of Ksharajala. (d) Evaporation of Ksharajala. (e) Last stage of Kshara Preparation. (f) Prepared Kshara stored in air tight glass bottle
Preparation of Kshara
All the three filtrates (of Ksharajala) were individually subjected to heat to evaporate the water content and to obtain Kshara, and by following this method; Kshara was prepared in five batches [Figure 2].
Physicochemical parameters
Preliminary physicochemical parameters like loss on drying at 110°C,[12] ash value,[13] acid insoluble ash,[13] pH value,[14] water soluble extractives[12] were carried out at pharmaceutical chemistry laboratory, Institute for Post Graduate Teaching and Research in Ayurveda (IPGT & RA), Jamnagar.
Atomic emission spectroscopy with inductively-coupled plasma
Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) is one of the most common techniques for elemental analysis and useful for standardization as well as to develop an analytical profile. In this technique, all samples of the trial drug were analyzed for ten elements.
Estimation of sodium and potassium ions by flame photometer
Estimation of sodium and potassium ions by flame photometer was carried out at the analytical laboratory.
OBSERVATION AND RESULTS
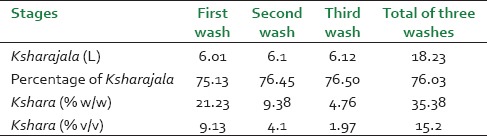
Apamarga panchanga burnt quickly and easily as it was completely dried. Comparatively, seeds took more time to burn. The powder of ash thus obtained was whitish with a characteristic taste. 59.81% Weight loss was observed after drying of Apamarga Panchanga and total 11.90% ash was obtained from dried Panchanga [Table 1]. After addition of water, the contents tasted salty, had characteristic odor and were yellowish in color. Total time required for preparation of Ksharajala was 4 h for a single wash. On an average, 76% Ksharajala was obtained after the wash [Table 2]. On evaporation of moisture on heat, the yellowish liquid was turned to brownish semisolid mass with aggregation and creaking sounds. The first wash yielded an average of 21.23% Kshara, while second and third washes yielded 9.38% and 4.76% respectively [Table 2]. This implies that the sediments at the end of first wash need not to be discarded and should be washed repeatedly. While attempts of further washings were failed to yield significant Kshara, hence after the third wash, the sediments were discarded.
Table 1.
Observations and results obtained during preparation of Apamarga ash
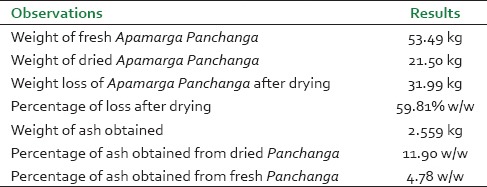
Table 2.
Results of Apamarga Kshara obtained in different washes (average of five batches)
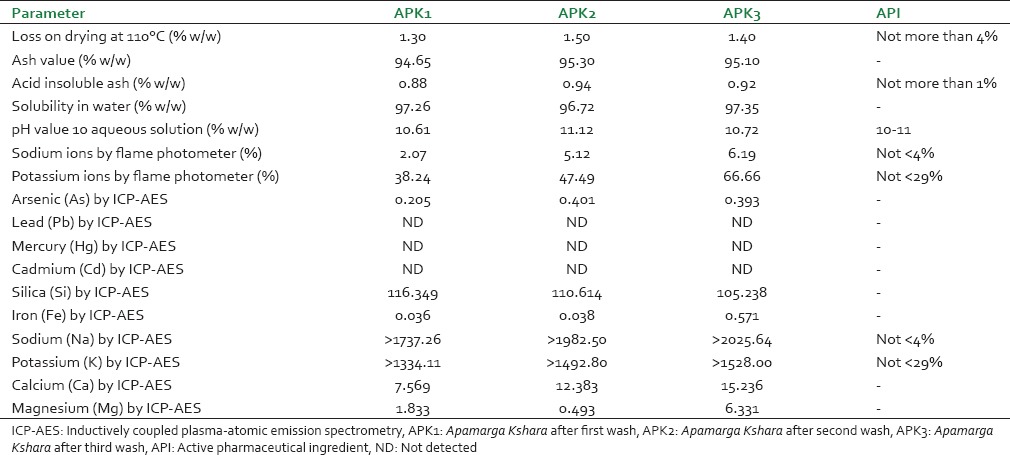
Physicochemical parameters of Apamarga Kshara, standard given in Ayurvedic Pharmacopeia of India,[15] Estimation of sodium and potassium ions[15] and results of AES-ICP were given in Table 3.
Table 3.
Comparative physicochemical parameters and ICP-AES analysis of all samples
DISCUSSION
In the present study, preparation of Aparmarga Kshara has been carried out as per reference of Rasa Tarangini.[7] Dried Panchanga should be made into small pieces for better drying. The plant should be burnt in a vessel to prevent contamination during burning. For proper burning, Panchanga should be added little by little into the fire. Ash and water were taken volumetrically by keeping constant weight for Ksharajala preparation. De-mineralized water was used to avoid any interference of inorganic salts present in tap water. Stainless steel or a suitable nonreactive vessel should be used to prevent possible chemical reactions.
Ash should be rubbed well in water for proper mixing and allowed to settle down for at least 3 h. Ksharajala is to be obtained very cautiously through the outlet without disturbing the vessel. Measures should be taken to avoid the entry of sediments. A clean cotton cloth should be used for this purpose. Acharyas also specified to filter the contents through multi-folded cloth to avoid sediments in the filtrate.
Initially, Ksharajala was yellowish colored clear liquid. Aggregation, vapors and crackling sounds were increased proportionally with temperature. Color was changed from yellowish to brownish gradually as the temperature raised. Kshara was started sticking to the vessel in final stages, and bumping was observed. It was stirred carefully to prevent bumping and sticking at this stage. Finally, white colored Kshara was obtained.
Kshara is considered as a water soluble ash, but all water soluble content cannot be obtained within a single wash; some of them may remain as residue. Considering this; total three washes were done to get maximum yield. Kshara obtained in the first wash was 21.23%, while at the end of the third wash the total Kshara obtained was 35.38% [Table 2]. Thus, current method is better and yielded more in comparison to commercials method used in pharmacy.
Organoleptic characters of APK1 (Apamarga Kshara after first wash), APK2 (Apamarga Kshara after second wash) and APK3 (Apamarga Kshara after third wash) like slimy touch, white color, salty taste, characteristic odor and fine powder appearance. Material absorbs moisture during the storage. In conjunction with a suitable temperature, moisture will lead to the activation of enzymes and given suitable condition to the proliferation of living organism. Hence, moisture contents may affect the quality of the drug. Although the weight loss in the samples is principally due to water, small amount of other volatile materials will also contribute to the weight loss. There was considerable difference observed in a loss on drying in APK1, APK2 and APK3 [Table 3].
Total ash is important and indicates to some extent the amount of care taken in the preparation of the drug. In the determination of total ash value, the carbon must be removed at as low temperature (450°C) as possible because alkali chlorides, which may be volatile at high temperature, would otherwise be lost. The pH value of a given sample expresses the degree of acidity or alkalinity of a sample solution. APK1, APK2, APK3 samples have pH 10.61, 11.12 and 10.72, respectively. The alkalinity of the drug indicates the site of absorption and action of the drug [Table 3].
Analysis of Ayurvedic medicines reveals a great deal about their elemental composition. ICP-AES analysis of all the samples revealed that sodium, potassium, calcium and magnesium are the main constituent of all samples may be due to Kshara present in all samples. Heavy metal like arsenic, lead, mercury and cadmium were not detected or within permissible limits. Silica and iron showed around same results in all sample of trial drugs. Silica was present in all samples. In the present study, it was observed that sodium, potassium, calcium and magnesium constantly increased in APK1, APK2, and APK3 [Table 3].
CONCLUSION
The residues after a first wash should never be discarded; they are to be processed further twice to obtain more Kshara. After three washes, maximum 35.38% yield was obtained. The current observations can be considered as a lead for future studies. The dimensions of a newly designed vessel are specific to the current batch size that will vary according to the quantity of raw material.
Financial support and sponsorship
IPGT and RA, Gujarat Ayurved University, Jamnagar.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bhanu PS, Zafar R, Panwar R. Herbal drug standardization. Indian Pharm. 2005;4:19–22. [Google Scholar]
- 2.Gautam V, Raman RM, Ashish K. Export-Import Bank of India. Mumbai: 2003. Exporting Indian Healthcare (Export potential of Ayurveda and Siddha Products and Services). Road Beyond Boundaries (The Case of Selected Indian Healthcare Systems) pp. 14–54. [Google Scholar]
- 3.World Health Organization, WHO; 2012. [accessed on: 09.06.2015]. WHO. Int. Available from: http://www.who.int/mediacentre/factsheets/2003/fs134/en/Last . [Google Scholar]
- 4.Vaidya AD, Devasagayam TP. Current status of herbal drugs in India: An overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambikadatta S, editor. Ch. 11, Ver. 13. Varanasi: Chaukhambha Sankrita Sansthan; 2010. Susruta Samhita of Maharsi Susruta, Sutra Sthana; Ksharapaka Vidhi; pp. 47–8. [Google Scholar]
- 6.Vidhyasagar PS, editor. 1st ed. Ch. 11, Ver. 101-104. Varanasi: Choukhamba Surbharti Praka; 2006. Sharangadhara Samhita of Sharangadhara, Madhyama Khand; p. 256. [Google Scholar]
- 7.Shastri K, editor. 11th ed. Ver. 59-61. New Delhi: Motilala Banarsidas; 2004. Rasa Tarangini of Sadanada Sharma, Taranga 14; p. 337. [Google Scholar]
- 8.6th ed. Ver. 102-104. Nagpur: Shree Baidhyanath Ayurveda Bhavan Li; 2013. Anonymous. Dravyaguna Vigyana of Yadavji Trikamji Acharya, Uttarardh, Adhyaya 2; p. 61. [Google Scholar]
- 9.Alahabad: Shree Baidhyanath Ayurveda Bhavan Li; 2013. Anonymous. Ayurved Sar Samgraha, Kshara-lavan-satva Prakarana; p. 697. [Google Scholar]
- 10.Jadav HR, Galib R, Prajapati PK, Harisha CR. Pharmacognostical study on flowers and fruits of Apamarga (Achyranthes aspera Linn.) Int J Green Pharm. 2013;7:136–9. [Google Scholar]
- 11.Jadav HR, Galib R, Harisha CR, Prajapati PK. Pharmacognostical evaluation of Apamarga (Achyranthes aspera Linn.) Int J Ayurveda Altern Med. 2014;2:53–9. [Google Scholar]
- 12.1st ed. I. Delhi: Govt of India, Ministry of Health and Family Welfare, Department of AYUSH; 2007. Anonymous. The Ayurvedic Pharmacopoeia of India, Part II; p. 141. [Google Scholar]
- 13.1st ed. I. Delhi: Govt of India, Ministry of Health and Family Welfare, Department of AYUSH; 2007. Anonymous. The Ayurvedic Pharmacopoeia of India, Part II; p. 140. [Google Scholar]
- 14.1st ed. I. Delhi: Govt of India, Ministry of Health and Family Welfare, Department of AYUSH; 2007. Anonymous. The Ayurvedic Pharmacopoeia of India, Part II; p. 191. [Google Scholar]
- 15.1st ed. I. Delhi: Govt of India, Ministry of Health and Family Welfare, Department of AYUSH (Formulations); 2008. Anonymous. The Ayurvedic Pharmacopoeia of India, Part II; pp. 147–8. [Google Scholar]




